Effect of Manual Lymphatic Drainage on the Concentrations of Selected Adipokines, Cytokines, C-Reactive Protein and Parameters of Carbohydrate and Lipid Metabolism in Patients with Abnormal Body Mass Index: Focus on Markers of Obesity and Insulin Resistance
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Patients with Normal Body Mass Index (Group I), Overweight Patients (Group II), Obese Patients (Group III) and Controls (Group IV)
2.2. Analysis of the Relationship between Biochemical Parameters and Anthropometric Indicators (BMI, WHR, VAT) of Patients in the Study Group and Those in the Control Group
2.2.1. Correlations between Biochemical Parameters and the BMI in the Study and Control Groups
2.2.2. Correlation between Biochemical Parameters and the WHR of Patients in the Study Group and the Control Group
2.2.3. Correlations between Biochemical Parameters and the VAT Value in the Study and Control Groups
2.3. Cut-Off Values of the Analyzed Biochemical Parameters in Identifying the Risk of Obesity in Patients in the Study and Control Groups
2.4. Cut-Off Values for the Analyzed Biochemical Parameters in Identifying the Risk of Insulin Resistance in Patients from the Study and Control Groups
2.5. Evaluation of the Impact of 10 MLD Sessions on Biochemical Parameters at Stages 0′ and 1′ in the Group of Patients with a Normal Body Mass Index (Group I), Overweight Patients (Group II), Obese Patients (Group III) and Control IV (without MLD Therapy)
3. Discussion
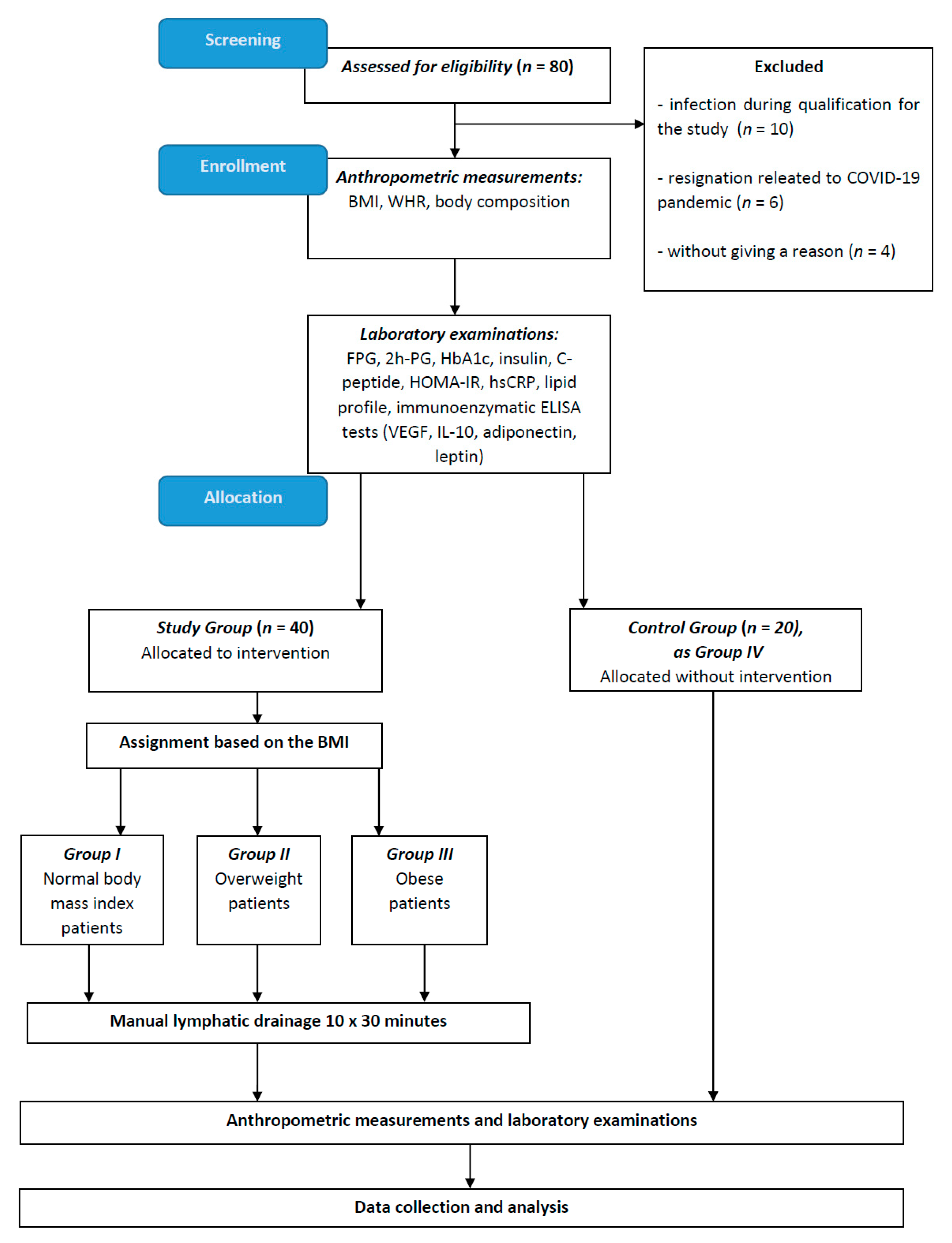
4. Materials and Methods
4.1. Data Collection
4.2. Anthropometric and Blood Pressure Measurements
4.3. Sample Collection and Laboratory Analyses
4.4. Intervention MLD
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| COVID-19 | coronavirus disease 2019 |
| eNOS | endothelial nitric oxide synthase |
| FFA | free fatty acids |
| FPG | fasting plasma glucose |
| GLUT4 | glucose transporter type 4 |
| HbA1c | glycated hemoglobin |
| HDL-C | high-density lipoprotein cholesterol |
| HOMA-IR | Homeostatic Model Assessment-Insulin Resistance |
| HDLECs | human dermal lymphatic endothelial cells |
| hsCRP | high-sensitivity C-reactive protein |
| IDF | International Diabetes Federation |
| IL-10 | interleukin-10 |
| INSRs | insulin receptors |
| IR | insulin resistance |
| LDL-C | low-density lipoprotein cholesterol |
| LECs | lymphoid endothelial cells |
| MLD | manual lymphatic drainage |
| TG | triglycerides |
| T2DM | type 2 diabetes mellitus |
| VAT | visceral adipose tissue |
| VEGF | vascular endothelial growth factor |
| WAT | white adipose tissue |
| WHR | waist–hip ratio |
| 2h-PG | 2h-post-load glucose |
References
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; World Health Organization: Genève, Switzerland, 2000; Volume 894.
- Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-Mass Index and Cause-Specific Mortality in 900 000 Adults: Collaborative Analyses of 57 Prospective Studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight: World Health Organization; World Health Organization: Geneva, Switzerland, 2019.
- Tong, Y.; Xu, S.; Huang, L.; Chen, C. Obesity and Insulin Resistance: Pathophysiology and Treatment. Drug Discov. Today 2022, 27, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin Resistance in Obesity: An Overview of Fundamental Alterations. Eat. Weight. Disord. 2019, 23, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3611. [Google Scholar] [CrossRef]
- Antoniak, K.; Hansdorfer-Korzon, R.; Mrugacz, M.; Zorena, K. Adipose Tissue and Biological Factors. Possible Link between Lymphatic System Dysfunction and Obesity. Metabolites 2021, 11, 617. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of Muscle Insulin Resistance and the Cross-Talk with Liver and Adipose Tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef]
- Fryk, E.; Olausson, J.; Mossberg, K.; Strindberg, L.; Schmelz, M.; Brogren, H.; Gan, L.-M.; Piazza, S.; Provenzani, A.; Becattini, B.; et al. Hyperinsulinemia and Insulin Resistance in the Obese May Develop as Part of a Homeostatic Response to Elevated Free Fatty Acids: A Mechanistic Case-Control and a Population-Based Cohort Study. EBioMedicine 2021, 65, 103264. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef]
- Czech, M.P. Mechanisms of Insulin Resistance Related to White, Beige, and Brown Adipocytes. Mol. Metab. 2020, 34, 27–42. [Google Scholar] [CrossRef]
- Olson, A.L. Regulation of GLUT4 and Insulin-Dependent Glucose Flux. ISRN Mol. Biol. 2012, 2012, 856987. [Google Scholar] [CrossRef]
- Si, Y.; Wang, A.; Yang, Y.; Liu, H.; Gu, S.; Mu, Y.; Lyu, Z. Fasting Blood Glucose and 2-h Postprandial Blood Glucose Predict Hypertension: A Report from the REACTION Study. Diabetes Ther. 2021, 12, 1117. [Google Scholar] [CrossRef]
- Antoniak, K.; Zorena, K.; Hansdorfer-Korzon, R.; Wojtowicz, D.; Kozí Nski, M. Favourable Changes in C-Peptide, C-Reactive Protein and Lipid Profile, and Improved Quality of Life in Patients with Abnormal Body Mass Index after the Use of Manual Lymphatic Drainage: A Case Series with Three-Month Follow-Up. Medicina 2022, 58, 273. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.; Daar, S.; Tzoulis, P.; Di Maio, S.; Kattamis, C. Oral glucose tolerance test: How to maximize its diagnostic value in children and adolescents. Acta BioMed. 2022, 93, e2022318. [Google Scholar] [CrossRef]
- Association, A.D. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S73–S84. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Meng, L.; Zheng, L.S. Association between Serum C-Peptide as a Risk Factor for Cardiovascular Disease and High-Density Lipoprotein Cholesterol Levels in Nondiabetic Individuals. PLoS ONE 2015, 10, e112281. [Google Scholar] [CrossRef]
- Jagannathan, R.; Neves, J.S.; Dorcely, B.; Chung, S.T.; Tamura, K.; Rhee, M.; Bergman, M. The Oral Glucose Tolerance Test: 100 Years Later. Diabetes Metab. Syndr. Obes. 2020, 13, 3787–3805. [Google Scholar] [CrossRef]
- Tang, N.; Ma, J.; Tao, R.; Chen, Z.; Yang, Y.; He, Q.; Lv, Y.; Lan, Z.; Zhou, J. The Effects of the Interaction between BMI and Dyslipidemia on Hypertension in Adults. Sci. Rep. 2022, 12, 927. [Google Scholar] [CrossRef]
- Feingold, K.R. Obesity and Dyslipidemia; Mdtext.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Franssen, R.; Monajemi, H.; Stroes, E.S.G.; Kastelein, J.J.P. Obesity and Dyslipidemia. Med. Clin. N. Am. 2011, 95, 893–902. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, Adiposity, and Dyslipidemia: A Consensus Statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The Role of Leptin/Adiponectin Ratio in Metabolic Syndrome and Diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Korda, M.; Kubant, R.; Patton, S.; Malinski, T. Leptin-Induced Endothelial Dysfunction in Obesity. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H1514–H1521. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Mukheem, A.; Kamarul, T. The Prevention and Treatment of Hypoadiponectinemia-Associated Human Diseases by up-Regulation of Plasma Adiponectin. Life Sci. 2015, 135, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo, M.P.; Hsu, E.; Hao, L.; Easley, K.; Martin, G.S.; Ziegler, T.R.; Alvarez, J.A. Relationships between Plasma Apelin and Adiponectin with Normal Weight Obesity, Body Composition, and Cardiorespiratory Fitness in Working Adults. J. Clin. Transl. Endocrinol. 2021, 24, 100257. [Google Scholar] [CrossRef]
- Sato, A.; Kamekura, R.; Kawata, K.; Kawada, M.; Jitsukawa, S.; Yamashita, K.; Sato, N.; Himi, T.; Ichimiya, S. Novel Mechanisms of Compromised Lymphatic Endothelial Cell Homeostasis in Obesity: The Role of Leptin in Lymphatic Endothelial Cell Tube Formation and Proliferation. PLoS ONE 2016, 11, 158408. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Liu, J.; Wang, G. Serum Vascular Endothelial Growth Factor Level Is Elevated in Patients with Impaired Glucose Tolerance and Type 2 Diabetes Mellitus. J. Int. Med. Res. 2019, 47, 5584–5592. [Google Scholar] [CrossRef]
- Clarkin, C.E.; Mahmoud, M.; Liu, B.; Sobamowo, E.O.; King, A.; Arthur, H.; Jones, P.M.; Wheeler-Jones, C.P. Modulation of Endoglin Expression in Islets of Langerhans by VEGF Reveals a Novel Regulator of Islet Endothelial Cell Function. BMC Res. Notes 2016, 9, 362. [Google Scholar] [CrossRef]
- Elias, I.; Franckhauser, S.; Bosch, F. New Insights into Adipose Tissue VEGF-A Actions in the Control of Obesity and Insulin Resistance. Adipocyte 2013, 2, 109–112. [Google Scholar] [CrossRef]
- Antoniak, K.; Zorena, K.; Jaskulak, M.; Hansdorfer-Korzon, R.; Mrugacz, M.; Koziński, M. Significant Decrease in Glycated Hemoglobin, 2h-Post-Load Glucose and High-Sensitivity C-Reactive Protein Levels in Patients with Abnormal Body Mass Index after Therapy with Manual Lymphatic Drainage. Biomedicines 2022, 10, 1730. [Google Scholar] [CrossRef]
- de Sire, A.; Inzitari, M.T.; Moggio, L.; Pinto, M.; de Sire, G.; Supervia, M.; Petraroli, A.; Rubino, M.; Carbotti, D.; Succurro, E.; et al. Effects of Intermittent Pneumatic Compression on Lower Limb Lymphedema in Patients with Type 2 Diabetes Mellitus: A Pilot Randomized Controlled Trial. Medicina 2021, 57, 1018. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Sokooti, S.; Kieneker, L.M.; de Borst, M.H.; Kobold, A.M.; Kootstra-Ros, J.E.; Gloerich, J.; van Gool, A.J.; Heerspink, H.J.L.; Gansevoort, R.T.; Dullaart, R.P.F.; et al. Plasma C-Peptide and Risk of Developing Type 2 Diabetes in the General Population. J. Clin. Med. 2020, 9, 3001. [Google Scholar] [CrossRef]
- Tohidi, M.; Ghasemi, A.; Hadaegh, F.; Derakhshan, A.; Chary, A.; Azizi, F. Age- and Sex-Specific Reference Values for Fasting Serum Insulin Levels and Insulin Resistance/Sensitivity Indices in Healthy Iranian Adults: Tehran Lipid and Glucose Study. Clin. Biochem. 2014, 47, 432–438. [Google Scholar] [CrossRef]
- Kumar, R.; Mal, K.; Razaq, M.K.; Magsi, M.; Memon, M.K.; Memon, S.; Afroz, M.N.; Siddiqui, H.F.; Rizwan, A. Association of Leptin With Obesity and Insulin Resistance. Cureus 2020, 12, e12178. [Google Scholar] [CrossRef]
- Moonishaa, T.M.; Nanda, S.K.; Shamraj, M.; Sivaa, R.; Sivakumar, P.; Ravichandran, K. Evaluation of Leptin as a Marker of Insulin Resistance in Type 2 Diabetes Mellitus. Int. J. Appl. Basic Med. Res. 2017, 7, 176. [Google Scholar] [CrossRef]
- Shih, Y.L.; Huang, T.C.; Shih, C.C.; Chen, J.Y. Relationship between Leptin and Insulin Resistance among Community-Dwelling Middle-Aged and Elderly Populations in Taiwan. J. Clin. Med. 2022, 11, 5357. [Google Scholar] [CrossRef]
- Iwamoto, J.; Takeda, T.; Sato, Y.; Matsumoto, H. Serum Leptin Concentration Positively Correlates with Body Weight and Total Fat Mass in Postmenopausal Japanese Women with Osteoarthritis of the Knee. Arthritis 2011, 2011, 580632. [Google Scholar] [CrossRef]
- Osegbe, I.; Okpara, H.; Azinge, E. Relationship between Serum Leptin and Insulin Resistance among Obese Nigerian Women. Ann. Afr. Med. 2016, 15, 14–19. [Google Scholar] [CrossRef]
- Frühbeck, G.; Salvador, J. Relation between Leptin and the Regulation of Glucose Metabolism. Diabetologia 2000, 43, 3–12. [Google Scholar] [CrossRef]
- Wang, J.; Obici, S.; Morgan, K.; Barzilai, N.; Feng, Z.; Rossetti, L. Overfeeding Rapidly Induces Leptin and Insulin Resistance. Diabetes 2001, 50, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Oshodi, T.; Ebuehi, O.A.T.; Ojewunmi, O.; Udenze, I.; Soriyan, T. Circulating Adipokine Levels in Type 2 Diabetes Mellitus in Lagos, Nigeria. Nig. Q. J. Hosp. Med. 2012, 22, 25–28. [Google Scholar] [PubMed]
- Gonzalez-Mejia, M.E.; Porchia, L.M.; Torres-Rasgado, E.; Ruiz-Vivanco, G.; Pulido-Pérez, P.; Báez-Duarte, B.G.; Pérez-Fuentes, R. C-Peptide Is a Sensitive Indicator for the Diagnosis of Metabolic Syndrome in Subjects from Central Mexico. Metab. Syndr. Relat. Disord. 2016, 14, 210–216. [Google Scholar] [CrossRef]
- Boyer, W.R.; Johnson, T.M.; Fitzhugh, E.C.; Richardson, M.R.; Churilla, J.R. The Associations Between Increasing Degrees of HOMA-IR and Measurements of Adiposity Among Euglycemic U.S. Adults. Metab. Syndr. Relat. Disord. 2016, 14, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.; Tavira, B.; Hofwimmer, K.; Gutsmann, B.; Massier, L.; Abildgaard, J.; Juul, A.; Rydén, M.; Arner, P.; Laurencikiene, J. Sex-Specific Regulation of IL-10 Production in Human Adipose Tissue in Obesity. Front. Endocrinol. 2022, 13, 996954. [Google Scholar] [CrossRef]
- Hu, R.Y.; He, Q.F.; Pan, J.; Wang, M.; Zhou, X.Y.; Yu, M. Association between body mass index changes and other risk factors for cardiovascular disease in patients with type 2 diabetes mellitus. Zhonghua Liu Xing Bing Xue Za Zhi 2021, 42, 1194–1199. [Google Scholar] [CrossRef]
- Gong, X.; You, L.; Li, F.; Chen, Q.; Chen, C.; Zhang, X.; Zhang, X.; Xuan, W.; Sun, K.; Lao, G.; et al. The Association of Adiponectin with Risk of Pre-Diabetes and Diabetes in Different Subgroups: Cluster Analysis of a General Population in South China. Endocr. Connect. 2021, 10, 1410–1419. [Google Scholar] [CrossRef]
- Akter, R.; Nessa, A.; Husain, M.F.; Wahed, F.; Khatun, N.; Yesmin, M.; Nasreen, S.; Tajkia, T. Effect of Obesity on Fasting Blood Sugar. Mymensingh Med. J. 2017, 26, 7–11. [Google Scholar]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Ahl, S.; Guenther, M.; Zhao, S.; James, R.; Marks, J.; Szabo, A.; Kidambi, S. Adiponectin Levels Differentiate Metabolically Healthy vs Unhealthy among Obese and Nonobese White Individuals. J. Clin. Endocrinol. Metab. 2015, 100, 4172–4180. [Google Scholar] [CrossRef]
- Xie, Y.; Huan, M.-T.; Sang, J.-J.; Luo, S.-S.; Kong, X.-T.; Xie, Z.-Y.; Zheng, S.-H.; Wei, Q.-B.; Wu, Y.-C. Clinical Effect of Abdominal Massage Therapy on Blood Glucose and Intestinal Microbiota in Patients with Type 2 Diabetes. Oxid. Med. Cell. Longev. 2022, 2022, 2286598. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New Insights into Oxidative Stress and Inflammation during Diabetes Mellitus-Accelerated Atherosclerosis. Redox Biol. 2019, 20, 247. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Jaldin-Fincati, J.R.; Pereira, R.V.S.; Bilan, P.J.; Klip, A. Insulin Uptake and Action in Microvascular Endothelial Cells of Lymphatic and Blood Origin. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E204–E217. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, W.; Nicolls, M.R.; Rockson, S.G. The Lymphatic System in Obesity, Insulin Resistance, and Cardiovascular Diseases. Front. Physiol. 2019, 10, 1402. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Z.; Chen, S.; Zhong, C.; Yan, M.; Wang, H.; Meng, M.; Liu, M. Abdominal Massage Alleviates Skeletal Muscle Insulin Resistance by Regulating the AMPK/SIRT1/PGC-1α Signaling Pathway. Cell Biochem. Biophys. 2021, 79, 895–903. [Google Scholar] [CrossRef]
- Rehal, S.; Kataru, R.P.; Hespe, G.E.; Baik, J.E.; Park, H.J.; Ly, C.; Shin, J.Y.; Mehrara, B.J. Regulation of Lymphatic Function and Injury by Nitrosative Stress in Obese Mice. Mol. Metab. 2020, 42, 101081. [Google Scholar] [CrossRef]
- Freire de Oliveira, M.M.; Costa Gurgel, M.S.; Pace do Amaral, M.T.; Amorim, B.J.; Ramos, C.D.; Almeida Filho, J.G.; de Rezende, L.F.; Zanatta Sarian, L.O. Manual Lymphatic Drainage and Active Exercise Effects on Lymphatic Function Do Not Translate into Morbidities in Women Who Underwent Breast Cancer Surgery. Arch. Phys. Med. Rehabil. 2017, 98, 256–263. [Google Scholar] [CrossRef]
- Miller, N.E.; Michel, C.C.; Nanjee, M.N.; Olszewski, W.L.; Miller, I.P.; Hazell, M.; Olivecrona, G.; Sutton, P.; Humphreys, S.M.; Frayn, K.N. Secretion of Adipokines by Human Adipose Tissue in Vivo: Partitioning between Capillary and Lymphatic Transport. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E659–E667. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, S.; Darbandi, M.; Mokarram, P.; Owji, A.-A.; Zhao, B.; Ghayor-Mobarhan, M.; Abdi, H.; Saberfiroozi, M.; Nematy, M.; Safarian, M.; et al. Effects of Body Electroacupuncture on Plasma Leptin Concentrations in Obese and Overweight People in Iran: A Randomized Controlled Trial. Altern. Ther. Health Med. 2013, 19, 24–31. [Google Scholar] [PubMed]
- Nazari, S.; Hassani, A.; Ardakanizadeh, M. Evaluation the Effect of Aerobic Training and LPG Massage, on Insulin Resistance and Leptin to Adiponectin Ratio in Sedentary Obese Women. Med. J. Mashhad Univ. Med. Sci. 2021, 64, 3902–3912. [Google Scholar] [CrossRef]
- Peng, J.; Yin, L.; Wang, X. Central and Peripheral Leptin Resistance in Obesity and Improvements of Exercise. Horm. Behav. 2021, 133, 105006. [Google Scholar] [CrossRef] [PubMed]
- Cromer, W.E.; Zawieja, S.D.; Tharakan, B.; Childs, E.W.; Newell, M.K.; Zawieja, D.C. The Effects of Inflammatory Cytokines on Lymphatic Endothelial Barrier Function. Angiogenesis 2014, 17, 395–406. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W. Nitric Oxide (NO) Side of Lymphatic Flow and Immune Surveillance. Proc. Natl. Acad. Sci. USA 2012, 109, 3–4. [Google Scholar] [CrossRef]
- Bouta, E.M.; Kuzin, I.; de Mesy Bentley, K.; Wood, R.W.; Rahimi, H.; Ji, R.C.; Ritchlin, C.T.; Bottaro, A.; Xing, L.; Schwarz, E.M. Brief Report: Treatment of Tumor Necrosis Factor-Transgenic Mice with Anti-Tumor Necrosis Factor Restores Lymphatic Contractions, Repairs Lymphatic Vessels, and May Increase Monocyte/Macrophage Egress. Arthritis Rheumatol. 2017, 69, 1187–1193. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Földi, M.; Strössenreuther, R.H.K. Foundations of Manual Lymph Drainage; Elsevier Mosby: Amsterdam, The Netherlands, 2005; ISBN 9780323070416. [Google Scholar]
| Parameter | All Patients with MLD Therapy (n = 60) | Group I Normal Body Mass Index Patients (n = 15) | Group II Overweight Patients (n = 15) | Group III Obese Patients (n = 10) | Group IV as Control/ without MLD Therapy (n = 20) | p | |
|---|---|---|---|---|---|---|---|
| Age [years] | 39 ± 11 | 39 ± 13 | 39 ± 11 | 39 ± 11 | 39 ± 12 | I vs. II | ns |
| I vs. III | ns | ||||||
| I vs. IV | ns | ||||||
| II vs. III | ns | ||||||
| II vs. IV | ns | ||||||
| III vs. IV | ns | ||||||
| SBP [mmHg] | 130 ± 8 | 121 ± 2 | 126 ± 3 | 140 ± 4 | 130 ± 5 | I vs. II | ns |
| I vs. III | 0.000000007 * | ||||||
| I vs. IV | ns | ||||||
| II vs. III | 0.0006 * | ||||||
| II vs. IV | ns | ||||||
| III vs. IV | 0.00000008 * | ||||||
| DBP [mmHg] | 82 ± 5 | 74 ± 6 | 81 ± 5 | 87 ± 7 | 84 ± 5 | I vs. II | ns |
| I vs. III | ns | ||||||
| I vs. IV | ns | ||||||
| II vs. III | ns | ||||||
| II vs. IV | ns | ||||||
| III vs. IV | ns | ||||||
| BMI [kg/m2] | 27 ± 5 | 22 ± 2 | 28 ± 2 | 34 ± 2 | 24 ± 4 | I vs. II | 0.018 * |
| I vs. III | 0.015 * | ||||||
| I vs. IV | ns | ||||||
| II vs. III | 0.0001 * | ||||||
| II vs. IV | 0.002 * | ||||||
| III vs. IV | 0.000000005 * | ||||||
| WHR | 0.9 ± 0.2 | 0.8 ± 0.06 | 1 ± 0.04 | 1.2 ± 0.08 | 0.8 ± 0.09 | I vs. II | 0.00001 * |
| I vs. III | 0.0000003 * | ||||||
| I vs. IV | ns | ||||||
| II vs. III | 0.0000002 * | ||||||
| II vs. IV | 0.00009 * | ||||||
| III vs. IV | 0.00000009 * | ||||||
| VAT [LVL] | 6 ± 4 | 4 ± 2 | 7 ± 3 | 9 ± 2 | 5 ± 3 | I vs. II | 0.002 * |
| I vs. III | 0.005 * | ||||||
| I vs. IV | ns | ||||||
| II vs. III | 0.008 | ||||||
| II vs. IV | ns | ||||||
| III vs. IV | 0.0003 * | ||||||
| Parameter | BMI [kg/m2] (n = 60) | WHR (n = 60) | VAT [LVL] (n = 60) |
|---|---|---|---|
| FPG [mg/dL] | r = 0.222 | r = 0.284 | r = 0.493 |
| p = 0.167 | p = 0.756 | p = 0.001 * | |
| 2h-PG [mg/dL] | r = 0.321 | r = 0.303 | r = 0.434 |
| p = 0.043 * | p = 0.056 | p = 0.005 * | |
| HbA1c [%] | r = 0.379 | r = 0.384 | r = 0.444 |
| p = 0.142 | p = 0.141 | p = 0.004 * | |
| Insulin [µIU/mL] | r = 0.465 | r = 0.433 | r = 0.396 |
| p = 0.002 * | p = 0.005 * | p = 0.005 * | |
| C-peptide [ng/mL] | r = 0.471 | r = 0.447 | r = 0.322 |
| p = 0.002 * | p = 0.003 * | p = 0.042 * | |
| HOMA-IR | r = 0.433 | r = 0.462 | r = 0.410 |
| p = 0.005 * | p = 0.002 * | p = 0.008 * | |
| hsCRP [mg/L] | r = 0.234 | r = 0.779 | r = 0.242 |
| p = 0.145 | p = 0.004 * | p = 0.132 | |
| Total cholesterol [mg/dL] | r = 0.999 | r = 0.909 | r = 0.757 |
| p = 0.001 * | p = 0.018 * | p = 0.050 * | |
| HDL-C [mg/dL] | r = −0.325 | r = −0.211 | r = −0.490 |
| p = 0.040* | p = 0.190 | p = 0.001 * | |
| LDL-C [mg/dL] | r = 0.990 | r = 0.709 | r = 0.757 |
| p = 0.001 * | p = 0.018 * | p = 0.017 * | |
| TG [mg/dL] | r = 0.291 | r = 0.316 | r = 0.500 |
| p = 0.067 | p = 0.046 * | p = 0.001 * | |
| VEGF [pg/mL] | r = 0.998 | r = 0.716 | r = 0.868 |
| p = 0.0002 * | p = 0.059 * | p = 0.027 * | |
| IL-10 [pg/mL] | r = 0.628 | r = 0.438 | r = 0.696 |
| p = 0.078 | p = 0.125 | p = 0.063 | |
| Adiponectin [μg/mL] | r = 0.683 | r = 0.531 | r = 0.810 |
| p = 0.066 | p = 0.101 | p = 0.039 * | |
| Leptin [ng/mL] | r = 0.718 | r = 0.733 | r = 0.581 |
| p = 0.0000001 * | p = 0.00000007 * | p = 0.00005 * |
| Variable | AUC | 95% CI for AUC | Specificity | Sensitivity | Optimal Cut-Off | p-Value |
|---|---|---|---|---|---|---|
| FPG | 48.65% | 32.86–64.66% | 43.43% | 51.60% | 98.5 [mg/dL] | 0.08 |
| 2h-PG | 65.2% | 47.21–83.18% | 29% | 58% | 106.5 [mg/dL] | 0.111 |
| HbA1c | 42.74% | 26.92–58.56% | 53.64% | 53.40% | 5.2 [%] | 0.369 |
| Insulin | 81.51% | 69.23–93.79% | 32.45% | 62.13% | 9.5 [µIU/mL] | 0.00009 * |
| C-peptide | 80.68% | 68.73–92.62% | 42.39% | 64.53% | 2.3 [ng/mL] | 0.0001 * |
| HOMA-IR | 79.97% | 67.16–92.78% | 33.16% | 69.53% | 1.8 | 0.0002 * |
| Total cholesterol | 66.30% | 49.61–82.99% | 42.98% | 55.47% | 195 [mg/dL] | 0.043 * |
| HDL-C | 37.42% | 21.86–52.97% | 57.57% | 41.09% | 59.5 [mg/dL] | 0.119 |
| LDL-C | 43.58% | 28.59–58.57% | 64.5% | 48.83% | 68 [mg/dL] | 0.426 |
| TG | 64.44% | 49.49–79.38% | 39.11% | 57.7% | 96.5 [mg/dL] | 0.073 |
| hsCRP | 68.22% | 53.62–82.33% | 16.28% | 38.09% | 3.4 [mg/L] | 0.024 * |
| VEGF | 44.92% | 28.75–61.10% | 51.88% | 48.47% | 215.3 [pg/mL] | 0.530 |
| IL-10 | 70.8% | 53.75–87.84% | 30.4% | 41.33% | 0.35 [pg/mL] | 0.029 * |
| Adiponectin | 41.46% | 25.65–57.27% | 53.53% | 47.36% | 3.5 [μg/mL] | 0.290 |
| Leptin | 82.79% | 71.51–94.08% | 41.92% | 27.22% | 17.7 [ng/mL] | 0.00004 * |
| Variable | AUC | 95% CI for AUC | Specificity | Sensitivity | Optimal Cut-Off | p-Value |
|---|---|---|---|---|---|---|
| FPG | 59.78% | 43.01–76.56% | 45.67% | 53.53% | 89.5 [mg/dL] | 0.232 |
| 2h-PG | 70.93% | 53.92–87.94% | 41.41% | 60.78% | 93 [mg/dL] | 0.028 * |
| HbA1c | 59.98% | 43.46–76.50% | 51.25% | 58.77% | 5.2 [%] | 0.223 |
| Insulin | 93.05% | 85.15–100% | 36.9% | 78% | 6.9 [µIU/mL] | 0.053 * |
| C-peptide | 89.35% | 78.93–99.77% | 40.79% | 77.56% | 1.8 [ng/mL] | 0.000001 * |
| Total cholesterol | 77.31% | 63.82–90.80% | 42.48% | 67.75% | 198 [mg/dL] | 0.0008 * |
| HDL-C | 41.53% | 25.67–57.39% | 50.84% | 42.15% | 59.5 [mg/dL] | 0.301 |
| LDL-C | 41.40% | 25.23–57.57% | 52.18% | 43.51% | 72 [mg/dL] | 0.294 |
| TG | 69.31% | 53.67–84.94% | 43.98% | 62.89% | 95 [mg/dL] | 0.018 * |
| hsCRP | 68.71% | 54.47–54.47% | 20.34% | 39.39% | 1.9 [mg/L] | 0.022 * |
| VEGF | 49.53% | 32.66–66.41% | 49.60% | 49.07% | 208.1 [pg/mL] | 0.954 |
| IL-10 | 47.60% | 28.98–66.21% | 35.6% | 32.66% | 0.85 [pg/mL] | 0.801 |
| Adiponectin | 42.72% | 24.93–60.51% | 52.21% | 44.99% | 3.6 [μg/mL] | 0.374 |
| Leptin | 79.76% | 66.30–93.21% | 39.77% | 69.44% | 17.6 [ng/mL] | 0.0002 * |
| Parameter | Group I (n = 15) | Group I p 0′–1′ | Group II (n = 15) | Group II p 0′–1′ | Group III (n = 10) | Group III p 0′–1′ | Group IV (n = 20) | Group IV p 0′–1′ | |
|---|---|---|---|---|---|---|---|---|---|
| FPG [mg/dL] | 0′ | 85 ± 8 | 0.814 | 97 ± 5 | 0.254 | 95 ± 22 | 0.671 | 90 ± 7 | 0.899 |
| 1′ | 86 ± 10 | 92 ± 5 | 91 ± 15 | 92 ± 7 | |||||
| 2h-PG [mg/dL] | 0′ | 79 ± 15 | 0.736 | 119 ± 7 | 0.049 * | 103 ± 31 | 0.998 | 100 ± 3 | 0.726 |
| 1′ | 77 ± 15 | 100 ± 5 | 103 ± 28 | 102 ± 3 | |||||
| HbA1c [%] | 0′ | 5.2 ± 0.5 | 0.751 | 5.2 ± 0.3 | 0.999 | 5.3 ± 0.6 | 0.665 | 5.2 ± 0.5 | 0.986 |
| 1′ | 5.2 ± 0.5 | 5.2 ± 0.3 | 5.3 ± 0.5 | 5.2 ± 0.5 | |||||
| Insulin [µIU/mL] | 0′ | 8 ± 2 | 0.039 * | 8 ± 4 | 0.899 | 9 ± 4 | 0.670 | 6 ± 4 | 0.968 |
| 1′ | 5 ± 2 | 9 ± 4 | 10 ± 4 | 6 ± 3 | |||||
| C-peptide [ng/mL] | 0′ | 1.9 ± 1.8 | 0.240 | 2 ± 0.8 | 0.878 | 2.2 ± 0.8 | 0.813 | 1.6 ± 0.5 | 0.865 |
| 1′ | 1.3 ± 0.3 | 2 ± 0.7 | 2.1 ± 0.8 | 1.7 ± 0.5 | |||||
| hsCRP [mg/L] | 0′ | 1 ± 0.1 | 0.998 | 4 ± 6.6 | 0.275 | 2 ± 2.4 | 0.899 | 2 ± 1.4 | 0.897 |
| 1′ | 1 ± 0.2 | 2 ± 1.5 | 3 ± 3.8 | 2 ± 1.3 | |||||
| Total cholesterol [mg/dL] | 0′ | 193 ± 34 | 0.881 | 208 ± 47 | 0.914 | 204 ± 7 | 0.661 | 178 ± 35 | 0.888 |
| 1′ | 194 ± 31 | 206 ± 47 | 208 ± 5 | 175 ± 36 | |||||
| HDL-C [mg/dL] | 0′ | 66 ± 10 | 0.712 | 58 ± 16 | 0.929 | 57 ± 15 | 0.942 | 62 ± 15 | 0.713 |
| 1′ | 64 ± 9 | 58 ± 17 | 57 ± 15 | 59 ± 15 | |||||
| LDL-C [mg/dL] | 0′ | 111 ± 28 | 0.674 | 120 ± 41 | 0.961 | 124 ± 20 | 0.912 | 98 ± 29 | 0.869 |
| 1′ | 116 ± 29 | 120 ± 41 | 130 ± 25 | 96 ± 32 | |||||
| TG [mg/dL] | 0′ | 78 ± 28 | 0.642 | 158 ± 32 | 0.901 | 114 ± 71 | 0.840 | 89 ± 46 | 0.856 |
| 1′ | 73 ± 24 | 153 ± 30 | 108 ± 46 | 93 ± 40 | |||||
| HOMA-IR | 0′ | 2 ± 2 | 0.003 * | 2.6 ± 0.9 | 0.049 * | 2.8 ± 1.5 | 0.169 | 1.4 ± 0.8 | 0.960 |
| 1′ | 1.1 ± 0.4 | 1.9 ± 0.7 | 2 ± 1 | 1.4 ± 0.6 | |||||
| VEGF [pg/mL] | 0′ | 252 ± 241 | 0.687 | 217 ± 137 | 0.598 | 230 ± 169 | 0.688 | 277 ± 181 | 0.803 |
| 1′ | 218 ± 215 | 191 ± 125 | 255 ± 105 | 290 ± 155 | |||||
| IL-10 [pg/mL] | 0′ | 0.5 ± 0.5 | 0.464 | 0.3 ± 0.2 | 0.807 | 0.7 ± 0.9 | 0.252 | 0.6 ± 0.3 | 0.822 |
| 1′ | 0.3 ± 0.3 | 0.3 ± 0.1 | 0.3 ± 0.01 | 0.6 ± 0.3 | |||||
| Adiponectin [μg/mL] | 0′ | 3.3 ± 1.0 | 0.883 | 2.9 ± 1.1 | 0.495 | 3.6 ± 2.5 | 0.975 | 3.6 ± 2.5 | 0.975 |
| 1′ | 3.3 ± 1.2 | 2.7 ± 1.1 | 3.6 ± 2.9 | 3.6 ± 2.9 | |||||
| Leptin [ng/mL] | 0′ | 9.6 ± 1.9 | 0.041 * | 18.1 ± 2.1 | 0.033 * | 28.0 ± 12.1 | 0.206 | 20.0 ± 10.5 | 0.823 |
| 1′ | 6.8 ± 1.4 | 13.0 ± 2.5 | 21.7 ± 9.2 | 21.3 ± 12.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniak-Pietrynczak, K.; Zorena, K.; Jaskulak, M.; Hansdorfer-Korzon, R.; Koziński, M. Effect of Manual Lymphatic Drainage on the Concentrations of Selected Adipokines, Cytokines, C-Reactive Protein and Parameters of Carbohydrate and Lipid Metabolism in Patients with Abnormal Body Mass Index: Focus on Markers of Obesity and Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 10338. https://doi.org/10.3390/ijms241210338
Antoniak-Pietrynczak K, Zorena K, Jaskulak M, Hansdorfer-Korzon R, Koziński M. Effect of Manual Lymphatic Drainage on the Concentrations of Selected Adipokines, Cytokines, C-Reactive Protein and Parameters of Carbohydrate and Lipid Metabolism in Patients with Abnormal Body Mass Index: Focus on Markers of Obesity and Insulin Resistance. International Journal of Molecular Sciences. 2023; 24(12):10338. https://doi.org/10.3390/ijms241210338
Chicago/Turabian StyleAntoniak-Pietrynczak, Klaudia, Katarzyna Zorena, Marta Jaskulak, Rita Hansdorfer-Korzon, and Marek Koziński. 2023. "Effect of Manual Lymphatic Drainage on the Concentrations of Selected Adipokines, Cytokines, C-Reactive Protein and Parameters of Carbohydrate and Lipid Metabolism in Patients with Abnormal Body Mass Index: Focus on Markers of Obesity and Insulin Resistance" International Journal of Molecular Sciences 24, no. 12: 10338. https://doi.org/10.3390/ijms241210338
APA StyleAntoniak-Pietrynczak, K., Zorena, K., Jaskulak, M., Hansdorfer-Korzon, R., & Koziński, M. (2023). Effect of Manual Lymphatic Drainage on the Concentrations of Selected Adipokines, Cytokines, C-Reactive Protein and Parameters of Carbohydrate and Lipid Metabolism in Patients with Abnormal Body Mass Index: Focus on Markers of Obesity and Insulin Resistance. International Journal of Molecular Sciences, 24(12), 10338. https://doi.org/10.3390/ijms241210338








