New Allosteric Modulators of AMPA Receptors: Synthesis and Study of Their Functional Activity by Radioligand-Receptor Binding Analysis
Abstract
1. Introduction
2. Results and Discussion
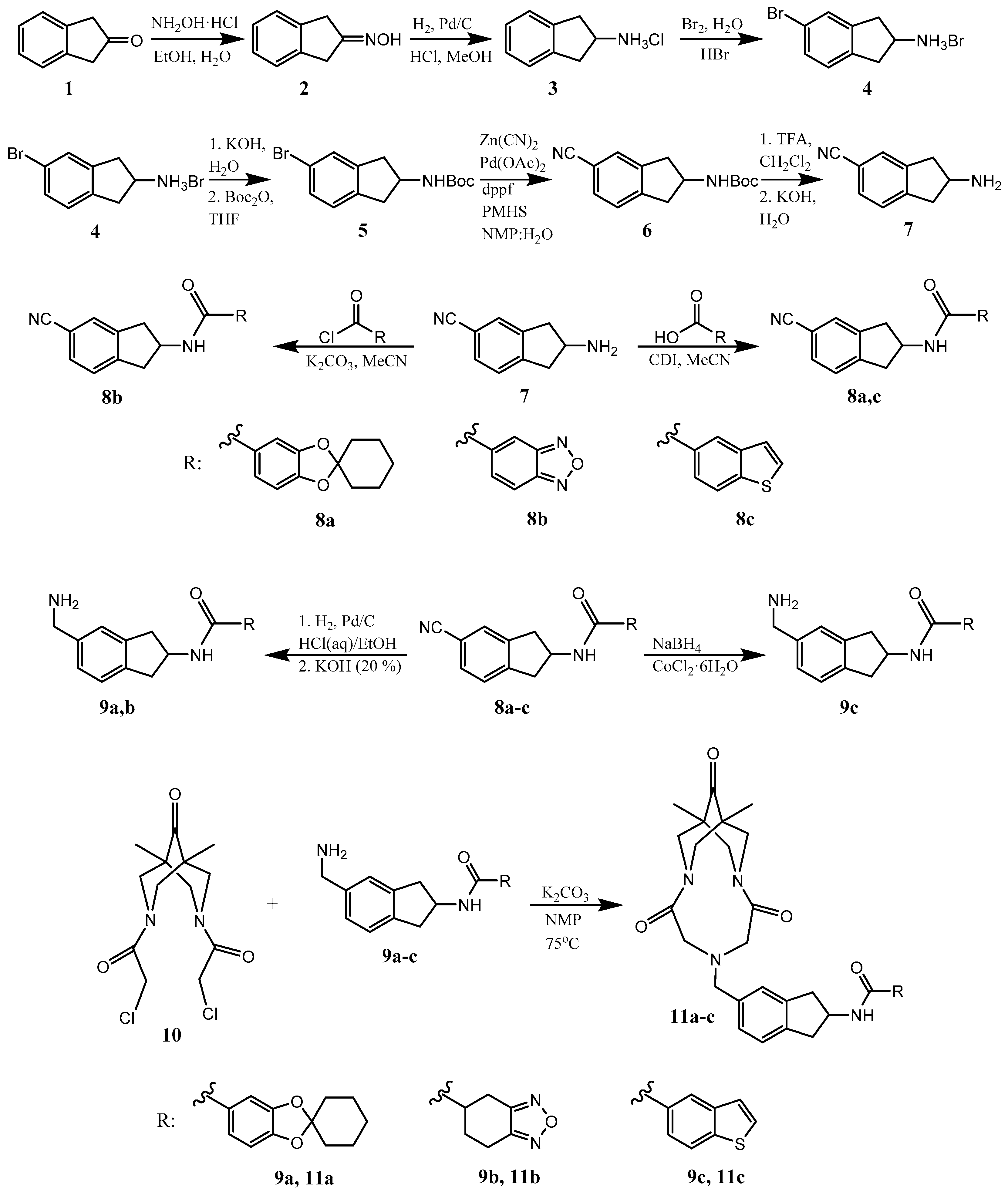
2.1. Synthesis of Target Compounds 11a–c
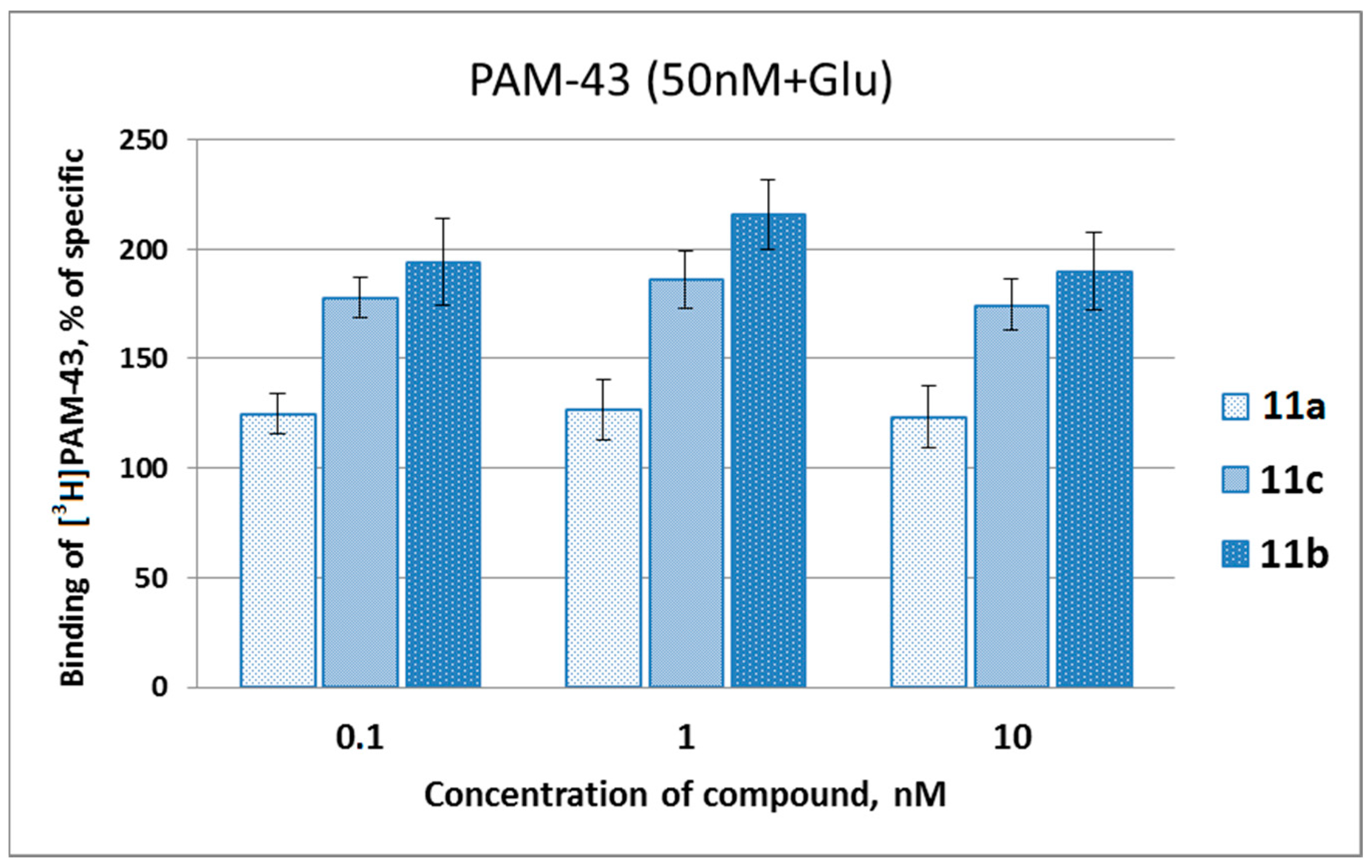
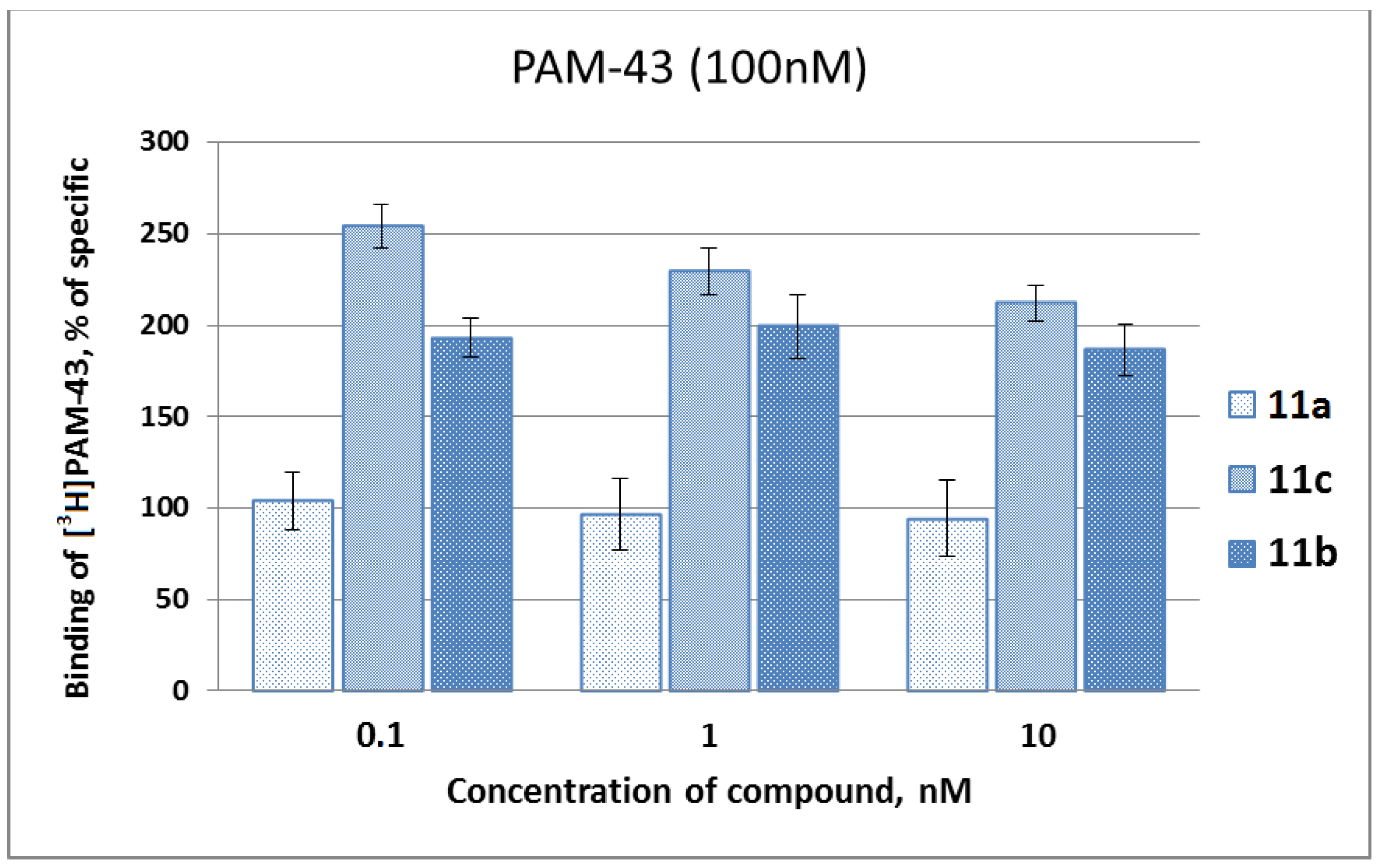
2.2. Biological Studies
3. Materials and Methods
3.1. Chemistry
3.2. Radioligand Binding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiner, A.; Levitz, J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Campiani, G.; Brindisi, M.; Butini, S. Allosteric modulation of ionotropic glutamate receptors: An outlook on new therapeutic approaches to treat central nervous system disorders. ACS Med. Chem. Lett. 2019, 10, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Partin, K.M. AMPA receptor potentiators: From drug design to cognitive enhancement. Curr. Opin. Pharmacol. 2015, 20, 46–53. [Google Scholar] [CrossRef]
- Lauterborn, J.C.; Palmer, L.C.; Jia, Y.; Pham, D.T.; Hou, B.; Wang, W.; Trieu, B.H.; Cox, C.D.; Kantorovich, S.; Gall, C.M.; et al. Chronic ampakine treatments stimulate dendritic growth and promote learning in middle-aged rats. J. Neurosci. 2016, 36, 1636–1646. [Google Scholar] [CrossRef]
- Twomey, E.C.; Sobolevsky, A.I. Structural mechanisms of gating in ionotropic glutamate receptors. Biochemistry 2018, 57, 267–276. [Google Scholar] [CrossRef]
- Chen, S.; Gouaux, E. Structure and mechanism of AMPA receptor—Auxiliary protein complexes. Curr. Opin. Struct. Biol. 2019, 54, 104–111. [Google Scholar] [CrossRef]
- Coombs, I.D.; Soto, D.; McGee, T.P.; Gold, M.G.; Farrant, M.; Cull-Candy, S.G. Homomeric GluA2(R) AMPA receptors can conduct when desensitized. Nat. Commun. 2019, 10, 4312. [Google Scholar] [CrossRef] [PubMed]
- Salazar, H.; Mischke, S.; Plested, A.J.R. Measurements of the timescale and conformational space of AMPA receptor desensitization. Biophys. J. 2020, 119, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- Pirotte, B.; Francotte, P.; Goffin, E.; de Tullio, P. AMPA receptor positive allosteric modulators: A patent review. Expert Opin. Ther. Pat. 2013, 23, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.E.; Pennicott, L.E.; Beswick, P. AMPA receptor-positive allosteric modulators for the treatment of schizophrenia: An overview of recent patent applications. Future Med. Chem. 2015, 7, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Reuillon, T.; Ward, S.E.; Beswick, P. AMPA receptor positive allosteric modulators: Potential for the treatment of neuropsychiatric and neurological disorders. Curr. Top. Med. Chem. 2016, 16, 3536–3565. [Google Scholar] [CrossRef]
- Matthews, P.M.; Pinggera, A.; Kampjut, D.; Greger, I.H. Biology of AMPA receptor interacting proteins—From biogenesis to synaptic plasticity. Neuropharmacology 2021, 197, 108709. [Google Scholar] [CrossRef]
- Díaz-Alonso, J.; Nicoll, R.A. AMPA receptor trafficking and LTP: Carboxy-termini, amino-termini and TARPs. Neuropharmacology 2021, 197, 108710. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Gao, Y.; Li, J.-T.; Ma, W.-Y.; Chen, N.-H. The role of AMPARs composition and trafficking in synaptic plasticity and diseases. Cell Mol. Neurobiol. 2022, 42, 2489–2504. [Google Scholar] [CrossRef] [PubMed]
- Cavalleri, L.; Merlo Pich, E.; Millan, M.J.; Chiamulera, C.; Kunath, T.; Spano, P.F.; Collo, G. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol. Psychiatry 2018, 23, 812–823. [Google Scholar] [CrossRef]
- Mozafari, N.; Shamsizadeh, A.; Fatemi, I.; Allahtavakoli, M.; Moghadam-Ahmadi, A.; Kaviani, E.; Kaeidi, A. CX691, as an AMPA receptor positive modulator, improves the learning and memory in a rat model of Alzheimer’s disease. Iran. J. Basic Med. Sci. 2018, 21, 724–730. [Google Scholar]
- Mendez-David, I.; Guilloux, J.-P.; Papp, M.; Tritschler, L.; Mocaer, E.; Gardier, A.M.; Bretin, S.; David, D.J. S47445 produces antidepressant- and anxiolytic-like effects through neurogenesis dependent and independent mechanisms. Front. Pharmacol. 2017, 8, 462. [Google Scholar] [CrossRef]
- Pilar-Cuellar, F.; Castro, E.; Bretin, S.; Mocaer, E.; Pazos, Á.; Díaz, Á. S 47445 counteracts the behavioral manifestations and hippocampal neuroplasticity changes in bulbectomized mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 205–213. [Google Scholar] [CrossRef]
- Kunugi, A.; Tajima, Y.; Kuno, H.; Sogabe, S.; Kimura, H. HBT1, a novel AMPA receptor potentiator with lower agonistic effect, avoided bell-shaped response in in vitro BDNF production. J. Pharmacol. Exp. Ther. 2018, 364, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Zarate, C.A.; Krystal, J.H.; Manji, H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008, 7, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.A.; Kroon, R.A.; Stein, M. A translational approach to evaluate the efficacy and safety of the novel AMPA receptor positive allosteric modulator org 26576 in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2012, 72, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Xie, F.; Xu, X.; Zhou, X. The impact and mechanism of ampakine CX1739 on protection against respiratory depression in rats. Future Med. Chem. 2020, 12, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kunugi, A.; Tajima, Y.; Suzuki, N.; Suzuki, M.; Toyofuku, M.; Kuno, H.; Sogabe, S.; Kosugi, Y.; Awasaki, Y.; et al. Strictly regulated agonist-dependent activation of AMPA-R is the key characteristic of TAK-653 for robust synaptic responses and cognitive improvement. Sci. Rep. 2021, 11, 14532. [Google Scholar] [CrossRef]
- Suzuki, A.; Hara, H.; Kimura, H. Role of the AMPA receptor in antidepressant effects of ketamine and potential of AMPA receptor potentiators as a novel antidepressant. Neuropharmacology 2022, 222, 109308. [Google Scholar] [CrossRef]
- Kadriu, B.; Musazzi, L.; Johnston, J.N.; Kalynchuk, L.E.; Caruncho, H.J.; Popoli, M.; Zarate, C.A. Positive AMPA receptor modulation in the treatment of neuropsychiatric disorders: A long and winding road. Drug Discov. Today 2021, 26, 2816–2838. [Google Scholar] [CrossRef]
- Cetin, S.; Knez, D.; Gobec, S.; Kos, J.; Pišlar, A. Cell models for Alzheimer’s and Parkinson’s disease: At the interface of biology and drug discovery. Biomed. Pharmacother. 2022, 149, 112924. [Google Scholar] [CrossRef]
- Banerjee, R.; Rai, A.; Iyer, S.M.; Narwal, S.; Tare, M. Animal models in the study of Alzheimer’s disease and Parkinson’s disease: A historical perspective. Anim. Model. Exp. Med. 2022, 5, 27–37. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, S.; Li, X.-J. Genetically modified large animal models for investigating neurodegenerative diseases. Cell Biosci. 2021, 11, 218. [Google Scholar] [CrossRef]
- Rapaka, D.; Adiukwu, P.C.; Bitra, V.R. Experimentally induced animal models for cognitive dysfunction and Alzheimer’s disease. MethodsX 2022, 9, 101933. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Wąsik, A. Advantages and limitations of animal schizophrenia models. Int. J. Mol. Sci. 2022, 23, 5968. [Google Scholar] [CrossRef] [PubMed]
- Karlov, D.S.; Lavrov, M.I.; Palyulin, V.A.; Zefirov, N.S. MMGBSA and MM-PBSA performance in activity evaluation of AMPA receptor positive allosteric modulators. J. Biomol. Struct. Dyn. 2018, 36, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Karlov, D.S.; Lavrov, M.I.; Palyulin, V.A.; Zefirov, N.S. Pharmacophore analysis of positive allosteric modulators of AMPA receptors. Russ. Chem. Bull. 2016, 65, 581–587. [Google Scholar] [CrossRef]
- Lavrov, M.I.; Karlov, D.S.; Palyulin, V.A.; Grigoriev, V.V.; Zamoyski, V.L.; Brkich, G.E.; Pyatigorskaya, N.V.; Zapolskiy, M.E. Novel positive allosteric modulator of AMPA-receptors based on tricyclic scaffold. Mendeleev Commun. 2018, 28, 311–313. [Google Scholar] [CrossRef]
- Lavrov, M.I.; Stroganov, O.V.; Zamoyski, V.L.; Grigoriev, V.V.; Zapolskiy, M.E.; Sysolyatin, S.V.; Malykhin, V.V.; Surmachev, V.N.; Palyulin, V.A. Synthesis of an allosteric modulator of ionotropic glutamate receptors. Mendeleev Commun. 2020, 30, 156–158. [Google Scholar] [CrossRef]
- Grigoriev, V.V.; Lavrov, M.I.; Zamoyski, V.L.; Garibova, T.L.; Palyulin, V.A.; Bachurin, S.O. New Positive Allosteric Modulator of AMPA Receptors: In vitro and in vivo Studies. Dokl. Biochem. Biophys. 2019, 488, 304–306. [Google Scholar] [CrossRef]
- Zapolsky, M.E.; Zefirov, N.S.; Palyulin, V.A.; Lavrov, M.I. Tricyclic Derivatives of N,N′-Substituted 3,7-diazabicyclo[3.3.1]nonanes and Drugs Based Thereon. U.S. Patent 9440985 B2, 13 September 2016. [Google Scholar]
- Golubeva, E.A.; Lavrov, M.I.; Veremeeva, P.N.; Bovina, E.M.; Radchenko, E.V.; Topchiy, M.A.; Asachenko, A.F.; Zamoiski, V.L.; Grigoriev, V.V.; Palyulin, V.A. New 1,11-dimethyl-3,6,9-triazatricyclo[7.3.1.13,11]tetradecane-4,8,12-trione derivative as an allosteric modulator of the glutamatergic system. Mendeleev Commun. 2023, 33, 70–72. [Google Scholar] [CrossRef]
- Gasperi, V.; Savini, I.; Catani, M.V. Assay of CB1 Receptor Binding. Methods Mol. Biol. 2023, 2576, 95–109. [Google Scholar] [CrossRef]
- Arai, K.; Homma, T.; Morikawa, Y.; Ubukata, N.; Tsuruoka, H.; Aoki, K.; Ishikawa, H.; Mizuno, M.; Sada, T. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur. J. Pharmacol. 2015, 761, 226–234. [Google Scholar] [CrossRef]
- Subramanian, N.; Kalkman, H.O. Receptor profile of P88-8991 and P95-12113, metabolites of the novel antipsychotic iloperidone. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 553–560. [Google Scholar] [CrossRef]
- Adamska-Bartłomiejczyk, A.; Lipiński, P.F.J.; Piekielna-Ciesielska, J.; Kluczyk, A.; Janecka, A. Pharmacological Profile and Molecular Modeling of Cyclic Opioid Analogues Incorporating Various Phenylalanine Derivatives. ChemMedChem 2020, 15, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Zvejniece, L.; Liepinsh, E.; Cirule, H.; Zharkova, O.; Veinberg, G.; Kalvinsh, I. Comparative pharmacological activity of optical isomers of phenibut. Eur. J. Pharmacol. 2008, 583, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zeilinger, M.; Pichler, F.; Nics, L.; Wadsak, W.; Spreitzer, H.; Hacker, M.; Mitterhauser, M. New approaches for the reliable in vitro assessment of binding affinity based on high-resolution real-time data acquisition of radioligand-receptor binding kinetics. EJNMMI Res. 2017, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Garzone, P.D.; Kroboth, P.D. Pharmacokinetics of the newer benzodiazepines. Clin. Pharmacokinet. 1989, 16, 337–364. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G.; Perez, J.A.; Pease, J.P. Comparison of biological effects of N-alkylated congeners of β-phenethylamine derived from 2-aminotetralin, 2-aminoindan, and 6-aminobenzocycloheptene. J. Med. Chem. 1980, 23, 745–749. [Google Scholar] [CrossRef]
- Levin, N.; Graham, B.E.; Kolloff, H.G. Physiologically active indanamines. J. Org. Chem. 1944, 9, 380–391. [Google Scholar] [CrossRef]
- Cere, V.; Dal Monte, D.; Sandri, E. Catalytic Hydrogenation of Benzo-2,l, 3.-oxadiazoles. Tetrahedron 1972, 28, 3271–3276. [Google Scholar] [CrossRef]
- Heinzman, S.W.; Ganem, B. Mechanism of sodium borohydride-cobaltous chloride reductions. J. Am. Chem. Soc. 1982, 104, 6801–6802. [Google Scholar] [CrossRef]
- Itsuno, S.; Sakurai, Y.; Ito, K. Reduction of some functional groups with zirconium tetrachloride/sodium borohydride. Synth. Commun. 1988, 12, 995–997. [Google Scholar] [CrossRef]
- Satoh, T.; Suzuki, S. Reduction of organic compounds with sodium borohydride-transition metal salt systems (1). Reduction of organic nitride, nitro and amide compounds to primary amines. Tetrahedron Lett. 1969, 52, 4555–4558. [Google Scholar] [CrossRef]
- Yamada, K.A.; Turetsky, D.M. Allosteric interactions between cyclothiazide and AMPA/kainate receptor antagonists. Br. J. Pharmacol. 1996, 117, 1663–1672. [Google Scholar] [CrossRef]
- Kessler, M.; Arai, A.; Quan, A.; Lynch, G. Effect of cyclothiazide on binding properties of AMPA-type glutamate receptors: Lack of competition between cyclothiazide and GYKI 52466. Mol. Pharmacol. 1996, 49, 123–131. [Google Scholar]
- Lindén, A.M.; Yu, H.; Zarrinmayeh, H.; Wheeler, W.J.; Skolnick, P. Binding of an AMPA receptor potentiator ([3H]LY395153) to native and recombinant AMPA receptors. Neuropharmacology 2001, 40, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Nagaev, I.Y.; Shevchenko, K.V.; Shevchenko, V.P.; Myasoedov, N.F.; Grigoriev, V.V.; Lavrov, M.I.; Bondarenko, E.V.; Kalashnikova, E.E. Synthesis of tritium-labeled PAM-43. Mendeleev Commun. 2018, 28, 64–65. [Google Scholar] [CrossRef]
- Vyunova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Grigoriev, V.V.; Palyulin, V.A.; Lavrov, M.I.; Bondarenko, E.V.; Kalashnikova, E.E.; Myasoedov, N.F. Characterization of a New Positive Allosteric Modulator of AMPA Receptors PAM-43: Specific Binding of the Ligand and its Ability to Potentiate AMPAR Currents. Curr. Mol. Pharmacol. 2020, 13, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O.; Grigorev, V.V.; Palyulin, V.A.; Lavrov, M.I.; Zefirov, N.S.; Garibova, T.L.; Voronina, T.A.; Roziev, R.A. N,N′-Substituted 3,7-diazabicyclo[3.3.1]nonanes, Pharmaceutical Compositions Based Thereon and Use Thereof. RU Patent 2613071, 29 October 2017. [Google Scholar]
- Bachurin, S.O.; Grigorev, V.V.; Zefirov, N.S.; Lavrov, M.I.; Lapteva, V.L.; Palyulin, V.A. N,N′-Substituted 3,7-diazabicyclo[3,3,1]nonanes with Pharmacological Effect, Pharmacological Compositions on Their Base, and Application Method. RU Patent 2333211, 10 September 2008. [Google Scholar]
- Golubeva, E.A.; Lavrov, M.I.; Radchenko, E.V.; Palyulin, V.A. Diversity of AMPA receptor ligands: Chemotypes, binding modes, mechanisms of action, and therapeutic effects. Biomolecules 2023, 13, 56. [Google Scholar] [CrossRef]
- Lyle, R.E.; Walsh, D.A. An improved synthesis of 1,4-dihydro-3-[2H]-isoquinolone. Org. Prep. Proced. Int. 1973, 5, 299. [Google Scholar] [CrossRef]
- Göksu, S.; Seçen, H. Concise syntheses of 2-aminoindans via indan-2-ol. Tetrahedron 2005, 61, 6801–6807. [Google Scholar] [CrossRef]
- Prashad, M.; Hu, B.; Har, D.; Repic, O.; Blacklock, T.J.; Acemoglub, M. Efficient and Practical Syntheses of (R)-(5-Amino-2,3-dihydro-1H-inden-2-yl)-carbamic Acid Methyl Ester. Adv. Synth. Catal. 2001, 343, 461–472. [Google Scholar] [CrossRef]
- Barvian, K.K.; Speake, J.D.; Cowan, D.J.; Larkin, A.L.; Szewczyk, J.R. Novel Heterocycle Compounds. U.S. Patent 20090054431A1, 26 February 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubeva, E.A.; Lavrov, M.I.; Veremeeva, P.N.; Vyunova, T.V.; Shevchenko, K.V.; Topchiy, M.A.; Asachenko, A.F.; Palyulin, V.A. New Allosteric Modulators of AMPA Receptors: Synthesis and Study of Their Functional Activity by Radioligand-Receptor Binding Analysis. Int. J. Mol. Sci. 2023, 24, 10293. https://doi.org/10.3390/ijms241210293
Golubeva EA, Lavrov MI, Veremeeva PN, Vyunova TV, Shevchenko KV, Topchiy MA, Asachenko AF, Palyulin VA. New Allosteric Modulators of AMPA Receptors: Synthesis and Study of Their Functional Activity by Radioligand-Receptor Binding Analysis. International Journal of Molecular Sciences. 2023; 24(12):10293. https://doi.org/10.3390/ijms241210293
Chicago/Turabian StyleGolubeva, Elena A., Mstislav I. Lavrov, Polina N. Veremeeva, Tatiana V. Vyunova, Konstantin V. Shevchenko, Maxim A. Topchiy, Andrey F. Asachenko, and Vladimir A. Palyulin. 2023. "New Allosteric Modulators of AMPA Receptors: Synthesis and Study of Their Functional Activity by Radioligand-Receptor Binding Analysis" International Journal of Molecular Sciences 24, no. 12: 10293. https://doi.org/10.3390/ijms241210293
APA StyleGolubeva, E. A., Lavrov, M. I., Veremeeva, P. N., Vyunova, T. V., Shevchenko, K. V., Topchiy, M. A., Asachenko, A. F., & Palyulin, V. A. (2023). New Allosteric Modulators of AMPA Receptors: Synthesis and Study of Their Functional Activity by Radioligand-Receptor Binding Analysis. International Journal of Molecular Sciences, 24(12), 10293. https://doi.org/10.3390/ijms241210293





