Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice
Abstract
1. Introduction
2. Results
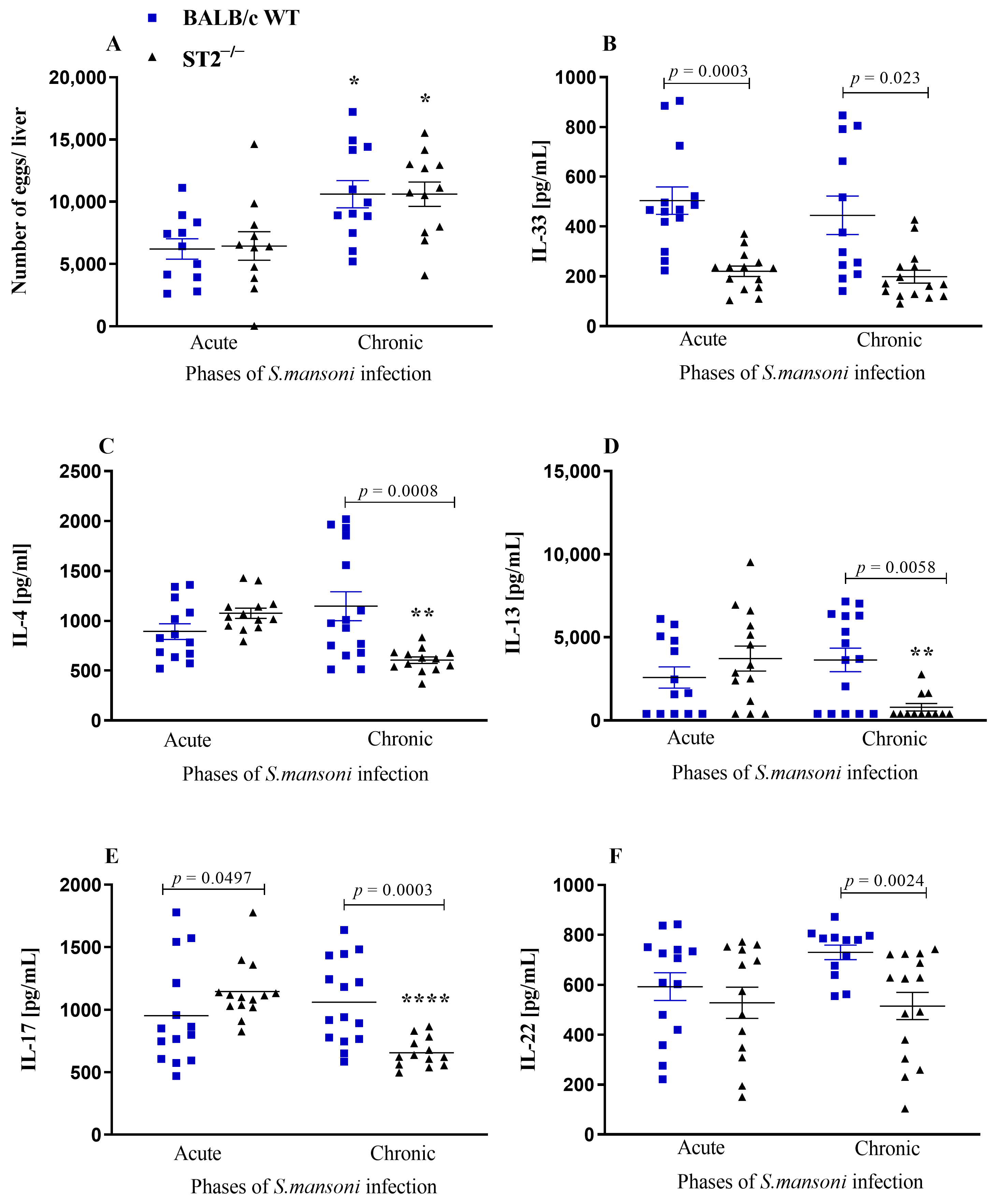
2.1. Systemic Immune Response
2.2. Liver Immune Response
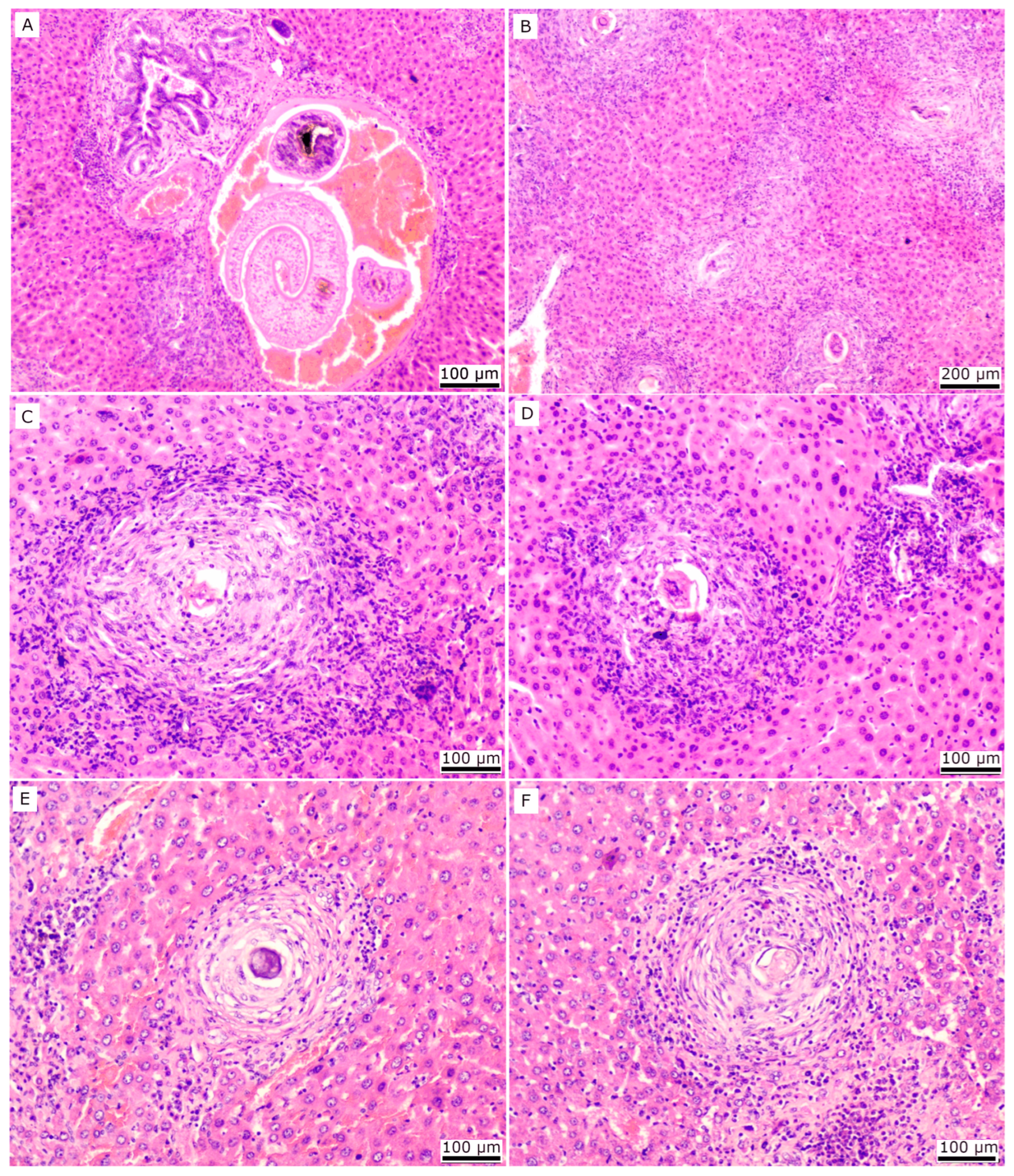
2.3. Histopathology
2.4. Hepatic Stellate Cell Differentiation
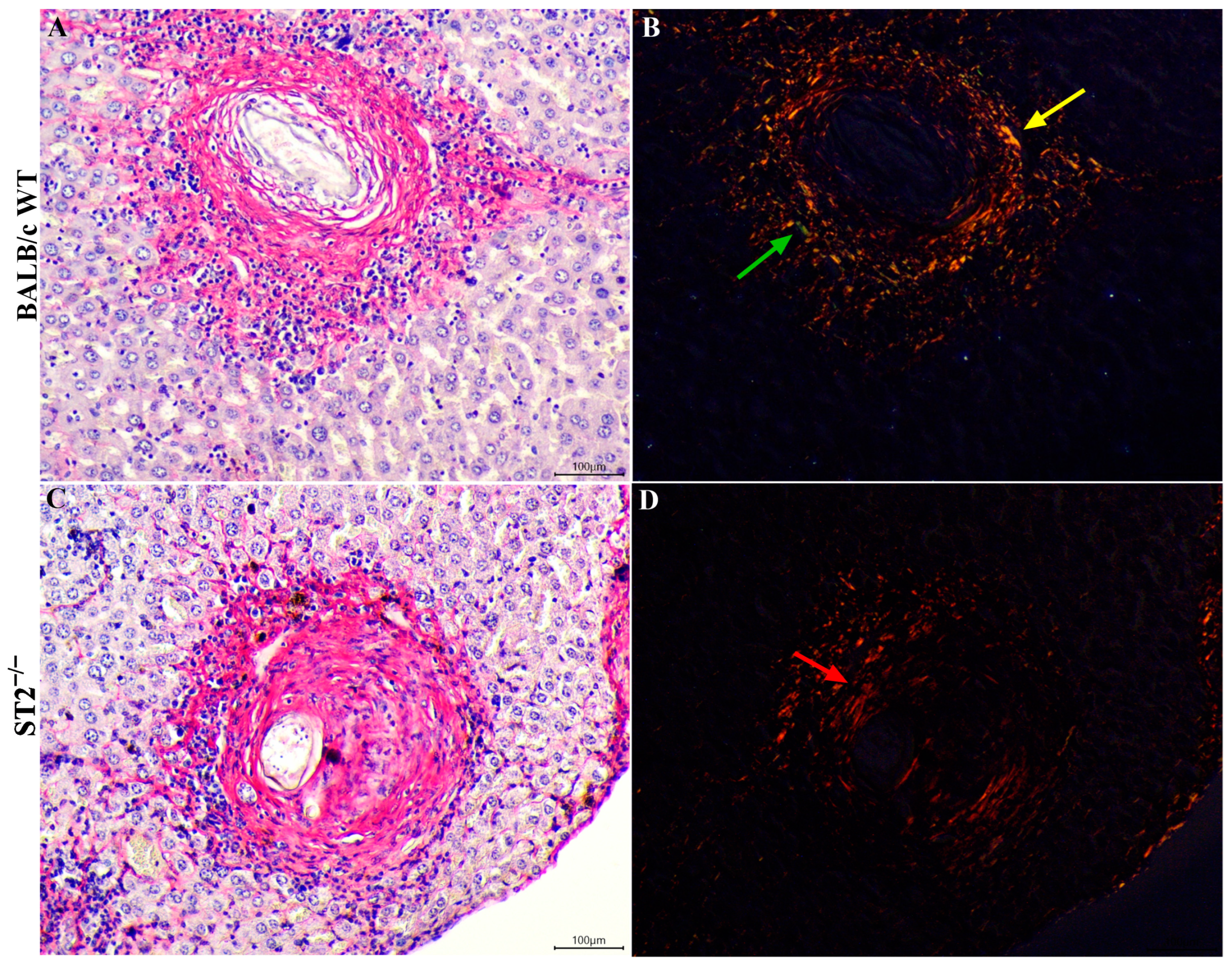
2.5. Collagen Deposition and Expression of Pro-Fibrotic Genes in Livers of S. mansoni-Infected Mice
3. Discussion
4. Methods
4.1. Mice
4.2. Parasite and Infection
4.3. Experimental Design
4.4. Cytokine and Chemokine Assay
4.5. Quantification of Hydroxyproline
4.6. Histopathological, Immunohistochemical, and Morphometric Analyses of Liver
4.7. Quantification of mRNA Expression by RT-qPCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.-F.; Feichtinger, J.; Zelger, B.; Sgonc, R.; Wolfram, D. The Immunology of Fibrosis. Annu. Rev. Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver Fibrosis and Repair: Immune Regulation of Wound Healing in a Solid Organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Backovic, A.; Rabensteiner, E.; Plank, N.; Schwentner, C.; Sgonc, R. The Immunology of Fibrosis: Innate and Adaptive Responses. Trends Immunol. 2010, 31, 110–119. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Organ and Tissue Fibrosis: Molecular Signals, Cellular Mechanisms and Translational Implications. Mol. Asp. Med. 2019, 65, 2–15. [Google Scholar] [CrossRef]
- Anthony, B.; Allen, J.T.; Li, Y.S.; McManus, D.P. Hepatic Stellate Cells and Parasite-Induced Liver Fibrosis. Parasites Vectors 2010, 3, 60. [Google Scholar] [CrossRef]
- Carson, J.P.; Ramm, G.A.; Robinson, M.W.; McManus, D.P.; Gobert, G.N. Schistosome-Induced Fibrotic Disease: The Role of Hepatic Stellate Cells. Trends Parasitol. 2018, 34, 524–540. [Google Scholar] [CrossRef]
- Kamdem, S.D.; Moyou-Somo, R.; Brombacher, F.; Nono, J.K. Host Regulators of Liver Fibrosis during Human Schistosomiasis. Front. Immunol. 2018, 9, 2781. [Google Scholar] [CrossRef]
- Chen, T.T.; Peng, S.; Wang, Y.; Hu, Y.; Shen, Y.; Xu, Y.; Yin, J.; Liu, C.; Cao, J. Improvement of Mitochondrial Activity and Fibrosis by Resveratrol Treatment in Mice with Schistosoma Japonicum Infection. Biomolecules 2019, 9, 658. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef]
- Chitsulo, L.; Engels, D.; Montresor, A.; Savioli, L. The Global Status of Schistosomiasis and Its Control. Acta Trop. 2000, 77, 41–51. [Google Scholar] [CrossRef]
- Masamba, P.; Adenowo, A.; Oyinloye, B.; Kappo, A. Universal Stress Proteins as New Targets for Environmental and Therapeutic Interventions of Schistosomiasis. Int. J. Environ. Res. Public Health 2016, 13, 972. [Google Scholar] [CrossRef]
- De Jesus, A.R.; Silva, A.; Santana, L.B.; Magalhães, A.; de Jesus, A.A.; de Almeida, R.P.; Rêgo, M.A.V.; Burattini, M.N.; Pearce, E.J.; Carvalho, E.M. Clinical and Immunologic Evaluation of 31 Patients with Acute Schistosomiasis Mansoni. J. Infect. Dis. 2002, 185, 98–105. [Google Scholar] [CrossRef]
- Hiatt, R.A.; Ottesen, E.A.; Sotomayor, Z.R.; Lawley, T.J. Serial Observations of Circulating Immune Complexes in Patients with Acute Schistosomiasis. J. Infect. Dis. 1980, 142, 665–670. [Google Scholar] [CrossRef]
- Abath, F.G.C.; Morais, C.N.L.; Montenegro, C.E.L.; Wynn, T.A.; Montenegro, S.M.L. Immunopathogenic Mechanisms in Schistosomiasis: What Can Be Learnt from Human Studies? Trends Parasitol. 2006, 22, 85–91. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H.; Mahana, N.; Dalton, J.P. Innate Immunogenicity and in vitro Protective Potential of Schistosoma mansoni Lung Schistosomula Excretory-Secretory Candidate Vaccine Antigens. Microbes Infect. 2010, 12, 700–709. [Google Scholar] [CrossRef]
- Egesa, M.; Lubyayi, L.; Tukahebwa, E.M.; Bagaya, B.S.; Chalmers, I.W.; Wilson, S.; Hokke, C.H.; Hoffmann, K.F.; Dunne, D.W.; Yazdanbakhsh, M.; et al. Schistosoma Mansoni Schistosomula Antigens Induce Th1/Pro-Inflammatory Cytokine Responses. Parasite Immunol. 2018, 40, e12592. [Google Scholar] [CrossRef]
- Okano, M.; Satoskar, A.R.; Nishizaki, K.; Abe, M.; Harn, D.A. Induction of Th2 Responses and IgE Is Largely Due to Carbohydrates Functioning as Adjuvants on Schistosoma mansoni Egg Antigens. J. Immunol. 1999, 163, 6712–6717. [Google Scholar] [CrossRef]
- Okano, M.; Satoskar, A.R.; Nishizaki, K.; Harn, D.A. Lacto-N-Fucopentaose III Found on Schistosoma mansoni Egg Antigens Functions as Adjuvant for Proteins by Inducing Th2-Type Response. J. Immunol. 2001, 167, 442–450. [Google Scholar] [CrossRef]
- Thomas, P.G.; Carter, M.R.; Atochina, O.; Da’Dara, A.A.; Piskorska, D.; McGuire, E.; Harn, D.A. Maturation of Dendritic Cell 2 Phenotype by a Helminth Glycan Uses a Toll-Like Receptor 4-Dependent Mechanism. J. Immunol. 2003, 171, 5837–5841. [Google Scholar] [CrossRef]
- Herbert, D.R.; Hölscher, C.; Mohrs, M.; Arendse, B.; Schwegmann, A.; Radwanska, M.; Leeto, M.; Kirsch, R.; Hall, P.; Mossmann, H.; et al. Alternative Macrophage Activation Is Essential for Survival during Schistosomiasis and Downmodulates T Helper 1 Responses and Immunopathology. Immunity 2004, 20, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Everts, B.; Perona-Wright, G.; Smits, H.H.; Hokke, C.H.; van der Ham, A.J.; Fitzsimmons, C.M.; Doenhoff, M.J.; van der Bosch, J.; Mohrs, K.; Haas, H.; et al. Omega-1, a Glycoprotein Secreted by Schistosoma mansoni Eggs, Drives Th2 Responses. J. Exp. Med. 2009, 206, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Costain, A.H.; MacDonald, A.S.; Smits, H.H. Schistosome Egg Migration: Mechanisms, Pathogenesis and Host Immune Responses. Front. Immunol. 2018, 9, 3042. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Mohrs, M.; Pearce, E.J. Regulatory T Cell Responses Develop in Parallel to Th Responses and Control the Magnitude and Phenotype of the Th Effector Populatio. J. Immunol. 2006, 176, 5839–5847. [Google Scholar] [CrossRef]
- Rutitzky, L.I.; Smith, P.M.; Stadecker, M.J. T-Bet Protects against Exacerbation of Schistosome Egg-Induced Immunopathology by Regulating Th17-Mediated Inflammation. Eur. J. Immunol. 2009, 39, 2470–2481. [Google Scholar] [CrossRef]
- Haeberlein, S.; Obieglo, K.; Ozir-Fazalalikhan, A.; Chayé, M.A.M.; Veninga, H.; van der Vlugt, L.E.P.M.; Voskamp, A.; Boon, L.; den Haan, J.M.M.; Westerhof, L.B.; et al. Schistosome Egg Antigens, Including the Glycoprotein IPSE/Alpha-1, Trigger the Development of Regulatory B Cells. PLoS Pathog. 2017, 13, e1006539. [Google Scholar] [CrossRef]
- Hoffmann, K.F.; Cheever, A.W.; Wynn, T.A. IL-10 and the Dangers of Immune Polarization: Excessive Type 1 and Type 2 Cytokine Responses Induce Distinct Forms of Lethal Immunopathology in Murine Schistosomiasis. J. Immunol. 2000, 164, 6406–6416. [Google Scholar] [CrossRef]
- Hesse, M.; Piccirillo, C.A.; Belkaid, Y.; Prufer, J.; Mentink-Kane, M.; Leusink, M.; Cheever, A.W.; Shevach, E.M.; Wynn, T.A. The Pathogenesis of Schistosomiasis Is Controlled by Cooperating IL-10-Producing Innate Effector and Regulatory T Cells. J. Immunol. 2004, 172, 3157–3166. [Google Scholar] [CrossRef]
- Ross, A.G.P.; Bartley, P.B.; Sleigh, A.C.; Olds, G.R.; Li, Y.; Williams, G.M.; McManus, D.P. Schistosomiasis. N. Engl. J. Med. 2002, 346, 1212–1220. [Google Scholar] [CrossRef]
- Chen, M.G. Schistosoma japonicum and S. japonicum-like Infections: Epidemiology, Clinical and Pathological Aspects. In Human Schistosomiasis; Jordan, P., Webbe, G., Sturrock, R.F., Eds.; CAB International: Wallingfordm, CT, USA, 1993; pp. 237–270. [Google Scholar]
- Chang, D.; Ramalho, L.N.Z.; Ramalho, F.S.; Martinelli, A.L.C.; Zucoloto, S. Hepatic Stellate Cells in Human Schistosomiasis Mansoni: A Comparative Immunohistochemical Study with Liver Cirrhosis. Acta Trop. 2006, 97, 318–323. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, Q.; Jiang, R.; Lv, L.; Shoto, S.S.; Maillet, I.; Quesniaux, V.; Tang, J.; Zhang, W.; Sun, B.; et al. Interleukin-33 Drives Hepatic Fibrosis through Activation of Hepatic Stellate Cells. Cell Mol. Immunol. 2018, 15, 388–398. [Google Scholar] [CrossRef]
- Hams, E.; Bermingham, R.; Wurlod, F.A.; Hogan, A.E.; O’Shea, D.; Preston, R.J.; Rodewald, H.; McKenzie, A.N.J.; Fallon, P.G. The Helminth T2 RNase Ω1 Promotes Metabolic Homeostasis in an IL-33- and Group 2 Innate Lymphoid Cell-dependent Mechanism. FASEB J. 2016, 30, 824–835. [Google Scholar] [CrossRef]
- Vannella, K.M.; Ramalingam, T.R.; Borthwick, L.A.; Barron, L.; Hart, K.M.; Thompson, R.W.; Kindrachuk, K.N.; Cheever, A.W.; White, S.; Budelsky, A.L.; et al. Combinatorial Targeting of TSLP, IL-25, and IL-33 in Type 2 Cytokine–Driven Inflammation and Fibrosis. Sci. Transl. Med. 2016, 8, 337ra65. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine That Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- He, X.; Xie, J.; Wang, Y.; Fan, X.; Su, Q.; Sun, Y.; Lei, N.; Zhang, D.; Gao, G.; Pan, W. Down-Regulation of MicroRNA-203-3p Initiates Type 2 Pathology during Schistosome Infection via Elevation of Interleukin-33. PLoS Pathog. 2018, 14, e1006957. [Google Scholar] [CrossRef]
- Schmitt, P.; Girard, J.-P.; Cayrol, C. Interleukin-33: From Biology to Potential Treatments. Med. Sci. 2019, 35, 440–451. [Google Scholar] [CrossRef]
- Mchedlidze, T.; Waldner, M.; Zopf, S.; Walker, J.; Rankin, A.L.; Schuchmann, M.; Voehringer, D.; McKenzie, A.N.J.; Neurath, M.F.; Pflanz, S.; et al. Interleukin-33-Dependent Innate Lymphoid Cells Mediate Hepatic Fibrosis. Immunity 2013, 39, 357–371. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic Stellate Cells as Key Target in Liver Fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Liu, Y.; Meyer, C.; Müller, A.; Herweck, F.; Li, Q.; Müllenbach, R.; Mertens, P.R.; Dooley, S.; Weng, H.-L. IL-13 Induces Connective Tissue Growth Factor in Rat Hepatic Stellate Cells via TGF-β–Independent Smad Signaling. J. Immunol. 2011, 187, 2814–2823. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Talvani, A.; Tafuri, W.L.; Lukacs, N.W.; Hellewell, P.G. Eosinophil Recruitment into Sites of Delayed-Type Hypersensitivity Reactions in Mice. J. Leukoc. Biol. 2001, 69, 353–360. [Google Scholar] [CrossRef]
- Hams, E.; Aviello, G.; Fallon, P.G. The Schistosoma Granuloma: Friend or Foe? Front. Immunol. 2013, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Deng, W.; Lei, J. Interleukin-33 Promotes Th2 Immune Responses in Infected Mice with Schistosoma Japonicum. Parasitol. Res. 2015, 114, 2911–2918. [Google Scholar] [CrossRef] [PubMed]
- da Paz, V.R.F.; Figueiredo-Vanzan, D.; dos Santos Pyrrho, A. Interaction and Involvement of Cellular Adhesion Molecules in the Pathogenesis of Schistosomiasis Mansoni. Immunol. Lett. 2019, 206, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guabiraba, R.; Besnard, A.-G.; Komai-Koma, M.; Jabir, M.S.; Zhang, L.; Graham, G.J.; Kurowska-Stolarska, M.; Liew, F.Y.; McSharry, C.; et al. IL-33 Promotes ST2-Dependent Lung Fibrosis by the Induction of Alternatively Activated Macrophages and Innate Lymphoid Cells in Mice. J. Allergy Clin. Immunol. 2014, 134, 1422–1432.e11. [Google Scholar] [CrossRef] [PubMed]
- Fanny, M.; Nascimento, M.; Baron, L.; Schricke, C.; Maillet, I.; Akbal, M.; Riteau, N.; le Bert, M.; Quesniaux, V.; Ryffel, B.; et al. The IL-33 Receptor ST2 Regulates Pulmonary Inflammation and Fibrosis to Bleomycin. Front. Immunol. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, L.; Zhu, C.; Feng, J.; Yin, J.; Lu, J.; Xu, Y.; Yang, H.; Huang, Y.; Zhang, Q. Helicobacter Hepaticus Infection-Induced IL-33 Promotes Hepatic Inflammation and Fibrosis through ST2 Signaling Pathways in BALB/c Mice. Biochem. Biophys. Res. Commun. 2020, 525, 654–661. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Q.; Li, X.; Liu, Z.; Shen, J.; Sun, R.; Wei, J.; Zhao, J.; Wu, X.; Feng, F.; et al. IL-33 Contributes to Schistosoma Japonicum-Induced Hepatic Pathology through Induction of M2 Macrophages. Sci. Rep. 2016, 6, 29844. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Xiao, L.; Lin, G.; Tang, J.; Chen, Y.; Chen, L.; Li, B.; Wu, M.; Liu, S.; Huang, C.; et al. Contribution of Tissue Transglutaminase to the Severity of Hepatic Fibrosis Resulting from Schistosoma Japonicum Infection through the Regulation of IL-33/ST2 Expression. Parasites Vectors 2019, 12, 302. [Google Scholar] [CrossRef]
- Maggi, L.; Rocha, I.C.; Camelo, G.M.A.; Fernandes, V.R.; Negrão-Corrêa, D. The IL-33/ST2 Pathway Is Not Essential to Th2 Stimulation but Is Key for Modulation and Survival during Chronic Infection with Schistosoma mansoni in Mice. Cytokine 2021, 138, 155390. [Google Scholar] [CrossRef]
- Bachem, M.G.; Meyer, D.; Melchior, R.; Sell, K.M.; Gressner, A.M. Activation of Rat Liver Perisinusoidal Lipocytes by Transforming Growth Factors Derived from Myofibroblastlike Cells. A Potential Mechanism of Self Perpetuation in Liver Fibrogenesis. J. Clin. Investig. 1992, 89, 19–27. [Google Scholar] [CrossRef]
- Geerts, A. Formation of Normal Desmin Intermediate Filaments in Mouse Hepatic Stellate Cells Requires Vimentin. Hepatology 2001, 33, 177–188. [Google Scholar] [CrossRef]
- Pearce, E.J.; MacDonald, A.S. The Immunobiology of Schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef]
- Smith, P.M.; Shainheit, M.G.; Bazzone, L.E.; Rutitzky, L.I.; Poltorak, A.; Stadecker, M.J. Genetic Control of Severe Egg-Induced Immunopathology and IL-17 Production in Murine Schistosomiasis. J. Immunol. 2009, 183, 3317–3323. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Wang, X.; Gu, P.; Lei, Z.; Tang, R.; Wei, C.; Xu, L.; Wang, C.; Chen, Y.; et al. Schistosome Eggs Stimulate Reactive Oxygen Species Production to Enhance M2 Macrophage Differentiation and Promote Hepatic Pathology in Schistosomiasis. PLoS Negl. Trop. Dis. 2021, 15, e0009696. [Google Scholar] [CrossRef]
- Wolk, K.; Kunz, S.; Witte, E.; Friedrich, M.; Asadullah, K.; Sabat, R. IL-22 Increases the Innate Immunity of Tissues. Immunity 2004, 21, 241–254. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Karow, M.; Flavell, R.A. Interleukin-22 but Not Interleukin-17 Provides Protection to Hepatocytes during Acute Liver Inflammation. Immunity 2007, 27, 647–659. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Flavell, R.A. Recent Advances in IL-22 Biology. Int. Immunol. 2011, 23, 159–163. [Google Scholar] [CrossRef]
- Xing, Z.; Wu, Y.; Liu, N. IL-22 Alleviates the Fibrosis of Hepatic Stellate Cells via the Inactivation of NLRP3 Inflammasome Signaling. Exp. Ther. Med. 2021, 22, 1088. [Google Scholar] [CrossRef]
- Lu, D.-H.; Guo, X.; Qin, S.; Luo, W.; Huang, X.; Chen, M.; Wang, J.; Ma, S.; Yang, X.; Jiang, H. Interleukin-22 Ameliorates Liver Fibrogenesis by Attenuating Hepatic Stellate Cell Activation and Downregulating the Levels of Inflammatory Cytokines. World J. Gastroenterol. 2015, 21, 1531–1545. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Zheng, W.-D.; Chen, Y.-X.; Huang, Y.-H.; Chen, Z.-X.; Zhang, S.-J.; Shi, M.-N.; Wang, X.-Z. Antifibrotic Effects of Interleukin-10 on Experimental Hepatic Fibrosis. Hepatogastroenterology 2007, 54, 2092–2098. [Google Scholar]
- Benyon, R.C.; Arthur, M.J.P. Extracellular Matrix Degradation and the Role of Hepatic Stellate Cells. Semin. Liver Dis. 2001, 21, 373–384. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A. Recent Advancement of Molecular Mechanisms of Liver Fibrosis. J. Hepatobiliary Pancreat Sci. 2015, 22, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Niles, E.G.; Verjovski-Almeida, S.; LoVerde, P.T. Schistosoma Mansoni TGF-β Receptor II: Role in Host Ligand-Induced Regulation of a Schistosome Target Gene. PLoS Pathog. 2006, 2, e54. [Google Scholar] [CrossRef] [PubMed]
- Kaviratne, M.; Hesse, M.; Leusink, M.; Cheever, A.W.; Davies, S.J.; McKerrow, J.H.; Wakefield, L.M.; Letterio, J.J.; Wynn, T.A. IL-13 Activates a Mechanism of Tissue Fibrosis That Is Completely TGF-β Independent. J. Immunol. 2004, 173, 4020–4029. [Google Scholar] [CrossRef]
- Chuah, C.; Jones, M.K.; Burke, M.L.; McManus, D.P.; Gobert, G.N. Cellular and Chemokine-Mediated Regulation in Schistosome-Induced Hepatic Pathology. Trends Parasitol. 2014, 30, 141–150. [Google Scholar] [CrossRef]
- Angeles, J.M.M.; Mercado, V.J.P.; Rivera, P.T. Behind Enemy Lines: Immunomodulatory Armamentarium of the Schistosome Parasite. Front. Immunol. 2020, 11, 1018. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGF-β in the Context of an Inflammatory Cytokine Milieu Supports De Novo Differentiation of IL-17-Producing T Cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Mangan, P.R.; Harrington, L.E.; O’Quinn, D.B.; Helms, W.S.; Bullard, D.C.; Elson, C.O.; Hatton, R.D.; Wahl, S.M.; Schoeb, T.R.; Weaver, C.T. Transforming Growth Factor-β Induces Development of the TH17 Lineage. Nature 2006, 441, 231–234. [Google Scholar] [CrossRef]
- Bai, Y.; Guan, F.; Zhu, F.; Jiang, C.; Xu, X.; Zheng, F.; Liu, W.; Lei, J. IL-33/ST2 Axis Deficiency Exacerbates Hepatic Pathology by Regulating Treg and Th17 Cells in Murine Schistosomiasis Japonica. J. Inflamm. Res. 2021, 14, 5981–5998. [Google Scholar] [CrossRef]
- Nady, S.; Shata, M.T.M.; Mohey, M.A.; El-Shorbagy, A. Protective Role of IL-22 against Schistosoma mansoni Soluble Egg Antigen-Induced Granuloma In Vitro. Parasite Immunol. 2017, 39, e12392. [Google Scholar] [CrossRef]
- Olds, G.R.; Griffin, A.; Kresina, T.F. Dynamics of Collagen Accumulation and Polymorphism in Murine Schistosoma Japonicum. Gastroenterology 1985, 89, 617–624. [Google Scholar] [CrossRef]
- Al Adnani, M.S. Concomitant Immunohistochemical Localization of Fibronectin and Collagen in Schistosome Granulomata. J. Pathol. 1985, 147, 77–85. [Google Scholar] [CrossRef]
- Hellerbrand, C.; Stefanovic, B.; Giordano, F.; Burchardt, E.R.; Brenner, D.A. The Role of TGFβ1 in Initiating Hepatic Stellate Cell Activation In Vivo. J. Hepatol. 1999, 30, 77–87. [Google Scholar] [CrossRef]
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M.; et al. Interleukin-17 Signaling in Inflammatory, Kupffer Cells, and Hepatic Stellate Cells Exacerbates Liver Fibrosis in Mice. Gastroenterology 2012, 143, 765–776.e3. [Google Scholar] [CrossRef]
- Millar, N.L.; Gilchrist, D.S.; Akbar, M.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Murrell, G.A.C.; Liew, F.Y.; Kurowska-Stolarska, M.; McInnes, I.B. MicroRNA29a Regulates IL-33-Mediated Tissue Remodelling in Tendon Disease. Nat. Commun. 2015, 6, 6774. [Google Scholar] [CrossRef]
- Ushiki, T. Collagen Fibers, Reticular Fibers and Elastic Fibers. A Comprehensive Understanding from a Morphological Viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126. [Google Scholar] [CrossRef]
- Spencer, M.; Yao-Borengasser, A.; Unal, R.; Rasouli, N.; Gurley, C.M.; Zhu, B.; Peterson, C.A.; Kern, P.A. Adipose Tissue Macrophages in Insulin-Resistant Subjects Are Associated with Collagen VI and Fibrosis and Demonstrate Alternative Activation. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E1016–E1027. [Google Scholar] [CrossRef]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Stolarski, B.; Kewin, P.; Murphy, G.; Corrigan, C.J.; Ying, S.; Pitman, N.; Mirchandani, A.; Rana, B.; van Rooijen, N.; et al. IL-33 Amplifies the Polarization of Alternatively Activated Macrophages That Contribute to Airway Inflammation. J. Immunol. 2009, 183, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Yu, T.; Yan, B.; Li, H. Interleukin-33 Promotes Obstructive Renal Injury via Macrophages. Mol. Med. Rep. 2019, 20, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.J.; Fallon, P.G.; Matthews, D.J.; Jolin, H.E.; McKenzie, A.N.J. T1/ST2-Deficient Mice Demonstrate the Importance of T1/ST2 in Developing Primary T Helper Cell Type 2 Responses. J. Exp. Med. 2000, 191, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, J.; Macedo, D.G. A Simplified Method for the Concentration of Cercariae. J. Parasitol. 1955, 41, 329. [Google Scholar] [CrossRef]
- Cheever, A.W. Conditions Affecting the Accuracy of Potassium Hydroxide Digestion Techniques for Counting Schistosoma mansoni Eggs in Tissues. Bull. World Health Organ. 1968, 39, 328–331. [Google Scholar]
- Reddy, G.K.; Enwemeka, C.S. A Simplified Method for the Analysis of Hydroxyproline in Biological Tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef]
- De Rezende, M.C.; Moreira, J.M.P.; Fernandes, L.L.M.; Rodrigues, V.F.; Negrão-Corrêa, D. Strongyloides Venezuelensis-Infection Alters the Profile of Cytokines and Liver Inflammation in Mice Co-Infected with Schistosoma Mansoni. Cytokine 2020, 127, 154931. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius Staining plus Polarization Microscopy, a Specific Method for Collagen Detection in Tissue Sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Junqueira, L.M.M. Técnicas Básicas de Citologia e Histologia; Santos Editora: São Paulo, Brazil, 1983. [Google Scholar]
- Melo, F.; Amaral, M.; Oliveira, P.; Lima, W.; Andrade, M.; Michalick, M.; Raso, P.; Tafuri, W.; Tafuri, W. Diffuse Intralobular Liver Fibrosis in Dogs Naturally Infected with Leishmania (Leishmania) chagasi. Am. J. Trop. Med. Hyg. 2008, 79, 198–204. [Google Scholar] [CrossRef]
- Alves, A.F.; Pereira, R.A.; Andrade, H.M.; Mosser, D.M.; Tafuri, W.L. Immunohistochemical Study of Renal Fibropoiesis Associated with Dogs Naturally and Experimentally Infected with Two Different Strains of Leishmania (L.) Infantum. Int. J. Exp. Pathol. 2019, 100, 222–233. [Google Scholar] [CrossRef]
- Caliari, M. V Princípios de Morfometria Digital: KS300 Para Iniciantes; Editora da UFMG: Belo Horizonte, Brazil, 1997. [Google Scholar]
- Schwartz, C.; Oeser, K.; da Costa, C.P.; Layland, L.E.; Voehringer, D. T Cell–Derived IL-4/IL-13 Protects Mice against Fatal Schistosoma mansoni Infection Independently of Basophils. J. Immunol. 2014, 193, 3590–3599. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, E.; Ilyas, G.; Lalazar, G.; Lin, Y.; Haseeb, M.; Tanaka, K.E.; Czaja, M.J. Impaired Macrophage Autophagy Increases the Immune Response in Obese Mice by Promoting Proinflammatory Macrophage Polarization. Autophagy 2015, 11, 271–284. [Google Scholar] [CrossRef]
- Wilson, M.S.; Elnekave, E.; Mentink-Kane, M.M.; Hodges, M.G.; Pesce, J.T.; Ramalingam, T.R.; Thompson, R.W.; Kamanaka, M.; Flavell, R.A.; Keane-Myers, A.; et al. IL-13Rα2 and IL-10 Coordinately Suppress Airway Inflammation, Airway-Hyperreactivity, and Fibrosis in Mice. J. Clin. Investig. 2007, 117, 2941–2951. [Google Scholar] [CrossRef]




| Gene | Sequence (5′–3′) | |
|---|---|---|
| GAPDH | Forward | AGG TCG GTG TGA ACG GAT TTG |
| [95] | Reverse | TGT AGA CCA TGT AGT TGA GGT CA |
| Col I | Forward | ACT GGA CTG TCC CAA CCC C |
| [95] | Reverse | CTT AGT TTG GAC AGG ATC TGG |
| Col III | Forward | AAC CTG GTT TCT TCT CAC CCT TC |
| [94] | Reverse | ACT CAT AGG ACT GAC CAA GGT GG |
| Col VI | Forward | CGC CCT TCC CAC TGA CAA |
| [96] | Reverse | GCG TTC CCT TTA AGA CAG TTG AG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggi, L.; Camelo, G.M.A.; Rocha, I.C.; Pereira Alves, W.; Moreira, J.M.P.; Almeida Pereira, T.; Tafuri, W.L.; Rabelo, É.M.L.; Correa, A., Jr.; Ecco, R.; et al. Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice. Int. J. Mol. Sci. 2023, 24, 10237. https://doi.org/10.3390/ijms241210237
Maggi L, Camelo GMA, Rocha IC, Pereira Alves W, Moreira JMP, Almeida Pereira T, Tafuri WL, Rabelo ÉML, Correa A Jr., Ecco R, et al. Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice. International Journal of Molecular Sciences. 2023; 24(12):10237. https://doi.org/10.3390/ijms241210237
Chicago/Turabian StyleMaggi, Laura, Genil Mororó Araújo Camelo, Izabella Chrystina Rocha, William Pereira Alves, João Marcelo Peixoto Moreira, Thiago Almeida Pereira, Wagner Luiz Tafuri, Élida Mara Leite Rabelo, Ary Correa, Jr., Roselene Ecco, and et al. 2023. "Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice" International Journal of Molecular Sciences 24, no. 12: 10237. https://doi.org/10.3390/ijms241210237
APA StyleMaggi, L., Camelo, G. M. A., Rocha, I. C., Pereira Alves, W., Moreira, J. M. P., Almeida Pereira, T., Tafuri, W. L., Rabelo, É. M. L., Correa, A., Jr., Ecco, R., & Negrão-Corrêa, D. A. (2023). Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice. International Journal of Molecular Sciences, 24(12), 10237. https://doi.org/10.3390/ijms241210237







