Evolution of a Cockroach Allergen into the Major Protein of Termite Royal Jelly
Abstract
1. Introduction
2. Results
2.1. Cellulose-Digesting Enzymes
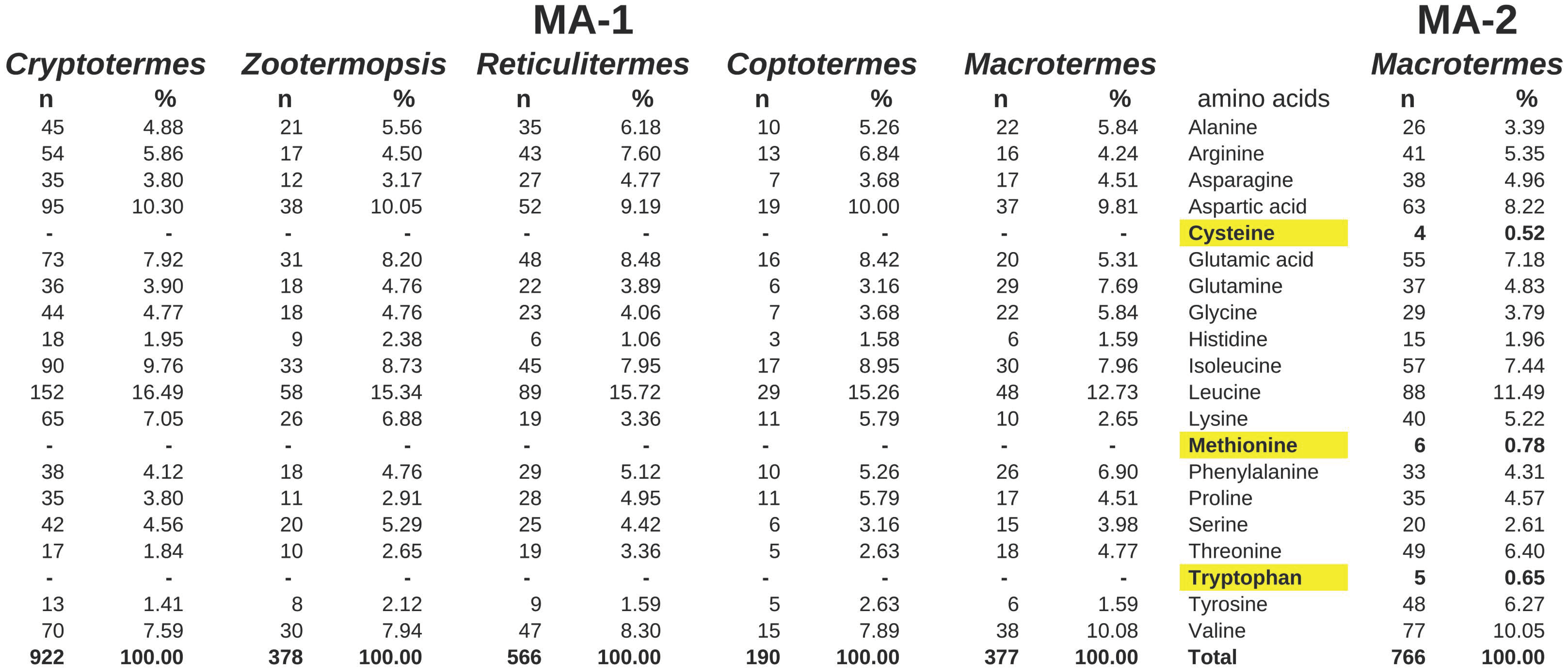
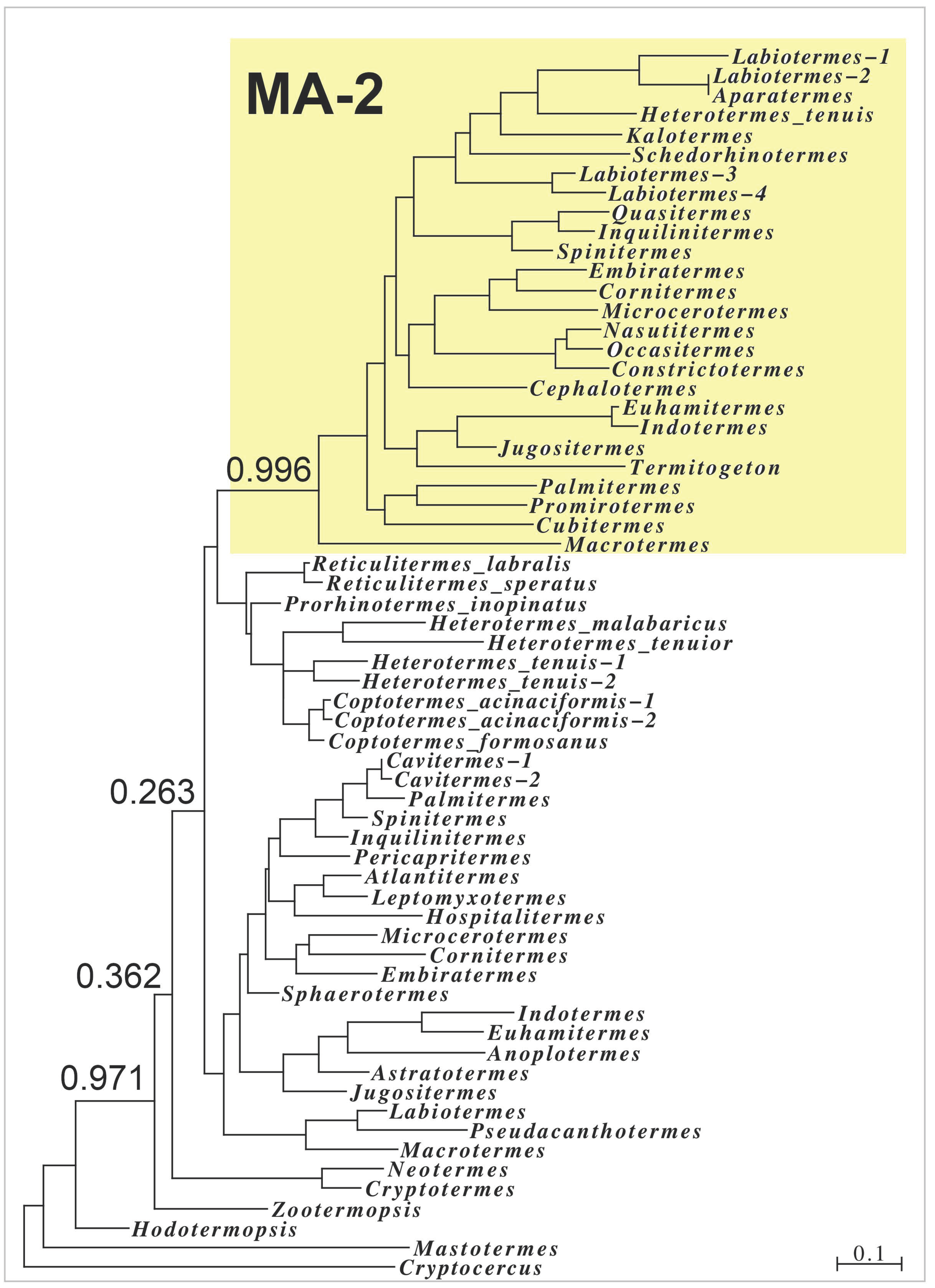
2.2. Major Allergens
2.3. Lysozymes
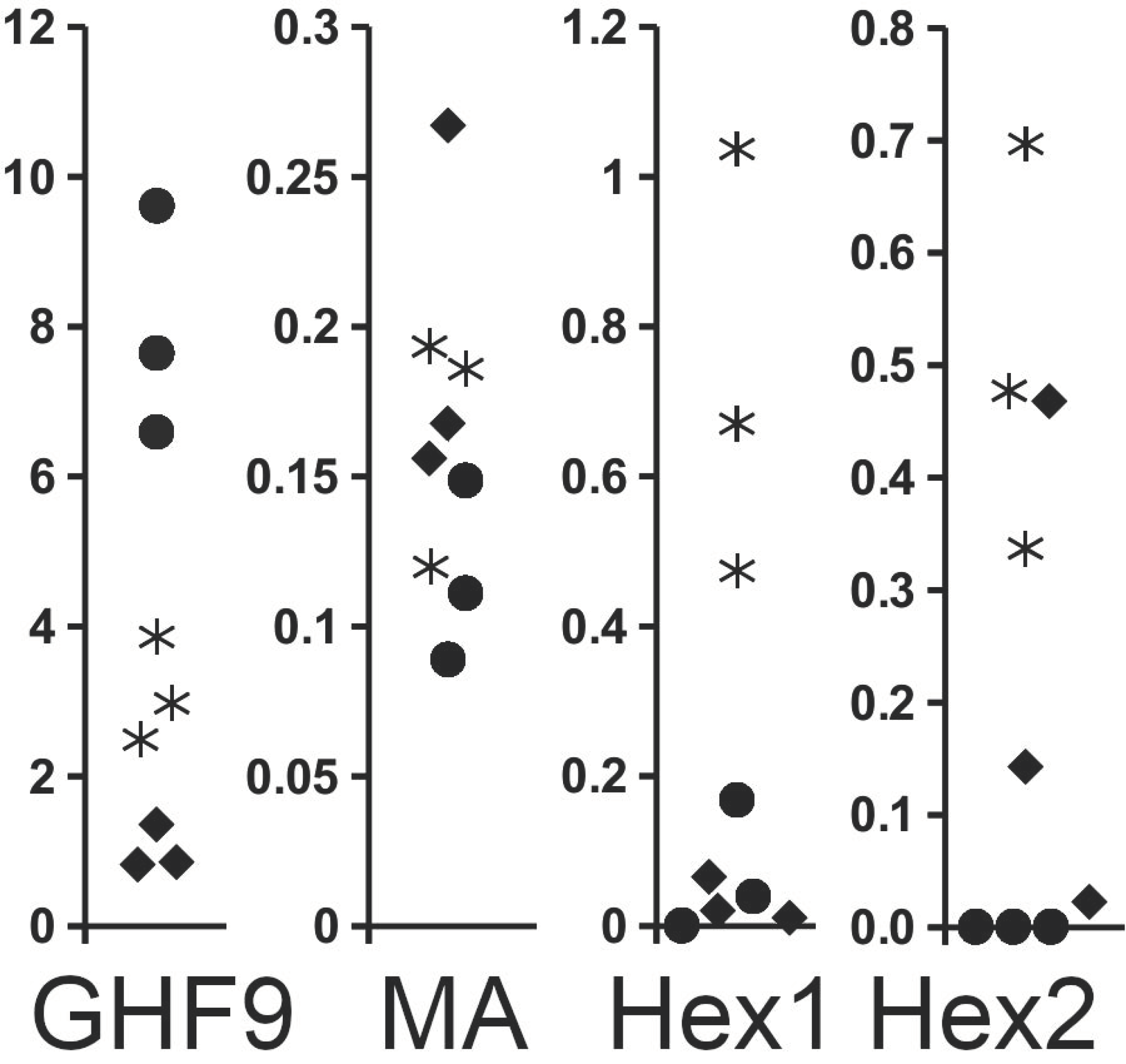
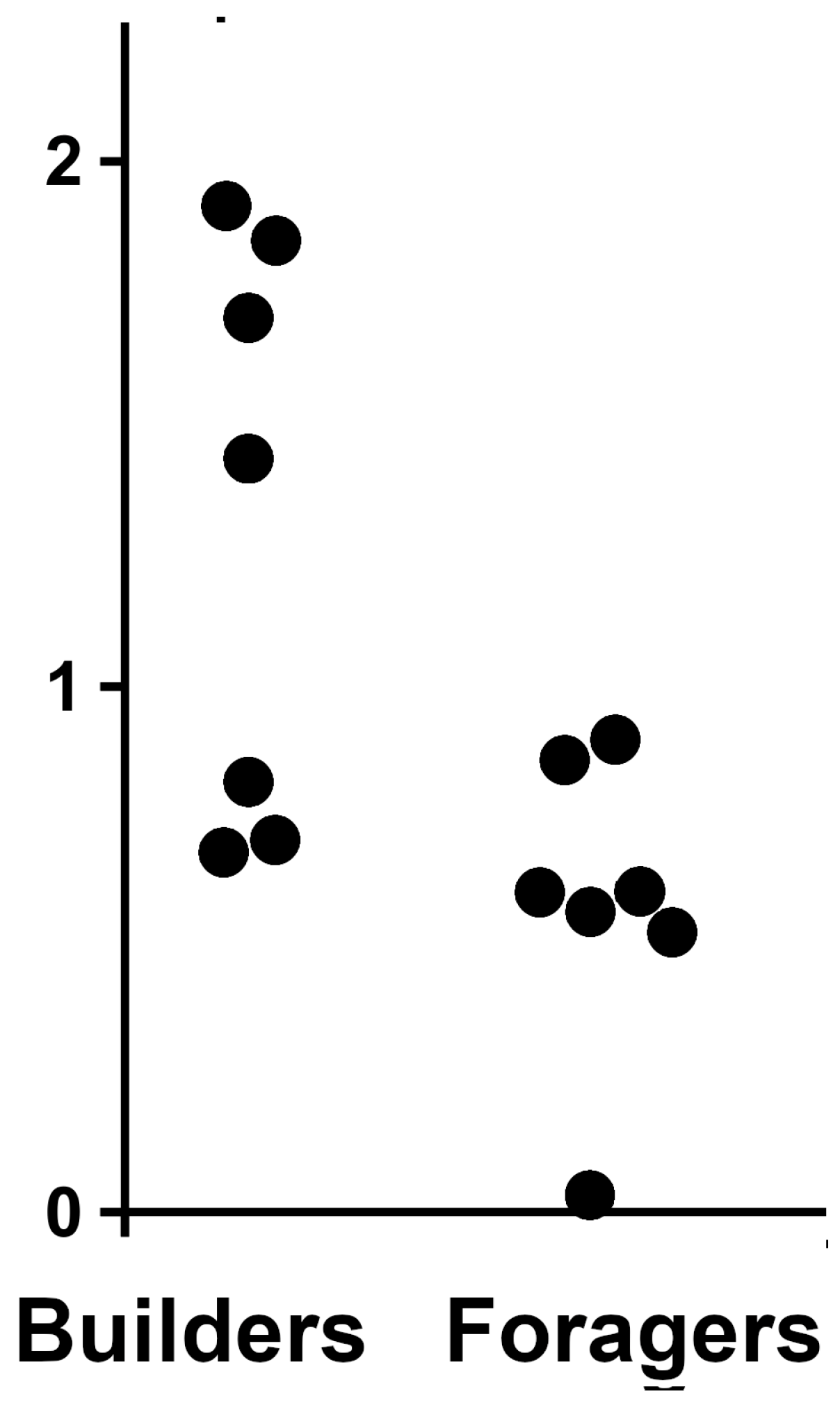
2.4. Expression
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.; Brown, M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Èntomol. 2006, 51, 1–24. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory Pathways Controlling Female Insect Reproduction. Annu. Rev. Èntomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Grassé, P.P. Termitologia Anatomie-Physiologie-Biologie-Systématique des Termites. Tome II. Fondation des Sociétés-Construction; Masson: Paris, France, 1984; p. 613. [Google Scholar]
- Badertscher, S.; Gerber, C.; Leuthold, R.H. Polyethism in food supply and processing in termite colonies of Macrotermes sub-hyalinus (Isoptera). Behav. Ecol. Sociobiol. 1983, 12, 115–119. [Google Scholar] [CrossRef]
- Gerber, C.; Badertscher, S.; Leuthold, R.H. Polyethism in Macrotermes bellicosus (Isoptera). Insectes Sociaux 1988, 35, 226–240. [Google Scholar] [CrossRef]
- Wilson, E.O. The Insect Societies; Harvard University Press: Cambridge, MA, USA; London, UK, 1971; p. 562. [Google Scholar]
- Grassé, P.P. Termitologia Anatomie-Physiologie-Biologie-Systématique des Termites. Tome I. Anatomie-Physiologie-Reproduction; Masson: Paris, France, 1982; p. 676. [Google Scholar]
- Veenstra, J.A. Differential expression of some termite neuropeptides and insulin/IGF-related hormones and their plausible functions in growth, reproduction and caste determination. PeerJ 2023, 11, e15259. [Google Scholar] [CrossRef]
- Sillam-Dussès, D.; Krasulová, J.; Vrkoslav, V.; Pytelková, J.; Cvačka, J.; Kutalová, K.; Bourguignon, T.; Miura, T.; Šobotník, J. Comparative study of the labial gland secretion in termites (Isoptera). PLoS ONE 2012, 7, e46431. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.F.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. Camb. Philos. Soc. 2014, 89, 255–269. [Google Scholar] [CrossRef]
- Watanabe, H.; Nakamura, M.; Tokuda, G.; Yamaoka, I.; Scrivener, A.M.; Noda, H. Site of secretion and properties of endogenous endo-β-1,4-glucanase components from Reticulitermes speratus (Kolbe), a Japanese subterranean termite. Insect Biochem. Mol. Biol. 1997, 27, 305–313. [Google Scholar] [CrossRef]
- Tokuda, G.; Lo, N.; Watanabe, H.; Slaytor, M.; Matsumoto, T.; Noda, H. Metazoan cellulase genes from termites: In-tron/exon structures and sites of expression. Biochim. Biophys. Acta 1999, 1447, 146–159. [Google Scholar] [CrossRef]
- Nakashima, K.; Watanabe, H.; Saitoh, H.; Tokuda, G.; Azuma, J.-I. Dual cellulose-digesting system of the wood-feeding termite, Coptotermes formosanus Shiraki. Insect Biochem. Mol. Biol. 2001, 32, 777–784. [Google Scholar] [CrossRef]
- Zhou, X.; Smith, J.A.; Oi, F.M.; Koehler, P.G.; Bennett, G.W.; Scharf, M.E. Correlation of cellulase gene expression and cellu-lolytic activity throughout the gut of the termite Reticulitermes flavipes. Gene 2007, 395, 29–39. [Google Scholar] [CrossRef]
- Zhang, D.; Lax, A.R.; Raina, A.K.; Bland, J. Differential cellulolytic activity of native-form and C-terminal tagged-form cellulase derived from Coptotermes formosanus and expressed in E. coli. Insect Biochem. Mol. Biol. 2009, 39, 516–522. [Google Scholar] [CrossRef]
- Zhang, D.; Lax, A.R.; Bland, J.M.; Allen, A.B. Characterization of a new endogenous endo-β-1,4-glucanase of Formosan sub-terranean termite (Coptotermes formosanus). Insect Biochem. Mol. Biol. 2011, 41, 211–218. [Google Scholar] [CrossRef]
- Zhang, D.; Allen, A.B.; Lax, A.R. Functional analyses of the digestive b-glucosidase of Formosan subterranean termites (Cop-totermes formosanus). J. Insect Physiol. 2012, 58, 205–210. [Google Scholar] [CrossRef]
- Tokuda, G.; Lo, N.; Watanabe, H.; Arakawa, G.; Matsumoto, T.; Noda, H. Major alteration of the expression site of endogenous cellulases in members of an apical termite lineage. Mol. Ecol. 2004, 13, 3219–3228. [Google Scholar] [CrossRef]
- Fujita, A.; Minamoto, T.; Shimizu, I.; Abe, T. Molecular cloning of lysozyme-encoding cDNAs expressed in the salivary gland of a wood-feeding termite, Reticulitermes speratus. Insect Biochem. Mol. Biol. 2002, 32, 1615–1624. [Google Scholar] [CrossRef]
- Pomés, A.; Melén, E.; Vailes, L.D.; Retief, J.D.; Arruda, L.K.; Chapman, M.D. Novel Allergen Structures with Tandem Amino Acid Repeats Derived from German and American Cockroach. J. Biol. Chem. 1998, 273, 30801–30807. [Google Scholar] [CrossRef]
- Gore, J.C.; Schal, C. Expression, production and excretion of Bla g 1, a major human allergen, in relation to food intake in the German cockroach, Blattella germanica. Med. Veter.-Èntomol. 2005, 19, 127–134. [Google Scholar] [CrossRef]
- Suazo, A.; Gore, C.; Schal, C. RNA interference-mediated knock-down of Bla g 1 in the German cockroach, Blattella germanica L., implicates this allergen-encoding gene in digestion and nutrient absorption. Insect Mol. Biol. 2009, 18, 727–736. [Google Scholar] [CrossRef]
- Suehiro, W.; Matsuura, K. Queen pheromone promotes production of salivary lysozyme by workers in a termite. Insectes Sociaux 2015, 62, 193–198. [Google Scholar] [CrossRef]
- Terrapon, N.; Li, C.; Robertson, H.M.; Ji, L.; Meng, X.; Booth, W.; Chen, Z.; Childers, C.P.; Glastad, K.M.; Gokhale, K.; et al. Molecular traces of alternative social organization in a termite genome. Nat. Commun. 2014, 5, 3636. [Google Scholar] [CrossRef] [PubMed]
- Korb, J.; Poulsen, M.; Hu, H.; Li, C.; Boomsma, J.J.; Zhang, G.; Liebig, J. A genomic comparison of two termites with different social complexity. Front. Genet. 2015, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.C.; Jongepier, E.; Robertson, H.M.; Arning, N.; Bitard-Feildel, T.; Chao, H.; Childers, C.P.; Dinh, H.; Doddapaneni, H.; Dugan, S.; et al. Hemimetabolous genomes reveal molecular basis of termite eusociality. Nat. Ecol. Evol. 2018, 2, 557–566. [Google Scholar] [CrossRef]
- Itakura, S.; Yoshikawa, Y.; Togami, Y.; Umezawa, K. Draft genome sequence of the termite, Coptotermes formosanus: Genetic insights into the pyruvate dehydrogenase complex of the termite. J. Asia-Pacific Èntomol. 2020, 23, 666–674. [Google Scholar] [CrossRef]
- Shigenobu, S.; Hayashi, Y.; Watanabe, D.; Tokuda, G.; Hojo, M.Y.; Toga, K.; Saiki, R.; Yaguchi, H.; Masuoka, Y.; Suzuki, R.; et al. Genomic and transcriptomic analyses of the subterranean termite Reticulitermes speratus: Gene duplication facilitates social evolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2110361119. [Google Scholar] [CrossRef]
- Shelomi, M.; Heckel, D.G.; Pauchet, Y. Ancestral gene duplication enabled the evolution of multifunctional cellulases in stick insects (Phasmatodea). Insect Biochem. Mol. Biol. 2016, 71, 1–11. [Google Scholar] [CrossRef]
- Shelomi, M.; Wipfler, B.; Zhou, X.; Pauchet, Y. Multifunctional cellulase enzymes are ancestral in Polyneoptera. Insect Mol. Biol. 2020, 29, 124–135. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Q.; Saelim, N.; Wang, L.; Nong, W.; Wan, A.T.; Shi, M.; Liu, X.; Cao, Q.; Hui, J.H.L.; et al. Genome assembly and annotation of Periplaneta americana reveal a comprehensive cockroach allergen profile. Allergy 2023, 78, 1088–1103. [Google Scholar] [CrossRef]
- Gore, J.C.; Schal, C. Gene Expression and Tissue Distribution of the Major Human Allergen Bla g 1 in the German Cockroach, Blattella germanica L. (Dictyoptera: Blattellidae). J. Med. Èntomol. 2004, 41, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Sookrung, N.; Khetsuphan, T.; Chaisri, U.; Indrawattana, N.; Reamtong, O.; Chaicumpa, W.; Tungtrongchitr, A. Specific B-cell Epitope of Per a 1: A Major Allergen of American Cockroach (Periplaneta americana) and Anatomical Localization. Allergy Asthma Immunol. Res. 2014, 6, 325–332. [Google Scholar] [CrossRef]
- Shao, L.; Devenport, M.; Fujioka, H.; Ghosh, A.; Jacobs-Lorena, M. Identification and characterization of a novel peritrophic matrix protein, Ae-Aper50, and the microvillar membrane protein, AEG12, from the mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2005, 35, 947–959. [Google Scholar] [CrossRef]
- Ye, C.; Rasheed, H.; Ran, Y.; Yang, X.; Xing, L.; Su, X. Transcriptome changes reveal the genetic mechanisms of the re-productive plasticity of workers in lower termites. BMC Genom. 2019, 20, 702. [Google Scholar] [CrossRef]
- Elsner, D.; Hartfelder, K.; Korb, J. Molecular underpinnings of division of labour among workers in a socially complex termite. Sci. Rep. 2021, 11, 18269. [Google Scholar] [CrossRef]
- Elsner, D.; Meusemann, K.; Korb, J. Longevity and transposon defense, the case of termite reproductives. Proc. Natl. Acad. Sci. USA 2018, 115, 5504–5509. [Google Scholar] [CrossRef]
- Séité, S.; Harrison, M.C.; Sillam-Dussès, D.; Lupoli, R.; Van Dooren, T.J.M.; Robert, A.; Poissonnier, L.A.; Lemainque, A.; Re-nault, D.; Acket, S.; et al. Lifespan pro-longing mechanisms and insulin upregulation without fat accumulation in long-lived reproductives of a higher termite. Commun. Biol. 2022, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Maekawam, K. Changes in endogenous cellulase gene expression levels and reproductive characteristics of primary and secondary reproductives with colony development of the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). J. Insect Physiol. 2010, 56, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, M.D.; Albert, S.; Kucharski, R.; Prusko, C.; Maleszka, R. Evolution of the Yellow/Major Royal Jelly Protein family and the emergence of social behavior in honey bees. Genome Res. 2006, 16, 1385–1394. [Google Scholar] [CrossRef]
- Rutherford, K.; Parkhill, J.; Crook, J.; Horsnell, T.; Rice, P.; Rajandream, M.-A.; Barrell, B. Artemis: Sequence visualization and annotation. Bioinformatics 2000, 16, 944–945. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Veenstra, J.A. Coleoptera genome and transcriptome sequences reveal numerous differences in neuropeptide signaling between species. PeerJ 2019, 7, e7144. [Google Scholar] [CrossRef]
- Veenstra, J.A. Arthropod IGF, relaxin and gonadulin, putative orthologs of Drosophila insulin-like peptides 6, 7 and 8, likely originated from an ancient gene triplication. PeerJ 2020, 8, e9534. [Google Scholar] [CrossRef] [PubMed]
- Billen, J.; Joye, L.; Leuthold, R.H. Fine structure of the labial gland in Macrotermes bellicosus (Isoptera, Termitidae). Acta Zoologica 1989, 70, 27–45. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veenstra, J.A. Evolution of a Cockroach Allergen into the Major Protein of Termite Royal Jelly. Int. J. Mol. Sci. 2023, 24, 10311. https://doi.org/10.3390/ijms241210311
Veenstra JA. Evolution of a Cockroach Allergen into the Major Protein of Termite Royal Jelly. International Journal of Molecular Sciences. 2023; 24(12):10311. https://doi.org/10.3390/ijms241210311
Chicago/Turabian StyleVeenstra, Jan A. 2023. "Evolution of a Cockroach Allergen into the Major Protein of Termite Royal Jelly" International Journal of Molecular Sciences 24, no. 12: 10311. https://doi.org/10.3390/ijms241210311
APA StyleVeenstra, J. A. (2023). Evolution of a Cockroach Allergen into the Major Protein of Termite Royal Jelly. International Journal of Molecular Sciences, 24(12), 10311. https://doi.org/10.3390/ijms241210311






