Effects of Environmental Conditions on the Individual Architectures and Photosynthetic Performances of Three Species in Drosera
Abstract
1. Introduction
2. Results
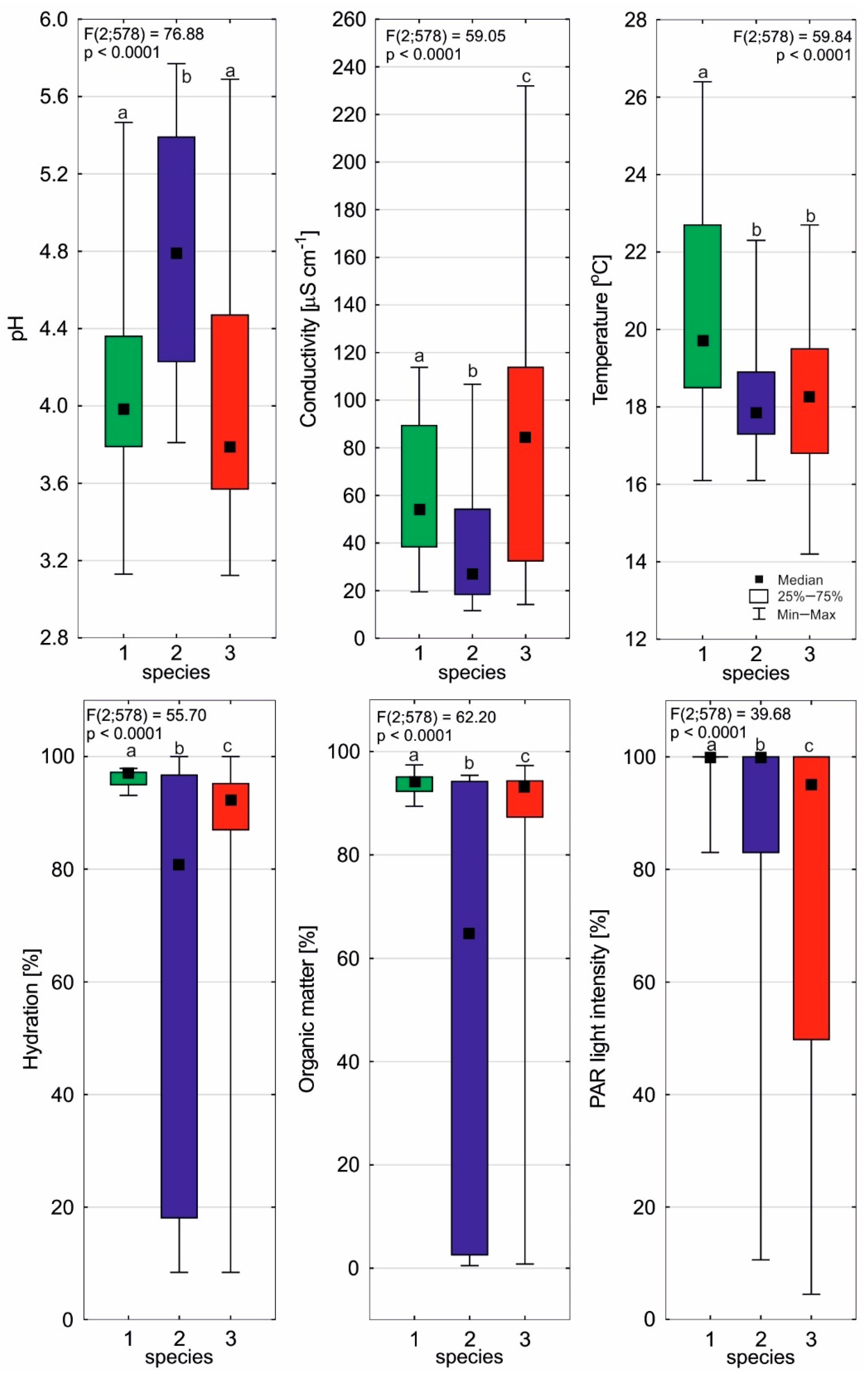
2.1. Environmental Conditions of Sundews
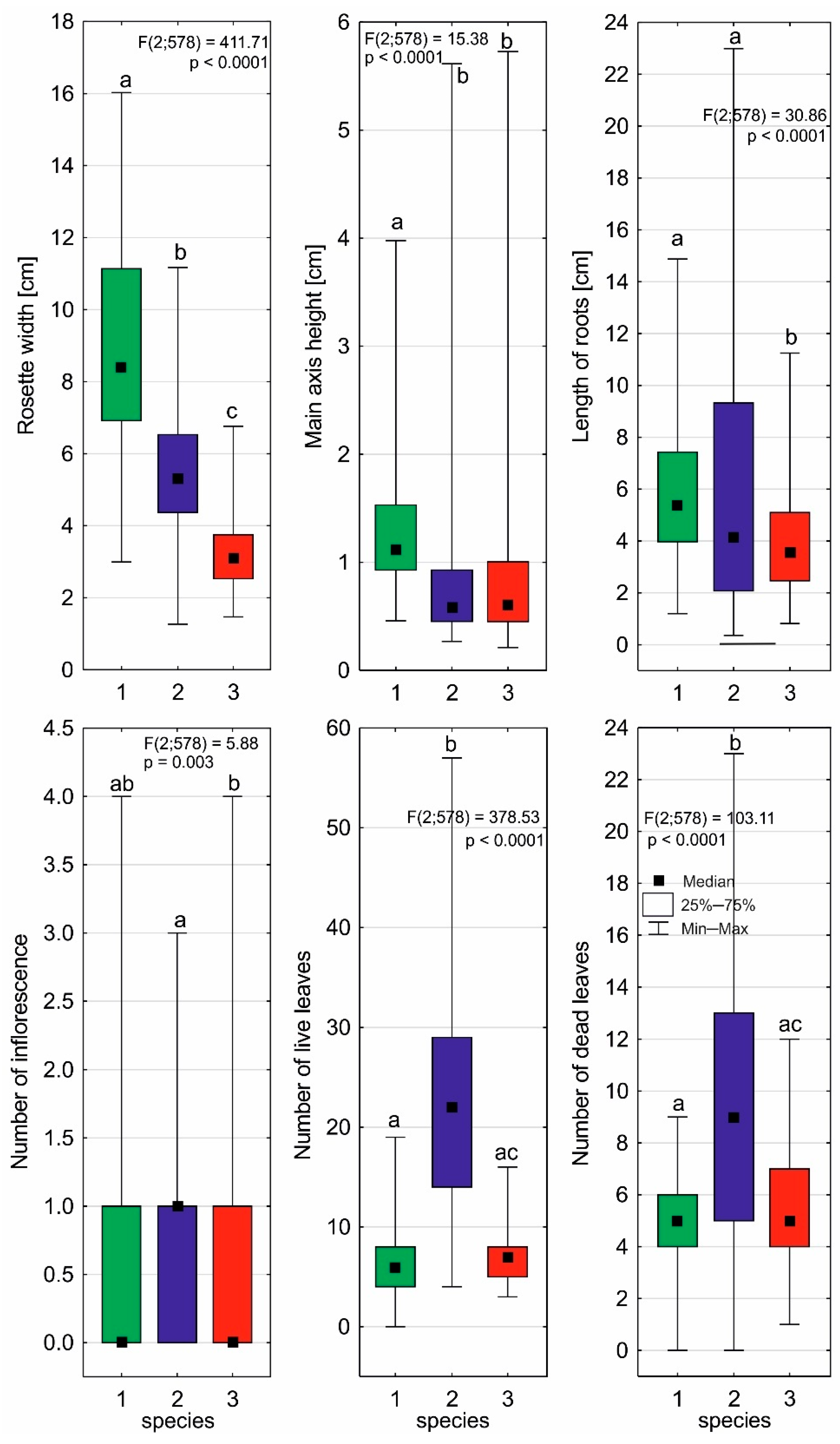
2.2. Individual Architectures of Sundews
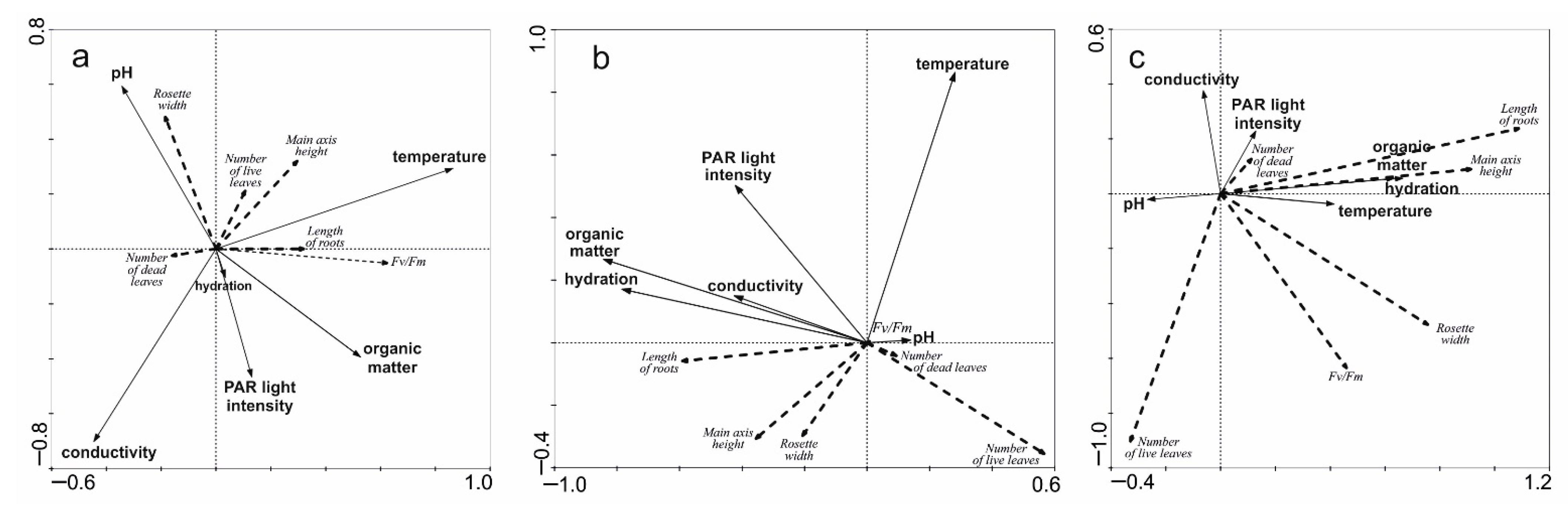
2.3. Influence of Environmental Factors on Individual Architecture
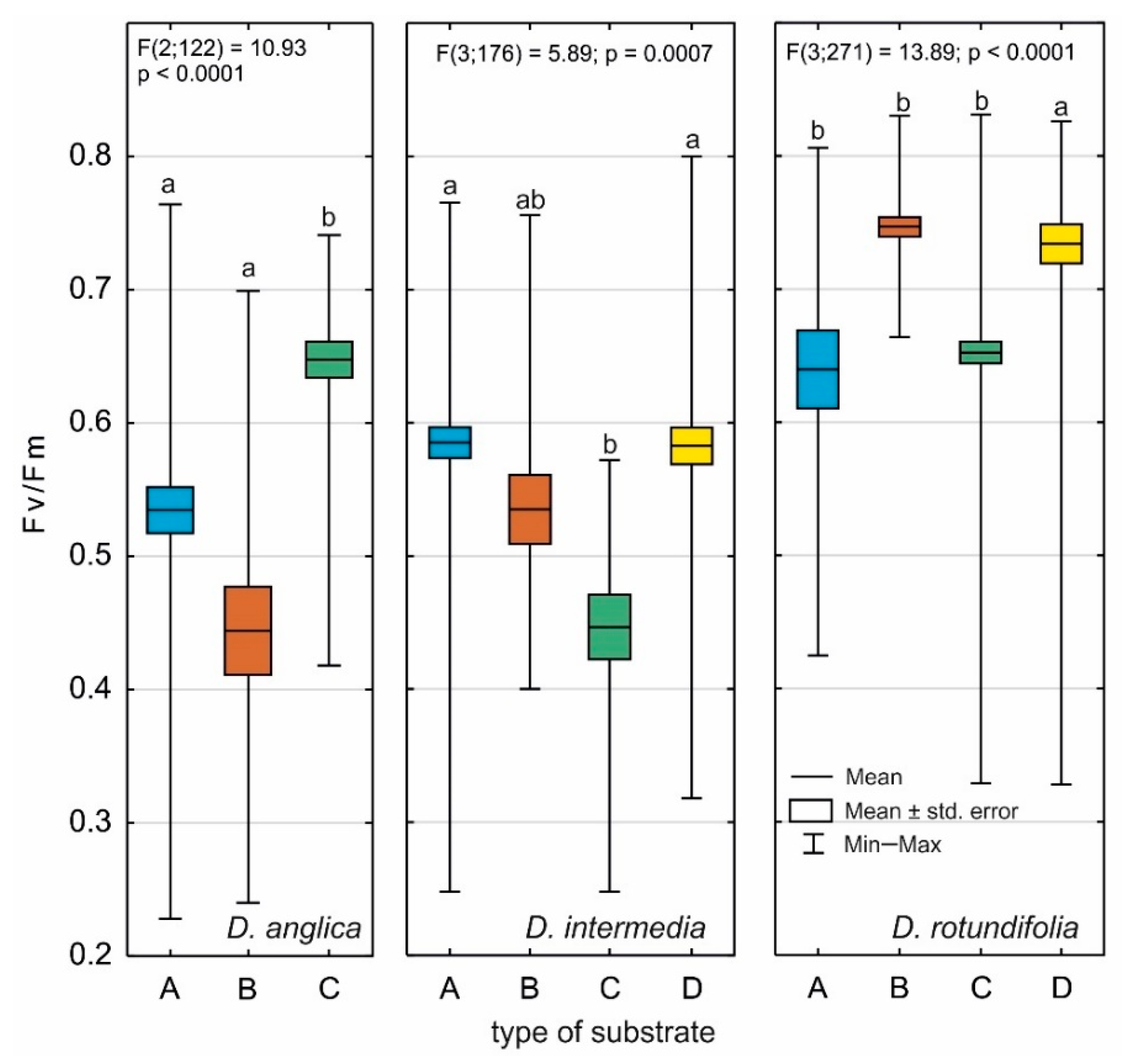
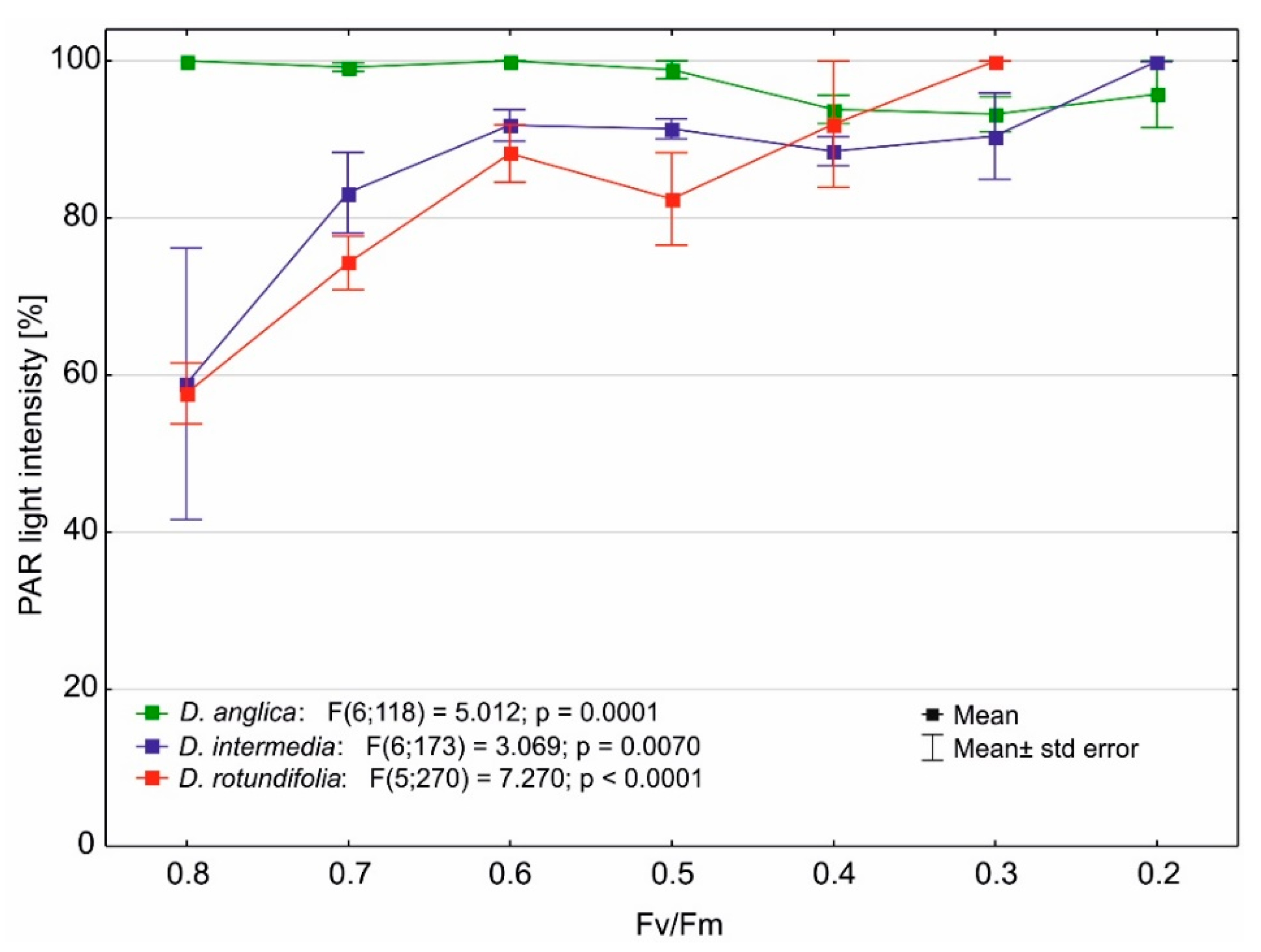
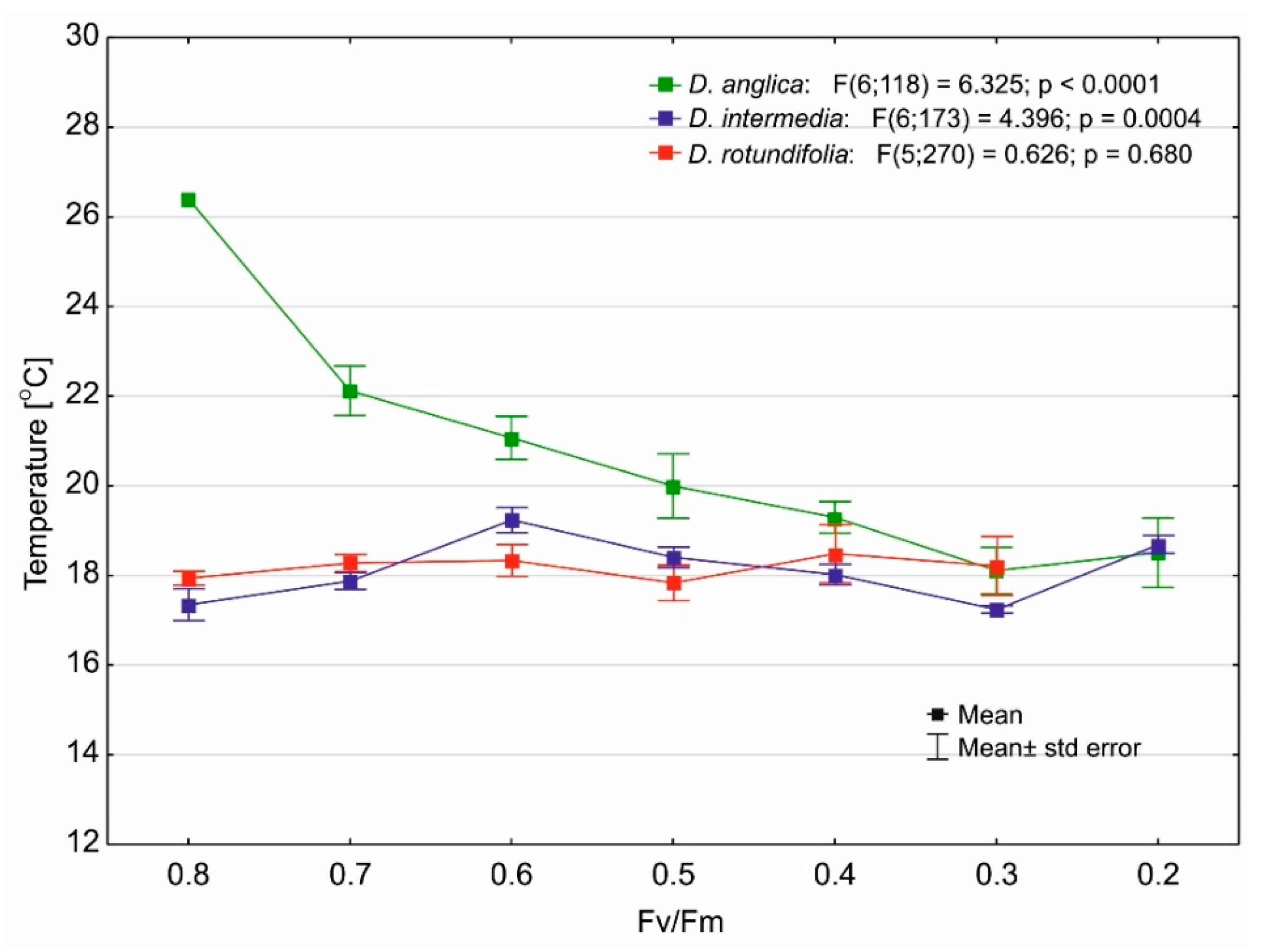
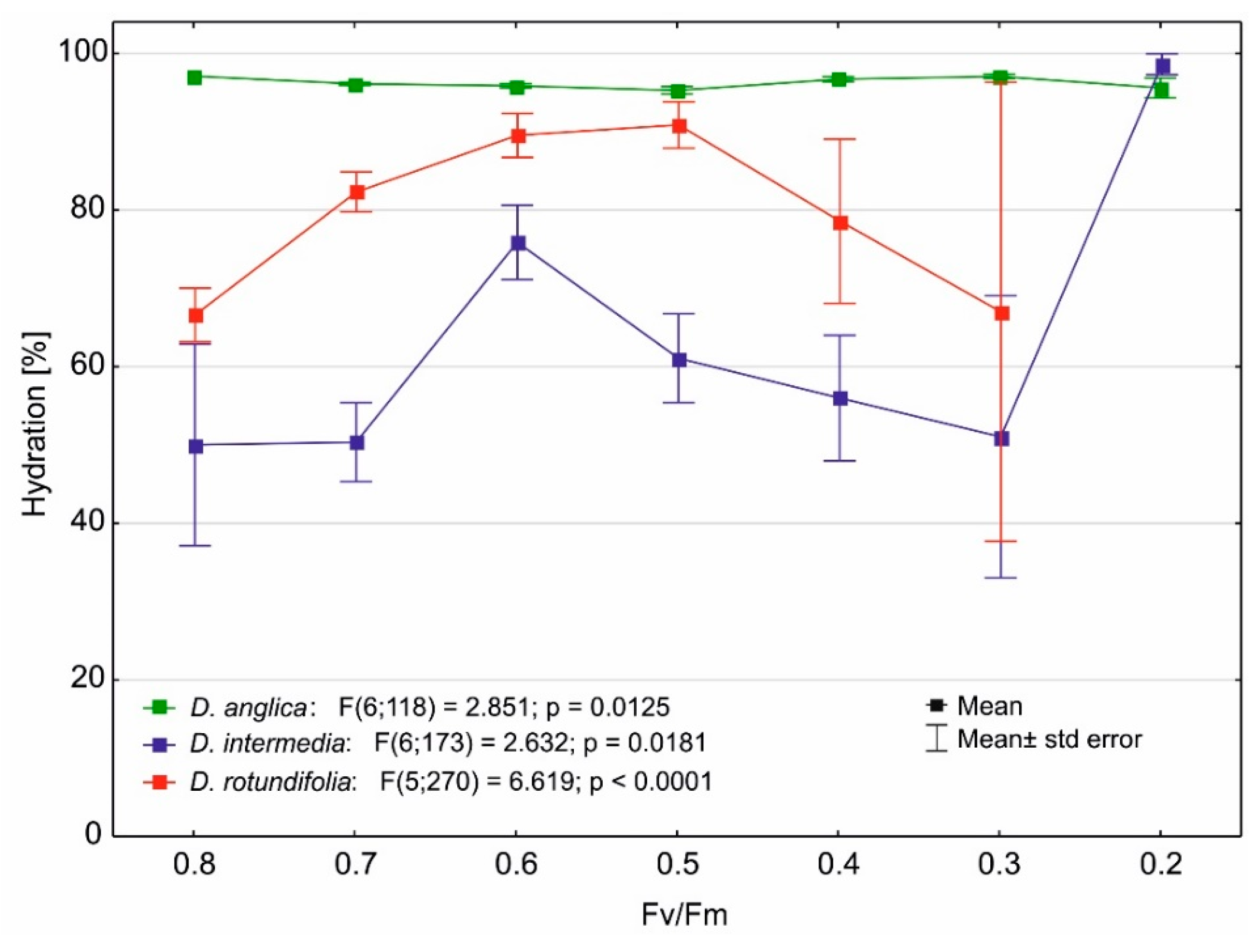
2.4. Photosynthetic Efficiency
3. Discussion
3.1. Variability of Individual Architectures and Photosynthetic Performance of Sundews
3.2. Effect of Light Stress on the Maximum Quantum Yield of Photosynthesis of Drosera
3.3. Effect of Temperature on the Maximum Quantum Yield of Photosynthesis of Drosera
3.4. Effect of Water Stress on the Maximum Quantum Yield of Photosynthesis of Drosera
4. Methods
4.1. Collection of Samples and Methods of the Analysis of Environmental Conditions
- -
- Photosynthetically active radiation (PAR) intensity was measured with an LI-250 light meter, and an average of five measurements at the sundews was taken, which was then converted for the plant site as a percentage of light reaching the site/peatland in a fully illuminated location;
- -
- pH and temperature—with a WTW 320/SET1 pH meter and a SENTIX 97T measuring electrode;
- -
- Electrolytic conductivity—with a WTW Cond 3210 SET 2 conductivity meter;
- -
- Substrate hydration—calculated as a percentage from the difference in the weight of fresh substrate, and the substrate dried to constant weight at 105 °C in a Binder FD115 laboratory dryer;
- -
- The organic matter content of the substrate was calculated as a percentage from the difference in weight between the dry substrate and the substrate roasted in a SEL 96C muffle furnace at 550 °C for 5 h.
- -
- Fv/Fm—maximum photochemical quantum yield of photosystem II (PSII);
- -
- Fv—fluorescence variable (Fm − Fo);
- -
- Fm—maximum fluorescence (fluorescence level when all photochemical traps are closed);
- -
- Fo—minimum fluorescence (fluorescence level when all photochemical traps are open).
4.2. Statistical Methods Used in Developing the Results
5. Conclusions
- RDOŚ-Gd-WOC.6205.46.2021.MaK.3—Żurawie Błota Nature Reserve;
- RDOŚ-Gd-WOC.6205.36.2022.JK.2—Żurawie Błota Nature Reserve, Krasne Lake Nature Reserve, Lisia Kępa Nature Reserve;
- RDOŚ-Gd-WOC.6205.28.2022.ATP.2—Małe Łowne Nature Reserve, Moczadło Lake Nature Reserve.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McPerson, S. Glistening Carnivores–The Sticky-Leaved Insect-Eating Plants; Redfern Natural History Productions: Poole, UK, 2008; 392p. [Google Scholar]
- WFO. World Flora Online. Drosera anglica Huds. Available online: http://www.worldfloraonline.org/taxon/wfo-0000945816 (accessed on 25 February 2023).
- Cheek, M. Drosera x eloisiana (Droseraceae) and the lectotypification and synonymisation of D. Belezeana. New J. Bot. 2016, 6, 58–59. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Goltsev, V.N.; Żuk-Gołaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications, 1st ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; 222p. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Kouzmanova, M.; Kalaji, H.M.; Yordanov, I.; Krasteva, V.; Alexandrov, V.; Stefanov, D.; Allakhverdiev, S.I.; et al. Drought- induced modifications of photosynthetic electron transport in intact leaves: Analysis and use of neural networks as a tool for a rapid non-invasive estimation. Biochim. Biophys. Acta 2012, 1817, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava., A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Srivastava, A.; Strasser, R.J. Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. Acta 1997, 1320, 95–106. [Google Scholar] [CrossRef]
- Bukhov, N.; Carpentier, R. Heterogeneity of photosystem II reaction centers as influenced by heat treatment of barley leaves. Physiol. Plant. 2000, 110, 279–285. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Angelini, G.; Ragni, P.; Esposito, D.; Giardi, P.; Pompili, M.L.; Moscardelli, R.; Giardi, M.T. A device to study the effect of space radiation on photosynthetic organisms. Phys. Med. 2001, 17 (Suppl. S1), 267–268. [Google Scholar]
- Ellison, A.M.; Gotelli, N.J. Evolutionary ecology of carnivorous plants. Trends Ecol. Evol. 2001, 16, 623–629. [Google Scholar] [CrossRef]
- Baranyai, B.; Joosten, H. Biology, ecology, use, conservation and cultivation of round-leaved sundew (Drosera rotundifolia L.): A review. Mires Peat 2016, 18, 1–28. [Google Scholar]
- Givnish, T.J.; Burkhardt, E.L.; Happel, R.E.; Weintraub, J.D. Carnivory in the bromeliad Brocchinia reducta with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient poor habitats. Am. Nat. 1984, 124, 479–497. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 2000, 151, 135–143. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J. Plant Physiol. 2000, 156, 46–51. [Google Scholar] [CrossRef]
- Hocking, P. Dry-matter production, mineral nutrient concentrations and nutrient distribution and redistribution in irrigated spring wheat. J. Plant Nutr. 1994, 17, 1289–1308. [Google Scholar] [CrossRef]
- Slack, A. Carnivorous Plants; MIT Press: Cambridge, UK, 2000. [Google Scholar]
- Adamec, L. Mineral nutrition of carnivorous plants: A review. Bot. Rev. 1997, 63, 273–299. [Google Scholar] [CrossRef]
- Farnsworth, E.J.; Ellison, A.M. Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. J. Ecol. 2008, 96, 213–221. [Google Scholar] [CrossRef]
- Pavlovič, A.; Krausko, M.; Libiakova, M.; Adamec, L. Feeding on prey increases photosynthetic efficiency in the in the carnivorous sundew Drosera capensis. Ann. Bot. 2014, 113, 69–78. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Y.; Berninger, F.; Li, C.Y. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Brestié, M.; Zivéak, M. PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: Protocols and applications. In Molecular Stress Physiology of Plants; Rout, G.M., Das, A.B., Eds.; Springer: Berlin, Gernamy, 2013; pp. 87–131. [Google Scholar]
- Royer, D.L.; McElwain, J.C.; Adams, J.M.; Wilf, P. Sensitivity of leaf size and shape to climate within Acer rubrum and Quercus kelloggii. New Phytol. 2008, 179, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Lowrie, A.; Robinson, A.; Nunn, R.; Ric, B. Drosera of the World, Oceania, Asia, Europe, North America; Robinson, A., Ed.; Redfern Natural History Productions Limited: Poole, UK, 2017; Volume 2, 546p. [Google Scholar]
- Crowder, A.A.; Pearson, M.C.; Grubb, P.J.; Langlois, P.H. Biological flora of the British Isles. J. Ecol. 1990, 78, 233–267. [Google Scholar] [CrossRef]
- Sprainaitytė, S. Review of Drosera intermedia herbarium specimens and new data on its distribution and ecology in Lithuania [Drosera intermedia Lietuvoje: Herbariumo pavyzdžių apžvalga ir nauji duomenys apiepaplitimą ir ekologiją]. Bot. Lith. 2015, 21, 39–45. [Google Scholar]
- Lindsay, R.A.; Riggall, J.; Bignal, E.M. Ombrogenous mires in Islay and Mull. Proc. R. Soc. Edinb.-Biol. 1983, 83, 341–371. [Google Scholar]
- Lindsay, R.; Rigall, J.; Burd, F. The use of small-scale surface patterns in the classification of British Peatlands. Aquil. Ser. Bot. 1985, 21, 67–79. [Google Scholar]
- Banaś, K.; Gos, K. Effect of peat-bog reclamtion on the physico-chemical characteristics of the ground water in peat. Pol. J. Ecol. 2004, 52, 69–74. [Google Scholar]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands; Oxford University Press: Oxford, UK, 2013; 382p. [Google Scholar]
- Baker, N.R.; Rosenquist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Percival, G.C.; Keary, I.P.; Al-Habsi, S. An assessment of the drought tolerance of Fraxinus genotypes for urban landscape plantings. Urban For. Urban Green. 2006, 5, 17–27. [Google Scholar] [CrossRef]
- Svensson, B.M. Competition between Sphagnum fuscum and Drosera rotundifolia: A case of ecosystem engineering. Oikos 1995, 74, 205–212. [Google Scholar] [CrossRef]
- Bruzzese, B.M.; Bowler, R.; Massicotte, H.B.; Fredeen, A.L. Photosynthetic light response in three carnivorous plant species: Drosera rotundifolia, D. capensis and Sarracenia leucophylla. Photosynthetica 2010, 48, 103–109. [Google Scholar] [CrossRef]
- Chmielewski, T.J.; Urban, D. Peatlands: Environmental functions. In Encyclopedia of Agrophysics; Glinski, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 548–551. [Google Scholar]
- Ilnicki, P. Peatlands and Peat; Wydawnictwo AR: Poznań, Poland, 2002; 606p. [Google Scholar]
- Wolf, E.; Gage, E.; Cooper, D.J. Drosera rotundifolia L. (Roundleaf Sundew): A Technical Conservation Assessment. USDA Forest Service, Rocky Mountain Region. 2006. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5250874.pdf (accessed on 23 February 2023).
- Stewart, N.C.; Nilsen, E.T. Drosera rotundifolia growth and nutrition in a natural population with special reference to the significance of insectivory. Can. J. Bot. 1992, 70, 1409–1416. [Google Scholar] [CrossRef]
- Hájek, T.; Adamec, L. Photosynthesis and dark respiration of leaves of terrestrial carnivorous plants. Biologia 2010, 65, 69–74. [Google Scholar] [CrossRef]
- Weng, X.-Y.; Xu, H.-X.; Jiang, D.-A. Characteristics of gas exchange, chlorophyll fluorescence and expression of key enzymes in photosynthesis during leaf senescence in rice plants. J. Integr. Plant Biol. 2005, 4–7, 560–566. [Google Scholar] [CrossRef]
- Kozaki, A.; Takeba, G. Photorespiration protects C3 plants from photooxidation. Nature 1996, 384, 557–560. [Google Scholar] [CrossRef]
- Baena-Gonzalez, E.; Barbato, R.; Aro, E. Role of phosphorylation in the repair cycle and oligomeric structure of photosystem II. Planta 1999, 208, 196–204. [Google Scholar] [CrossRef]
- Bailey, S.; Walters, R.G.; Jansson, S.; Horton, P. Acclimation of Arabidopsis thaliana to the light environment: The existence of separate low light and high light responses. Planta 2001, 213, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Hoch, W.A.; Singsaas, E.L.; Mccown, B.H. Resorption protection: Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiol. 2003, 133, 1296–1305. [Google Scholar] [CrossRef]
- Tkalec, M.; Dobos, M.; Babic, M.; Jurak, E. The acclimation of carnivorous round-leaved sundew (Drosera rotundifolia L.) to solar radiation. Acta Physiol. Plant. 2015, 37, 78. [Google Scholar] [CrossRef]
- Mckersie, B.D.; Leshem, Y.Y. Stress and Stress Coping in Cultivated Plants; Kluwer Academic: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Banaś, K. The hydrochemistry of peatland lakes as a result of the morphological characteristics of their basins. Oceanol. Hydrobiol. Stud. 2013, 42, 28–39. [Google Scholar] [CrossRef]
- Lazar, D.; Ilik, P. High temperature induced chlorophyll fluorescence changes in barley leaves: Comparison of the critical temperatures derived from fluorescence induction and fluorescence temperature curves. Plant Sci. 1997, 124, 159–164. [Google Scholar] [CrossRef]
- Frolec, J.; Ilik, P.; Krchnak, P.; Susila, P.; Naus, J. Irreversible changes in barley leaf chlorophyll fluorescence detected by the fluorescence temperature curve in a linear heating/cooling regime. Photosynthetica 2008, 46, 537–546. [Google Scholar] [CrossRef]
- Savitch, L.; Gray, G.; Huner, N.A. Feedback-limited photosynthesis and regulation of sucrose-starch accumulation during cold acclimation and low-temperature stress in a spring and winter wheat. Planta 1997, 201, 18–26. [Google Scholar] [CrossRef]
- Rapacz, M. Chlorophyll a fluorescence transient during freezing and recovery in winter wheat. Photosynthetica 2007, 45, 409–418. [Google Scholar] [CrossRef]
- Goodde, D.; Bornman, J.S. Regulation of photosynthesis in higher plants. In Molecular to Global Photosynthesis; Archer, M.D., Barber, J., Eds.; London Imperial College Press: London, UK, 2004; pp. 49–51. [Google Scholar]
- Thorén, L.M.; Tuomi, J.; Kämäräinen, T.; Laine, K. Resource availability affects investment in carnivory in Drosera rotundifolia. New Phytol. 2003, 159, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.H.; Lai, M.F. Estimating heat tolerance among plant species by two chlorophyll fluorescence parameters. Photosynthetica 2005, 43, 439–444. [Google Scholar] [CrossRef]
- Havaux, M.; Tardy, F.; Ravenel, J.; Chanu, D.; Parot, P. Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: Influence of the xanthophyll content. Plant Cell Environ. 1996, 19, 1359–1368. [Google Scholar] [CrossRef]
- Kóta, Z.; Horvath, L.I.; Droppa, M.; Horvath, G.; Farkas, T.; Pali, T. Protein assembly and heat stability in developing thylakoid membranes during greening. Proc. Natl. Acad. Sci. USA 2002, 99, 12149–12154. [Google Scholar] [CrossRef]
- Van Heerden, P.D.R.; Swanepoel, J.W.; Kruger, G.H.I. Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation. Environ. Exp. Bot. 2007, 61, 124–136. [Google Scholar] [CrossRef]
- Lauriano, J.A.; Ramalho, J.C.; Lidon, F.C.; Ceu Matos, M. Mechanisms of energy dissipation in peanut under water stress. Photosynthetica 2006, 44, 404–410. [Google Scholar] [CrossRef]
- Cornic, G.; Massacci, A. Leaf photosynthesis under drought stress. In Photosynthesis and the Environment; Baker, N.R., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1996; pp. 347–366. [Google Scholar]
- Razavi, E.; Pollet, B.; Steppe, K.; Labeke, M.C. Chlorophyll fluorescence as a tool for evaluation of drought stress in strawberry. Photosynthetica 2008, 46, 631–633. [Google Scholar] [CrossRef]
- Kaiser, W.M. Effects of water deficit on photosynthetic capacity. Physiol. Plant. 1987, 71, 142–149. [Google Scholar] [CrossRef]
- Naumann, J.C.; Young, D.R.; Anderson, J.E. Leaf chlorophyll fluorescence, reflectance, and physiological response to freshwater and saltwater flooding in the evergreen shrub, Myrica cerifera. Environ. Exp. Bot. 2008, 63, 402–409. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Li, M.; Yang, D.; Li, W. Leaf gas exchange characteristics and chlorophyll fluorescence of three wetland plants in response to long-term soil flooding. Photosynthetica 2007, 45, 222–228. [Google Scholar] [CrossRef]
- Aksmann, A.; Pokora, W.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Tukaj, Z. High hydrogen peroxide production and antioxidative enzymes expression in the Chlamydomonas reinhardtii cia3 mutant with an increased tolerance to cadmium and anthracene. Phycol. Res. 2016, 64, 300–311. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.10); Microcomputer Power: Ithaca, NY, USA, 2018; 536p. [Google Scholar]



| Summary of RDA analysis | ||||||
| D. anglica | D. intermedia | D. rotundifolia | ||||
| Axes | 1 | 2 | 1 | 2 | 1 | 2 |
| Eigenvalues | 0.108 | 0.064 | 0.237 | 0.064 | 0.141 | 0.052 |
| Species–environment correlations | 0.714 | 0.558 | 0.825 | 0.547 | 0.761 | 0.477 |
| Cumulative percentage variance | ||||||
| of species data | 10.8 | 17.2 | 23.7 | 30.1 | 14.1 | 19.3 |
| of species-environment relation | 51.6 | 81.9 | 67.3 | 85.6 | 63.4 | 86.8 |
| Sum of all canonical eigenvalues | 0.210 | 0.351 | 0.223 | |||
| Summary of RDA analysis | ||||||
| D. anglica | D. intermedia | D. rotundifolia | ||||
| Axes | 1 | 2 | 1 | 2 | 1 | 2 |
| Eigenvalues | 0.108 | 0.064 | 0.237 | 0.064 | 0.141 | 0.052 |
| Species–environment correlations | 0.714 | 0.558 | 0.825 | 0.547 | 0.761 | 0.477 |
| Cumulative percentage variance | ||||||
| of species data | 10.8 | 17.2 | 23.7 | 30.1 | 14.1 | 19.3 |
| of species-environment relation | 51.6 | 81.9 | 67.3 | 85.6 | 63.4 | 86.8 |
| Sum of all canonical eigenvalues | 0.210 | 0.351 | 0.223 | |||

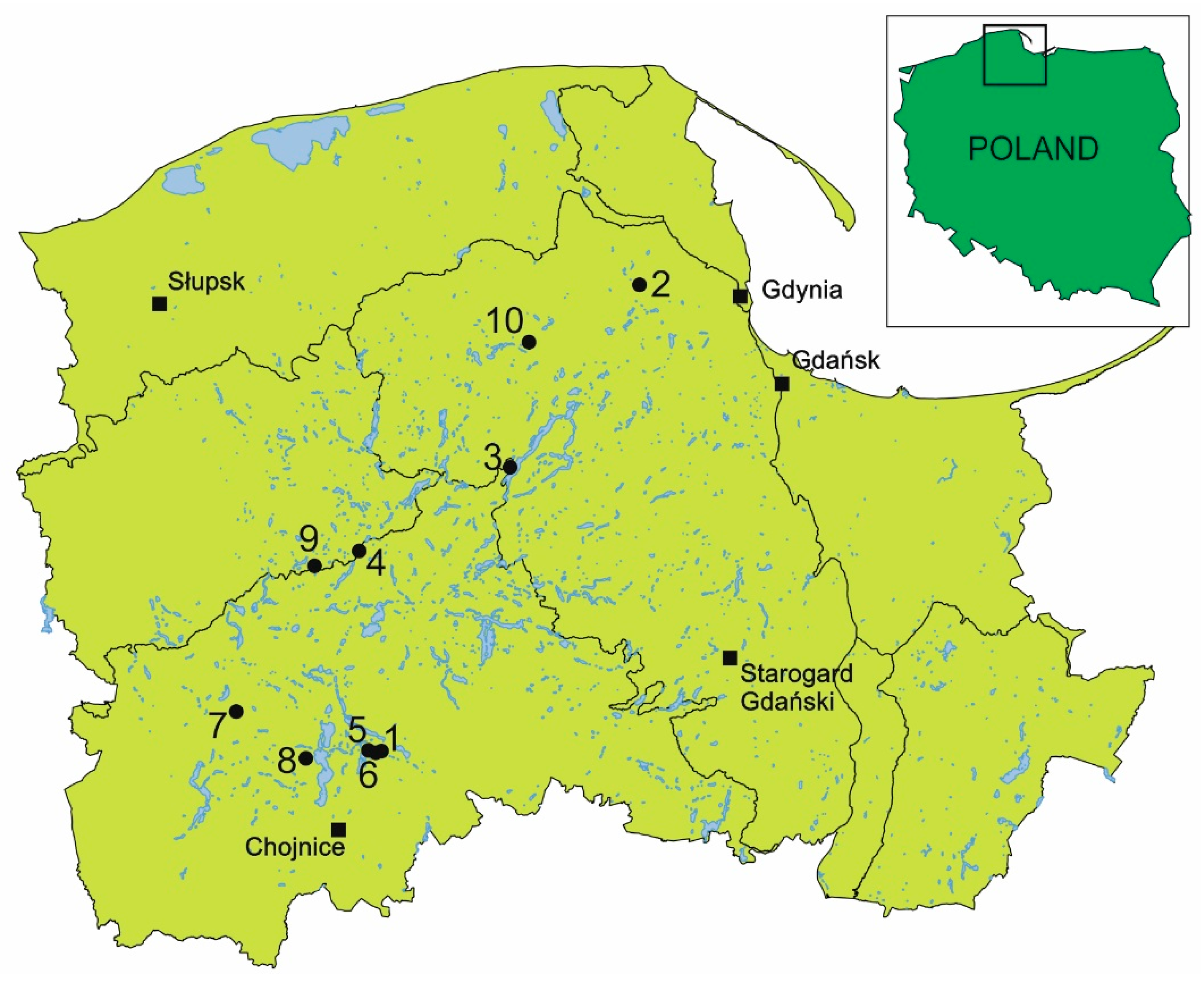
| No. | Coordinates | D. rotundifolia | D. anglica | D. intermedia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W | P | M | S | W | P | M | S | W | P | M | S | ||
| 1. | 53°48′52.9″ N 17°38′27.7″ E | + | + | + | + | + | |||||||
| 2. | 54°30′31.8″ N 18°16′40.7″ E | + | + | + | |||||||||
| 3. | 54°14′17.3″ N 17°57′37.0″ E | + | + | + | |||||||||
| 4. | 54°06′25.8″ N 17°34′55.8″ E | + | + | + | + | + | + | ||||||
| 5. | 53°48′58.3″ N 17°36′58.1″ E | + | + | + | |||||||||
| 6. | 53°48′51.2″ N 17°38′00.7″ E | + | + | ||||||||||
| 7. | 53°52′03.8″ N 17°16′54.5″ E | + | + | ||||||||||
| 8. | 53°48′10.1″ N 17°27′29.8″ E | + | |||||||||||
| 9. | 54°05′11.4″ N 17°28′09.6″ E | + | + | + | + | + | |||||||
| 10. | 54°25′27.5″ N 18°00′06.5″ E | + | |||||||||||
| No. of plants | 18 | 30 | 174 | 54 | 83 | 18 | 24 | 0 | 66 | 12 | 12 | 90 | |
| D. anglica | D. intermedia | D. rotundifolia | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| PAR light intensity [%] | 97.6 | 6.0 | 87.9 | 22.3 | 72.9 | 34.9 |
| Temperature [°C] | 20.6 | 3.1 | 18.4 | 1.6 | 18.1 | 1.97 |
| pH | 4.09 | 0.57 | 4.78 | 0.66 | 4.05 | 0.67 |
| Conductivity [mS cm−1] | 64.3 | 31.3 | 38.7 | 27.5 | 82.7 | 53.1 |
| Hydration [%] | 96.2 | 1.5 | 61.6 | 37.1 | 78.9 | 28.3 |
| Organic matter [%] | 93.6 | 2.1 | 51.6 | 42.7 | 75.4 | 33.4 |
| D. anglica | D. intermedia | D. rotundifolia | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Rosette width [cm] | 8.88 | 2.92 | 5.55 | 1.82 | 3.26 | 1.01 |
| Main axis height [cm] | 1.33 | 0.65 | 0.84 | 0.69 | 0.94 | 0.90 |
| Length of roots [cm] | 5.95 | 2.75 | 5.95 | 4.81 | 3.86 | 1.80 |
| Number of inflorescences | 0.6 | 0.84 | 0.7 | 0.8 | 0.5 | 0.7 |
| Number of flowers | 2.3 | 2.87 | 2.6 | 2.9 | 2.0 | 3.3 |
| Number of live leaves | 6.2 | 3.3 | 22.7 | 10.8 | 7.1 | 2.6 |
| Number of dead leaves | 4.8 | 1.7 | 9.4 | 5.2 | 5.4 | 2.0 |
| Species | N | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|
| D. anglica | 125 | 0.543 | 0.154 | 0.597 | 0.228 | 0.764 |
| D. intermedia | 180 | 0.571 | 0.118 | 0.572 | 0.248 | 0.800 |
| D. rotundifolia | 275 | 0.677 | 0.111 | 0.706 | 0.328 | 0.831 |
| Total | 580 | 0.616 | 0.137 | 0.645 | 0.228 | 0.831 |
| Species | Environmental Feature | |||||
|---|---|---|---|---|---|---|
| pH | Conductivity | Temperature | PAR Light Intensity | Hydration | Organic Matter | |
| D. anglica | −0.259 | −0.152 | 0.468 | 0.380 | −0.146 | 0.146 |
| D. intermedia | −0.171 | −0.115 | −0.067 | −0.176 | −0.007 | 0.027 |
| D. rotundifolia | 0.071 | −0.034 | −0.069 | −0.314 | −0.230 | −0.267 |
| Environmental Traits | ||||||
|---|---|---|---|---|---|---|
| Type of Substrate | pH | Conductivity | Temperature | PAR Light Intensity | Hydration | Organic Matter |
| D. anglica | ||||||
| water | −0.023 | −0.576 | 0.654 | 0.397 | −0.065 | 0.140 |
| peat | −0.552 | 0.675 | 0.533 | 0.352 | −0.457 | −0.587 |
| sphagnum | −0.811 | 0.950 | 0.290 | −0.495 | 0.155 | |
| D. intermedia | ||||||
| water | −0.751 | 0.599 | −0.681 | 0.710 | −0.056 | −0.143 |
| peat | −1.0 | 1.0 | 1.0 | 1.0 | −1.0 | −1.0 |
| sphagnum | 1.0 | 1.0 | −1.0 | −1.0 | −1.0 | |
| sand | −0.573 | 0.012 | 0.069 | −0.290 | 0.191 | 0.540 |
| D. rotundifolia | ||||||
| water | 0.172 | −0.762 | 0.207 | 0.199 | 0.937 | 0.747 |
| peat | 0.222 | 0.314 | 0.031 | −0.632 | −0.556 | −0.398 |
| sphagnum | 0.125 | 0.103 | −0.117 | −0.008 | −0.146 | 0.486 |
| sand | −0.590 | 0.087 | −0.052 | −0.569 | 0.463 | 0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaś, K.; Ronowski, R.; Marciniak, P. Effects of Environmental Conditions on the Individual Architectures and Photosynthetic Performances of Three Species in Drosera. Int. J. Mol. Sci. 2023, 24, 9823. https://doi.org/10.3390/ijms24129823
Banaś K, Ronowski R, Marciniak P. Effects of Environmental Conditions on the Individual Architectures and Photosynthetic Performances of Three Species in Drosera. International Journal of Molecular Sciences. 2023; 24(12):9823. https://doi.org/10.3390/ijms24129823
Chicago/Turabian StyleBanaś, Krzysztof, Rafał Ronowski, and Paweł Marciniak. 2023. "Effects of Environmental Conditions on the Individual Architectures and Photosynthetic Performances of Three Species in Drosera" International Journal of Molecular Sciences 24, no. 12: 9823. https://doi.org/10.3390/ijms24129823
APA StyleBanaś, K., Ronowski, R., & Marciniak, P. (2023). Effects of Environmental Conditions on the Individual Architectures and Photosynthetic Performances of Three Species in Drosera. International Journal of Molecular Sciences, 24(12), 9823. https://doi.org/10.3390/ijms24129823






