The Changes in Mitochondrial Morphology and Physiology Accompanying Apoptosis in Galleria mellonella (Lepidoptera) Immunocompetent Cells during Conidiobolus coronatus (Entomophthorales) Infection
Abstract
1. Introduction
2. Results
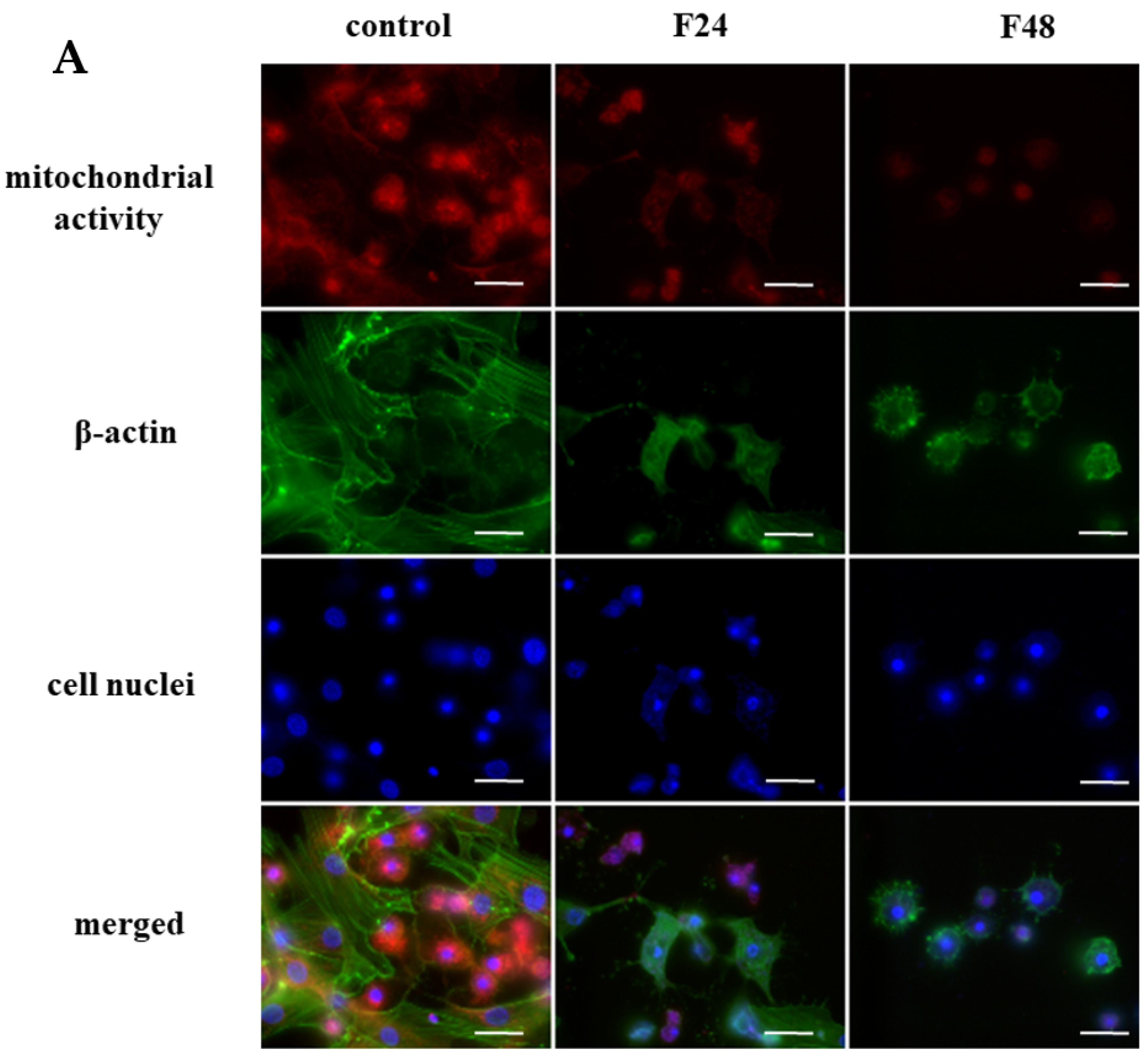
2.1. The Changes in Mitochondrial Activity in G. mellonella Hemocytes after Fungal Infection
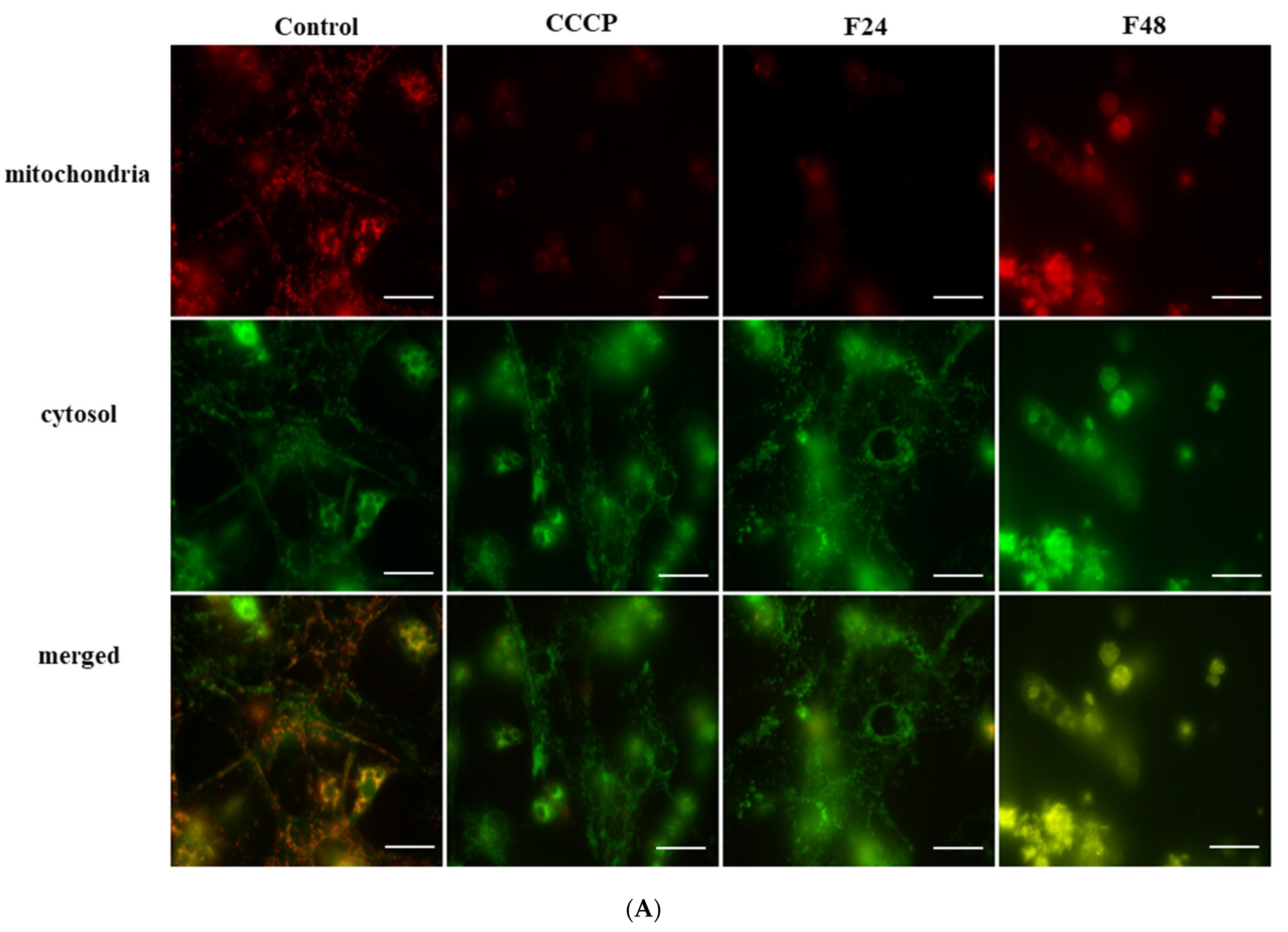
2.2. The Translocation of Cytochrome c-like Protein from Mitochondrial to Cytosol Fraction in G. mellonella Hemocytes during Fungal Infection
2.3. The Flux of Ca2+ Level in G. mellonella Hemocytes after Fungal Infection
2.4. Detection of Changes in ADP/ATP Ratio in G. mellonella Hemocytes after Fungal Infection
2.5. Changes in Caspase-9-like Protein Activity in G. mellonella Hemocytes after Fungal Infection
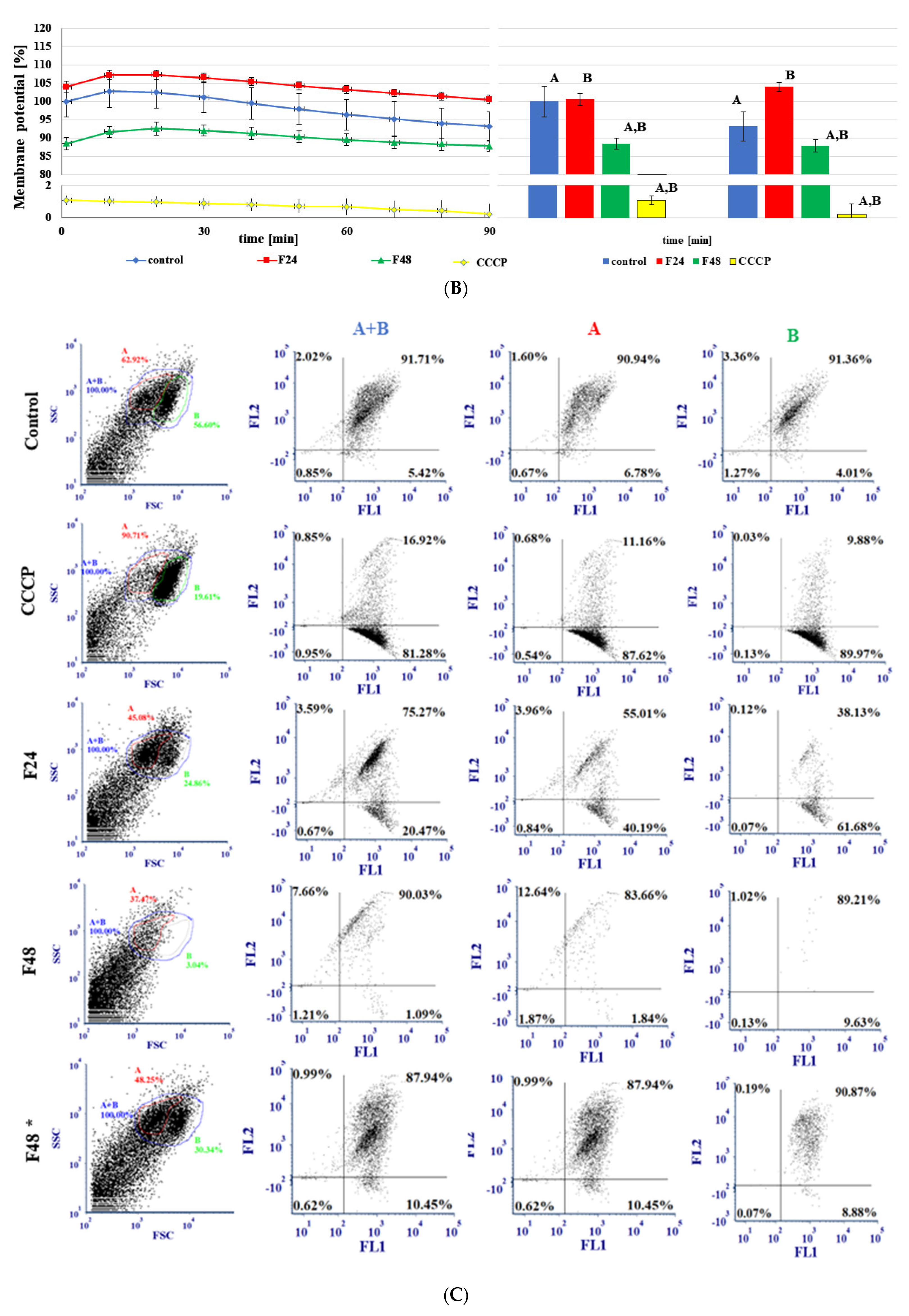
2.6. Changes in Membrane Potential in G. mellonella Hemocytes after Fungal Infection
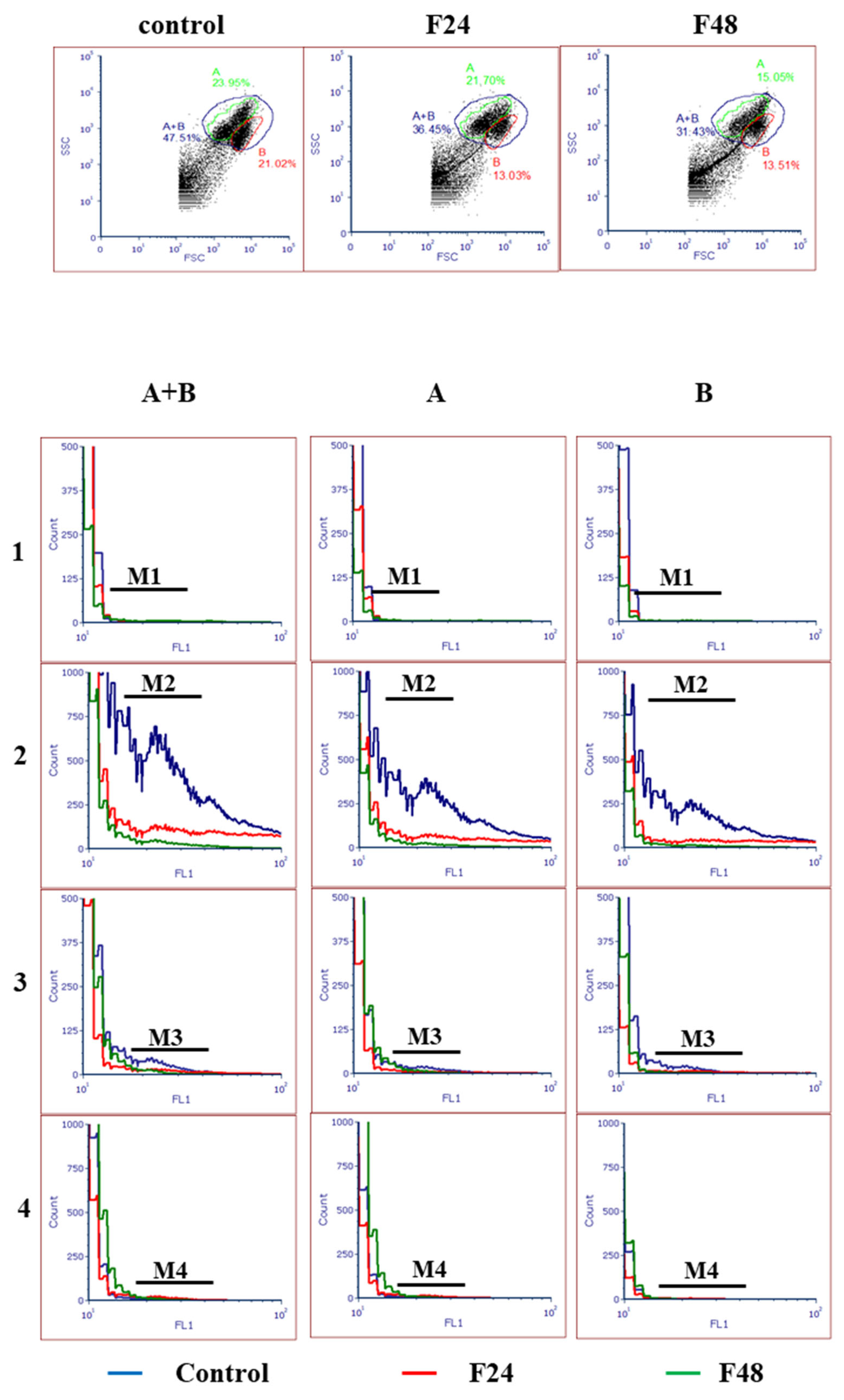
2.7. Detection of Mitochondrial Permeability Transition Pore (MPTP) Opening in G. mellonella Hemocytes after Fungal Infection
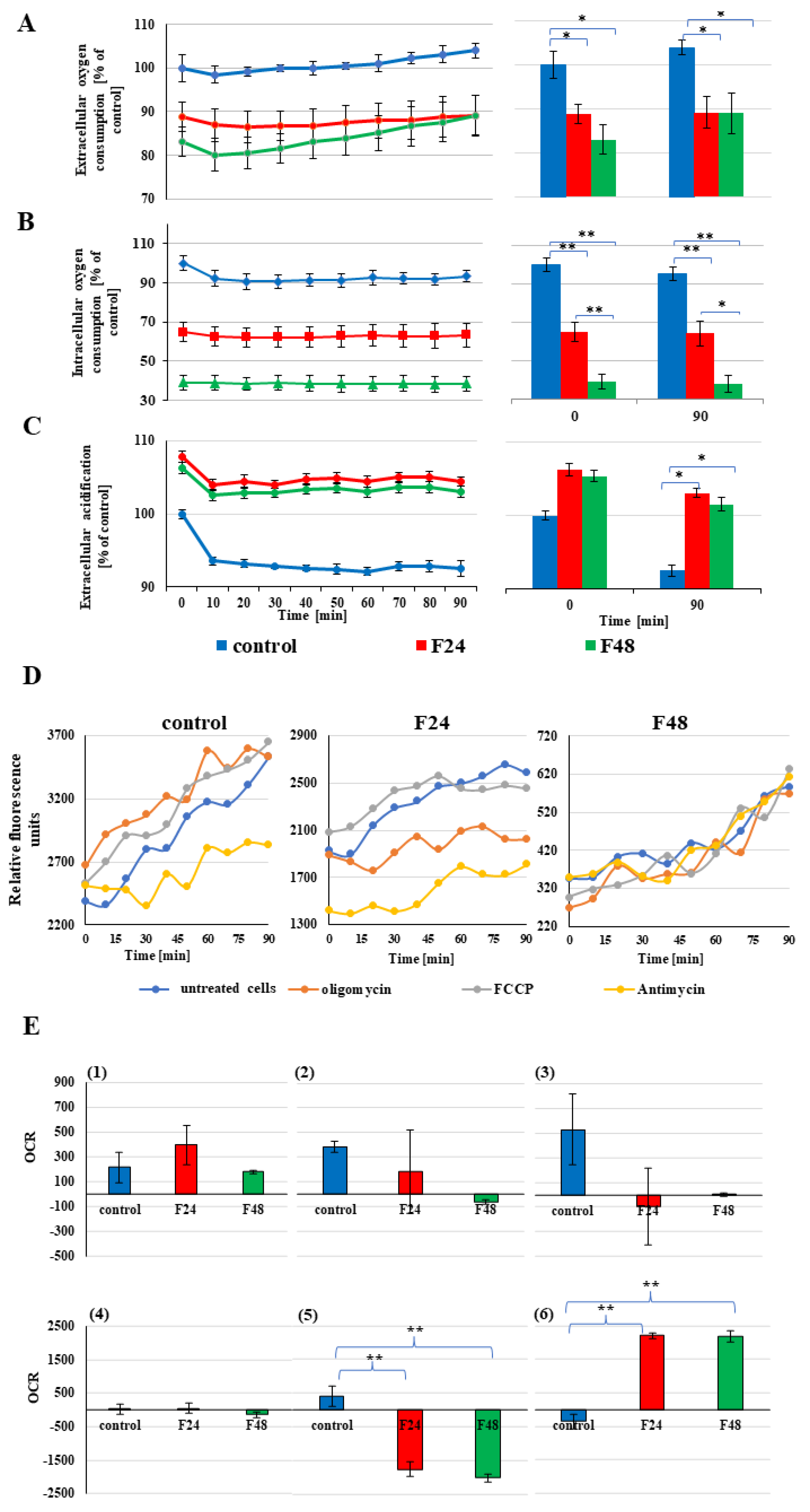
2.8. The Changes in Oxygen Consumption in G. mellonella Hemocytes after Fungal Infection
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Fungus
4.3. Infection of Insects with C. coronatus
4.4. Larval Hemolymph Collection
4.5. Staining of Mitochondria in Fluorescent Microscopy
4.6. The Calculation of Ca2+ Concentration in Hemocytes
4.7. The Detection of Changes in ADP/ATP Ratio
4.8. The Measurement of Protein Concentration—The Bicinchoninic Acid Assay (BCA Method)
4.9. Measurement of Caspase Activity
4.10. Detection of the Changes in Membrane Potential in G. mellonella Hemocyte Mitochondria
4.11. Detection of Mitochondrial Permeability Transition Pore (MPTP) Opening
4.12. The Detection of Changes in Oxygen Consumption
4.13. Isolation of Cytochrome c-like Protein from G. mellonella Larvae’ Hemolymph
4.14. Detection of Cytochrome c-like Protein in the Mitochondrial and Cytosolic Fractions (Western Blot Method)
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.J.; Gonzales-Huerta, L.E.; Armstrong-James, D. Fungal-induced programmed cell death. J. Fungi 2021, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C.; Yurdagul, A.; Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020, 20, 254–267. [Google Scholar] [CrossRef]
- Zhang, A.; Wu, Y.; Lai, H.W.L.; Yew, D.T. Apoptosis—A brief review. Neuroembryol. Aging 2004, 3, 47–59. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Seol, D.W. The role of mitochondria in apoptosis. BMB Rep. 2008, 41, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Will, Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today 2007, 12, 777–785. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Marques, C.A.; Bonert, A.; Frey, C.; Schüssel, K.; Müller, W.E. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer’s disease. Biochem. Pharmacol. 2003, 66, 1627–1634. [Google Scholar] [CrossRef]
- Ibata-Ombetta, S.; Idziorek, T.; Trinel, P.A.; Poulain, D.; Jouault, T. Candida albicans phospholipomannan promotes survival of phagocytosed yeasts through modulation of bad phosphorylation and macrophage apoptosis. J. Biol. Chem. 2003, 278, 13086–13093. [Google Scholar] [CrossRef]
- Wu, H.; Downs, D.; Ghosh, K.; Ghosh, A.K.; Staib, P.; Monod, M.; Tang, J. Candida albicans secreted aspartic proteases 4–6 induce apoptosis of epithelial cells by a novel Trojan horse mechanism. FASEB J. 2013, 27, 2132–2144. [Google Scholar] [CrossRef]
- Wagener, J.; Weindl, G.; de Groot, P.W.J.; de Boer, A.D.; Kaesler, S.; Thavaraj, S.; Bader, O.; Mailänder-Sanchez, D.; Borelli, C.; Weig, M.; et al. Glycosylation of Candida albicans cell wall proteins is critical for induction of innate immune responses and apoptosis of epithelial cells. PLoS ONE 2012, 7, e50518. [Google Scholar] [CrossRef] [PubMed]
- Monari, C.; Paganelli, F.; Bistoni, F.; Kozel, T.R.; Vecchiarelli, A. Capsular polysaccharide induction of apoptosis by intrinsic and extrinsic mechanisms. Cell. Microbiol. 2008, 10, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Ben-Abdallah, M.; Sturny-Leclère, A.; Avé, P.; Louise, A.; Moyrand, F.; Weih, F.; Janbon, G.; Mémet, S. Fungal-induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-κB. PLoS Pathog. 2012, 8, e1002555. [Google Scholar] [CrossRef]
- Geissler, A.; Haun, F.; Frank, D.O.; Wieland, K.; Simon, M.M.; Idzko, M.; Davis, R.J.; Maurer, U.; Borner, C. Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ. 2013, 20, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, M.; Orciuolo, E.; Lewis, R.; Kontoyiannis, D.P.; Martins, S.L.R.; St John, L.S.; Komanduri, K.V. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 2005, 105, 2258–2265. [Google Scholar] [CrossRef]
- Pardo, J.; Urban, C.; Galvez, E.M.; Ekert, P.G.; Müller, U.; Kwon-Chung, J.; Lobigs, M.; Müllbacher, A.; Wallich, R.; Borner, C.; et al. The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J. Cell Biol. 2006, 174, 509–519. [Google Scholar] [CrossRef]
- Abdelwahid, E.; Yokokura, T.; Krieser, R.J.; Balasundaram, S.; Fowle, W.H.; White, K. Mitochondrial disruption in Drosophila apoptosis. Dev. Cell 2007, 12, 793–806. [Google Scholar] [CrossRef]
- Clavier, A.; Rincheval-Arnold, A.; Colin, J.; Mignotte, B.; Guénal, I. Apoptosis in Drosophila: Which role for mitochondria? Apoptosis 2016, 21, 239–251. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Seth, R.K.; Dwarakanath, B.S.; Chandna, S. Mitochondrial regulation of insect cell apoptosis: Evidence for permeability transition pore-independent cytochrome-c release in the Lepidopteran Sf9 cells. Int. J. Biochem. Cell Biol. 2009, 41, 1430–1440. [Google Scholar] [CrossRef]
- Huang, J.; Lv, C.; Hu, M.; Zhong, G. The mitochondria-mediate apoptosis of lepidopteran cells induced by azadirachtin. PLoS ONE 2013, 8, e58499. [Google Scholar] [CrossRef]
- Liu, K.; Shu, D.; Song, N.; Gai, Z.; Yuan, Y.; Li, J.; Li, M.; Guo, S.; Peng, J.; Hong, H. The role of cytochrome c on apoptosis induced by Anagrapha falcifera multiple nuclear polyhedrosis virus in insect Spodoptera litura cells. PLoS ONE 2012, 7, e40877. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wu, K.H.; Pang, H.L.; Xu, P.Z.; Li, M.W.; Zhang, G.Z. Study on the role of cytc in response to BmNPV infection in silkworm, Bombyx mori (Lepidoptera). Int. J. Mol. Sci. 2019, 20, 4325. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Liu, K.; Peng, J.; Yao, H.; Li, Y.; Hong, H. Mitochondria are involved in apoptosis induced by ultraviolet radiation in lepidopteran Spodoptera litura cell line. Insect Sci. 2009, 16, 485–491. [Google Scholar] [CrossRef]
- Wrońska, A.K.; Kaczmarek, A.; Kazek, M.; Boguś, M.I. Infection of Galleria mellonella (Lepidoptera) larvae with the entomopathogenic fungus Conidiobolus coronatus (Entomophthorales) induces apoptosis of hemocytes and affects the concentration of eicosanoids in the hemolymph. Front. Physiol. 2022, 12, 774086. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A. Wpływ Metabolitów Entomopatogennego Grzyba Conidiobolus coronatus: Kwasu Oktanowego, Kwasu 2-Oktenowego oraz 2,6-Dimetylofenolu na Wybrane Procesy Fizjologiczne Galleria mellonella oraz Lucilia sericata; Witold Stefanski Institute of Parasitology: Warsaw, Poland, 2018. [Google Scholar]
- Kazek, M.; Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I. Conidiobolus coronatus induces oxidative stress and autophagy response in Galleria mellonella larvae. PLoS ONE 2020, 15, e0228407. [Google Scholar] [CrossRef]
- Deak, L.; Mudalagiriyappa, S.; Ballin, A.; Saxton, D.; Chakrabarti, A. A rhinofacial Conidiobolus coronatus fungal infection presenting as an intranasal tumour. Sultan Qaboos Univ. Med. J. 2018, 18, e549–e552. [Google Scholar] [CrossRef]
- Gupta, P.; Pilania, R.K.; Kaur, H.; Rudramurthy, S.M.; Soni, R.; Batra, N.; Verma, R.; Singh, S.; Chatterjee, D. Pediatric case of conidiobolomycosis: A rare entity. Pediatr. Dermatol. 2022, 39, 149–150. [Google Scholar] [CrossRef]
- Purohit, G.; Sable, M.; Rudramurthy, S.M.; Sarkar, S.; Parida, P.; Deshmukh, V.; Hallur, V. A rare case of condiobolomycosis due to Conidiobolus coronatus presenting with dysphagia. Indian J. Med. Microbiol. 2021, 39, 558–560. [Google Scholar] [CrossRef]
- Wüppenhorst, N.; Lee, M.K.; Rappold, E.; Kayser, G.; Beckervordersandforth, J.; De With, K.; Serr, A. Rhino-orbitocerebral zygomycosis caused by Conidiobolus incongruus in an immunocompromised patient in Germany. J. Clin. Microbiol. 2010, 48, 4322–4325. [Google Scholar] [CrossRef]
- Smith, M.F.; Callaghan, A.A. Quantitative survey of Conidiobolus and Basidiobolus in soils and litter. Trans. Br. Mycol. Soc. 1987, 89, 179–185. [Google Scholar] [CrossRef]
- Subramanian, C.; Sobel, J.D. A case of Conidiobolus coronatus in the vagina. Med. Mycol. 2011, 49, 427–429. [Google Scholar] [CrossRef]
- Shaikh, N.; Hussain, K.A.; Petraitiene, R.; Schuetz, A.N.; Walsh, T.J. Entomophthoramycosis: A neglected tropical mycosis. Clin. Microbiol. Infect. 2016, 22, 688–694. [Google Scholar] [CrossRef]
- Boguś, M.I.; Scheller, K. Extraction of an insecticidal protein fraction from the parasitic fungus Conidiobolus coronatus (Entomophthorales). Acta Parasitol. 2002, 47, 66–72. [Google Scholar]
- Boguś, M.I.; Wieloch, W.; Ligȩza-Żuber, M. Coronatin-2 from the entomopathogenic fungus Conidiobolus coronatus kills Galleria mellonella larvae and incapacitates hemocytes. Bull. Entomol. Res. 2017, 107, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, A.K.; Boguś, M.I.; Włóka, E.; Kazek, M.; Kaczmarek, A.; Zalewska, K. Cuticular fatty acids of Galleria mellonella (Lepidoptera) inhibit fungal enzymatic activities of pathogenic Conidiobolus coronatus. PLoS ONE 2018, 13, e0192715. [Google Scholar] [CrossRef] [PubMed]
- Bania, J.; Samborski, J.; Bogus, M.; Polanowski, A. Specificity of an extracellular proteinase from Conidiobolus coronatus and its inhibition by an inhibitor from insect hemolymph. Arch. Insect Biochem. Physiol. 2006, 62, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, V.Y.; Kosman, E.; Tomilova, O.; Polenogova, O.; Rotskaya, U.; Tyurin, M.; Alikina, T.; Yaroslavtseva, O.; Kabilov, M.; Glupov, V. Interplay between fungal infection and bacterial associates in the wax moth Galleria mellonella under different temperature conditions. J. Fungi 2020, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Jemel, S.; Guillot, J.; Kallel, K.; Botterel, F.; Dannaoui, E. Galleria mellonella for the evaluation of antifungal efficacy against medically important fungi, a narrative review. Microorganisms 2020, 8, 390. [Google Scholar] [CrossRef]
- Curtis, A.; Binder, U.; Kavanagh, K. Galleria mellonella larvae as a model for investigating fungal—Host interactions. Front. Fungal Biol. 2022, 3, 893494. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Wrońska, A.K.; Kazek, M.; Boguś, M.I. Octanoic acid—An insecticidal metabolite of Conidiobolus coronatus (Entomopthorales) that affects two majors antifungal protection systems in Galleria mellonella (Lepidoptera): Cuticular lipids and hemocytes. Int. J. Mol. Sci. 2022, 23, 5204. [Google Scholar] [CrossRef]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Galleria mellonella: An invertebrate model to study pathogenicity in correctly defined fungal species. Fungal Biol. 2016, 120, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Courtiade, J.; Pauchet, Y.; Vogel, H.; Heckel, D.G. A comprehensive characterization of the caspase gene family in insects from the order Lepidoptera. BMC Genom. 2011, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yan, X.; Yan, L.; Zhao, J.; Song, J.; Peng, R.; Yang, Y.; Peng, J.; Liu, K. Identification and functional analysis of apoptotic protease activating factor-1 (Apaf-1) from Spodoptera litura. Insects 2021, 12, 64. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Lubawy, J.; Chowański, S.; Adamski, Z.; Słocińska, M. Mitochondria as a target and central hub of energy division during cold stress in insects. Front. Zool. 2022, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Angajala, A.; Lim, S.; Phillips, J.B.; Kim, J.H.; Yates, C.; You, Z.; Tan, M. Diverse roles of mitochondria in immune responses: Novel insights into immuno-metabolism. Front. Immunol. 2018, 9, 1605. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.J.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Balaban, R.S. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 2012, 51, 2959–2973. [Google Scholar] [CrossRef]
- Gherardi, G.; Monticelli, H.; Rizzuto, R.; Mammucari, C. The mitochondrial Ca2+ uptake and the fine-tuning of aerobic metabolism. Front. Physiol. 2020, 11, 554904. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef]
- Pinton, P.; Ferrari, D.; Rapizzi, E.; Di Virgilio, F.; Pozzan, T.; Rizzuto, R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: Significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001, 20, 2690–2701. [Google Scholar] [CrossRef]
- Scorrano, L.; Oakes, S.A.; Opferman, J.T.; Cheng, E.H.; Sorcinelli, M.D.; Pozzan, T.; Korsmeyer, S.J. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science 2003, 300, 135–139. [Google Scholar] [CrossRef]
- Hajnóczky, G.; Csordás, G.; Das, S.; Garcia-Perez, C.; Saotome, M.; Roy, S.S.; Yi, M. Mitochondrial calcium signalling and cell death: Approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 2006, 40, 553–560. [Google Scholar] [CrossRef]
- Xiu, M.; Peng, J.; Hong, H. Mitochondrial response and calcium ion change in apoptotic insect cells induced by SfaMNPV. Chin. Sci. Bull. 2005, 50, 1191–1198. [Google Scholar] [CrossRef]
- Wiesel, E.; Kaltofen, S.; Hansson, B.S.; Wicher, D. Homeostasis of mitochondrial Ca2+ stores is critical for signal amplification in Drosophila melanogaster olfactory sensory neurons. Insects 2022, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, D.; Parone, P.; Martinou, J.C.; Antonsson, B.; Estaquier, J.; Ameisen, J.C. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J. Cell Biol. 2002, 159, 923–929. [Google Scholar] [CrossRef]
- Dorstyn, L.; Mills, K.; Lazebnik, Y.; Kumar, S. The two cytochrome c species, DC3 and DC4, are not required for caspase activation and apoptosis in Drosophila cells. J. Cell Biol. 2004, 167, 405–410. [Google Scholar] [CrossRef]
- Arama, E.; Agapite, J.; Steller, H. Caspase activity and a specific cytochrome c are required for sperm differentiation in Drosophila. Dev. Cell 2003, 4, 687–697. [Google Scholar] [CrossRef]
- Wang, X.Y.; Ding, X.Y.; Chen, Q.Y.; Zhang, K.X.; Zhao, C.X.; Tang, X.D.; Wu, Y.C.; Li, M.W. Bmapaf-1 is involved in the response against bmnpv infection by the mitochondrial apoptosis pathway. Insects 2020, 11, 647. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L.; Zhang, Y.; Mao, L.; Jiang, H. Mitochondria response to camptothecin and hydroxycamptothecine-induced apoptosis in Spodoptera exigua cells. Pestic. Biochem. Physiol. 2017, 140, 97–104. [Google Scholar] [CrossRef]
- Sahdev, S.; Taneja, T.K.; Mohan, M.; Sah, N.K.; Khar, A.K.; Hasnain, S.E.; Athar, M. Baculoviral p35 inhibits oxidant-induced activation of mitochondrial apoptotic pathway. Biochem. Biophys. Res. Commun. 2003, 307, 483–490. [Google Scholar] [CrossRef]
- Mohan, M.; Taneja, T.K.; Sahdev, S.; Mohareer, K.; Begum, R.; Athar, M.; Sah, N.K.; Hasnain, S.E. Antioxidants prevent UV-induced apoptosis by inhibiting mitochondrial cytochrome c release and caspase activation in Spodoptera frugiperda (Sf9) cells. Cell Biol. Int. 2003, 27, 483–490. [Google Scholar] [CrossRef]
- Huang, J.F.; Tian, M.; Lv, C.J.; Li, H.Y.; Muhammad, R.-u.-H.; Zhong, G.H. Preliminary studies on induction of apoptosis by abamectin in Spodoptera frugiperda (Sf9) cell line. Pestic. Biochem. Physiol. 2011, 100, 256–263. [Google Scholar] [CrossRef]
- Sehnal, F. Critical study of the bionomics and biometrics of the wax moth (Galleria mellonella) reared under different conditions. Z. Wiss. Zool. 1966, 174, 53–82. [Google Scholar]

| Fraction | Sample | Protein Concentration [µg/µL] |
|---|---|---|
| mitochondrial | control (Cm) | 8.41 |
| F24m | 7.76 | |
| F48m | 6.97 | |
| cytosol | control (Cc) | 4.18 |
| F24c | 7.76 | |
| F48c | 4.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I. The Changes in Mitochondrial Morphology and Physiology Accompanying Apoptosis in Galleria mellonella (Lepidoptera) Immunocompetent Cells during Conidiobolus coronatus (Entomophthorales) Infection. Int. J. Mol. Sci. 2023, 24, 10169. https://doi.org/10.3390/ijms241210169
Kaczmarek A, Wrońska AK, Boguś MI. The Changes in Mitochondrial Morphology and Physiology Accompanying Apoptosis in Galleria mellonella (Lepidoptera) Immunocompetent Cells during Conidiobolus coronatus (Entomophthorales) Infection. International Journal of Molecular Sciences. 2023; 24(12):10169. https://doi.org/10.3390/ijms241210169
Chicago/Turabian StyleKaczmarek, Agata, Anna Katarzyna Wrońska, and Mieczysława Irena Boguś. 2023. "The Changes in Mitochondrial Morphology and Physiology Accompanying Apoptosis in Galleria mellonella (Lepidoptera) Immunocompetent Cells during Conidiobolus coronatus (Entomophthorales) Infection" International Journal of Molecular Sciences 24, no. 12: 10169. https://doi.org/10.3390/ijms241210169
APA StyleKaczmarek, A., Wrońska, A. K., & Boguś, M. I. (2023). The Changes in Mitochondrial Morphology and Physiology Accompanying Apoptosis in Galleria mellonella (Lepidoptera) Immunocompetent Cells during Conidiobolus coronatus (Entomophthorales) Infection. International Journal of Molecular Sciences, 24(12), 10169. https://doi.org/10.3390/ijms241210169







