In Vitro Functional Characterization of Type-I Taste Bud Cells as Monocytes/Macrophages-like Which Secrete Proinflammatory Cytokines
Abstract
1. Introduction
2. Results
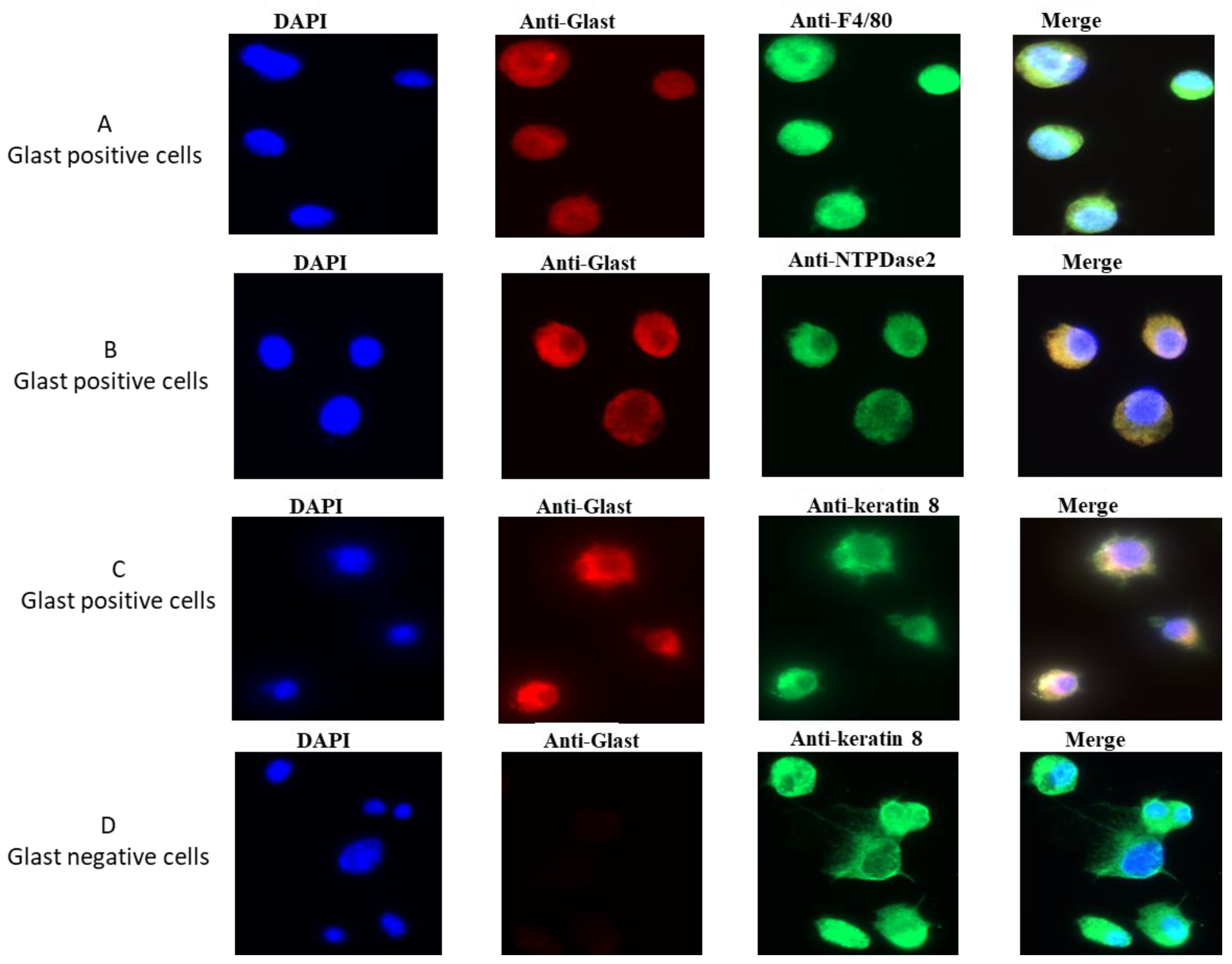
2.1. GLAST-Positive Cells Express F4/80
2.2. GLAST-Positive Gustatory Cells Share Similarities with Macrophages
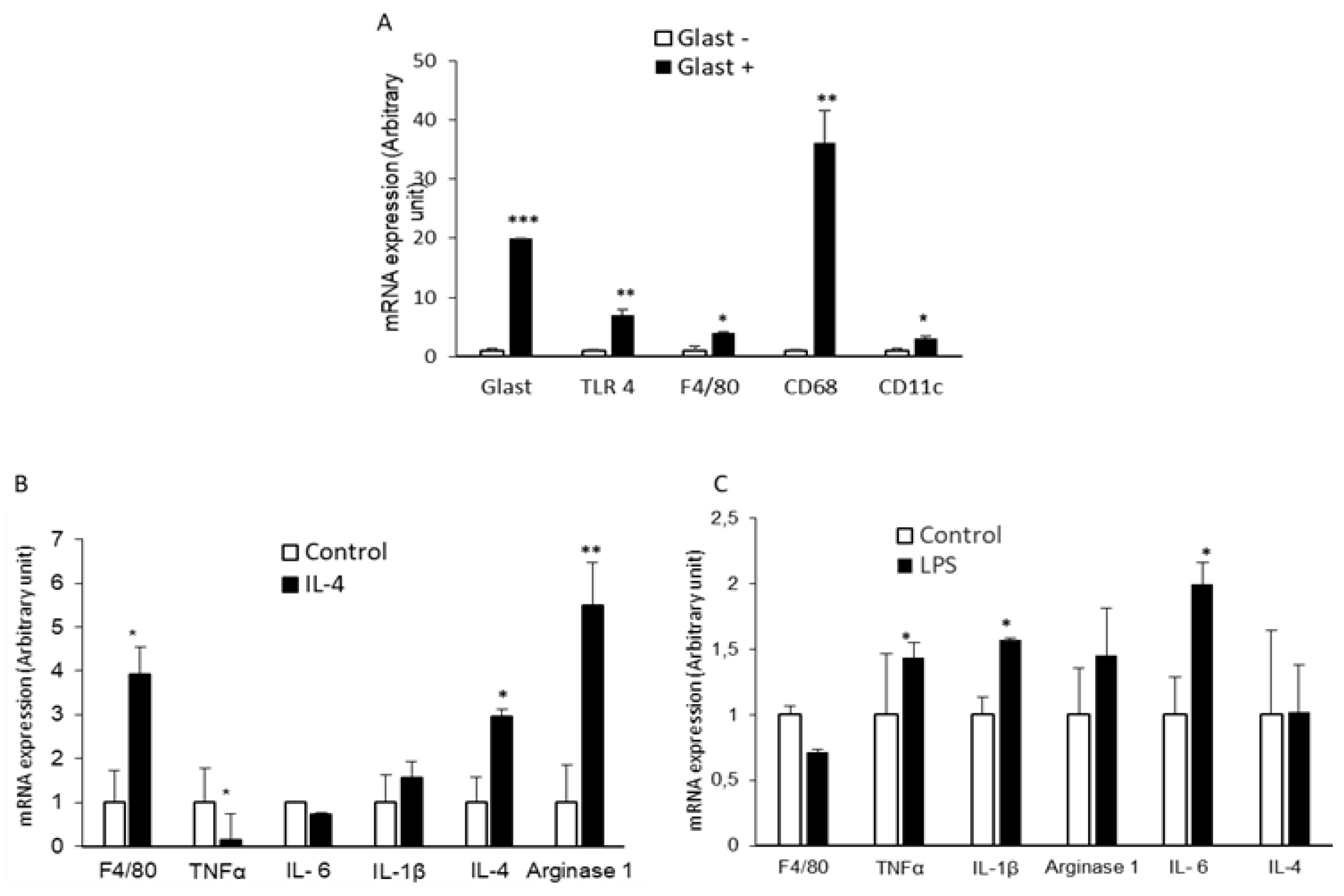
2.3. GLAST-Positive Gustatory Cells Express mRNA of Macrophage/Glial Cell Markers
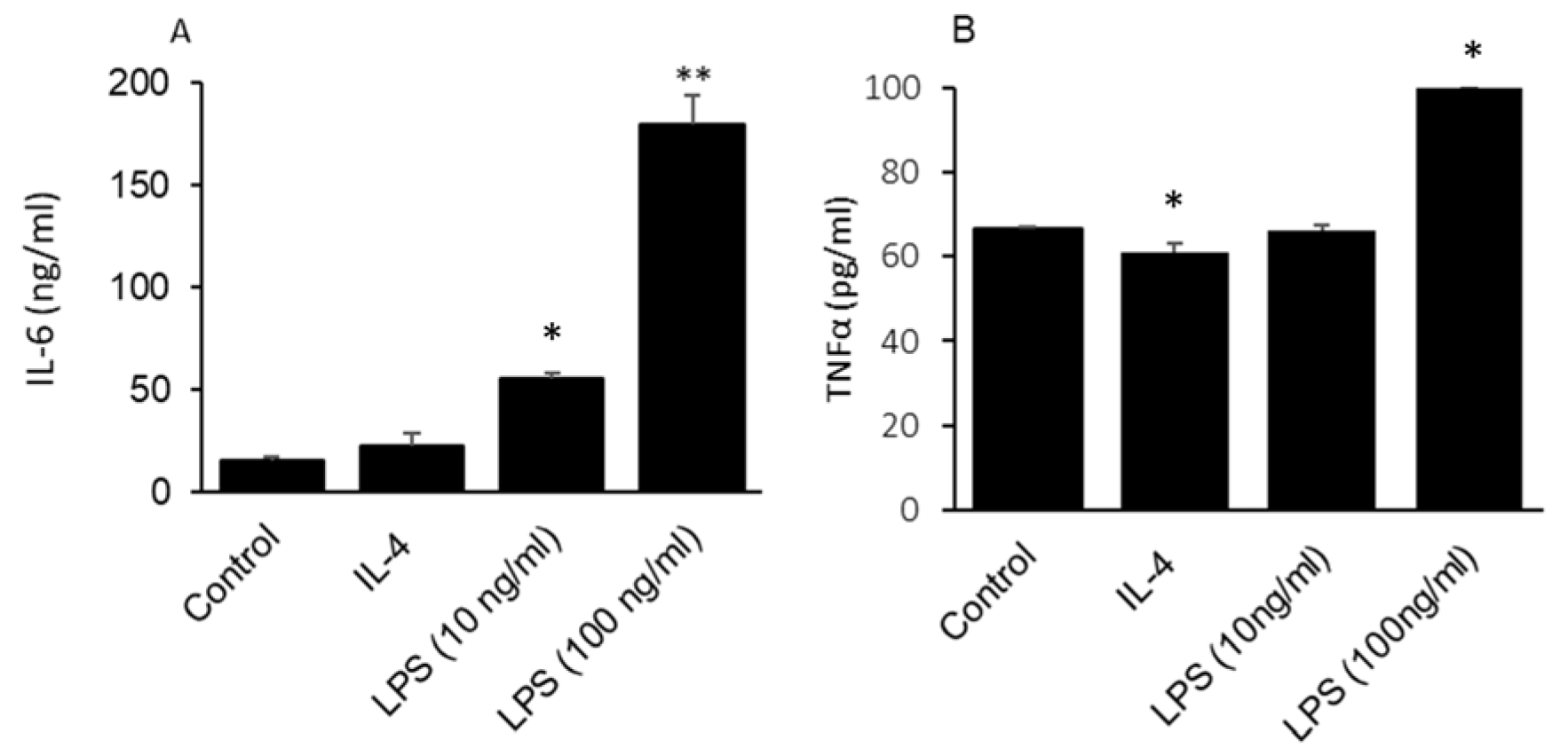
2.4. GLAST-Positive Gustatory Cells Can Be Differentiated under In Vitro Conditions into a Phenotype Similar to M1 or M2 Macrophages
2.5. GLAST-Positive Gustatory Cells Are Differentiated into M1-Macrophages in Obesity
3. Discussion
4. Materials and Methods
4.1. Animal and Diet
4.2. Isolation of Taste Papillae
4.3. Culture of Mouse Taste Bud Cells (TBC)
4.4. Magnetic Labeling and Isolation of GLAST Positives Cells
4.5. Differentiation into M1 and M2 Macrophages-like Cells
4.6. Immunocytochemistry of Type I Gustatory Cells
4.7. Flow Cytometry Analysis
4.8. Determination of mRNA Expression
4.9. Determination of Pro-Inflammatory Cytokines Levels
4.10. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, I.J. Anatomy of the peripherol taste system. In Handbook of Olfaction Gustation; Handbook of Clinical Neurology; John Wiley & Sons: Hoboken, NJ, USA, 1995; pp. 521–547. [Google Scholar]
- Mela, D.J.; Mattes, R.D. The chemical senses and nutrition: Part I. Nutr. Today 1988, 23, 19–24. [Google Scholar] [CrossRef]
- Gilbertson, T.A.; Khan, N.A. Cell signaling mechanisms of oro-gustatory detection of dietary fat: Advances and challenges. Prog. Lipid. Res. 2014, 53, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Bigiani, A. Mouse taste cells with glialike membrane properties. J. Neurophysiol. 2001, 85, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.A.; Maruyama, Y.; Stimac, R.; Roper, S.D. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J. Physiol. 2008, 586, 2903–2912. [Google Scholar] [CrossRef]
- Roper, S.D. The cell biology of vertebrate taste receptors. Annu. Rev. Neurosci. 1989, 12, 329–353. [Google Scholar] [CrossRef]
- Delay, R.J.; Kinnamon, J.C.; Roper, S.D. Ultrastructure of mouse vallate taste buds: II. Cell types and cell lineage. J. Comp. Neurol. 1986, 253, 242–252. [Google Scholar] [CrossRef]
- Berthoud, H.; Bereiter, D.; Trimble, E.; Siegel, E.; Jeanrenaud, B. Cephalic phase, reflex insulin secretion neuroanatomical and physiological characterization. Diabetologia 1981, 20, 393–401. [Google Scholar] [CrossRef]
- Gaillard, D.; Laugerette, F.; Darcel, N.; El-Yassimi, A.; Passilly-Degrace, P.; Hichami, A.; Khan, N.A.; Montmayeur, J.P.; Besnard, P. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008, 22, 1458–1468. [Google Scholar] [CrossRef]
- Ozdener, M.H.; Subramaniam, S.; Sundaresan, S.; Sery, O.; Hashimoto, T.; Asakawa, Y.; Besnard, P.; Abumrad, N.A.; Khan, N.A. CD36-and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 2014, 146, 995–1005. [Google Scholar] [CrossRef]
- Berrichi, M.; Hichami, A.; Addou-Klouche, L.; Sayed Khan, A.; Khan, N.A. CD36 and GPR120 methylation associates with orosensory detection thresholds for fat and bitter in algerian young obese children. J. Clin. Med. 2020, 9, 1956. [Google Scholar] [CrossRef]
- Stewart, J.E.; Seimon, R.V.; Otto, B.; Keast, R.S.; Clifton, P.M.; Feinle-Bisset, C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am. J. Clin. Nutr. 2011, 93, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Cohn, Z.J.; Kim, A.; Huang, L.; Brand, J.; Wang, H. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci. 2010, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Feng, P.; Ohkuri, T.; Sauers, D.; Cohn, Z.J.; Chai, J.; Nelson, T.; Bachmanov, A.A.; Huang, L.; Wang, H.J.P.O. Defects in the peripheral taste structure and function in the MRL/lpr mouse model of autoimmune disease. PLoS ONE 2012, 7, e35588. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Appel, S.H.; Zhao, W.; Beers, D.R.; Henkel, J.S. The microglial-motoneuron dialogue in ALS. Acta Myol. 2011, 30, 4–8. [Google Scholar]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Morris, L.; Graham, C.F.; Gordon, S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development 1991, 112, 517–526. [Google Scholar] [CrossRef]
- Austyn, J.M.; Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981, 11, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Akinrinmade, O.A.; Chetty, S.; Daramola, A.K.; Islam, M.-U.; Thepen, T.; Barth, S. CD64: An attractive immunotherapeutic target for M1-type macrophage mediated chronic inflammatory diseases. Biomedicines 2017, 5, 56. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Brand, J.; Huang, L. Inflammation and taste disorders: Mechanisms in taste buds. Ann. N. Y. Acad. Sci. 2009, 1170, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Okayama, N.; Fujimura, K.; Suehiro, Y.; Hamanaka, Y.; Fujiwara, M.; Matsubara, T.; Maekawa, T.; Hazama, S.; Oka, M.; Nohara, H. Simple genotype analysis of the Asp299Gly polymorphism of the Toll-like receptor-4 gene that is associated with lipopolysaccharide hyporesponsiveness. J. Clin. Lab. Anal. 2002, 16, 56–58. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, H.; Chai, J.; Huang, L.; Wang, H. Expression and secretion of TNF-α in mouse taste buds: A novel function of a specific subset of type II taste cells. PLoS ONE 2012, 7, e35588. [Google Scholar] [CrossRef]
- Louis, C.A.; Mody, V.; Henry, W.L., Jr.; Reichner, J.S.; Albina, J.E. Regulation of arginase isoforms I and II by IL-4 in cultured murine peritoneal macrophages. Am. J. Physiol. 1999, 276, R237–R242. [Google Scholar] [CrossRef]
- Gordon, J.W.; Ruddle, F.H. Integration and stable germ line transmission of genes injected into mouse pronuclei. Science 1981, 214, 1244–1246. [Google Scholar] [CrossRef]
- Ezekowitz, R.; Austyn, J.; Stahl, P.D.; Gordon, S. Surface properties of bacillus Calmette-Guérin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J. Exp. Med. 1981, 154, 60–76. [Google Scholar] [CrossRef]
- Sheng, J.; Ruedl, C.; Karjalainen, K. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity 2015, 43, 382–393. [Google Scholar] [CrossRef]
- Lin, H.H.; Faunce, D.E.; Stacey, M.; Terajewicz, A.; Nakamura, T.; Zhang-Hoover, J.; Kerley, M.; Mucenski, M.L.; Gordon, S.; Stein-Streilein, J. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J. Exp. Med. 2005, 201, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Saidi, H.; Bounihi, A.; Bouazza, A.; Hichami, A.; Koceir, E.H.A.; Khan, N.A. Spirulina reduces diet-induced obesity through downregulation of lipogenic genes expression in Psammomys obesus. Arch. Physiol. Biochem. 2022, 128, 1001–1009. [Google Scholar] [CrossRef]
- Kaufman, A.; Choo, E.; Koh, A.; Dando, R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018, 16, e2001959. [Google Scholar] [CrossRef]
- Saidi, H.; Murtaza, B.; Khan, A.S.; Koceir, E.A.; Hichami, A.; Khan, N.A. DHA induces Jurkat T-cell arrest in G2/M phase of cell cycle and modulates the plasma membrane expression of TRPC3/6 channels. Biochimie 2021, 181, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Diet Composition of the Scheme g/100g A03 SAFE | Normal Diet | High-Fat Diet (60%) | ||
|---|---|---|---|---|
| g | kcal | g | kcal | |
| Carbohydrate | 52.00 | 208 | 35.43 | 141.73 |
| Protein | 21.40 | 85.6 | 14.58 | 58.33 |
| Lipids | 5.10 | 45.9 | 35.34 | 318.02 |
| Fibers | 4 | 8 | 2,73 | 5.45 |
| Minerals Ash | 5.40 | 0 | 3.68 | 0 |
| water | 12.10 | 0 | 8.24 | 0 |
| Gene | Primer Sequence | Accession Number |
|---|---|---|
| GLAST | F: 5′ ACCAAAAGCAACGGAGAAGAG 3′ R: 5′ GGCATTCCGAAACAGGTAACTC 3′ | NM_148938.3 |
| CD68 | F: 5′ CTTCCCACAGGCAGCACAG 3′ R: 5′ AATGATGAGAGGCAGCAAGAGG 3′ | NM_001291058.1 |
| CD11c | F: 5′ GTGCCCATCAGTTCCTTACA 3′ R: 5′ GAGAAGAACTGTGGAGCTGAC 3′ | NM_021334.3 |
| TLR4 | F: 5′ AGCTCCTGACCTTGGTCTTG 3′ R: 5′ CGCAGGGGAACTCAATGAGG 3′ | NM_025817.4 |
| F4/80 | F: 5′ TGACTCACCTTGTGGTCCTAA 3′ R: 5′ CTTCCCAGAATCCAGTCTTTCC 3′ | NM_001355722.1 |
| Arginase 1 | F: 5′ CTCCAAGCCAAAGTCCTTAGAG 3′ R: 5′ AGGAGCTGTCATTAGGGACATC 3′ | NM_007482.3 |
| TNFα | F: 5′ CTGTTCTCATTCCTGCTTGTGG 3′ R: 5′ AATCGGCTGACGGTGTGG 3′ | NM_001278601.1 |
| IL-4 | F: 5′ CGAGCTCACTCTCTGTGGTG 3′ R: 5′ TGAACGAGGTCACAGGAGAA 3′ | NM_021283.2 |
| IL-6 | F: 5′ TAGTCCTTCCTACCCCAATTTCC 3′ R: 5′ TTGGTCCTTAGCCACTCCTTC 3′ | NM_001314054.1 |
| IL-1β | F: 5′ CACAGCAGCACATCAACAAG 3′ R: 5′ GTGCTCATGTCCTCATCCTG 3′ | NM_008361.4 |
| β-Actin | F: 5′ CTGTGTTGTCCCTGTATGCG 3′ R: 5′ TAGATGGGCACAGTGTGGGT 3′ | NM_007393.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hichami, A.; Saidi, H.; Khan, A.S.; Degbeni, P.; Khan, N.A. In Vitro Functional Characterization of Type-I Taste Bud Cells as Monocytes/Macrophages-like Which Secrete Proinflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 10325. https://doi.org/10.3390/ijms241210325
Hichami A, Saidi H, Khan AS, Degbeni P, Khan NA. In Vitro Functional Characterization of Type-I Taste Bud Cells as Monocytes/Macrophages-like Which Secrete Proinflammatory Cytokines. International Journal of Molecular Sciences. 2023; 24(12):10325. https://doi.org/10.3390/ijms241210325
Chicago/Turabian StyleHichami, Aziz, Hamza Saidi, Amira Sayed Khan, Pernelle Degbeni, and Naim Akhtar Khan. 2023. "In Vitro Functional Characterization of Type-I Taste Bud Cells as Monocytes/Macrophages-like Which Secrete Proinflammatory Cytokines" International Journal of Molecular Sciences 24, no. 12: 10325. https://doi.org/10.3390/ijms241210325
APA StyleHichami, A., Saidi, H., Khan, A. S., Degbeni, P., & Khan, N. A. (2023). In Vitro Functional Characterization of Type-I Taste Bud Cells as Monocytes/Macrophages-like Which Secrete Proinflammatory Cytokines. International Journal of Molecular Sciences, 24(12), 10325. https://doi.org/10.3390/ijms241210325






