Expression of Cell-Adhesion Molecules in E. coli: A High Throughput Screening to Identify Paracellular Modulators
Abstract
1. Introduction
2. Results and Discussion
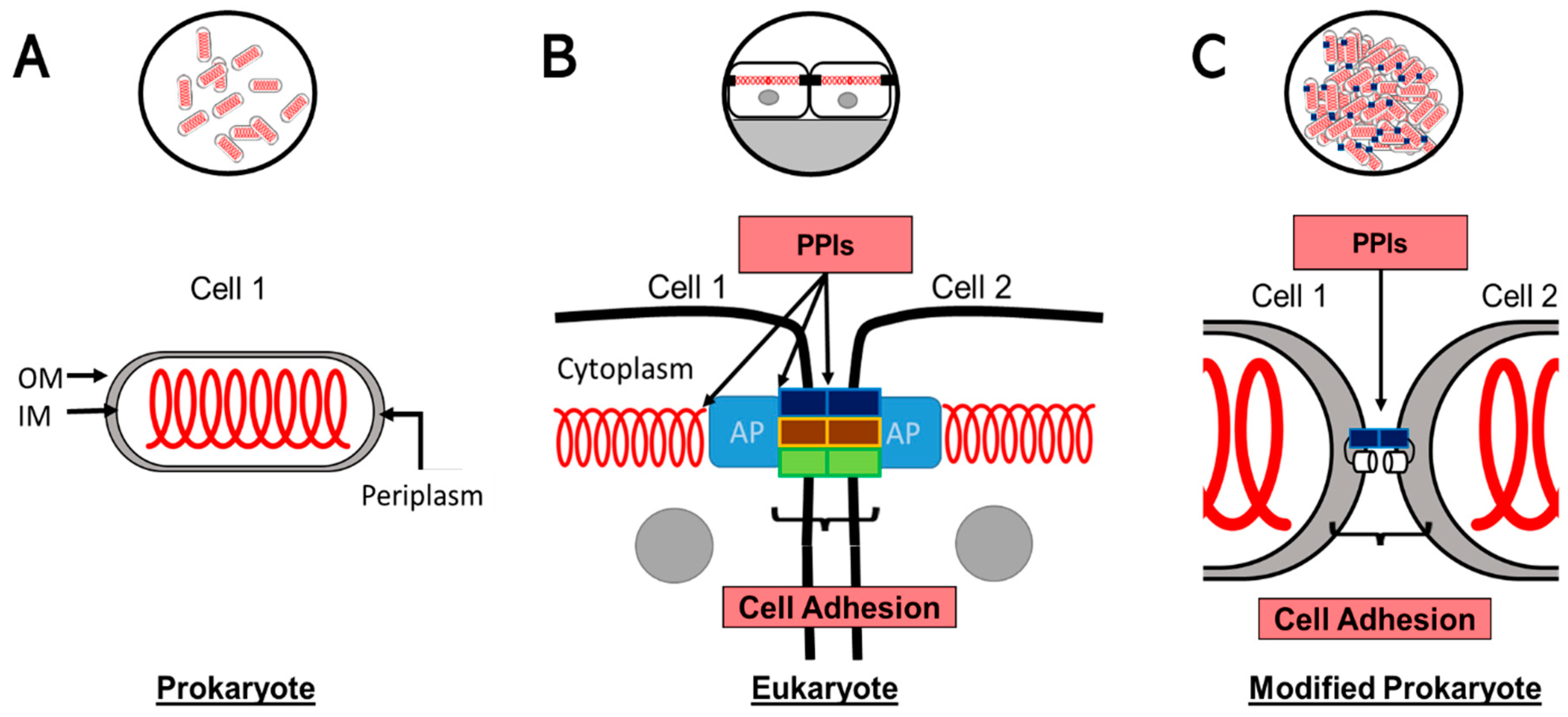
2.1. Conceptual Model
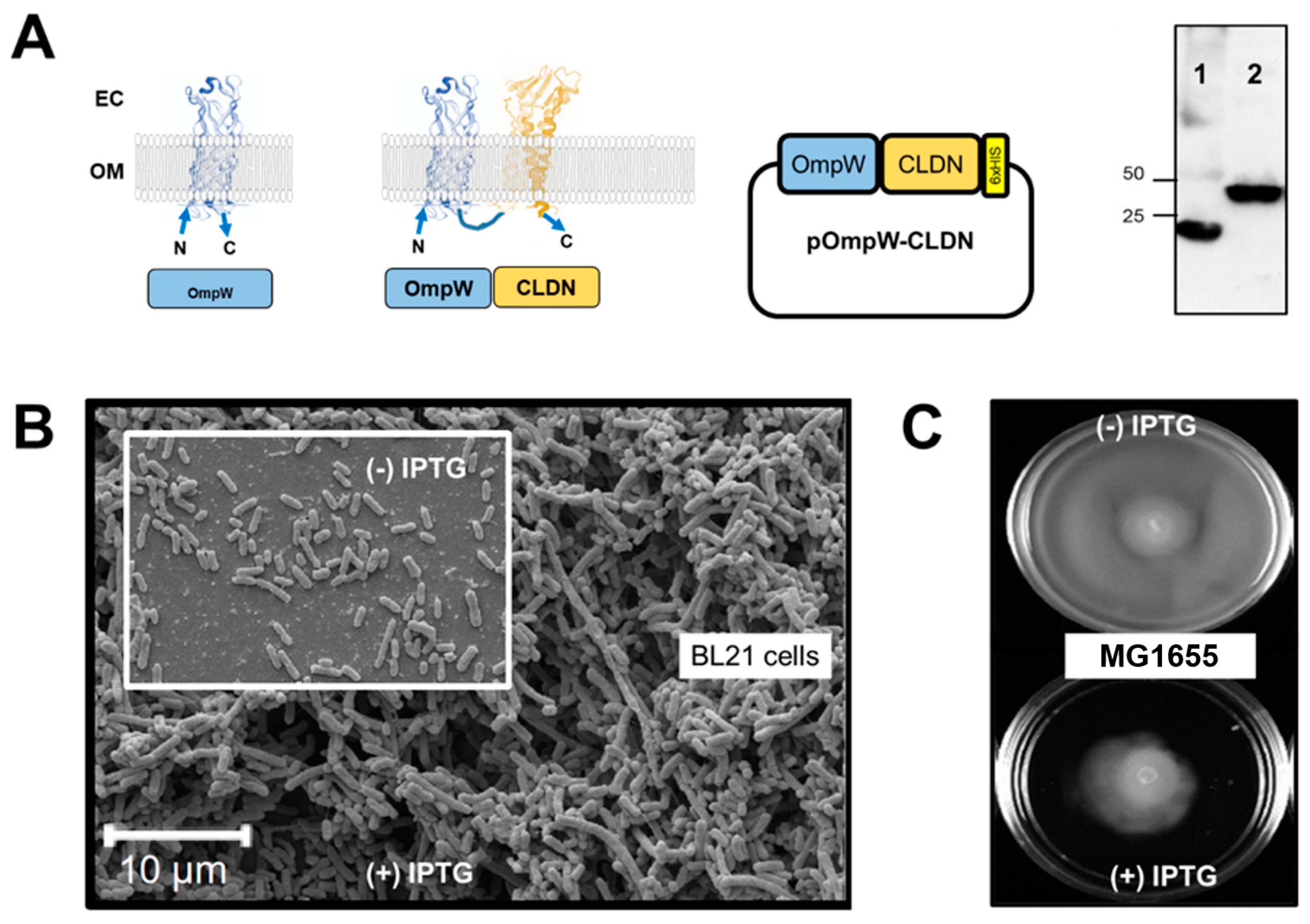
2.2. Expression System Design
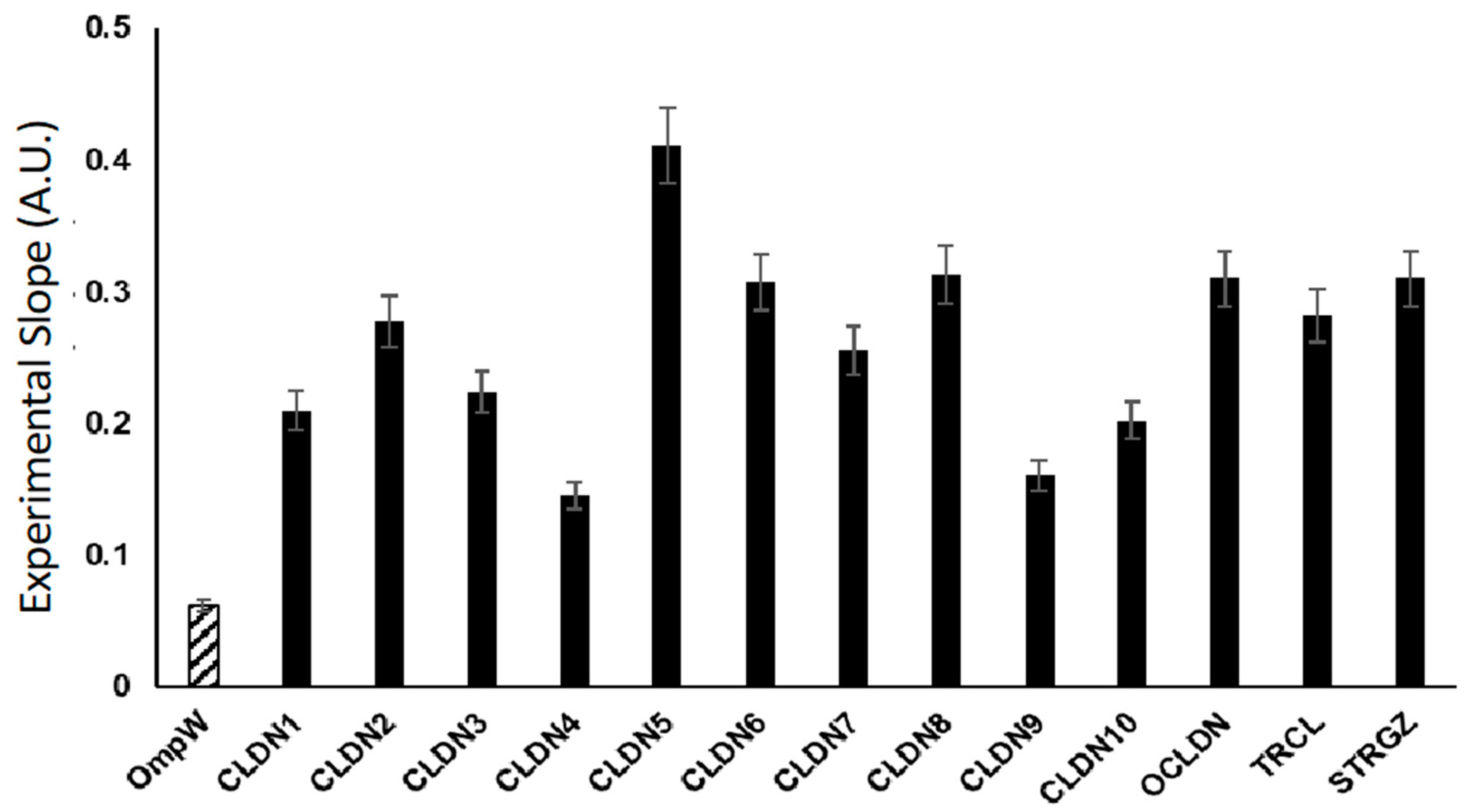
2.3. Flow Cytometry of Bacterial Cells Expressing 4-α-Helix CAMs
2.4. Ion Permeable CLDNs and the Hofmeister Effect
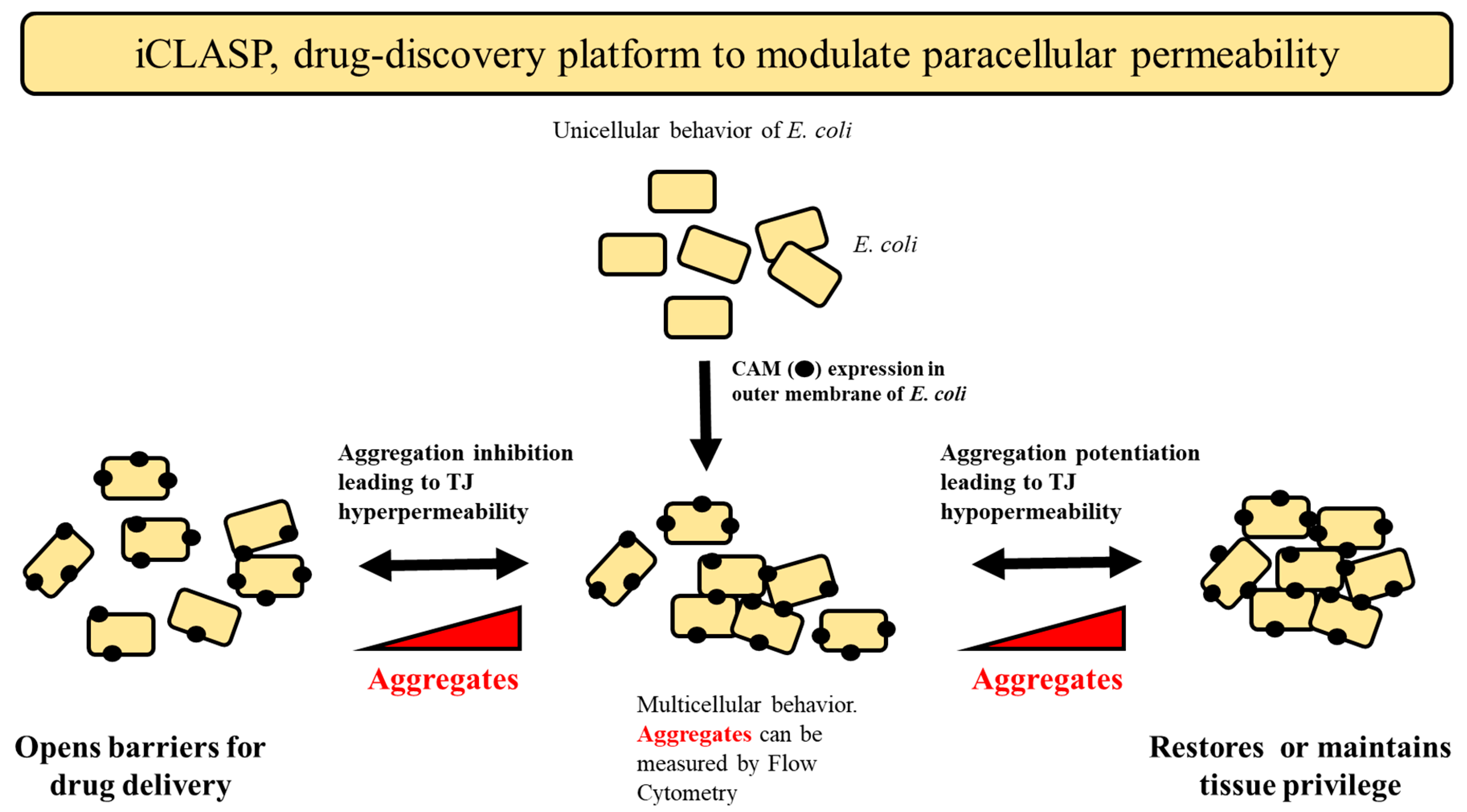
2.5. iCLASP Model
2.6. Paracellular Modulators of CLDN2 in Mammalian Cells A549
3. Materials and Methods
3.1. Reagents and Genes
3.2. Transformation, Cell Growth, and Protein Expression (LB, Fresh Transformations, Controls)
3.3. Flow Cytometry Data Collection and Analysis
3.4. Cell Proliferation Assay
3.5. Statistical Analysis
3.6. ChemBridge DIVERSet Library
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Ponce, J.; Brea, D.; Carrascal, M.; Guirao, V.; DeGregorio-Rocasolano, N.; Sobrino, T.; Castillo, J.; Dávalos, A.; Gasull, T. The effect of simvastatin on the proteome of detergent-resistant membrane domains: Decreases of specific proteins previously related to cytoskeleton regulation, calcium homeostasis and cell fate. Proteomics 2010, 10, 1954–1965. [Google Scholar] [CrossRef]
- Cabeen, M.T.; Jacobs-Wagner, C. Bacterial cell shape. Nat. Rev. Microbiol. 2005, 3, 601–610. [Google Scholar] [CrossRef]
- Young, K.D. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 2006, 70, 660–703. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.J.; Carballido-Lopez, R.; Errington, J. Control of cell shape in bacteria: Helical, actin-like filaments in Bacillus subtilis. Cell 2001, 104, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Drubin, D.G.; Nelson, W.J. Origins of cell polarity. Cell 1996, 84, 335–344. [Google Scholar] [CrossRef]
- Marshall, W.F. Pattern Formation and Complexity in Single Cells. Curr. Biol. 2020, 30, R544–R552. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, G.; Myat, M.M. Microtubule Motors in Establishment of Epithelial Cell Polarity. Cold Spring Harb. Perspect. Biol. 2018, 10, a027896. [Google Scholar] [CrossRef]
- Naftalin, R.J.; Tripathi, S. The roles of paracellular and transcellular pathways and submucosal space in isotonic water absorption by rabbit ileum. J. Physiol. 1986, 370, 409–432. [Google Scholar] [CrossRef]
- Zuo, L.; Kuo, W.T.; Turner, J.R. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef]
- Aplin, A.E.; Howe, A.; Alahari, S.K.; Juliano, R.L. Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol. Rev. 1998, 50, 197–263. [Google Scholar] [PubMed]
- Kassa, E.G.; Zlotkin-Rivkin, E.; Friedman, G.; Ramachandran, R.P.; Melamed-Book, N.; Weiss, A.M.; Belenky, M.; Reichmann, D.; Breuer, W.; Pal, R.R.; et al. Enteropathogenic Escherichia coli remodels host endosomes to promote endocytic turnover and breakdown of surface polarity. PLoS Pathog. 2019, 15, e1007851. [Google Scholar] [CrossRef] [PubMed]
- Cavenagh, J.D.; Cahill, M.R.; Kelsey, S.M. Adhesion molecules in clinical medicine. Crit. Rev. Clin. Lab. Sci. 1998, 35, 415–459. [Google Scholar] [CrossRef] [PubMed]
- Cid i Xutgla, M.C.; Coll-Vincent i Puig, B.; Grau i Junyent, J.M. Adhesion molecules in the interactions of leukocytes, endothelium, and extracellular matrix (II). Relevance in human clinical medicine and potential therapeutic applications. Med. Clin. 1997, 108, 503–511. [Google Scholar]
- Harjunpaa, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Harris, T.J.; Tepass, U. Adherens junctions: From molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 502–514. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. Occludin and claudins in tight-junction strands: Leading or supporting players? Trends Cell Biol. 1999, 9, 268–273. [Google Scholar] [CrossRef]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Zahraoui, A.; Louvard, D.; Galli, T. Tight junction, a platform for trafficking and signaling protein complexes. J. Cell Biol. 2000, 151, F31–F36. [Google Scholar] [CrossRef]
- Adil, M.S.; Narayanan, S.P.; Somanath, P.R. Cell-cell junctions: Structure and regulation in physiology and pathology. Tissue Barriers 2021, 9, 1848212. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, U.; Schuetz, A. Structural Features of Tight-Junction Proteins. Int. J. Mol. Sci. 2019, 20, 6020. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, T.; Kummer, D.; Ebnet, K. Junctional adhesion molecule-A: Functional diversity through molecular promiscuity. Cell. Mol. Life Sci. 2018, 75, 1393–1409. [Google Scholar] [CrossRef]
- Joseph-Silverstein, J.; Silverstein, R.L. Cell adhesion molecules: An overview. Cancer Investig. 1998, 16, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Lustig, M.; Grumet, M.; Shirai, T. Cell adhesion molecules regulate guidance of dorsal root ganglion axons in the marginal zone and their invasion into the mantle layer of embryonic spinal cord. Dev. Biol. 1997, 192, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G.; Borsig, L. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Borsig, L. Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis. Front. Immunol. 2019, 10, 2120. [Google Scholar] [CrossRef]
- Yin, M.; Li, C.; Jiang, J.; Le, J.; Luo, B.; Yang, F.; Fang, Y.; Yang, M.; Deng, Z.; Ni, W.; et al. Cell adhesion molecule-mediated therapeutic strategies in atherosclerosis: From a biological basis and molecular mechanism to drug delivery nanosystems. Biochem. Pharmacol. 2021, 186, 114471. [Google Scholar] [CrossRef]
- Di Giovanna, A.P.; Tibo, A.; Silvestri, L.; Mullenbroich, M.C.; Costantini, I.; Allegra Mascaro, A.L.; Sacconi, L.; Frasconi, P.; Pavone, F.S. Whole-Brain Vasculature Reconstruction at the Single Capillary Level. Sci. Rep. 2018, 8, 12573. [Google Scholar] [CrossRef]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Tsukita, S. Paracellular barrier and channel functions of TJ claudins in organizing biological systems: Advances in the field of barriology revealed in knockout mice. Semin. Cell Dev. Biol. 2014, 36, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.; Walsh, D.M.; O’Malley, T.; Hudson, N.; Crosbie, D.E.; Loftus, T.; Sheehan, F.; McDaid, J.; Humphries, M.M.; Callanan, J.J.; et al. Autoregulated paracellular clearance of amyloid-beta across the blood-brain barrier. Sci. Adv. 2015, 1, e1500472. [Google Scholar] [CrossRef]
- Venugopal, S.; Anwer, S.; Szaszi, K. Claudin-2: Roles beyond Permeability Functions. Int. J. Mol. Sci. 2019, 20, 5655. [Google Scholar] [CrossRef]
- Matsumoto, K.; Imasato, M.; Yamazaki, Y.; Tanaka, H.; Watanabe, M.; Eguchi, H.; Nagano, H.; Hikita, H.; Tatsumi, T.; Takehara, T.; et al. Claudin 2 deficiency reduces bile flow and increases susceptibility to cholesterol gallstone disease in mice. Gastroenterology 2014, 147, 1134–1145.e1110. [Google Scholar] [CrossRef]
- Milatz, S. A Novel Claudinopathy Based on Claudin-10 Mutations. Int. J. Mol. Sci. 2019, 20, 5396. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269, G467–G475. [Google Scholar] [CrossRef]
- Panwar, S.; Sharma, S.; Tripathi, P. Role of Barrier Integrity and Dysfunctions in Maintaining the Healthy Gut and Their Health Outcomes. Front. Physiol. 2021, 12, 715611. [Google Scholar] [CrossRef]
- Si, Y.; Liu, S.; Liu, X.; Jacobs, J.L.; Cheng, M.; Niu, Y.; Jin, Q.; Wang, T.; Yang, W. A human claudin-1-derived peptide inhibits hepatitis C virus entry. Hepatology 2012, 56, 507–515. [Google Scholar] [CrossRef]
- Sladojevic, N.; Stamatovic, S.M.; Johnson, A.M.; Choi, J.; Hu, A.; Dithmer, S.; Blasig, I.E.; Keep, R.F.; Andjelkovic, A.V. Claudin-1-Dependent Destabilization of the Blood-Brain Barrier in Chronic Stroke. J. Neurosci. 2019, 39, 743–757. [Google Scholar] [CrossRef]
- Staat, C.; Coisne, C.; Dabrowski, S.; Stamatovic, S.M.; Andjelkovic, A.V.; Wolburg, H.; Engelhardt, B.; Blasig, I.E. Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers. Biomaterials 2015, 54, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Wasielewska, J.M.; White, A.R. Focused Ultrasound-mediated Drug Delivery in Humans—A Path Towards Translation in Neurodegenerative Diseases. Pharm. Res. 2022, 39, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Fishman, P.S.; Eisenberg, H.M.; Kaplitt, M.; Baltuch, G.; Chang, J.W.; Chang, W.C.; Martinez Fernandez, R.; Del Alamo, M.; Halpern, C.H.; et al. Trial of Globus Pallidus Focused Ultrasound Ablation in Parkinson’s Disease. N. Engl. J. Med. 2023, 388, 683–693. [Google Scholar] [CrossRef]
- Engevik, A.C.; Krystofiak, E.S.; Kaji, I.; Meyer, A.R.; Weis, V.G.; Goldstein, A.; Coutts, A.W.; Melkamu, T.; Saqui-Salces, M.; Goldenring, J.R. Recruitment of Polarity Complexes and Tight Junction Proteins to the Site of Apical Bulk Endocytosis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 59–80. [Google Scholar] [CrossRef]
- Otani, T.; Nguyen, T.P.; Tokuda, S.; Sugihara, K.; Sugawara, T.; Furuse, K.; Miura, T.; Ebnet, K.; Furuse, M. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J. Cell Biol. 2019, 218, 3372–3396. [Google Scholar] [CrossRef]
- Citi, S. The mechanobiology of tight junctions. Biophys. Rev. 2019, 11, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Warner, M.; Mendoza, C.; Memmott, C.; LeCheminant, T.; Bailey, S.; Christensen, C.; Keller, J.; Suli, A.; Mizrachi, D. Chimeric Claudins: A New Tool to Study Tight Junction Structure and Function. Int. J. Mol. Sci. 2021, 22, 4947. [Google Scholar] [CrossRef]
- Nasako, H.; Akizuki, R.; Takashina, Y.; Ishikawa, Y.; Shinoda, T.; Shirouzu, M.; Asai, T.; Matsunaga, T.; Endo, S.; Ikari, A. Claudin-2 binding peptides, VPDSM and DSMKF, down-regulate claudin-2 expression and anticancer resistance in human lung adenocarcinoma A549 cells. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118642. [Google Scholar] [CrossRef]
- McAlister, M.S.; Mott, H.R.; van der Merwe, P.A.; Campbell, I.D.; Davis, S.J.; Driscoll, P.C. NMR analysis of interacting soluble forms of the cell-cell recognition molecules CD2 and CD48. Biochemistry 1996, 35, 5982–5991. [Google Scholar] [CrossRef]
- van der Merwe, P.A.; Barclay, A.N. Analysis of cell-adhesion molecule interactions using surface plasmon resonance. Curr. Opin. Immunol. 1996, 8, 257–261. [Google Scholar] [CrossRef] [PubMed]
- McEver, R.P.; Cummings, R.D. Perspectives series: Cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Investig. 1997, 100, 485–491. [Google Scholar] [CrossRef]
- Choi, W.; Acharya, B.R.; Peyret, G.; Fardin, M.A.; Mege, R.M.; Ladoux, B.; Yap, A.S.; Fanning, A.S.; Peifer, M. Remodeling the zonula adherens in response to tension and the role of afadin in this response. J. Cell Biol. 2016, 213, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytonen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef] [PubMed]
- Cacace, M.G.; Landau, E.M.; Ramsden, J.J. The Hofmeister series: Salt and solvent effects on interfacial phenomena. Q. Rev. Biophys. 1997, 30, 241–277. [Google Scholar] [CrossRef]
- Koch, A.W.; Pokutta, S.; Lustig, A.; Engel, J. Calcium binding and homoassociation of E-cadherin domains. Biochemistry 1997, 36, 7697–7705. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Nagidi, S.H.; Collett, K.; McKell, J.; Mizrachi, D. Calcium regulates the interplay between the tight junction and epithelial adherens junction at the plasma membrane. FEBS Lett. 2021, 596, 219–231. [Google Scholar] [CrossRef]
- Rollauer, S.E.; Sooreshjani, M.A.; Noinaj, N.; Buchanan, S.K. Outer membrane protein biogenesis in Gram-negative bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20150023. [Google Scholar] [CrossRef]
- Jeeves, M.; Knowles, T.J. A novel pathway for outer membrane protein biogenesis in Gram-negative bacteria. Mol. Microbiol. 2015, 97, 607–611. [Google Scholar] [CrossRef]
- Wang, S.; Wingreen, N.S. Cell shape can mediate the spatial organization of the bacterial cytoskeleton. Biophys. J. 2013, 104, 541–552. [Google Scholar] [CrossRef]
- McVey, M.J.; Spring, C.M.; Kuebler, W.M. Improved resolution in extracellular vesicle populations using 405 instead of 488 nm side scatter. J. Extracell. Vesicles 2018, 7, 1454776. [Google Scholar] [CrossRef]
- Kishigami, S.; Kanaya, E.; Kikuchi, M.; Ito, K. DsbA-DsbB interaction through their active site cysteines. Evidence from an odd cysteine mutant of DsbA. J. Biol. Chem. 1995, 270, 17072–17074. [Google Scholar] [CrossRef] [PubMed]
- Missiakas, D.; Georgopoulos, C.; Raina, S. Identification and characterization of the Escherichia coli gene dsbB, whose product is involved in the formation of disulfide bonds in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 7084–7088. [Google Scholar] [CrossRef] [PubMed]
- Whitley, P.; von Heijne, G. The DsbA-DsbB system affects the formation of disulfide bonds in periplasmic but not in intramembraneous protein domains. FEBS Lett. 1993, 332, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cierpicki, T.; Jimenez, R.H.; Lukasik, S.M.; Ellena, J.F.; Cafiso, D.S.; Kadokura, H.; Beckwith, J.; Bushweller, J.H. NMR solution structure of the integral membrane enzyme DsbB: Functional insights into DsbB-catalyzed disulfide bond formation. Mol. Cell 2008, 31, 896–908. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, W.; Nicoll, R.A.; Bredt, D.S. Stargazin is an AMPA receptor auxiliary subunit. Proc. Natl. Acad. Sci. USA 2005, 102, 485–490. [Google Scholar] [CrossRef]
- Alves, M.G.; Oliveira, P.F.; Socorro, S.; Moreira, P.I. Impact of diabetes in blood-testis and blood-brain barriers: Resemblances and differences. Curr. Diabetes Rev. 2012, 8, 401–412. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef]
- Del Vecchio, G.; Tscheik, C.; Tenz, K.; Helms, H.C.; Winkler, L.; Blasig, R.; Blasig, I.E. Sodium caprate transiently opens claudin-5-containing barriers at tight junctions of epithelial and endothelial cells. Mol. Pharm. 2012, 9, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Karrberg, L.; Suljovic, D.; Seeliger, F.; Soderberg, M.; Perez-Alcazar, M.; Van Zuydam, N.; Abrahamsson, B.; Hugerth, A.M.; Davies, N.; et al. Impact of Intestinal Concentration and Colloidal Structure on the Permeation-Enhancing Efficiency of Sodium Caprate in the Rat. Mol. Pharm. 2022, 19, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, W.A.; Fu, P.; Chakraborty, T.; Hussain, M.; Guthikonda, M.; Rafols, J.A.; Ding, Y. At low doses ethanol maintains blood-brain barrier (BBB) integrity after hypoxia and reoxygenation: A brain slice study. Neurol. Res. 2013, 35, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Gunzel, D.; Conrad, M.P.; Lee, I.F.; Amasheh, S.; Fromm, M.; Yu, A.S. Charge-selective claudin channels. Ann. N. Y. Acad. Sci. 2012, 1257, 20–28. [Google Scholar] [CrossRef]
- Hani, F.M.; Cole, A.E.; Altman, E. The ability of salts to stabilize proteins in vivo or intracellularly correlates with the Hofmeister series of ions. Int. J. Biochem. Mol. Biol. 2019, 10, 23–31. [Google Scholar] [PubMed]
- Xie, W.J.; Gao, Y.Q. A Simple Theory for the Hofmeister Series. J. Phys. Chem. Lett. 2013, 4, 4247–4252. [Google Scholar] [CrossRef]
- Leberman, R. The Hofmeister series and ionic strength. FEBS Lett. 1991, 284, 293–294. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P.S. The inverse and direct Hofmeister series for lysozyme. Proc. Natl. Acad. Sci. USA 2009, 106, 15249–15253. [Google Scholar] [CrossRef]
- Muto, S.; Hata, M.; Taniguchi, J.; Tsuruoka, S.; Moriwaki, K.; Saitou, M.; Furuse, K.; Sasaki, H.; Fujimura, A.; Imai, M.; et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl. Acad. Sci. USA 2010, 107, 8011–8016. [Google Scholar] [CrossRef]
- Breiderhoff, T.; Himmerkus, N.; Stuiver, M.; Mutig, K.; Will, C.; Meij, I.C.; Bachmann, S.; Bleich, M.; Willnow, T.E.; Muller, D. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc. Natl. Acad. Sci. USA 2012, 109, 14241–14246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, J.; Li, L.; Wang, Y.; Li, Y.; Zhang, S. Claudin Family Participates in the Pathogenesis of Inflammatory Bowel Diseases and Colitis-Associated Colorectal Cancer. Front. Immunol. 2019, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Nusrat, A. Claudin switching: Physiological plasticity of the Tight Junction. Semin. Cell Dev. Biol. 2015, 42, 22–29. [Google Scholar] [CrossRef]
- Capaldo, C.T. Claudin Barriers on the Brink: How Conflicting Tissue and Cellular Priorities Drive IBD Pathogenesis. Int. J. Mol. Sci. 2023, 24, 8562. [Google Scholar] [CrossRef] [PubMed]
- Hichino, A.; Okamoto, M.; Taga, S.; Akizuki, R.; Endo, S.; Matsunaga, T.; Ikari, A. Down-regulation of Claudin-2 Expression and Proliferation by Epigenetic Inhibitors in Human Lung Adenocarcinoma A549 Cells. J. Biol. Chem. 2017, 292, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Tabaries, S.; Annis, M.G.; Lazaris, A.; Petrillo, S.K.; Huxham, J.; Abdellatif, A.; Palmieri, V.; Chabot, J.; Johnson, R.M.; Van Laere, S.; et al. Claudin-2 promotes colorectal cancer liver metastasis and is a biomarker of the replacement type growth pattern. Commun. Biol. 2021, 4, 657. [Google Scholar] [CrossRef]
- Ikari, A.; Watanabe, R.; Sato, T.; Taga, S.; Shimobaba, S.; Yamaguchi, M.; Yamazaki, Y.; Endo, S.; Matsunaga, T.; Sugatani, J. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim. Biophys. Acta 2014, 1843, 2079–2088. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, Q.; del Rincon, S.V.; Lovato, A.; Marques, M.; Witcher, M. Gallotannin imposes S phase arrest in breast cancer cells and suppresses the growth of triple-negative tumors in vivo. PLoS ONE 2014, 9, e92853. [Google Scholar] [CrossRef]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.A.; Shao, P.; Farag, M.A. Recent Advances in Tannic Acid (Gallotannin) Anticancer Activities and Drug Delivery Systems for Efficacy Improvement; A Comprehensive Review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef]
- Barnes, R.C.; Kim, H.; Fang, C.; Bennett, W.; Nemec, M.; Sirven, M.A.; Suchodolski, J.S.; Deutz, N.; Britton, R.A.; Mertens-Talcott, S.U.; et al. Body Mass Index as a Determinant of Systemic Exposure to Gallotannin Metabolites during 6-Week Consumption of Mango (Mangifera indica L.) and Modulation of Intestinal Microbiota in Lean and Obese Individuals. Mol. Nutr. Food Res. 2019, 63, e1800512. [Google Scholar] [CrossRef]
- Erakovic Haber, V.; Spaventi, R. Discovery and Development of Novel Drugs. Prog. Mol. Subcell. Biol. 2017, 55, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Leeson, P.D. Mapping the Efficiency and Physicochemical Trajectories of Successful Optimizations. J. Med. Chem. 2018, 61, 6421–6467. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.; Rosch, J.; Putnam, D. Concepts, technologies, and practices for drug delivery past the blood-brain barrier to the central nervous system. J. Control. Release 2016, 240, 251–266. [Google Scholar] [CrossRef]
- Gallen, C.C. Strategic challenges in neurotherapeutic pharmaceutical development. NeuroRx 2004, 1, 165–180. [Google Scholar] [CrossRef]
- Schmidt, B.J.; Papin, J.A.; Musante, C.J. Mechanistic systems modeling to guide drug discovery and development. Drug Discov. Today 2013, 18, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Elbakary, B.; Badhan, R.K.S. A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Sci. Rep. 2020, 10, 3788. [Google Scholar] [CrossRef]
- Rautio, J.; Laine, K.; Gynther, M.; Savolainen, J. Prodrug approaches for CNS delivery. AAPS J. 2008, 10, 92–102. [Google Scholar] [CrossRef]
- Shugarts, S.; Benet, L.Z. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm. Res. 2009, 26, 2039–2054. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollins, J.; Worthington, T.; Dransfield, A.; Whitney, J.; Stanford, J.; Hooke, E.; Hobson, J.; Wengler, J.; Hope, S.; Mizrachi, D. Expression of Cell-Adhesion Molecules in E. coli: A High Throughput Screening to Identify Paracellular Modulators. Int. J. Mol. Sci. 2023, 24, 9784. https://doi.org/10.3390/ijms24129784
Rollins J, Worthington T, Dransfield A, Whitney J, Stanford J, Hooke E, Hobson J, Wengler J, Hope S, Mizrachi D. Expression of Cell-Adhesion Molecules in E. coli: A High Throughput Screening to Identify Paracellular Modulators. International Journal of Molecular Sciences. 2023; 24(12):9784. https://doi.org/10.3390/ijms24129784
Chicago/Turabian StyleRollins, Jay, Tyler Worthington, Allison Dransfield, Jordan Whitney, Jordan Stanford, Emily Hooke, Joseph Hobson, Jacob Wengler, Sandra Hope, and Dario Mizrachi. 2023. "Expression of Cell-Adhesion Molecules in E. coli: A High Throughput Screening to Identify Paracellular Modulators" International Journal of Molecular Sciences 24, no. 12: 9784. https://doi.org/10.3390/ijms24129784
APA StyleRollins, J., Worthington, T., Dransfield, A., Whitney, J., Stanford, J., Hooke, E., Hobson, J., Wengler, J., Hope, S., & Mizrachi, D. (2023). Expression of Cell-Adhesion Molecules in E. coli: A High Throughput Screening to Identify Paracellular Modulators. International Journal of Molecular Sciences, 24(12), 9784. https://doi.org/10.3390/ijms24129784






