Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends
Abstract
1. Introduction
2. Concepts of Electrochemical CO2 Reduction Reaction
Concepts of Electrochemical CO2 Reduction Reaction
3. Product Selectivity Parameters
3.1. Electrocatalyst Materials and Their Morphology
3.1.1. Metals
3.1.2. Metal Oxides
3.1.3. Two-Dimensional Materials
3.1.4. Functional Microorganisms
4. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, J.P.; Magnan, A.; Billé, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting Futures for Ocean and Society from Different Anthropogenic CO2 Emissions Scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, J.B.; Miller, D.J.; Ager, J.W.; Houle, F.A.; Sharp, I.D. The Technical and Energetic Challenges of Separating (Photo) Electrochemical Carbon Dioxide Reduction Products. Joule 2018, 2, 381–420. [Google Scholar] [CrossRef]
- Mostafa, M.M.M.; Shawky, A.; Zaman, S.F.; Narasimharao, K.; Abdel Salam, M.; Alshehri, A.A.; Khdary, N.H.; Al-Faifi, S.; Chowdhury, A.D. Visible-Light-Driven CO2 Reduction into Methanol Utilizing Sol-Gel-Prepared CeO2-Coupled Bi2O3 Nanocomposite Heterojunctions. Catalysts 2022, 12, 1479. [Google Scholar] [CrossRef]
- Gwóźdź, M.; Brzęczek-Szafran, A. Carbon-Based Electrocatalyst Design with Phytic Acid—A Versatile Biomass-Derived Modifier of Functional Materials. Int. J. Mol. Sci. 2022, 23, 11282. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, D.; Papanikolaou, G.; Perathoner, S.; Centi, G.; Lanzafame, P. Comparing Molecular Mechanisms in Solar NH3 Production and Relations with CO2 Reduction. Int. J. Mol. Sci. 2020, 22, 139. [Google Scholar] [CrossRef]
- Khdary, N.H.; Ghanem, M.A. Metal–Organic–Silica Nanocomposites: Copper, Silver Nanoparticles–Ethylenediamine–Silica Gel and Their CO2 Adsorption Behaviour. J. Mater. Chem. 2012, 22, 12032–12038. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Liu, L. Electrochemical Reduction of Carbon Dioxide to Value-Added Products: The Electrocatalyst and Microbial Electrosynthesis. Chem. Rec. 2019, 19, 1272–1282. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An Overview of CO2 Capture Technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The Role of CO2 Capture and Utilization in Mitigating Climate Change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Dong Zhu, D.; Long Liu, J.; Zhang Qiao, S.; Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon Capture and Storage Update. Energy Environ. Sci. 2013, 7, 130–189. [Google Scholar] [CrossRef]
- Campeau, A.; Bishop, K.; Amvrosiadi, N.; Billett, M.F.; Garnett, M.H.; Laudon, H.; Öquist, M.G.; Wallin, M.B. Current Forest Carbon Fixation Fuels Stream CO2 Emissions. Nat. Commun. 2019, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Lamparelli, D.H.; Grimaldi, I.; Martínez-Carrión, A.; Bravo, F.; Kleij, A.W. Supercritical CO2 as an Efficient Medium for Macromolecular Carbonate Synthesis through Ring-Opening Co- and Teroligomerization. ACS Sustain. Chem. Eng. 2023, 11, 8193–8198. [Google Scholar] [CrossRef]
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. New Trends in the Development of Heterogeneous Catalysts for Electrochemical CO2 Reduction. Catal. Today 2016, 270, 19–30. [Google Scholar] [CrossRef]
- Hu, J.; Al-Salihy, A.; Zhang, B.; Li, S.; Xu, P. Mastering the D-Band Center of Iron-Series Metal-Based Electrocatalysts for Enhanced Electrocatalytic Water Splitting. Int. J. Mol. Sci. 2022, 23, 15405. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The Teraton Challenge. A Review of Fixation and Transformation of Carbon Dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Caillé, F.; Chevalier, A.; Loreau, O.; Horkka, K.; Halldin, C.; Schou, M.; Camus, N.; Kessler, P.; Kuhnast, B.; et al. Late-Stage Isotopic Carbon Labeling of Pharmaceutically Relevant Cyclic Ureas Directly from CO2. Angew. Chem. Int. Ed. 2018, 57, 9744–9748. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Razzaq, S.; Wang, Z.; Shagdar, E.; Zhao, J. A Techno-Economic Study of Commercial Electrochemical CO2 Reduction into Diesel Fuel and Formic Acid. J. Electrochem. Sci. Technol. 2022, 13, 148–158. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Pei, Y.; Zhong, H.; Jin, F. A Brief Review of Electrocatalytic Reduction of CO2—Materials, Reaction Conditions, and Devices. Energy Sci. Eng. 2021, 9, 1012–1032. [Google Scholar] [CrossRef]
- Ding, M.; Chen, Z.; Liu, C.; Wang, Y.; Li, C.; Li, X.; Zheng, T.; Jiang, Q.; Xia, C. Electrochemical CO2 Reduction: Progress and Opportunity with Alloying Copper. Mater. Rep. Energy 2023, 3, 100175. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, T.; Tao, H.; Fan, Q.; Han, B. Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials. Chem 2017, 3, 560–587. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Dufek, E.J.; Lister, T.E.; Stone, S.G.; McIlwain, M.E. Operation of a Pressurized System for Continuous Reduction of CO2. J. Electrochem. Soc. 2012, 159, F514–F517. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A Review of High Temperature Co-Electrolysis of H2O and CO2 to Produce Sustainable Fuels Using Solid Oxide Electrolysis Cells (SOECs): Advanced Materials and Technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef] [PubMed]
- Gabardo, C.M.; Seifitokaldani, A.; Edwards, J.P.; Dinh, C.T.; Burdyny, T.; Kibria, M.G.; O’Brien, C.P.; Sargent, E.H.; Sinton, D. Combined High Alkalinity and Pressurization Enable Efficient CO2 Electroreduction to CO. Energy Environ. Sci. 2018, 11, 2531–2539. [Google Scholar] [CrossRef]
- Gong, Q.; Ding, P.; Xu, M.; Zhu, X.; Wang, M.; Deng, J.; Ma, Q.; Han, N.; Zhu, Y.; Lu, J.; et al. Structural Defects on Converted Bismuth Oxide Nanotubes Enable Highly Active Electrocatalysis of Carbon Dioxide Reduction. Nat. Commun. 2019, 10, 2807. [Google Scholar] [CrossRef]
- Lv, J.-J.; Jouny, M.; Luc, W.; Zhu, W.; Zhu, J.-J.; Jiao, F.; Lv, J.; Jouny, M.; Luc, W.; Zhu, W.L.; et al. A Highly Porous Copper Electrocatalyst for Carbon Dioxide Reduction. Adv. Mater. 2018, 30, 1803111. [Google Scholar] [CrossRef]
- Lamaison, S.; Wakerley, D.; Blanchard, J.; Montero, D.; Rousse, G.; Mercier, D.; Marcus, P.; Taverna, D.; Giaume, D.; Mougel, V.; et al. High-Current-Density CO2-to-CO Electroreduction on Ag-Alloyed Zn Dendrites at Elevated Pressure. Joule 2020, 4, 395–406. [Google Scholar] [CrossRef]
- Wang, Z.L.; Choi, J.; Xu, M.; Hao, X.; Zhang, H.; Jiang, Z.; Zuo, M.; Kim, J.; Zhou, W.; Meng, X.; et al. Optimizing Electron Densities of Ni-N-C Complexes by Hybrid Coordination for Efficient Electrocatalytic CO2 Reduction. ChemSusChem 2020, 13, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Khojin, A.; Jhong, H.R.M.; Rosen, B.A.; Zhu, W.; Ma, S.; Kenis, P.J.A.; Masel, R.I. Nanoparticle Silver Catalysts That Show Enhanced Activity for Carbon Dioxide Electrolysis. J. Phys. Chem. C 2013, 117, 1627–1632. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, C.; Yamauchi, Y. Nanostructured Nonprecious Metal Catalysts for Electrochemical Reduction of Carbon Dioxide. Nano Today 2016, 11, 373–391. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Peng, C.; Priest, C.; Mei, Y.; Wu, G.; Zhu, Y.; Peng, C.; Mei, Y.; Yang, X.; et al. Carbon-Supported Single Metal Site Catalysts for Electrochemical CO2 Reduction to CO and Beyond. Small 2021, 17, 2005148. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-Carbon Fuels. Chem. Soc. Rev. 2013, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Bharath, G.; Rambabu, K.; Aubry, C.; Abu Haija, M.; Nadda, A.K.; Ponpandian, N.; Banat, F. Self-Assembled Co3O4 Nanospheres on N-Doped Reduced Graphene Oxide (Co3O4/N-RGO) Bifunctional Electrocatalysts for Cathodic Reduction of CO2 and Anodic Oxidation of Organic Pollutants. ACS Appl. Energy Mater. 2021, 4, 11408–11418. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhu, H.L.; Zheng, Y.Q. Surface Modification of CuO Nanoflake with Co3O4 Nanowire for Oxygen Evolution Reaction and Electrocatalytic Reduction of CO2 in Water to Syngas. Electrochim. Acta 2019, 299, 281–288. [Google Scholar] [CrossRef]
- Zhong, X.; Liang, S.; Yang, T.; Zeng, G.; Zhong, Z.; Deng, H.; Zhang, L.; Sun, X. Sn Dopants with Synergistic Oxygen Vacancies Boost CO2 Electroreduction on CuO Nanosheets to CO at Low Overpotential. ACS Nano 2022, 16, 19210–19219. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, X.; Malkani, A.S.; Yang, X.; Thompson, L.; Jiao, F.; Xu, B. Speciation of Cu Surfaces during the Electrochemical CO Reduction Reaction. J. Am. Chem. Soc. 2020, 142, 9735–9743. [Google Scholar] [CrossRef]
- Geioushy, R.A.; Khaled, M.M.; Alhooshani, K.; Hakeem, A.S.; Rinaldi, A. Graphene/ZnO/Cu2O Electrocatalyst for Selective Conversion of CO2 into n-Propanol. Electrochim. Acta 2017, 245, 456–462. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, Q.; Zhang, J.; Moioli, E.; Zhao, K.; Züttel, A. Electrochemical Reconstruction of ZnO for Selective Reduction of CO2 to CO. Appl. Catal. B 2020, 273, 119060. [Google Scholar] [CrossRef]
- Windle, C.D.; Perutz, R.N. Advances in Molecular Photocatalytic and Electrocatalytic CO2 Reduction. Coord. Chem. Rev. 2012, 256, 2562–2570. [Google Scholar] [CrossRef]
- Liu, J.Y.; Gong, X.Q.; Li, R.; Shi, H.; Cronin, S.B.; Alexandrova, A.N. (Photo) Electrocatalytic CO2 Reduction at the Defective Anatase TiO2 (101) Surface. ACS Catal. 2020, 10, 4048–4058. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, K.; Zhang, L.; Zhang, Y.; Lei, Y.; Sun, X.; Liang, F.; Zhang, K.; Zhang, Y.; Zhang, L.; et al. Recent Development of Electrocatalytic CO2 Reduction Application to Energy Conversion. Small 2021, 17, 2100323. [Google Scholar] [CrossRef] [PubMed]
- Khdary, N.H.; Alayyar, A.S.; Alsarhan, L.M.; Alshihri, S.; Mokhtar, M. Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review. Catalysts 2022, 12, 300. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, L.; Ge, Y.; Huang, Z.; Shi, Z.; Liu, J.; Zhai, W.; Liang, J.; Zhang, H. Wet-Chemical Synthesis of Two-Dimensional Metal Nanomaterials for Electrocatalysis. Natl. Sci. Rev. 2022, 9, nwab142. [Google Scholar] [CrossRef]
- Cai, F.; Hu, X.; Gou, F.; Chen, Y.; Xu, Y.; Qi, C.; Ma, D.K. Ultrathin ZnIn2S4 Nanosheet Arrays Activated by Nitrogen-Doped Carbon for Electrocatalytic CO2 Reduction Reaction toward Ethanol. Appl. Surf. Sci. 2023, 611, 155696. [Google Scholar] [CrossRef]
- Ao, C.; Feng, B.; Qian, S.; Wang, L.; Zhao, W.; Zhai, Y.; Zhang, L. Theoretical Study of Transition Metals Supported on G-C3N4 as Electrochemical Catalysts for CO2 Reduction to CH3OH and CH4. J. CO2 Util. 2020, 36, 116–123. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Lu, S.; Lou, F.; Yu, Z. Recent Progress in Two-Dimensional Materials for Electrocatalytic CO2 Reduction. Catalysts 2022, 12, 228. [Google Scholar] [CrossRef]
- Luan, L.; Ji, X.; Guo, B.; Cai, J.; Dong, W.; Huang, Y.; Zhang, S. Bioelectrocatalysis for CO2 Reduction: Recent Advances and Challenges to Develop a Sustainable System for CO2 Utilization. Biotechnol. Adv. 2023, 63, 108098. [Google Scholar] [CrossRef]
- Singh, S.; Noori, M.T.; Verma, N. Efficient Bio-Electroreduction of CO2 to Formate on a Iron Phthalocyanine-Dispersed CDC in Microbial Electrolysis System. Electrochim. Acta 2020, 338, 135887. [Google Scholar] [CrossRef]
- Shafaat, H.S.; Yang, J.Y. Uniting Biological and Chemical Strategies for Selective CO2 Reduction. Nat. Catal. 2021, 4, 928–933. [Google Scholar] [CrossRef]
- Lekshmi, G.S.; Bazaka, K.; Ramakrishna, S.; Kumaravel, V. Microbial Electrosynthesis: Carbonaceous Electrode Materials for CO2 Conversion. Mater. Horiz. 2023, 10, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Loiudice, A.; Lobaccaro, P.; Kamali, E.A.; Thao, T.; Huang, B.H.; Ager, J.W.; Buonsanti, R. Tailoring Copper Nanocrystals towards C2 Products in Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2016, 55, 5789–5792. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly Selective Plasma-Activated Copper Catalysts for Carbon Dioxide Reduction to Ethylene. Nat. Commun. 2016, 7, 12123. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. A Review of the Aqueous Electrochemical Reduction of CO2 to Hydrocarbons at Copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Zhou, Y.; Che, F.; Liu, M.; Zou, C.; Liang, Z.; De Luna, P.; Yuan, H.; Li, J.; Wang, Z.; Xie, H.; et al. Dopant-Induced Electron Localization Drives CO2 Reduction to C2 Hydrocarbons. Nat. Chem. 2018, 10, 974–980. [Google Scholar] [CrossRef]
- Eilert, A.; Cavalca, F.; Roberts, F.S.; Osterwalder, J.; Liu, C.; Favaro, M.; Crumlin, E.J.; Ogasawara, H.; Friebel, D.; Pettersson, L.G.M.; et al. Subsurface Oxygen in Oxide-Derived Copper Electrocatalysts for Carbon Dioxide Reduction. J. Phys. Chem. Lett. 2017, 8, 285–290. [Google Scholar] [CrossRef]
- Albo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the Electrochemical Conversion of Carbon Dioxide into Methanol. Green Chem. 2015, 17, 2304–2324. [Google Scholar] [CrossRef]
- Babin, V.; Sallustrau, A.; Loreau, O.; Caillé, F.; Goudet, A.; Cahuzac, H.; Del Vecchio, A.; Taran, F.; Audisio, D. A General Procedure for Carbon Isotope Labeling of Linear Urea Derivatives with Carbon Dioxide. Chem. Commun. 2021, 57, 6680–6683. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How Copper Catalyzes the Electroreduction of Carbon Dioxide into Hydrocarbon Fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Quan, Y.; Zhu, J.; Zheng, G. Electrocatalytic Reactions for Converting CO2 to Value-Added Products. Small Sci. 2021, 1, 2100043. [Google Scholar] [CrossRef]
- Ponsard, L.; Nicolas, E.; Tran, N.H.; Lamaison, S.; Wakerley, D.; Cantat, T.; Fontecave, M. Coupling Electrocatalytic CO2 Reduction with Thermocatalysis Enables the Formation of a Lactone Monomer. ChemSusChem 2021, 14, 2198–2204. [Google Scholar] [CrossRef]
- Liang, S.; Huang, L.; Gao, Y.; Wang, Q.; Liu, B. Electrochemical Reduction of CO2 to CO over Transition Metal/N-Doped Carbon Catalysts: The Active Sites and Reaction Mechanism. Adv. Sci. 2021, 8, 2102886. [Google Scholar] [CrossRef]
- Garg, S.; Li, M.; Weber, A.Z.; Ge, L.; Li, L.; Rudolph, V.; Wang, G.; Rufford, T.E. Advances and Challenges in Electrochemical CO2 Reduction Processes: An Engineering and Design Perspective Looking beyond New Catalyst Materials. J. Mater. Chem. A Mater. 2020, 8, 1511–1544. [Google Scholar] [CrossRef]
- Nielsen, D.U.; Hu, X.M.; Daasbjerg, K.; Skrydstrup, T. Chemically and Electrochemically Catalysed Conversion of CO2 to CO with Follow-up Utilization to Value-Added Chemicals. Nat. Catal. 2018, 1, 244–254. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Talbot, A.; Caillé, F.; Chevalier, A.; Sallustrau, A.; Loreau, O.; Destro, G.; Taran, F.; Audisio, D. Carbon Isotope Labeling of Carbamates by Late-Stage [11C], [13C] and [14C]Carbon Dioxide Incorporation. Chem. Commun. 2020, 56, 11677–11680. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic Process of CO Selectivity in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Johnson, D.; Qiao, Z.; Djire, A. Progress and Challenges of Carbon Dioxide Reduction Reaction on Transition Metal Based Electrocatalysts. ACS Appl. Energy Mater. 2021, 4, 8661–8684. [Google Scholar] [CrossRef]
- Monteiro, M.C.O.; Philips, M.F.; Schouten, K.J.P.; Koper, M.T.M. Efficiency and Selectivity of CO2 Reduction to CO on Gold Gas Diffusion Electrodes in Acidic Media. Nat. Commun. 2021, 12, 4943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, L.; Libretto, N.J.; Li, X.; Li, C.; Wan, Y.; He, C.; Lee, J.; Gregg, J.; Zong, H.; et al. Ensemble Effect in Bimetallic Electrocatalysts for CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 16635–16642. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Qian, C.; Dong, Y.; Li, Y.F.; Wang, H.; Ghoussoub, M.; Butler, K.T.; Walsh, A.; Ozin, G.A. Heterogeneous Catalytic Hydrogenation of CO2 by Metal Oxides: Defect Engineering—Perfecting Imperfection. Chem. Soc. Rev. 2017, 46, 4631–4644. [Google Scholar] [CrossRef] [PubMed]
- Overa, S.; Ko, B.H.; Zhao, Y.; Jiao, F. Electrochemical Approaches for CO2 Conversion to Chemicals: A Journey toward Practical Applications. Acc. Chem. Res. 2022, 55, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Su, Y.; Deng, P.; Qi, R.; Zhu, J.; Chen, J.; Wang, Z.; Zhou, L.; Guo, X.; Xia, B.Y. Highly Selective Carbon Dioxide Electroreduction on Structure-Evolved Copper Perovskite Oxide toward Methane Production. ACS Catal. 2020, 10, 4640–4646. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, P.; Li, A.; Wei, B.; Si, K.; Wei, Y.; Wang, X.; Zhu, G.; Chen, Q.; Gu, X.; et al. Electrochemical CO2 Reduction to Ethylene by Ultrathin CuO Nanoplate Arrays. Nat. Commun. 2022, 13, 1877. [Google Scholar] [CrossRef]
- Anzai, A.; Liu, M.H.; Ura, K.; Noguchi, T.G.; Yoshizawa, A.; Kato, K.; Sugiyama, T.; Yamauchi, M. Cu Modified TiO2 Catalyst for Electrochemical Reduction of Carbon Dioxide to Methane. Catalysts 2022, 12, 478. [Google Scholar] [CrossRef]
- Asadi, M.; Kumar, B.; Behranginia, A.; Rosen, B.A.; Baskin, A.; Repnin, N.; Pisasale, D.; Phillips, P.; Zhu, W.; Haasch, R.; et al. Robust Carbon Dioxide Reduction on Molybdenum Disulphide Edges. Nat. Commun. 2014, 5, 4470. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured Transition Metal Dichalcogenide Electrocatalysts for CO2 Reduction in Ionic Liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef]
- Abbasi, P.; Asadi, M.; Liu, C.; Sharifi-Asl, S.; Sayahpour, B.; Behranginia, A.; Zapol, P.; Shahbazian-Yassar, R.; Curtiss, L.A.; Salehi-Khojin, A. Tailoring the Edge Structure of Molybdenum Disulfide toward Electrocatalytic Reduction of Carbon Dioxide. ACS Nano 2017, 11, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Elnabawy, A.O.; Schimmenti, R.; Song, P.; Wang, J.; Peng, Z.; Yao, S.; Deng, R.; Song, S.; Lin, Y.; et al. Bismuthene for Highly Efficient Carbon Dioxide Electroreduction Reaction. Nat. Commun. 2020, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, Q.; Chu, N.; Hao, W.; Zhang, L.; Zhan, G.; Li, D.; Zeng, R.J. A Slurry Electrode Integrated with Membrane Electrolysis for High-Performance Acetate Production in Microbial Electrosynthesis. Sci. Total Environ. 2020, 741, 140198. [Google Scholar] [CrossRef] [PubMed]
- De la Puente, C.; Carrillo-Peña, D.; Pelaz, G.; Morán, A.; Mateos, R. Microbial Electrosynthesis for CO2 Conversion and Methane Production: Influence of Electrode Geometry on Biofilm Development. Greenh. Gases Sci. Technol. 2022, 13, 173–185. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Han, T.H.; Cho, M.H. Metal-Free Carbon-Based Materials: Promising Electrocatalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. Int. J. Mol. Sci. 2016, 18, 25. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, K.; Xu, B.; Li, J.; Hu, C.; Yuan, J.S.; Dai, S.Y. Chem-Bio Interface Design for Rapid Conversion of CO2 to Bioplastics in an Integrated System. Chem 2022, 8, 3363–3381. [Google Scholar] [CrossRef]
- Liu, H.; Song, T.; Fei, K.; Wang, H.; Xie, J. Microbial Electrosynthesis of Organic Chemicals from CO2 by Clostridium Scatologenes ATCC 25775T. Bioresour. Bioprocess. 2018, 5, 7. [Google Scholar] [CrossRef]
- Chatzipanagiotou, K.R.; Soekhoe, V.; Jourdin, L.; Buisman, C.J.N.; Bitter, J.H.; Strik, D.P.B.T.B. Catalytic Cooperation between a Copper Oxide Electrocatalyst and a Microbial Community for Microbial Electrosynthesis. Chempluschem 2021, 86, 763–777. [Google Scholar] [CrossRef]
- Bai, X.; Chen, W.; Zhao, C.; Li, S.; Song, Y.; Ge, R.; Wei, W.; Sun, Y. Exclusive Formation of Formic Acid from CO2 Electroreduction by a Tunable Pd-Sn Alloy. Angew. Chem. Int. Ed. 2017, 56, 12219–12223. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, X.G.; Jiang, K.; Wu, D.Y.; Cai, W. Bin Boosting Formate Production in Electrocatalytic CO2 Reduction over Wide Potential Window on Pd Surfaces. J. Am. Chem. Soc. 2018, 140, 2880–2889. [Google Scholar] [CrossRef]
- Bok, J.; Lee, S.Y.; Lee, B.H.; Kim, C.; Nguyen, D.L.T.; Kim, J.W.; Jung, E.; Lee, C.W.; Jung, Y.; Lee, H.S.; et al. Designing Atomically Dispersed Au on Tensile-Strained Pd for Efficient CO2 Electroreduction to Formate. J. Am. Chem. Soc. 2021, 143, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Cho, N.H.; Nam, K.T.; Hwang, Y.J.; Min, B.K. Cyclic Two-Step Electrolysis for Stable Electrochemical Conversion of Carbon Dioxide to Formate. Nat. Commun. 2019, 10, 3919. [Google Scholar] [CrossRef] [PubMed]

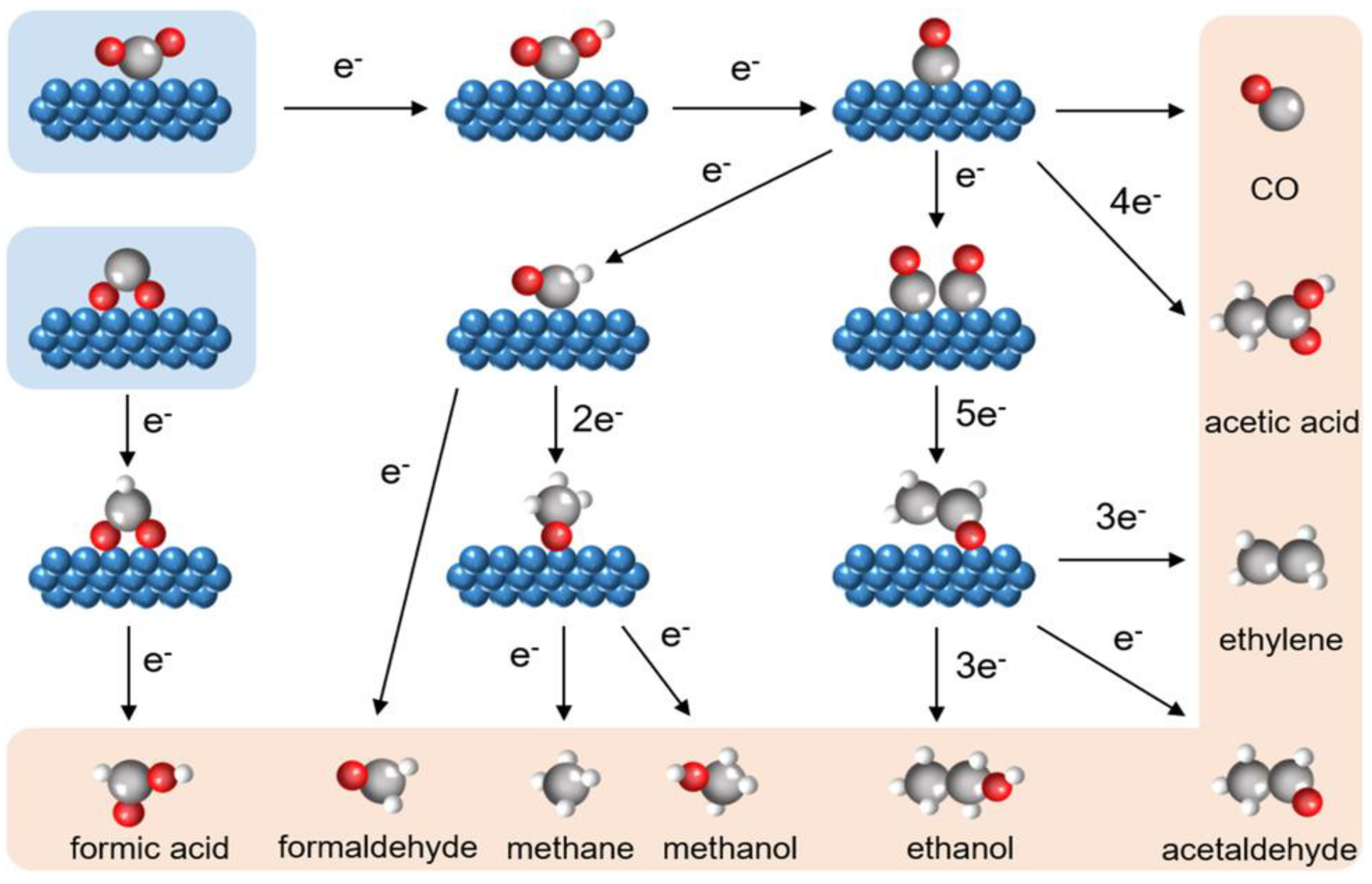

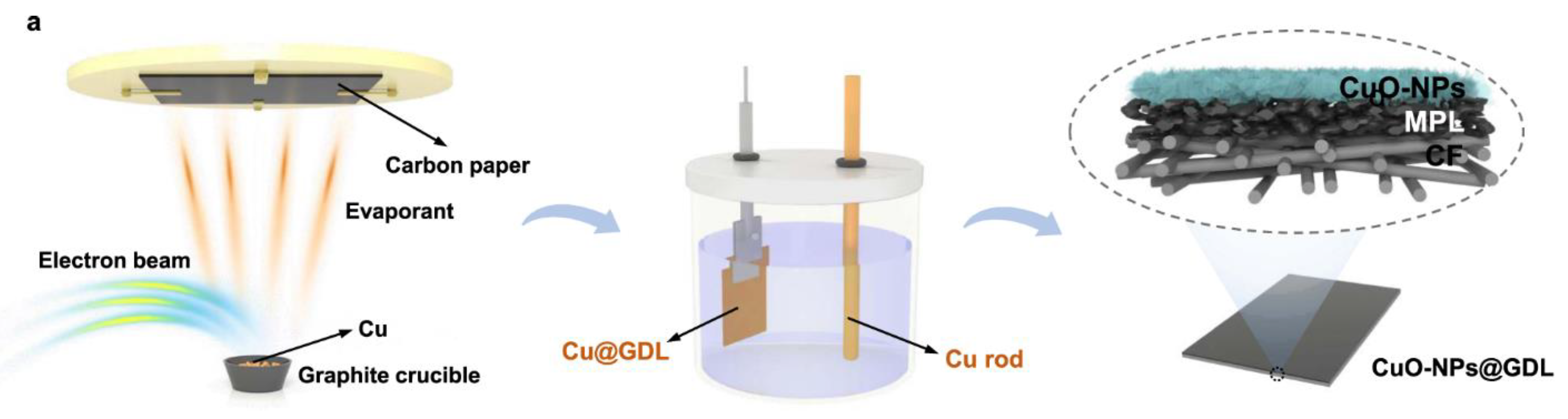
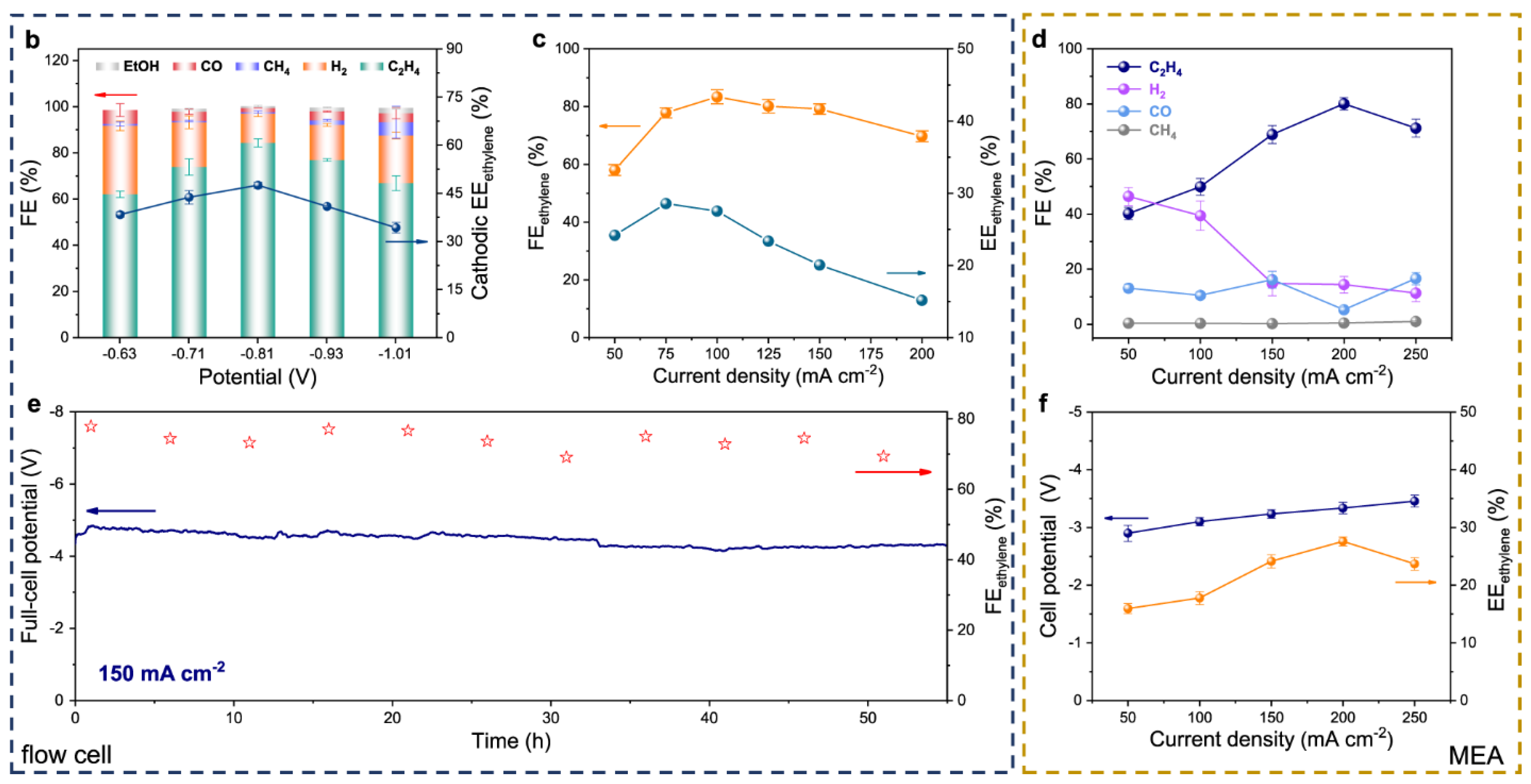
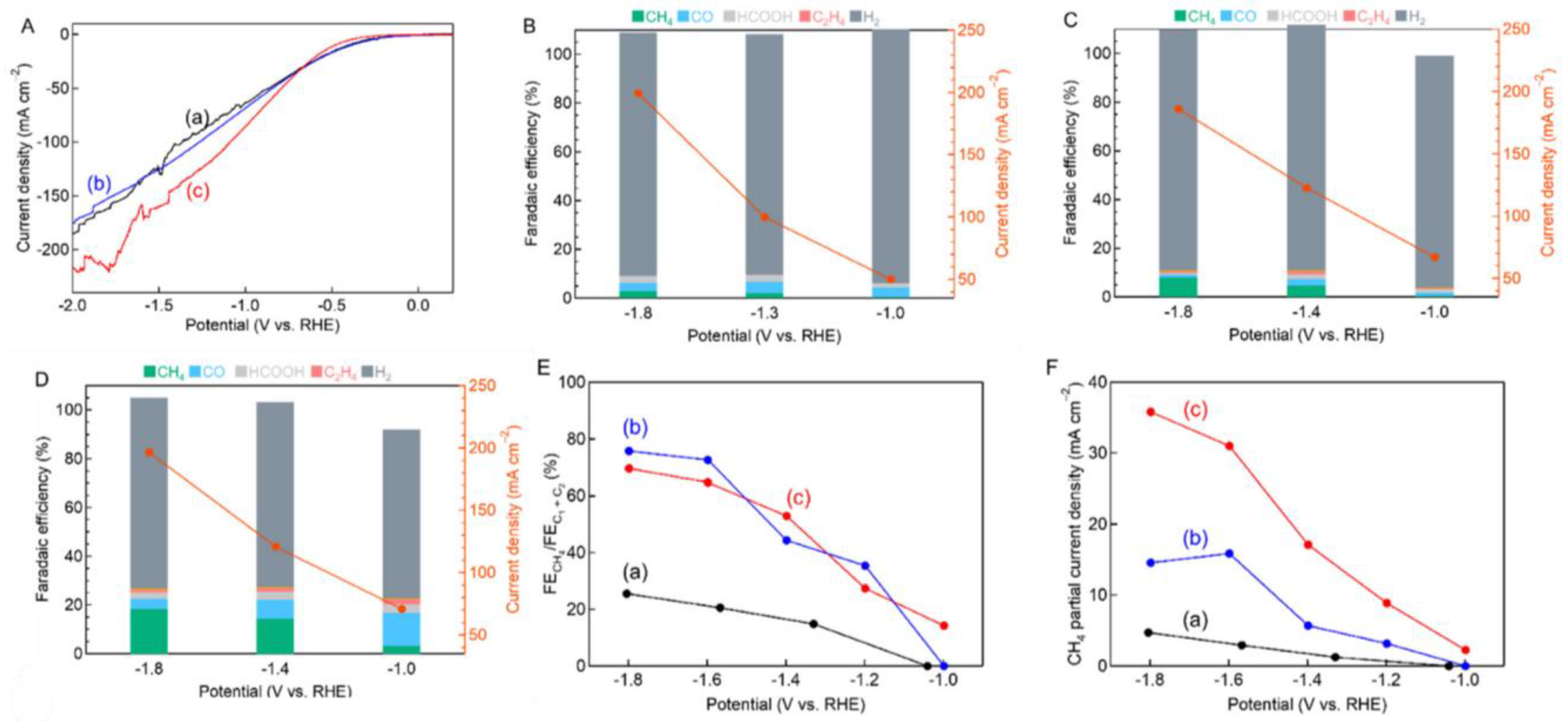
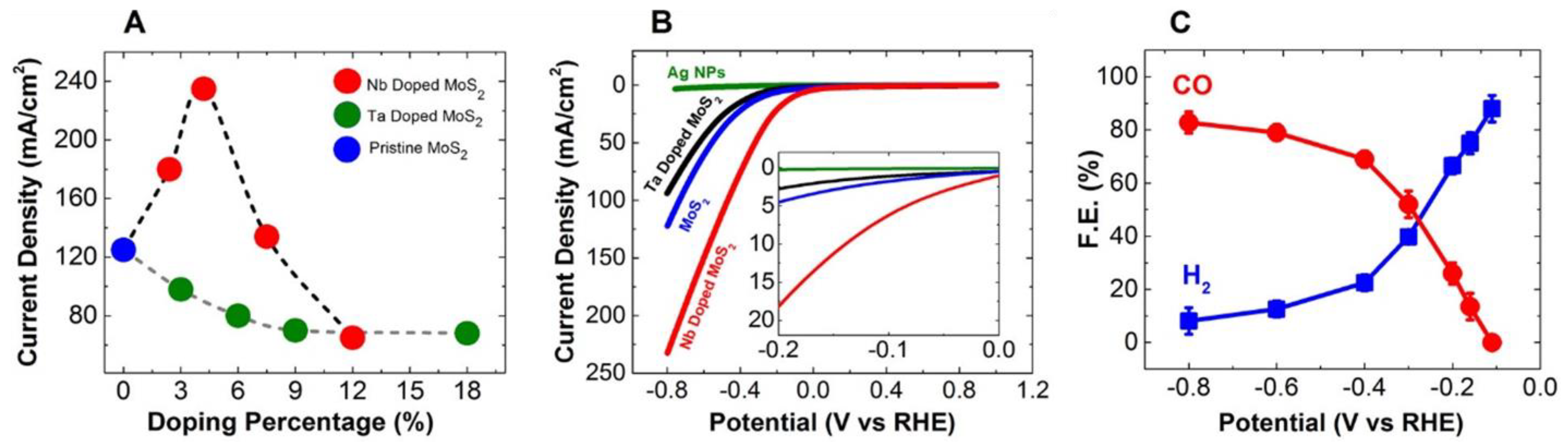

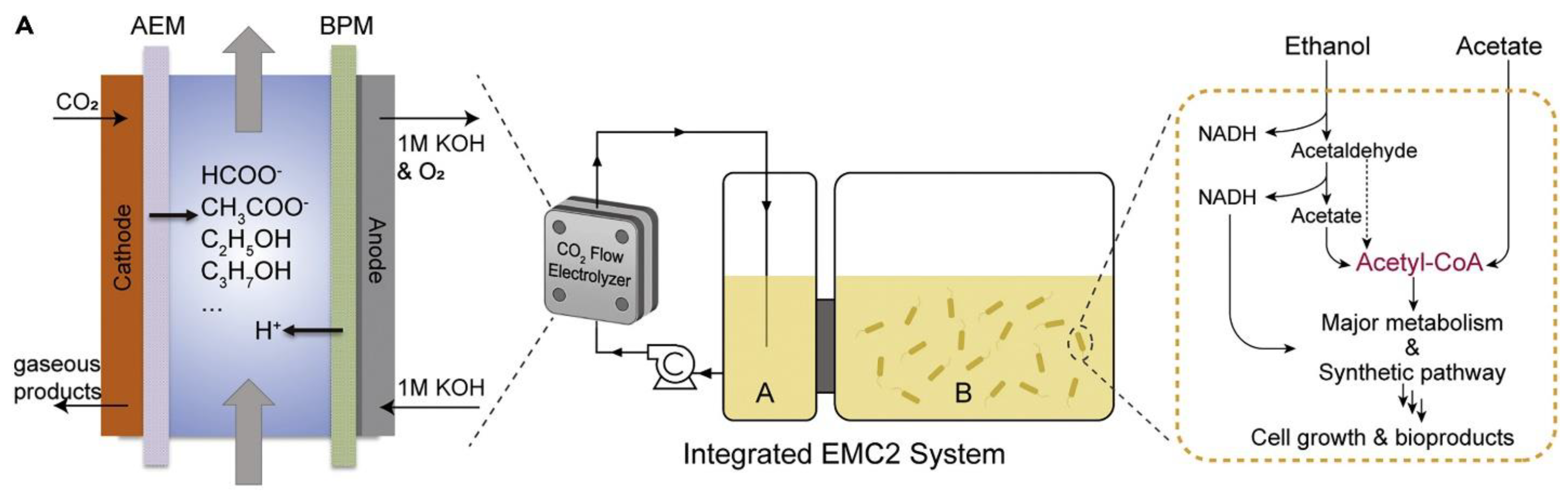
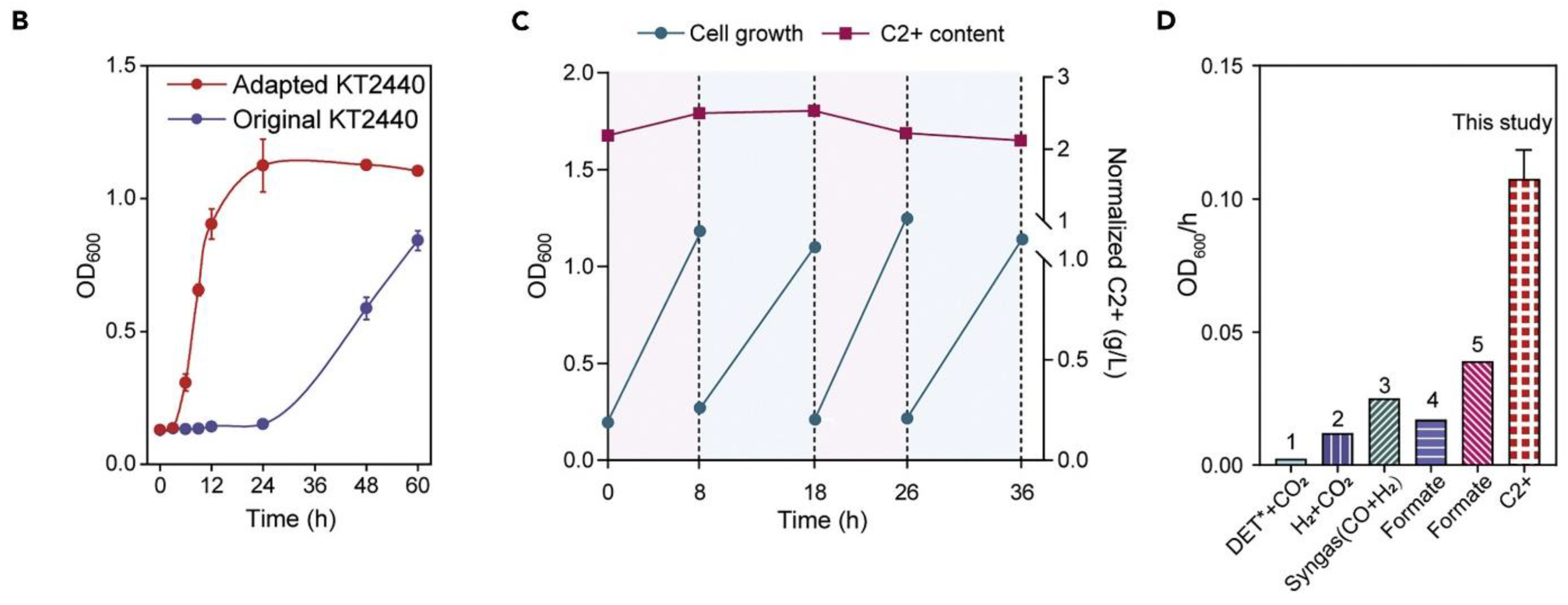
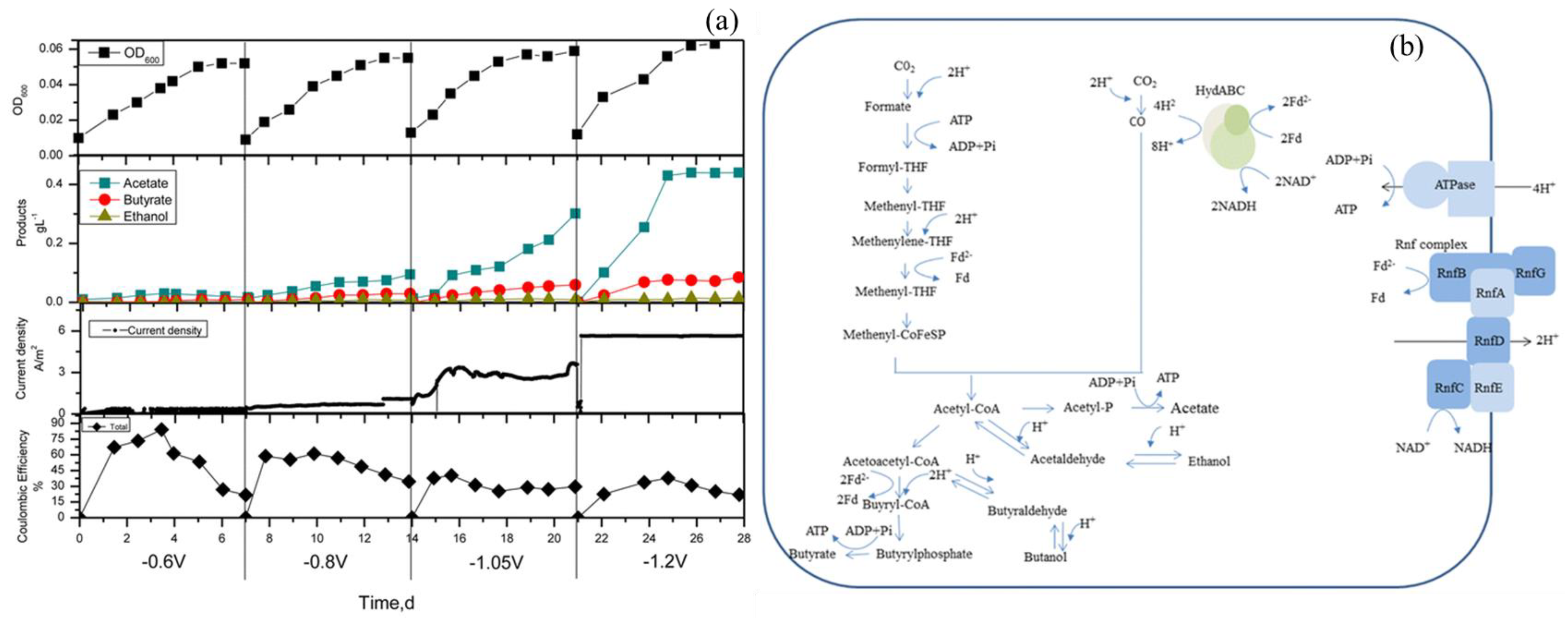
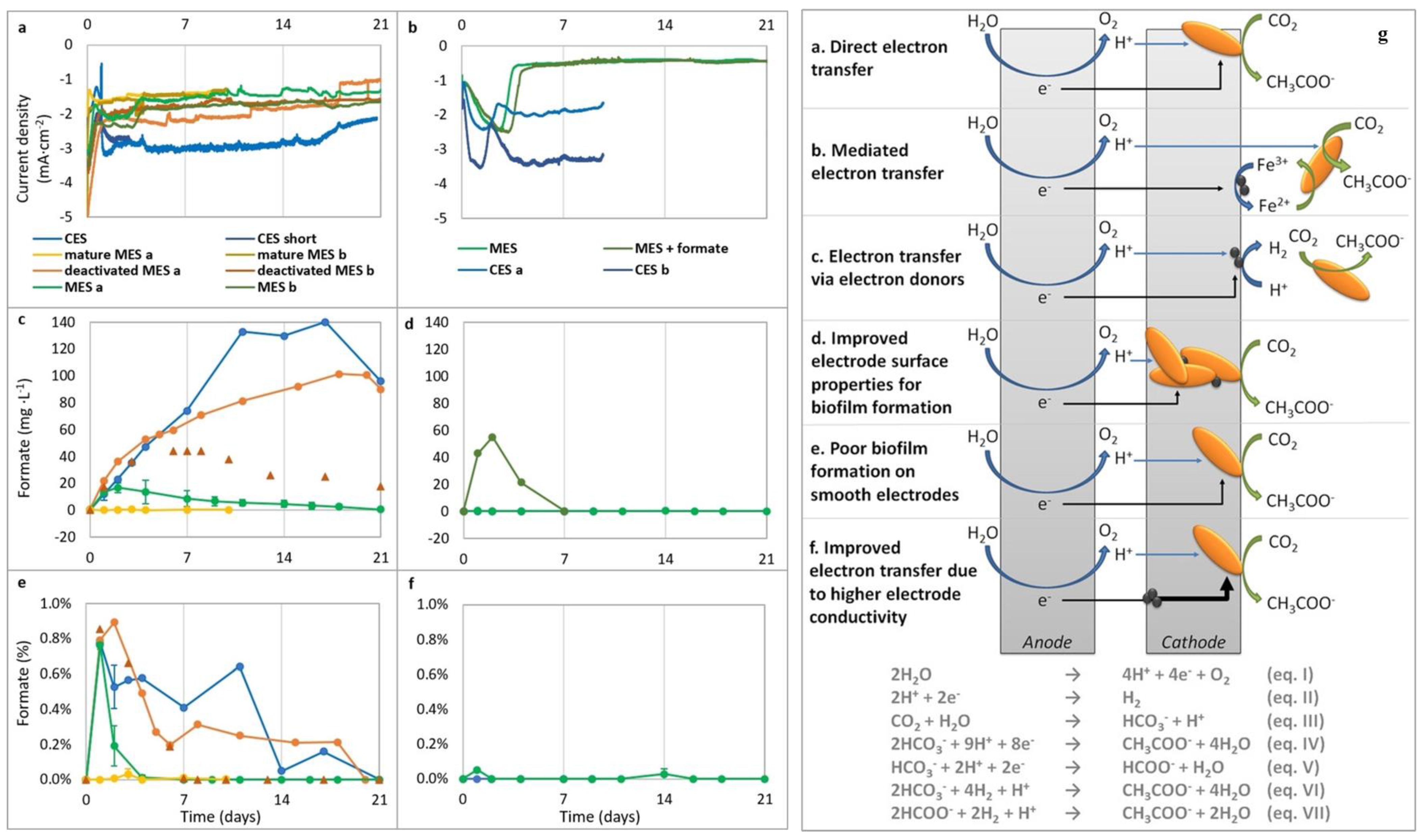
| Produces | Acidic Electrolyte | Alkaline Electrolyte | ||
|---|---|---|---|---|
| Chemical Reactions | Potential | Chemical Reactions | Potential | |
| Ethanol | 2CO2 + 12H+ + 12e− → CH3CH2OH + 3H2O | 0.084 | 2CO2 + 9H2O + 12e− → CH3CH2OH + 12OH− | −0.744 |
| Ethylene | 2CO2 + 12H+ + 12e− → C2H4 + 4H2O | 0.085 | 2CO2 + 8H2O + 12e− → C2H4 + 12OH− | −0.743 |
| Formic acid | CO2 + 2H+ + 2e− → HCOOH | −0.171 | CO2 + H2O + 2e− → HCOO− + OH− | −0.639 |
| Methanol | CO2 + 6H+ + 6e− → CH3OH + H2O | 0.016 | CO2 + 5H2O + 6e− → CH3OH + 6OH− | −0.812 |
| Methane | CO2 + 8H+ + 8e− → CH4 + 2H2O | 0.169 | CO2 + 6H2O + 8e− → CH4 + 8OH− | −0.659 |
| Carbon monoxide | CO2 + 2H+ + 2e− → CO + H2O | −0.104 | CO2 + H2O + 2e− → CO + 2OH− | −0.932 |
| Acetic acid | 2CO2 + 8H+ + 8e− → CH3COOH + 2H2O | 0.098 | 2CO2 + 5H2O + 8e− → CH3COO− + 7OH− | −0.653 |
| Electrocatalyst | Catalyst Loading | Electrolyte | Main Product | Total Current Density | FE (%) | Reference |
|---|---|---|---|---|---|---|
| PdSn/C | 0.5 mg cm−2 | 0.5 M KHCO3 | HCOOH | 2 mA cm−2 at −0.43 V vs. RHE | 99 | 2017/[89] |
| Pd-B/C | 100 μg cm−2 | 0.5 M KHCO3 | Formate | 10 mA cm−2 at −0.5 V vs. RHE | 70 | 2018/[90] |
| MOF-AuPd | 1.0 mg cm−2 | 0.5 M KHCO3 | HCOOH | 7 mA cm−2 at −0.25 V vs. RHE | 99 | 2021/[91] |
| Pd80Ag20/C | - | 0.5 M NaHCO3 /0.5 M NaClO4 | HCOO− | 11 mA cm−2 at −0.18 V vs. RHE | 97.8 | 2019/[92] |
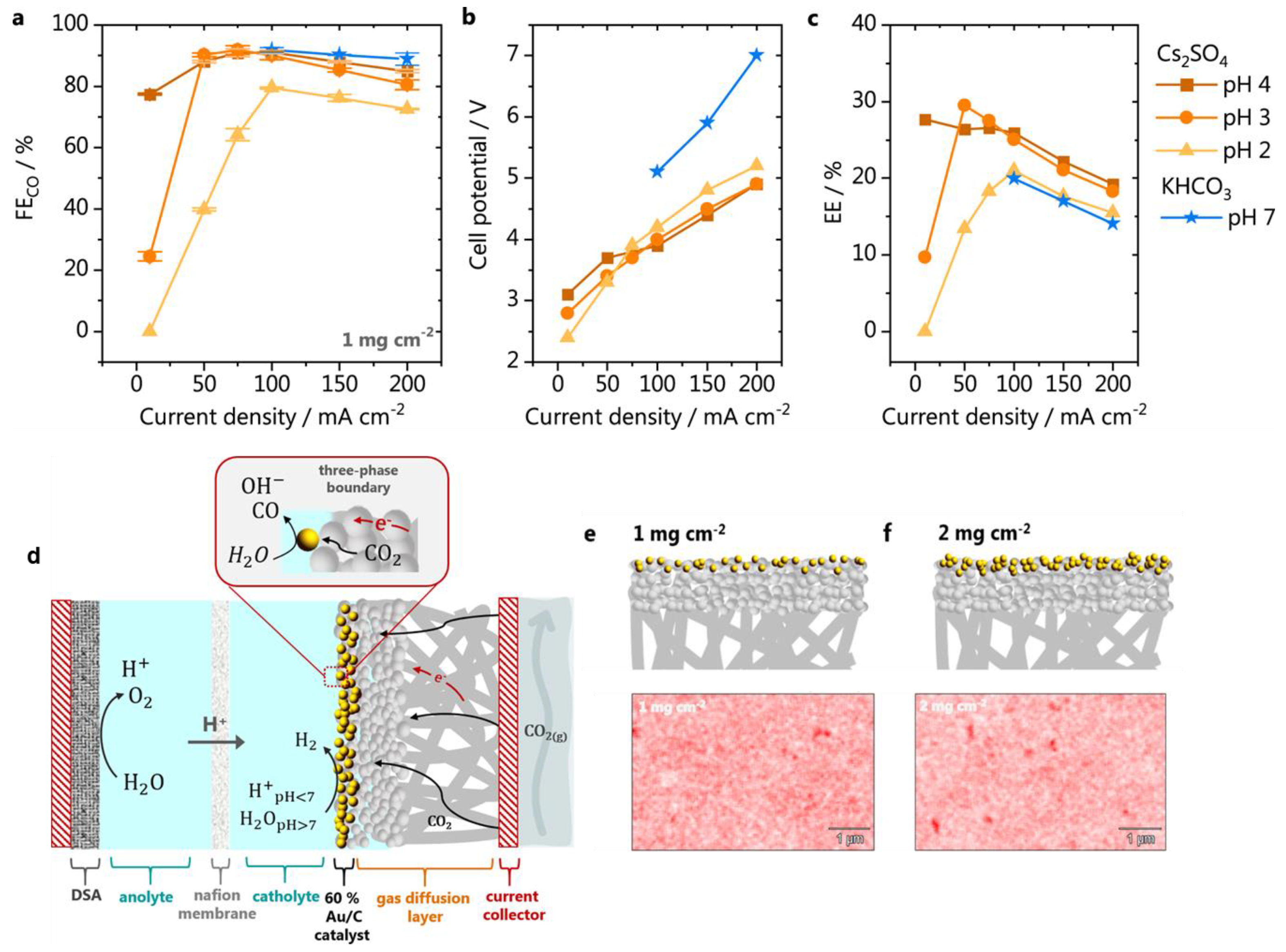
| Gold gas-diffusion electrode | 1 mg cm−2 | 1 M KHCO3 | CO | 100 mA cm−2 at −0.18 V vs. RHE | 90 | 2021/[72] |
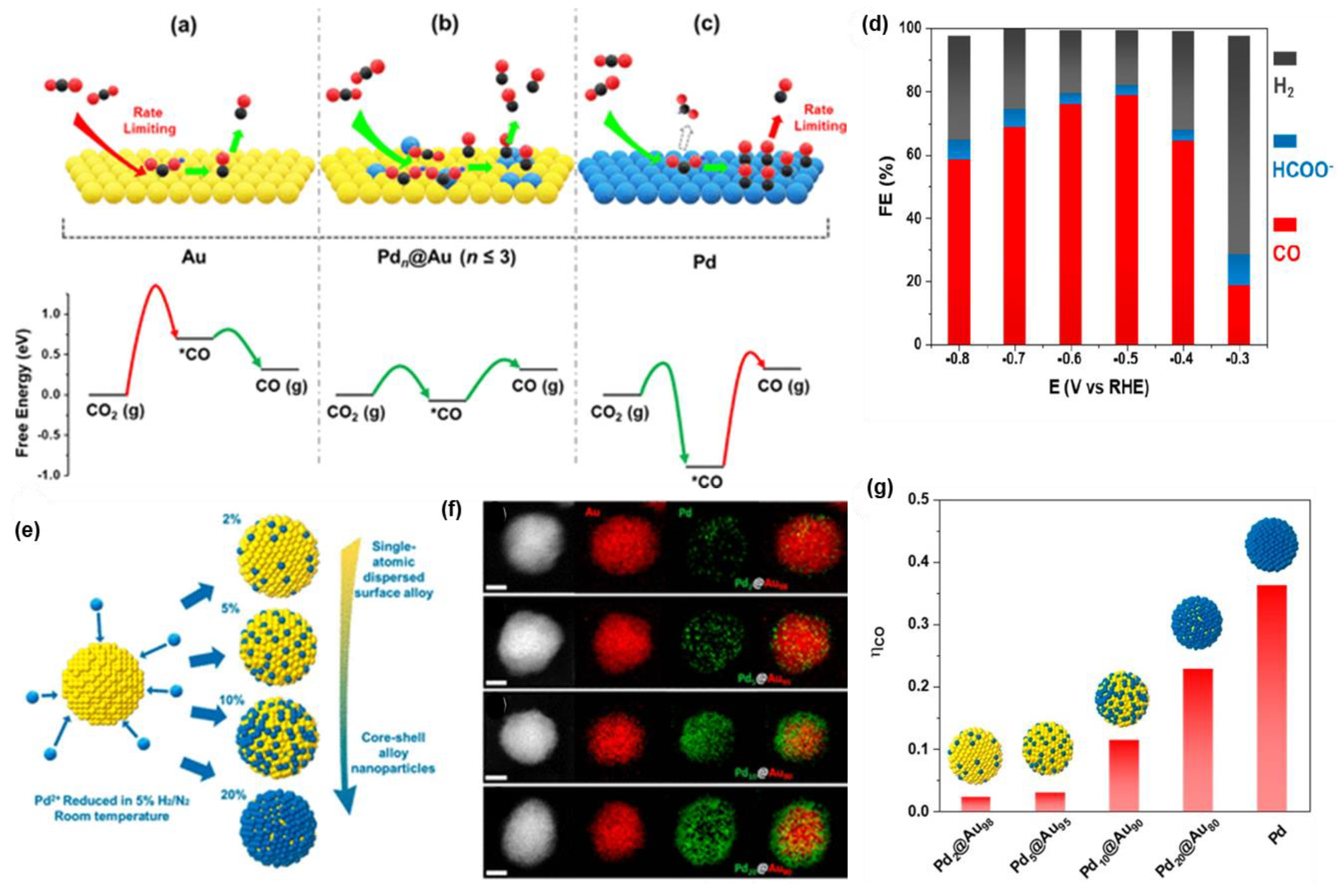
| Pd5@Au95 | 10 μg cm−2 | 0.1 M KHCO3 | CO | 1.6 mA cm−2 at −0.5 V vs. RHE | 80 | 2019/[73] |
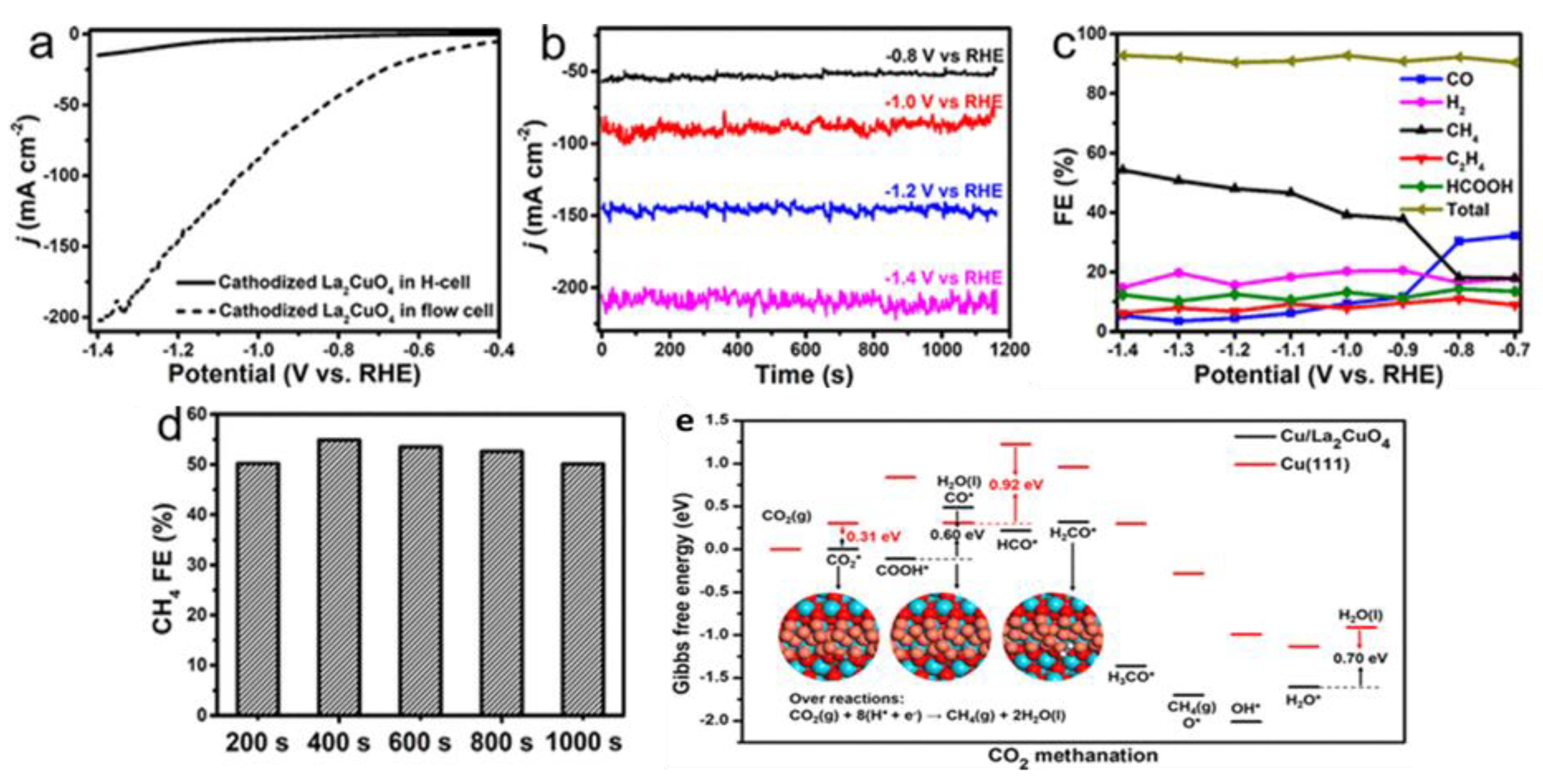
| Cu/La2CuO4 | 0.12 mg cm−2 | 1 M KOH | CH4 | 12 mA cm−2 at −1.4 V vs. RHE | 56.3 | 2020/[76] |
| Cu/Cu2O | - | 1.0 M NaOH | C2H4 | 200 mA cm−2 at −0.81 V vs. RHE | 84.5 | 2022/[77] |
| Cu-TiO2 | - | 1 M KOH | CH4 | 117 mA cm−2 at −1.8 V vs. RHE | 70 | 2022/[78] |
| β-Bi2O3 | 1 mg cm−2 | 1 M KOH | Formate | 288 mA cm−2 at −0.61 V vs. RHE | 98 | 2019/[29] |
| VaMo0.95 Nb0.05S2 | 1 mg cm−2 | EMIM-BF4 | CO | 237 mA cm−2 at −0.8 V vs. RHE | 83 | 2017/[81] |
| BiNSs | 0.39 mg cm2 | 0.5 M KHCO3 | HCOO− | 2.5 mA cm−2 at −0.58 V vs. RHE | 98 | 2020/[82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masoumi, Z.; Tayebi, M.; Tayebi, M.; Masoumi Lari, S.A.; Sewwandi, N.; Seo, B.; Lim, C.-S.; Kim, H.-G.; Kyung, D. Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends. Int. J. Mol. Sci. 2023, 24, 9952. https://doi.org/10.3390/ijms24129952
Masoumi Z, Tayebi M, Tayebi M, Masoumi Lari SA, Sewwandi N, Seo B, Lim C-S, Kim H-G, Kyung D. Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends. International Journal of Molecular Sciences. 2023; 24(12):9952. https://doi.org/10.3390/ijms24129952
Chicago/Turabian StyleMasoumi, Zohreh, Meysam Tayebi, Mahdi Tayebi, S. Ahmad Masoumi Lari, Nethmi Sewwandi, Bongkuk Seo, Choong-Sun Lim, Hyeon-Gook Kim, and Daeseung Kyung. 2023. "Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends" International Journal of Molecular Sciences 24, no. 12: 9952. https://doi.org/10.3390/ijms24129952
APA StyleMasoumi, Z., Tayebi, M., Tayebi, M., Masoumi Lari, S. A., Sewwandi, N., Seo, B., Lim, C.-S., Kim, H.-G., & Kyung, D. (2023). Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends. International Journal of Molecular Sciences, 24(12), 9952. https://doi.org/10.3390/ijms24129952







