Abstract
Global warming is posing a threat to animals. As a large group of widely distributed poikilothermal animals, insects are liable to heat stress. How insects deal with heat stress is worth highlighting. Acclimation may improve the heat tolerance of insects, but the underlying mechanism remains vague. In this study, the high temperature of 39 °C was used to select the third instar larvae of the rice leaf folder Cnaphalocrocis medinalis, an important insect pest of rice, for successive generations to establish the heat-acclimated strain (HA39). The molecular mechanism of heat acclimation was explored using this strain. The HA39 larvae showed stronger tolerance to 43 °C than the unacclimated strain (HA27) persistently reared at 27 °C. The HA39 larvae upregulated a glucose dehydrogenase gene, CmGMC10, to decrease the reactive oxygen species (ROS) level and increase the survival rate under heat stress. The HA39 larvae maintained a higher activity of antioxidases than the HA27 when confronted with an exogenous oxidant. Heat acclimation decreased the H2O2 level in larvae under heat stress which was associated with the upregulation of CmGMC10. The rice leaf folder larvae may acclimate to global warming via upregulating CmGMC10 to increase the activity of antioxidases and alleviate the oxidative damage of heat stress.
1. Introduction
Temperature crucially affects the growth, survival, and reproduction of insects [1,2]. Global warming causes a significant increase in average temperature and extremely high temperatures [3], which poses a greater threat to insects, a huge group of terrestrial ectotherms [4,5]. Exposure to 40 °C for 2 h led to the death of 100% Bradysia odoriphaga larvae within 5 days [6], and exposure to 42 °C for 5 d reduced the adult survival rate of the flour beetle Tribolium castaneum by approximately 30% and of pupae by 100% [5]. However, acclimation to a mild heat may increase the tolerance capacity of insects to an extremely high temperature [7] by inducing heat shock proteins and altering oxidoreductase activity [8,9,10,11].
Oxidoreductase catalyzes redox which is involved in basic metabolism. Oxidoreductases are closely related to the reproduction of insects. Glucose dehydrogenase (GDH) facilitates sperm uptake and release through the spermathecal ducts of the female Drosophila melanogaster, and GDH-mutant females store only one-third as much sperm as wild-type females [12]. The GDH-silenced small brown planthopper females laid 48.6% fewer eggs than the control females [13]. Oxidoreductase may affect insect immunity. The upregulated glucose–methanol–choline (GMC) oxidoreductase GMCβ genes increase the survival rate of the silkworm when infected with pathogens [14]. A high level of FAD-glucose dehydrogenase-specific activity is induced by abiotic implants (sterile latex) in the larvae of Manduca sexta [15]. Oxidoreductases are also related to biological and environmental stress pressed on organisms. Heat stress affects the metabolism of animals [16,17,18], which may be mediated by oxidoreductases.
Under environmental stress, a large number of reactive oxygen species (ROS) will be produced, which bring about oxidative damage to organisms. Multiple antioxidant enzymes in organisms inhibit the accumulation of ROS [19]. Superoxide dismutase (SOD) can protect cells from damage by oxygen radical generation in the livers of rats [20]. When whiteflies, Bemisia tabaci, were exposed to 4 °C and 40 °C, SOD activity was significantly increased [21]. In the citrus red mite Panonychus citri, the activities of SOD and glutathione-S-transferase (GST) were significantly increased when the mite was undergoing thermal stress [22].
RNA interference (RNAi) based on dsRNA is an economical, convenient, and powerful technique for exploring gene function. It has been widely used in Coleoptera, Hemiptera, Hymenoptera, etc. [23,24,25,26]. Although the interference efficiency using dsRNA is not very stable in Lepidoptera insects, depending on species and genes, the RNAi is still an alternative method and has been used well in the silkworm Bombyx mori, diamondback moth Plutella xylostella, and rice leaf folder Cnaphalocrocis medinalis, etc. [27,28,29,30,31]. The dsRNase in insects can degrade dsRNA, which decreases the interference efficiency of the target gene [32,33]. The CmdsRNase gene has been cloned in C. medinalis, and injection of dsCmCHS with dsCmdsRNase increased the interference efficiency of CmCHS by 27.17% [34]. Therefore, the improved RNAi will be used in Lepidoptera insects until a convenient gene editing technique is developed and becomes popular.
The rice leaf folder is a serious pest of rice in Asia whose populations have a migratory habit. In summer, populations migrate stepwise from southwest towards northeast China and then return in autumn [35]. This migratory habit can help adults avoid regional high temperatures in summer. However, the pest populations are unable to escape from global warming, which leads to the rise of the average temperature and extremely high temperatures. Although the rice leaf folder is liable to high temperatures [36,37], under the threat of global warming, the population density and outbreak frequency increased in the 21st century [38,39,40]. Previous studies found that the rice leaf folder larvae could generate a heat-acclimated strain via multigenerational selection at a high temperature [8,10,41], and heat acclimation-induced differentially expressed genes enriched in the GO term of oxidoreductase activity [10]. Therefore, here we hypothesized that heat acclimation altered the expression profile of oxidoreductase activity genes, which increased the antioxidant capacity of larvae to tolerate heat stress. In this study, the roles of two glucose dehydrogenase genes belonging to the GMC family, CmGMC10 and CmGMC38, were studied using heat-acclimated and unacclimated strains, and we found that CmGMC10 mediated the heat acclimation of larvae via acting on antioxidases and reducing ROS levels under heat stress. The results addressed the molecular mechanism of an insect’s ability to adapt to heat stress.
2. Results
2.1. Heat Tolerance of the Heat-Acclimated Larvae
Heat acclimation significantly increased the tolerance of larvae to a high temperature which the larvae had not previously experienced. The survival rate of the HA39 was much higher than that of the HA27 when they were exposed to 43 °C for 0.5 h (t = 3.13, df = 4, p = 0.035; Figure 1A) and 1h (t = 13.338, df = 4, p < 0.0001; Figure 1B). Although the survival rates of larvae between HA27 and HA39 at 2 d (t = 1.250, df = 4, p = 0.279) and 4 d (t = 1.581, df = 4, p = 0.189) after exposure to 43 °C for 0.5 h were similar (Figure 1A), the survival rates of HA39 were still significantly higher than that of the HA27 at 2 d (t = 28.778, df = 4, p < 0.0001) and 4 d (t = 23.398, df = 4, p < 0.0001) after exposure to 43 °C for 1 h (Figure 1B).
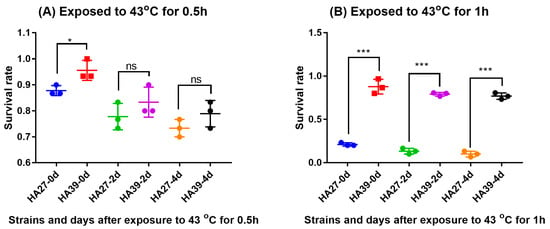
Figure 1.
Survival rates of the heat-acclimated (HA39) and unacclimated (HA27) strains after exposure to 43 °C for 0.5 h (A) and 1h (B). * and *** indicate significant differences between HA27 and HA39 at p = 0.05 and 0.001 levels, respectively.
2.2. Effect of Heat-Acclimation on Expression Levels of Oxidoreductase Genes
When larvae were exposed to 41 °C for 1 h, 4 out of 21 putative oxidoreductase genes were differentially expressed between the HA27 and HA39 (Figure 2A), and only 121117 was significantly upregulated in the HA39, compared to the HA27 (t = 4.223, df = 4, p = 0.013), whereas the other three, 121220 (t = 2.978, df = 4, p = 0.041), 71513 (t = 9.441, df = 4, p = 0.001), and 74602 (t = 9.705, df = 4, p = 0.001), were significantly downregulated (Figure 2A). Based on the genome of C. medinalis, 14 putative genes were clustered into 5 GMC family genes, and 74602 and 71513 were the same gene (Figure 2B). 121117 and 71513 were clustered in CmGMC10 and CmGMC38, respectively (Figure 2B). A partial sequence of CmGMC10 and the full length of CmGMC38 were cloned (Figure S2). Sequence alignment in NCBI showed that the two genes belong to the subfamily of glucose dehydrogenases in the GMC gene family.
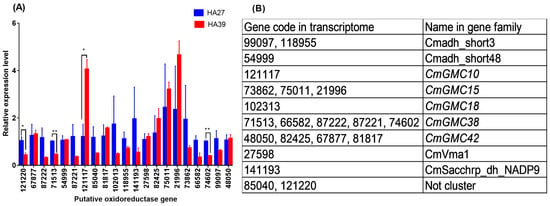
Figure 2.
Relative expression levels of 21 putative oxidoreductase genes of the heat-acclimated (HA39) and unacclimated larvae (HA27) exposed to 41 °C for 1 h (A) and their classification in the gene family (B). * and ** indicate significant differences between HA27 and HA39 at the p = 0.05 and 0.01 levels, respectively.
2.3. Spatiotemporal Expression Patterns of Two Oxidoreductase Genes
The relative expression levels of CmGMC10 of HA27 (F4, 10 = 0.605, p = 0.668) and HA39 (F4, 10 = 0.486, p = 0.746) were not significantly different among the larvae exposed to 39 °C for 0, 0.5, 1, 2, and 3 h (Figure 3A). Similarly, the relative expression levels of CmGMC38 of HA27 (F4, 10 = 0.523, p = 0.721) and HA39 (F4, 9 = 0.512, p = 0.729) were also affected little by the exposure duration of 0–3 h at 39 °C (Figure 3B). However, these two oxidoreductase activity genes exhibited an obvious tissue-specific expression profile in the HA27 and HA39 larvae (Figure 3C,D). The CmGMC10 gene was specifically expressed in the body wall of the HA39 larvae (F5, 12 = 7.257, p = 0.002), whereas it was expressed mainly in the silk gland and body wall of the HA27 larvae (F5, 11 = 5.938, p = 0.007; Figure 3C). The CmGMC38 was expressed specifically in the head of HA27 larvae (F5, 11 = 3.495, p = 0.039), and it was expressed mainly in the body wall and head of HA39 larvae (F5, 12 = 5.129, p = 0.010; Figure 3D). The body wall and head-specific and stable expression profiles in the HA27 and HA39 imply that these two oxidoreductase genes might be associated with the heat acclimation of larvae because the body wall and head are the organs that first feel heat stress.
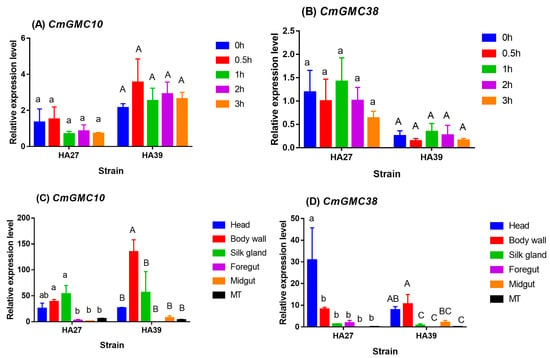
Figure 3.
Spatiotemporal expression profiles of two oxidoreductase genes, CmGMC10 and CmGMC38, of the HA27 and HA39 larvae. The relative expression levels of CmGMC10 (A) and CmGMC38 (B) in the HA27 and HA39 strains exposed to 39 °C for 0–3 h. The relative expression levels of CmGMC10 (C) and CmGMC38 (D) in different body parts of the HA27 and HA39 larvae. MT: Malpighian tubule. Different lowercase and uppercase letters indicate significant differences among different exposure durations or body parts of the HA27 and HA39 larvae, respectively.
2.4. Effects of Knockdown of CmGMC10 and CmGMC38 on Heat Tolerance
Injection of dsCmGMC10 and dsCmGMC38 into the HA27 and HA39 larvae decreased the expression levels of CmGMC10 and CmGMC38, respectively (Figure 4A,B,E,F). Interference of CmGMC38 or CmGMC10 decreased the survival rates of both the HA27 (Figure 4C,G) and HA39 larvae (Figure 4D,H) after exposure to 39 °C for 3 h. The survival rates of HA27 larvae with knocked down CmGMC38 by injection of dsCmGMC38 (F2, 6=6.251, p = 0.034) or dsCmdsRNase+dsCmGMC38 (F2, 7 = 7.381, p = 0.019) were significantly lower than larvae injected with dsGFP after heat exposure to 39 °C for 3h, whereas knockdown of CmGMC10 did not affect the survival rate of HA27 (Figure 4C,G). Knockdown of CmGMC38 using dsCmGMC38 decreased the survival rate of HA39 after heat exposure (F2, 6 = 29.859, p = 0.001; Figure 4D), and knockdown of CmGMC10 using dsCmdsRNase+dsCmGMC10 also significantly decreased the survival rate of HA39 (F2, 6 = 8.330, p = 0.019; Figure 4H). The expression levels of CmGMC10 and CmGMC38 were associated with the heat tolerance of the larvae (Figure 4).
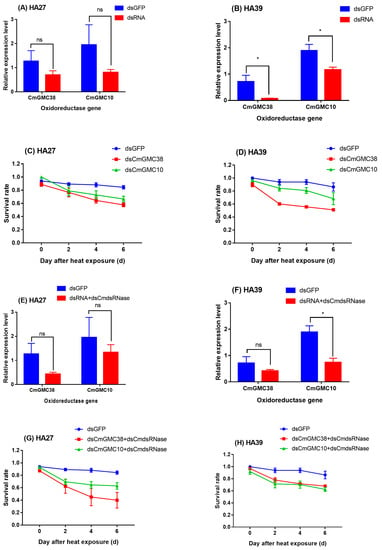
Figure 4.
Knockdown of CmGMC38 and CmGMC10 using injection of dsRNA (A,B) and dsRNA+dsCmdsRNase (E,F) and its effect on the survival rates of the HA27 and HA39 larvae at 0–6 d after 3 h heat exposure to 39 °C. (A–D) Larvae injected with dsRNA and (E–H) larvae injected with dsRNA+dsCmdsRNase (1:1). * Indicates significant difference between the relative expression levels in the larvae injected with dsGFP and dsRNA or dsRNA+dsCmdsRNase. ns: not significant.
2.5. Enzyme Activity of Larvae after Knockdown of an Oxidoreductase Gene
Knockdown of CmGMC10 or CmGMC38 did not significantly affect the enzyme activity of SOD, CAT, GST, and total antioxidant capacity (T-AOC) of the HA27 (SOD: F2, 15 = 0.877, p = 0.436; CAT: F2, 14 = 1.175, p = 0.337; GST: F2, 16 = 1.073, p = 0.365; T-AOC: F2, 16 = 0.618, p = 0.551) and HA39 (SOD: F2, 13 = 2.121, p = 0.160; CAT: F2, 15 = 0.089, p = 0.915; GST: F2, 15 = 0.267, p = 0.770; T-AOC: F2, 15 = 1.639, p = 0.227) larvae when exposed to 39 °C for 3 h, but these activities in the HA39 were lower than in the HA27 (Figure 5A–D). The ROS level in the HA27 larvae under heat exposure to 39 °C for 3 h was not significantly affected by the knockdown of CmGMC38 or CmGMC10 (F2, 7 = 0.439, p = 0.662, Figure 5E), but the knockdown of CmGMC10 resulted in a significant increase in the ROS level in the HA39 larvae under heat exposure (F2, 15 = 4.694, p = 0.026, Figure 5E). When the HA27 and HA39 larvae without knockdown of these two genes were exposed to 41 °C for 0–2 h, the ROS levels in the HA27 were increased significantly as the exposure duration extended (F3, 10 = 4.499, p = 0.03), but the ROS levels in the HA39 did not change (F3, 11 = 0.344, p = 0.794; Figure 5F). The oxidoreductase gene CmGMC10 regulated the ROS level of the heat-acclimated larvae under heat stress.
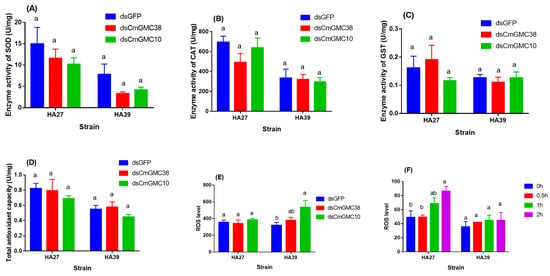
Figure 5.
Enzyme activities of SOD (A), CAT (B), and GST (C), total antioxidant capacity (D), and ROS levels (E) of the HA27 and HA39 larvae exposed to 39 °C for 3 h after knockdown of CmGMC10 or CmGMC38 genes, and ROS levels of the HA27 and HA39 larvae exposed to 41 °C for 0–2 h (F). Different letters indicate significant differences among knockdown of GFP, CmGMC10, and CmGMC38 of the HA27 and HA39 larvae. SOD: Superoxide dismutase, CAT: Catalase, GST: Glutathione transferase, ROS: Reactive oxygen species.
2.6. Effects of the Exogenous Oxidant and Antioxidant on the Enzyme Activity
Exogenous oxidant H2O2 induced a significant increase in the SOD activity in the HA27 larvae at 27 °C (F2, 6 = 77.378, p < 0.001) but did not affect the HA39 (F2, 5 = 4.843, p = 0.068; Figure 6A). The SOD activity of HA39 larvae was significantly higher than that of the HA27 at 27 °C when larvae were injected with H2O (t = 6.174, df = 4, p = 0.003) and NAC (t = 16.947, df = 3, p < 0.001), but the activity became similar when larvae were injected with H2O2 (t = 1.789, df = 4, p = 0.148; Figure 6A). Injection of NAC and H2O2 did not affect the CAT activity of HA27 (F2, 6 = 2.204, p = 0.192) and HA39 larvae (F2, 5 = 1.103, p = 0.401) at 27 °C (Figure 6B). The CAT activity in the HA27 and HA39 at 27 °C was similar when these larvae were injected with H2O (t = 0.923, df = 4, p = 0.408) and H2O2 (t = 1.466, df = 4, p = 0.217), but the activity in the HA39 was significantly higher than that in the HA27 when injected with NAC (t = 4.216, df = 3, p = 0.024; Figure 6B). Injection of H2O2 decreased the GST activity of HA27 larvae (F2, 6 = 5.273, p = 0.048) but did not affect the HA39 (F2, 5 = 3.891, p = 0.096) larvae at 27 °C (Figure 6C). The GST activity in the HA39 at 27 °C was significantly higher than that in the HA27 when they were injected with H2O (t = 22.176, df = 4, p < 0.001) and H2O2 (t = 6.599, df = 4, p = 0.022), but there was no difference when injected with NAC (t = 4.869, df = 3, p = 0.135; Figure 6C). When larvae were exposed to 41 °C for 2 h, the HA39 showed similar activities of SOD and CAT to the HA27 when they were injected with H2O and H2O2, but the activities were significantly lower in the HA27 than that in the HA39 when injected with NAC (SOD: t = 15.682, df = 4, p < 0.001; CAT: t = 5.432, df = 4, p = 0.006; Figure 6D,E). Under exposure to 41 °C, the GST activity in the HA39 was significantly higher than that in the HA27 when they were injected with H2O (t = 3.013, df = 4, p = 0.039), NAC (t = 5.914, df = 4, p = 0.004), and H2O2 (t = 13.589, df = 3, p = 0.001; Figure 6F). The heat-acclimated larvae exhibited higher levels of enzyme activity of SOD, CAT, or GST to confront NAC or H2O2 (Figure 6).
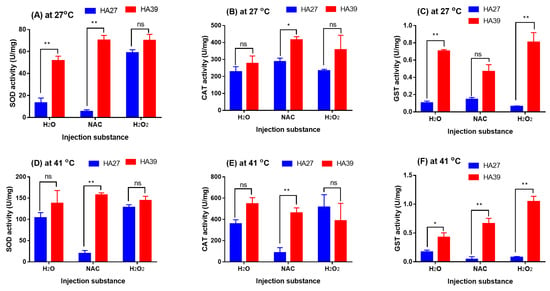
Figure 6.
The activity of SOD, CAT, and GST in the HA27 and HA39 at 27 °C (A–C) and 41 °C of heat exposure for 2 h (D–F) after injection of antioxidant NAC and oxidant H2O2. * and ** indicate significant difference between HA27 and HA39 at p = 0.05 and p = 0.01 levels, respectively. ns: not significant.
2.7. The Titer of H2O2 in the HA27 and HA39 Larvae under Heat Stress
At 27 °C, knockdown of CmGMC38 or CmGMC10 did not affect the level of H2O2 in the HA27 and HA39 larvae (Figure 7A), but at 39 °C for 3 h, knockdown of CmGMC38 (t = 3.346, df = 5, p = 0.040) and CmGMC10 (t = 3.915, df = 5, p = 0.022) increased the H2O2 level in the HA39 larvae, whereas it did not affect the HA27 (Figure 7B). Heat acclimation affected the accumulation of H2O2 in larvae exposed to a high temperature (Figure 7C). The titer of H2O2 in the HA27 larvae was significantly higher than that in the HA39 when they were exposed to 41 °C for 2 h (t = 2.509, df = 8, p = 0.036) and 3 h (t = 2.744, df = 8, p = 0.025), although there were no significant differences between these two strains when exposed to 41 °C for 0–1 h and 4–5 h (Figure 7). Heat acclimation decreased the accumulation of H2O2 under a certain degree of heat stress.
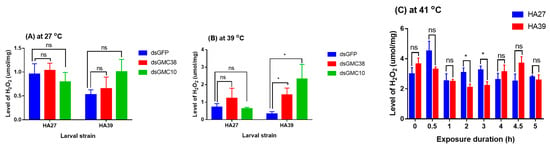
Figure 7.
RNAi of CmGMC10 or CmGMC38 (A,B) and heat exposure to 41 °C (C) affect the levels of H2O2 in the HA27 and HA39 larvae. * indicates a significant difference between the HA27 and HA39. ns: not significant at p = 0.05 level.
3. Discussion
Temperature plays an important role in the physiology and life history of insects [42,43]. Insects also show a certain degree of adaptability to temperature, including extreme temperature [41,44,45]. The responses of organisms to thermal change involve enzyme activity. A previous study has shown that differentially expressed genes between HA27 and HA39 of C. medinalis when exposed to 41 °C were enriched in the GO term of oxidoreductase activity [10]. Here, we found that two glucose dehydrogenase genes, CmGMC10 and CmGMC38, were expressed differentially between the HA27 and HA39 under heat stress. The expression level of CmGMC10 was significantly higher in the HA39 than in the HA27 under heat stress, suggesting this gene may be associated with heat acclimation. The comparison of transcriptomes showed that differentially expressed genes between thermo-sensitive and thermo-resistant strains of B. mori were significantly enriched in the GO terms, metabolic process, extracellular region, and serine-type peptidase activity when larvae were exposed to 35 °C [46]. In Tribolium castaneum, acclimation to a sublethal high temperature enhanced the heat tolerance of adults, and the upregulated genes between different temperature-acclimated adults exposed to 50 °C for 25 min were enriched in these categories, the terms of membrane part, metabolic process, and catalytic activity [47]. The wa`rm-acclimated rainbow trout Salmo gairdneri would decrease the GDH activity in the liver when the temperature decreased, but the long-time cold-acclimated did not [48], suggesting that changing temperature affects glucose dehydrogenase. Temperature significantly affected the enzyme activities and gene expression of hepatic glucokinase, glucose-6-phosphatase, and glucose-6-phosphate dehydrogenase in the tilapia Oreochromis niloticus [49]. In this study, a glucose dehydrogenase gene, CmGMC10, was more highly expressed in the body wall of the heat-acclimated larvae when confronted with heat stress, implying that the glucose dehydrogenase gene may mediate heat acclimation.
The result of RNAi further confirmed that the CmGMC10 regulated heat acclimation of the rice leaf folder larvae. Although the interference efficiency when using dsRNA or dsRNA+dsCmdsRNase was not very high, the heat tolerance of larvae injected with dsCmGMC10 or dsCmGMC38 was significantly decreased. RNAi of CmGMC10 led to a significant reduction in the survival rate of the HA39 larvae under heat stress. The RNAi using dsRNA is not a stable method to unfold the gene function of lepidopteran insects, but it is an alternative method now prior to the establishment of a simple and economical gene-editing method such as CRISPR/Cas9 [28,30,50]. In this study, we injected dsRNA or dsRNA+dsCmdsRNase to knock down the CmGMC gene, and similar results were attained, suggesting that the RNAi method was relatively effective for these two genes. RNAi of CmGMC10 resulted in a decrease in the larval survival rate and an increase in the ROS level of HA39 larvae under heat stress, but injection of dsCmGMC10 or dsCmGMC38 did not result in a change in ROS in the HA27. The result indicated that the CmGMC10 was related to the ROS level of larvae under heat stress.
The CmGMC10 and CmGMC38 belong to the oxidoreductase gene, which regulates the activity of antioxidant enzymes, such as SOD, CAT, and GST. The CAT, GST, and total antioxidant capacity were significantly increased in the ladybeetle Propylaea japonica when exposed to 35–43 °C, and the SOD was also increased when the temperature was above 37 °C [51]. An increase in temperature to 28 °C elevated the enzyme activity of SOD and CAT in aphids, and the antioxidant enzyme system protected aphids from high temperatures [52]. In the leaf folder larvae, the SOD and GST in the HA39 were significantly higher than that in the HA27 at 27 °C, and the GST was still higher at 41 °C. The relative expression level of CmGMC10 of the HA39 was higher than that of the HA27 at 41 °C. Overexpression of CmGMC10 in the heat-acclimated larvae enhanced enzyme activity and antioxidant defenses, and consequently conferred higher heat tolerance to the heat-acclimated larvae. SOD can convert superoxide anion into oxygen and H2O2, and GST can remove the hydroperoxide from cells [53,54,55]. The level of H2O2 in the HA39 larvae was lower than that in the HA27 when exposed to 41 °C for 2 or 3 h, and the ROS level increased in the HA39 larvae under heat stress when their CmGMC10 was knocked down, but these results were not found in the HA27. These results further confirmed that heat acclimation was mediated by the highly expressed glucose dehydrogenase gene CmGMC10, which reduced the level of oxidative stress in larvae under high temperatures (Figure 8).
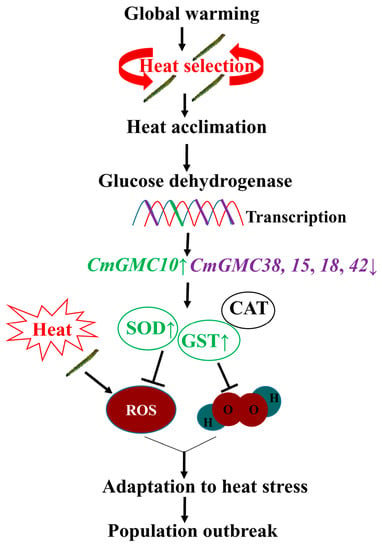
Figure 8.
A mode of glucose dehydrogenase-mediated heat acclimation of larvae to deal with global warming. ↑ means the up-regulated gene or enzyme activity. ↓ means the down-regulated gene.
Global warming is a threat to insects [56,57], while it may act as a selection stress driving the evolution of heat adaptation. Multigenerational selection under a high temperature increased heat tolerance to certain higher heat stress in the rice leaf folder [8,10], and this heat tolerance had transgenerational effects [41]. The slowly increasing ambient temperature from year to year may be similar to multigenerational selection and induce the heat acclimation of insects. In this study, we found that heat acclimation was mediated by the overexpression of a glucose dehydrogenase gene, suggesting a phenotypic plasticity (Figure 8). Transgenerational plasticity affects the heat tolerance of the offspring of the exposed generation [58,59,60]. Therefore, the population of rice leaf folder can adapt to increasing heat stress, and population outbreak will continue under global warming conditions.
4. Materials and Methods
4.1. Establishment of the Heat-Acclimated Strain
A population of about 600 larvae of C. medinalis was collected from a rice field in Nanjing, China in 2010. They were reared in the laboratory using wheat seedlings at 27 °C, 60% Rh, and a photoperiod of 14L:10D [61]. From the population, a part of the third instar larvae was exposed to 39 °C for 3 h per day at 11:00 a.m. (local time) for 3 d, and then all heat-treated larvae were reared at 27 °C. The same heat treatment was performed in the following generations. After more than 5 generations, the heat-treated population was considered as the heat-acclimated strain, HA39 [8,10,28]. The other part of the larvae was persistently reared at 27 °C and considered as the unacclimated strain (HA27).
4.2. Heat Tolerance of Larvae
Thirty third instar larvae from the HA27 (reared for about 123 generations at 27 °C in the laboratory) and HA39 (heat acclimated to 39 °C for about 54 generations) were transferred into a plastic cup with 30 wheat seedlings. Those larvae were exposed to 43 °C for 0.5 and 1 h, and then their survival was examined. All the surviving larvae were transferred into a 27 °C climate chamber and reared using new wheat seedlings. After 2 and 4 days, the survival of all larvae was examined again. Therefore, survival rates of tested larvae at the 0, 2nd, and 4th day after heat exposure were computed. The experiments were replicated three times for the HA27 and HA39.
4.3. Examination of the Relative Expression Level of Oxidoreductase Gene
In the transcriptome analysis, 21 differentially expressed putative genes between HA27 and HA39 after exposure to 41 °C for 1 h were enriched in the oxidoreductase activity GO term [10]. The relative expression levels of these putative genes of the HA27 (reared for 96 generations) and HA39 (heat-acclimated for 27 generations) were examined using the qPCR method. Ten third instar larvae of the HA27 and HA39 reared on a cup of wheat seedlings were exposed to 41 °C for 1 h in a climate chamber, and then three larvae were collected as a sample and quickly frozen in liquid nitrogen. Three samples were collected for the HA27 and HA39 and maintained at −80 °C until being used to extract RNA for the qPCR. We found 4 out of 21 putative genes were expressed differentially, including one upregulated and three downregulated putative genes in the HA39, compared to HA27. Therefore, we selected the one upregulated 121117 and one downregulated 71513 genes to examine the spatiotemporal expression in the HA27 and HA39 under different heat exposure durations of 0, 0.5, 1, 2, and 3 h to 39 °C and in different tissues or parts of the third instar larvae at 27 °C. Three replicates were performed, but one sample in the HA39 that was exposed for 2 h failed the qPCR for the CmGMC38 gene. These tissue samples from the HA27 (reared for 98 generations) and HA39 (heat acclimated for 29 generations) were collected from the head, silk gland, epidermis, foregut, midgut, and Malpighian tubules. Each sample was dissected from 10 third instar larvae and stored in a 1.5 mL centrifuge tube at −80 °C after quick freezing in liquid nitrogen. Three samples for each tissue were collected and examined, except the foregut of HA27, in which one sample failed qPCR.
RNA of the sample was extracted using the TRI-ZOL method (Takara, Dalian, China). The cDNA synthesis was performed using a PrimeScriptTM RT Reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Dalian, China) according to the instructions. The synthesized cDNA was stored at −80 °C for qPCR. The test kit for qPCR was a TB GREEN Premix Ex Taq Kit (Takara, Dalian, China). qPCR was performed in the ABI 7500 (Applied Biosystems, Carlsbad, CA, USA) using these primers listed in Table S1. Actin and RPs15 were used as the internal reference genes because they were expressed stably in the larvae under heat treatment (Figure S1). The relative expression levels of two oxidoreductase genes were calculated using the 2−ΔΔCt method based on two reference genes [62]. Three technical replicates were performed for each RNA sample.
4.4. Clone of Two Oxidoreductase Activity Genes
The full length of the gene 71513 and a partial sequence of 121117 were cloned using the SMARTer RACE 5 ‘/3’ Kit (Takara, Dalian, China) based on the primers in Table S2. The gene clone method is shown in the supplementary information. Based on the sequence (Figure S2) and the genome of the rice leaf folder [63], two oxidoreductase activity genes, 121117 and 71513, were identified as CmGMC10 and CmGMC38, respectively (Figure 1).
4.5. Knockdown of Two Oxidoreductase Activity Genes
The 300-400 bp dsRNA of CmGMC10 and CmGMC38 was designed using the online software E-RNAi Design version 3.2 (https://www.dkfz.de/signaling/e-rnai3/, accessed on 8 May 2020). The synthesis of dsRNA was performed according to instructions of the T7RiboMAXTM Express RNAi System (Promega, Madison, WI, USA) based on primers in Table S3. Two dsRNAs were designed for the CmGMC38 and their mixture reagents were used for RNAi. Only one dsRNA was designed for the CmGMC10 and GFP. The sequenced gel recycling products were used as templates to synthesize dsRNA. The reaction system included 10 μL RiboMAXTM Express T7 2x Buffer, 1–8 μL templates, 0–7 μL nuclease-free water, and 2 μL enzyme Mix T7 Express. The integrity of the synthesized dsRNA was detected using 1.2% agarose gel electrophoresis, and its concentration and purity were measured by spectrophotometer. All dsRNA samples were stored at −80 °C. It has been known that dsRNA degradation enzymes in lepidopteran insects lead to a low interference efficiency of RNAi [64] and the dsCmdsRNase increased the interference efficiency of the chitin synthase gene of C. medinalis (CmCHS) by 27.17% [34]. Therefore, we synthesized the dsCmdsRNase to improve the interference efficiency. The dsCmdsRNase was injected after mixing with the dsRNA of a target gene (1:1).
To determine the optimal interference efficiency of RNAi, the dsCmGMC38-1 and dsCmGMC38-2 were mixed (1:1) as dsCmGMC38. The third instar larvae of HA27 (reared for about 119 generations) and HA39 (heat acclimated for 50 generations) were collected, and 100, 200, 300, 400, and 500 µg of dsCmGMC38 were injected at the 6–8th segment of the abdomen under the Micro4/nanoliter microinjection system (World Precision Instruments, Sarasota, FL, USA). Forty larvae were injected for each dose of dsCmGMC38. After injection, the larvae were reared using fresh wheat seedlings at 27 °C for 24, 48, and 72 h, and then three larvae were collected to extract RNA for the examination of the relative expression level of CmGMC38 using qPCR. Based on the relative expression levels, we found that the interference efficiency was the highest 48 h after injection of 400 µg of dsRNA (Figure S3). Therefore, in the RNAi experiments, 400 µg of dsRNA was used. The third instar larvae from both the HA27 (reared for 144 generations) and HA39 (heat acclimated for 75 generations) were divided into three groups. One group injected dsGFP (CK), another injected dsCmGMC38 or dsCmGMC10, and the other injected dsCmdsRNase+dsCmGMC38 or +dsCmGMC10. Each group injected 30 larvae. Forty-eight hours after injection, the expression level of the CmGMC38 or CmGMC10 genes of 3 larvae was detected using qPCR to determine the interference efficiency, and the other injected larvae were transferred into tubes (25–30 larvae per tube) for exposure to 39 °C in a water bath for 3 h. After the exposure, the survival rate of the larvae was examined, and then all the heat-exposed larvae were reared on the wheat seedlings at 27 °C. The survival rates of larvae were surveyed at 2, 4, and 6 days after heat exposure. Injection of the larvae of HA27 and HA39 with each dsRNA was performed in three or four replicates.
4.6. Detection of Enzyme Activity and ROS Level
After dsRNA injection and heat exposure to 39 °C for 3h, three larvae were collected from the HA27 (reared for 144 generations) and HA39 (heat acclimated for 75 generations) strains reared in a cup of wheat seedlings as a sample. Three or four replicates were performed for the HA27 and five to seven replicates were performed for the HA39. Larval samples for each treatment of RNAi were collected to examine the enzyme activity. One milliliter of PBS was added to each sample and then ground. The supernatant after centrifugation at 4 °C, 12,000 rpm for 15 min was the crude enzyme solution to measure the content of protein, the activity of SOD, CAT, and GST, and total antioxidant capacity (T-AOC) using the BCA Protein (Beyotime, Shanghai, China), SOD, CAT, GST, and T-AOC Assay Kits (Solarbio, Beijing, China), respectively. When measuring the level of ROS after RNAi and heat exposure, three larvae were ground using 1 mL cell lysis buffer (Beyotime, China), and then centrifuged at 12,000 rpm at 4 °C for 15 min. The supernatant was used to measure the protein content and ROS level using the BCA Protein and Reactive Oxygen Species Assay Kits (Beyotime, China), respectively. The levels of ROS in the HA27 (reared for 134 generations) and HA39 (heat acclimated for 65 generations) larvae exposed to 41 °C for 0, 0.5, 1, and 2 h were also examined using the same method as above, and three or four replicates were performed.
4.7. Enzyme Activity of Larvae Injected with Exogenous Oxidant and Antioxidant
The antioxidant N-Acetyl-L-cysteine (NAC) can inhibit the ROS [65] and the oxidant H2O2 can increase the ROS level in insects [66]. To explore the relationship of oxidant and antioxidant with enzyme activity, 200 nL exogenous oxidant H2O2 (75 mg/mL) or antioxidant NAC (12.5 mg/mL) were injected into the third instar larva of HA27 (reared for 137 generations) and HA39 (heat acclimated for 68 generations) at the 6–8th segment of the abdomen using the Micro4/nanoliter microinjection system (World Precision Instruments, Sarasota, FL, USA), and injection of 200 nL water was the control. Sixty to ninety larvae were injected for each of the exogenous substances and then reared using wheat seedlings at 27 °C. After 24 h, 15–30 injected larvae on a cup of wheat seedlings were exposed to 41 °C for 2 h, and the 30 injected larvae kept at 27 °C were used as control. After heat treatment, 3 larvae from a cup of wheat seedlings were collected as a sample for examination of the SOD, CAT, and GST activity. Three replicates were performed for each combination of injection and heat treatment, but a sample failed to be examined for each the HA39 larvae injected with NAC at 27 °C and those injected with H2O2 at 41 °C.
4.8. Examination of the H2O2 Level in Larvae
The H2O2 levels in the third instar larvae of HA27 (reared for 144 generations) and HA39 (heat acclimated for 75 generations) injected with dsCmGMC10 and dsCmGMC38 after 48 h were detected when they were exposed to 27 °C and 39 °C for 3 h. Four replicates were performed, but one replicate failed in the HA39 knockdown of CmGMC10 or CmGMC38.
Twenty third instar larvae of the HA27 and HA39 reared on a cup of wheat seedlings were transferred into tubes and exposed to 41 °C for 0, 0.5, 1, 2, 3, 4, 4.5, and 5 h, respectively. Three surviving larvae were transferred into a 1.5 mL centrifuge tube and then transferred to a refrigerator at −80 °C for examination of the H2O2 level after rapid freezing in liquid nitrogen. Five replicates were performed. Each sample had 300 µL acetone added and was homogenized in ice bath, centrifuged at 8000 rpm at 4 °C for 10 min, and the supernatant was placed on ice for examination using the BCA protein detection kit (Beyotime, China) and H2O2 Assay Kits (Cominbio, Suzhou, China) to examine the protein concentration and H2O2 level, respectively.
4.9. Data Analysis
The standard statistical analyses, the Student’s t-test, followed by the Bonferroni correction and ANOVA, followed by post hoc Tukey’s test, were used to analyze the data except those specially mentioned below. The log transformation for the relative expression levels of genes after RNAi was used before the statistical analysis because the data did not meet the normal distribution. The survival rates of larvae with knocked down CmGMC10 or CmGMC38 after heat exposure were analyzed using the repeated-measures ANOVA in the GLM model, and the survival rates measured at 0, 2, 4, and 6 d after exposure were considered as repeated measurements. All analyses were performed in IBM SPSS Statistics software V25.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241210146/s1.
Author Contributions
Conceptualization, X.-D.L. and P.-Q.Q.; methodology, P.-Q.Q.; formal analysis, P.-Q.Q.; investigation, P.-Q.Q. and J.-R.L.; resources, X.-D.L. and P.-Q.Q.; data curation, P.-Q.Q. and J.-R.L.; writing—original draft preparation, P.-Q.Q.; writing—review and editing, X.-D.L.; visualization, X.-D.L.; supervision, X.-D.L.; project administration, X.-D.L.; funding acquisition, X.-D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant no 31871960.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying all the results presented in the paper have been archived in figshare: 10.6084/m9.figshare.22546621 [67].
Acknowledgments
We thank Ling-Ling Gu and Ming-Zhu Li for establishing the heat acclimated strain.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Levy, G.; David, D.; Degani, G. Effect of environmental temperature on growth- and reproduction-related hormones gene expression in the female blue gourami (Trichogaster trichopterus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 160, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, J.M.; Lazzari, C.R.; Lahondère, C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: A review. Insects 2018, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson Delmotte, V., Pörtner, H.-O., Roberts, D.C., Eds.; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Hoffmann, A.A.; Chown, S.L.; Clusella-Trullas, S. Upper thermal limits in terrestrial ectotherms: How constrained are they? Funct. Ecol. 2013, 27, 934–949. [Google Scholar] [CrossRef]
- Sales, K.; Vasudeva, R.; Gage, M.J.G. Fertility and mortality impacts of thermal stress from experimental heatwaves on different life stages and their recovery in a model insect. R. Soc. Open Sci. 2021, 8, 201717. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, S.; Hu, J.; Zhang, Y. Effects of non-lethal high-temperature stress on Bradysia odoriphaga (Diptera: Sciaridae) larval development and offspring. Insects 2020, 11, 159. [Google Scholar] [CrossRef]
- Bourg, E.L. A mild heat stress increases resistance to heat of dFOXO Drosophila melanogaster mutants but less in wild-type flies. Biogerontology 2021, 22, 237–251. [Google Scholar] [CrossRef]
- Gu, L.L.; Li, M.Z.; Wang, G.R.; Liu, X.D. Multigenerational heat acclimation increases thermal tolerance and expression levels of Hsp70 and Hsp90 in the rice leaf folder larvae. J. Therm. Biol. 2019, 81, 103–109. [Google Scholar] [CrossRef]
- Guz, N.; Dageri, A.; Altincicek, B.; Aksoy, S. Molecular characterization and expression patterns of heat shock proteins in Spodoptera littoralis, heat shock or immune response? Cell Stress Chaperones 2021, 26, 29–40. [Google Scholar] [CrossRef]
- Quan, P.Q.; Li, M.Z.; Wang, G.R.; Gu, L.L.; Liu, X.D. Comparative transcriptome analysis of the rice leaf folder (Cnaphalocrocis medinalis) to heat acclimation. BMC Genom. 2020, 21, 450. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Zhang, W.; Zhang, B.; Ma, C.S. Dynamics of heat shock protein responses to thermal stress changes after metamorphosis in a lepidopteran insect. Arch. Insect Biochem. Physiol. 2021, 107, e21791. [Google Scholar] [CrossRef]
- Iida, K.; Cavener, D.R. Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. J. Exp. Biol. 2004, 207, 675–681. [Google Scholar] [CrossRef]
- Ding, J.; Wu, Y.; You, L.-L.; Xu, B.; Ge, L.-Q.; Yang, G.-Q.; Wu, J.-C. Jinggangmycin-suppressed reproduction in the small brown planthopper (SBPH), Laodelphax striatellus (Fallen), is mediated by glucose dehydrogenase (GDH). Pestic. Biochem. Physiol. 2017, 139, 73–78. [Google Scholar] [CrossRef]
- Sun, W.; Shen, Y.-H.; Yang, W.-J.; Cao, Y.-F.; Xiang, Z.-H.; Zhang, Z. Expansion of the silkworm GMC oxidoreductase genes is associated with immunity. Insect Biochem. Mol. Biol. 2012, 42, 935–945. [Google Scholar] [CrossRef]
- Cox-Foster, D.L.; Stehr, J.E. Induction and localization of FAD-glucose dehydrogenase (GLD) during encapsulation of abiotic implants in Manduca sexta larvae. J. Insect Physiol. 1994, 40, 235–249. [Google Scholar] [CrossRef]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.-H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef]
- Schulte, P.M. The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Dryer, S.E.; Dryer, R.L.; Autor, A.P. Enhancement of mitochondrial, cyanide-resistant superoxide dismutase in the livers of rats treated with 2,4-dinitrophenol. J. Biol. Chem. 1980, 255, 1054–1057. [Google Scholar] [CrossRef]
- Li, J.-M.; Su, Y.-L.; Gao, X.-L.; He, J.; Liu, S.S.; Wang, X.-W. Molecular characterization and oxidative stress response of an intracellular Cu/Zn superoxide dismutase (CuZnSOD) of the whitefly, Bemisia tabaci. Arch. Insect Biochem. Physiol. 2011, 77, 118–133. [Google Scholar] [CrossRef]
- Yang, L.-H.; Huang, H.; Wang, J.-J. Antioxidant responses of citrus red mite, Panonychus citri (McGregor) (Acari: Tetranychidae), exposed to thermal stress. J. Insect Physiol. 2010, 56, 1871–1876. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, M.; Zhang, Q.; Fu, J.; Loiacono, F.V.; Yang, Y.; Wang, Z.; Li, S.; Chang, L.; Bock, R.; et al. Control of a sap-sucking insect pest by plastid-mediated RNA interference. Mol. Plant 2022, 15, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Laudani, F.; Strano, C.P.; Edwards, M.G.; Malacrinò, A.; Campolo, O.; Abd El Halim, H.M.; Gatehouse, A.M.R.; Palmeri, V. RNAi-mediated gene silencing in Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae). Open Life Sci. 2017, 12, 214–222. [Google Scholar] [CrossRef]
- Li-Byarlay, H.; Li, Y.; Stroud, H.; Feng, S.; Newman, T.C.; Kaneda, M.; Hou, K.K.; Worley, K.C.; Elsik, C.G.; Wickline, S.A.; et al. RNA interference knockdown of DNA methyl-transferase 3 affects gene alternative splicing in the honey bee. Proc. Natl. Acad. Sci. USA 2013, 110, 12750–12755. [Google Scholar] [CrossRef]
- Yu, J.-L.; An, Z.-F.; Liu, X.-D. Wingless gene cloning and its role in manipulating the wing dimorphism in the white-backed planthopper, Sogatella furcifera. BMC Mol. Biol. 2014, 15, 20. [Google Scholar] [CrossRef]
- Cai, L.J.; Li, T.P.; Lin, X.J.; Huang, Y.P.; Qin, J.M.; Xu, W.; You, M.S. Identification and characterization of zero population growth (zpg) gene in Plutella xylostella. Physiol. Entomol. 2022, 47, 46–54. [Google Scholar] [CrossRef]
- Guo, P.L.; Guo, Z.Q.; Liu, X.D. Cuticular protein genes involve heat acclimation of insect larvae under global warming. Insect Mol. Biol. 2022, 31, 519–532. [Google Scholar] [CrossRef]
- Shi, G.; Zhou, Y.; Ren, F. Identification and function analysis of BmPxtA in the immune response regulated by PGE2 of silkworm, Bombyx mori. Dev. Comp. Immunol. 2022, 130, 104358. [Google Scholar] [CrossRef]
- Sun, S.-F.; Zeng, F.-F.; Yi, S.-C.; Wang, M.-Q. Molecular screening of behaviorally active compounds with CmedOBP14 from the rice leaf folder Cnaphalocrocis medinalis. J. Chem. Ecol. 2019, 45, 849–857. [Google Scholar] [CrossRef]
- Yang, W.; Yang, H.; Zhang, Y.; Wang, X.; Cong, Y.; Fan, D. RNA interference of β-N-acetylglucosaminidase from the oriental armyworm moth, Mythimna separata. Bull. Insectology 2019, 72, 297–307. [Google Scholar]
- Arimatsu, Y.; Kotani, E.; Sugimura, Y.; Furusawa, T. Molecular characterization of a cDNA encoding extracellular dsRNase and its expression in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2007, 37, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, Q.; Luan, J.; Chung, S.H.; Van Eck, J.; Turgeon, R.; Douglas, A.E. Towards an understanding of the molecular basis of effective RNAi against a global insect pest, the whitefly Bemisia tabaci. Insect Biochem. Mol. Biol. 2017, 88, 21–29. [Google Scholar] [CrossRef]
- Li, J.; Du, J.; Li, S.; Wang, X. Identification and characterization of a double-stranded RNA degrading nuclease influencing RNAi efficiency in the rice leaf folder Cnaphalocrocis medinalis. Int. J. Mol. Sci. 2022, 23, 3961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Geng, J.G.; Zhou, W.J. Migration regularity of Cnaphalocrocis medinalis Guenée in China. J. Nanjing Agric. Coll. 1981, 3, 43–54. [Google Scholar]
- Liao, H.J.; Qian, Q.; Liu, X.D. Heat shock suppresses mating and sperm transfer in the rice leaf folder Cnaphalocrocis medinalis. Bull. Entomol. Res. 2014, 104, 383–392. [Google Scholar] [CrossRef]
- Qian, Q.; Gu, L.-L.; Liu, X.-D. Can the young larvae of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) tolerate the heat stress in summer? Environ. Entomol. 2017, 46, 125–130. [Google Scholar]
- Guo, R.; Han, M.; Su, F. Tactics and methods to control rice pests using less pesticides. China Plant Prot. 2013, 33, 38–41. [Google Scholar]
- Kwon, Y.S.; Chung, N.; Bae, M.J.; Li, F.; Chon, T.S.; Park, Y.S. Effects of meteorological factors and global warming on rice insect pests in Korea. J. Asia-Pac. Entomol. 2012, 15, 507–515. [Google Scholar] [CrossRef]
- Pan, Q.B.; Yang, Q.; Xu, J.; Zhang, Y.; Chen, Y.L. Population outbreak reasons of the fifth generation of rice leaf folders and control. J. Zhejiang Agric. Sci. 2014, 5, 706–708. [Google Scholar]
- Li, M.Z.; Liu, X.D. Transgenerational effect of heat adaptation induced by heat acclimation in larvae of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Crambidae). Acta Entomol. Sin. 2022, 65, 1314–1323. [Google Scholar]
- Jeffs, C.T.; Leather, S.R. Effects of extreme, fluctuating temperature events on life history traits of the grain aphid, Sitobion avenae. Entomol. Exp. Et Appl. 2014, 150, 240–249. [Google Scholar] [CrossRef]
- Laughton, A.M.; O’Connor, C.O.; Knell, R.J. Responses to a warming world: Integrating life history, immune investment, and pathogen resistance in a model insect species. Ecol. Evol. 2017, 7, 9699–9710. [Google Scholar] [CrossRef]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Perez, R.; Aron, S. Adaptations to thermal stress in social insects: Recent advances and future directions. Biol. Rev. 2020, 95, 1535–1553. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, P.; Xiao, J.; Wang, L.; Liu, T.; Wu, Y.; Dong, F.; Jiang, Y.; Pan, M.; Zhang, Y.; et al. Comparative transcriptome profiling of a thermal resistant vs. sensitive silkworm strain in response to high temperature under stressful humidity condition. PLoS ONE 2017, 12, e0177641. [Google Scholar] [CrossRef]
- Lü, J.; Huo, M. Transcriptome analysis reveals heat tolerance of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) adults. J. Stored Prod. Res. 2018, 78, 59–66. [Google Scholar] [CrossRef]
- Tranulis, M.A.; Christophersen, B.; Blom, A.K.; Borrebaek, B. Glucose dehydrogenase, glucose-6-phosphate dehydrogenase and hexokinase in liver of rainbow trout (Salmo gairdneri). Effects of starvation and temperature variations. Comp. Biochem. Physiol. Part B: Comp. Biochem. 1991, 99, 687–691. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Wang, H.; Kpundeh, M.D.; Xu, P.; Zhu, Z.X. Temperature modulates hepatic carbohydrate metabolic enzyme activity and gene expression in juvenile GIFT tilapia (Oreochromis niloticus) fed a carbohydrate-enriched diet. J. Therm. Biol. 2014, 40, 25–31. [Google Scholar] [CrossRef]
- Chaitanya, B.N.; Asokan, R.; Sita, T.; Rebijith, K.B.; Kumar, P.; Kumar, N.K.K. Silencing of JHEH and EcR genes of Plutella xylostella (Lepidoptera: Plutellidae) through double stranded RNA oral delivery. J. Asia-Pac. Entomol. 2017, 20, 637–643. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, W.; Li, N.; Zhang, F.; Liu, T.X. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef]
- Durak, R.; Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, T. Changes in antioxidative, oxidoreductive and detoxification enzymes during development of aphids and temperature increase. Antioxidants 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Duval, D.L.; Weinhold, L.C.; Pardini, R.S. Cabbage looper antioxidant enzymes: Tissue specificity. Insect Biochem. 1991, 21, 563–572. [Google Scholar] [CrossRef]
- Lyakhovich, V.V.; Vavilin, V.A.; Zenkov, N.K.; Menshchikova, E.B. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry 2006, 71, 962–1183. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, P.; O’Brien, P.J. Selenium-independent glutathione peroxidase activity in rabbit liver. Can. J. Biochem. 1980, 58, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tweksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef]
- Crill, W.D.; Huey, R.B.; Gilchrist, G.W. With- and Between-generation effects of temperature on the morphology and physiology of Drosophila melanogaster. Evolution 1996, 50, 1205–1218. [Google Scholar]
- Gonzalez-Tokman, D.; Cordoba-Aguilar, A.; Dattilo, W.; Lira-Noriega, A.; Sanchez-Guillen, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Schiffer, M.; Hangartner, S.; Hoffmann, A.A. Assessing the relative importance of environmental effects, carry-over effects and species differences in thermal stress resistance: A comparison of Drosophilids across field and laboratory generations. J. Exp. Biol. 2013, 216, 3790–3798. [Google Scholar] [CrossRef]
- Zhu, A.-X.; Qian, Q.; Liu, X.-D. A method for rearing the rice leaf folder (Cnaphalocrocis medinalis) using wheat seedlings. Chin. J. Appl. Entomol. 2015, 52, 883–889. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, H.; He, K.; Shi, Z.; Chen, X.; Ye, X.; Mei, Y.; Yang, Y.; Li, M.; Gao, L.; et al. A chromosome-level genome assembly of rice leaffolder, Cnaphalocrocis medinalis. Mol. Ecol. Resour. 2021, 21, 561–572. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, G.-H.; Wang, K.; Chen, J.; Liu, X.; Wu, M.; Zhao, C.; Xiao, H.; Palli, S.R.; Han, Z. Knockout of SldsRNase1 and SldsRNase2 revealed their function in dsRNA degradation and contribution to RNAi efficiency in the tobacco cutworm, Spodoptera litura. J. Pest Sci. 2021, 94, 1449–1460. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Wang, T.; Lin, X.-W.; Denlinger, D.L.; Xu, W.H. Reactive oxygen species extend insect life span using components of the insulin-signaling pathway. Proc. Natl. Acad. Sci. USA 2017, 114, E7832–E7840. [Google Scholar] [CrossRef]
- Chen, P.; Hu, Y.-F.; Wang, L.; Xiao, W.-F.; Bao, X.-Y.; Pan, C.; Yi, H.-S.; Chen, X.-Y.; Pan, M.-H.; Lu, C. Mitochondrial apoptotic pathway is activated by H2O2-mediated oxidative stress in BmN-SWU1 cells from Bombyx mori ovary. PLoS ONE 2015, 10, e0134694. [Google Scholar] [CrossRef]
- Liu, X.D. Glucose Dehydrogenases-Mediated Heat Acclimation.xlsx; Figshare LLP: London, UK, 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).