Abstract
Despite the known influence of DNA methylation from lipopolysaccharide (LPS) activation, data on the O6-methylguanine-DNA methyltransferase (MGMT, a DNA suicide repair enzyme) in macrophages is still lacking. The transcriptomic profiling of epigenetic enzymes from wild-type macrophages after single and double LPS stimulation, representing acute inflammation and LPS tolerance, respectively, was performed. Small interfering RNA (siRNA) silencing of mgmt in the macrophage cell line (RAW264.7) and mgmt null (mgmtflox/flox; LysM-Crecre/−) macrophages demonstrated lower secretion of TNF-α and IL-6 and lower expression of pro-inflammatory genes (iNOS and IL-1β) compared with the control. Macrophage injury after a single LPS dose and LPS tolerance was demonstrated by reduced cell viability and increased oxidative stress (dihydroethidium) compared with the activated macrophages from littermate control mice (mgmtflox/flox; LysM-Cre−/−). Additionally, a single LPS dose and LPS tolerance also caused mitochondrial toxicity, as indicated by reduced maximal respiratory capacity (extracellular flux analysis) in the macrophages of both mgmt null and control mice. However, LPS upregulated mgmt only in LPS-tolerant macrophages but not after the single LPS stimulation. In mice, the mgmt null group demonstrated lower serum TNF-α, IL-6, and IL-10 than control mice after either single or double LPS stimulation. Suppressed cytokine production resulting from an absence of mgmt in macrophages caused less severe LPS-induced inflammation but might worsen LPS tolerance.
1. Introduction
Sepsis is a potentially life-threatening condition arising in response to severe infection, regardless of the organismal causes (bacteria, viruses, fungi, and parasites) [1,2,3], partly due to the simultaneous immunological imbalance between hyperinflammation and immune exhaustion (immune paralysis) [4,5] in the same patient [6]. Sepsis-induced hyperinflammation results in severe sepsis, while immune exhaustion leads to inadequate inflammation for microbial control, causing secondary infection [7]. The blockage and enhancement of inflammation during sepsis hyperinflammation and immune exhaustion, respectively, may be helpful [8,9,10,11,12,13,14,15], despite the improvement of supportive care in sepsis [16]. Among several sepsis mechanisms, the responses against lipopolysaccharide (LPS) during sepsis are extensively studied [17,18,19,20]. The presence of LPS, a major cell wall component of Gram-negative bacteria in the blood (endotoxemia) during sepsis, may be due to Gram-negative bacteremia or the translocation of LPS from the gut because of the high abundance of Gram-negative bacteria in the gut [21,22,23]. Because monocytes and macrophages are important for controlling microbial molecules, both acute responses to LPS and the reduced cytokine production after the first LPS stimulation (LPS tolerance), are possible during prolonged endotoxemia [24,25]. The causes of LPS tolerance in macrophages might be due to epigenetic modifications, chromatin remodeling, and interferences on cell energy status [26,27,28]. Following LPS stimulation, epigenetics (phenotypic alterations without changes in the DNA sequence [29,30]) are responsible for the switch-on and switch-off of DNA transcription through DNA methylation (methyl group added onto the DNA), histone modifications, and noncoding RNA (microRNA) [2]. Of these, the DNA methylation and histone modification are mediated by three groups of enzymes, including (i) the writers (methylation, acetylation, phosphorylation, and ubiquitination), (ii) the erasers (removal of the modifications), and (iii) the readers (binding to different covalent modifications by the writers to mediate physiological outcomes) [3]. Within DNA methylation, the epigenetic regulation of DNA repair is profoundly interesting.
Any forms of electrophilic species (oxidative stress) from several cellular processes, including regular cell activities (stress, cell adaptation, tissue integrity, and remodeling to adapt to the microenvironment), metabolic activation (such as LPS stimulation), and exposure to alkylating agents, induce DNA methylation, especially the purine N -methylation and O6-methylguanine (O6MeG) [31]. Some of these methylation events, particularly O6MeG, are common processes of DNA damage that can trigger point mutations with high mutagenicity and carcinogenicity [32]. Because polymerase enzymes frequently mis insert thymine instead of cytosine (O6MeG:T mismatch) due to the roughly equal strength of the hydrogen bonds to cytosine and thymine; O6MeG, which is commonly activated by the alkylating agent, is mutagenic [33]. However, O6MeG is produced not only by alkylating agents and environmental compounds but also by several endogenous factors during regular cell activities, especially oxidative stress [34]. Indeed, normal metabolic processes, such as hydrolysis, deamination, alkylation, and oxidation, result in several forms of DNA damage, including base damage, single-strand breaks (SSB), double-strand breaks (DSB), and inter-strand cross-links with roughly more than 50,000 lesions per cell daily (approximately 30,000 nucleoside sites in DNA per cell [35]). Although modifications at the O6 position of guanine (O6MeG) might be not high compared with the total number of DNA lesions [36], an abundance of O6MeG might originate from high oxidative stress during sepsis or LPS response. Indeed, chronic inflammation can induce both cancer mutation and DNA methylation [37]. Because of the easy point mutation of O6MeG, O6MeG might cause more severe DNA damage and cell death compared to other types of DNA methylation [38].
To maintain genome stability, DNA repair is necessary, partly through the removal of methyl groups on the DNA by base excision repair initiated by the alkyladenine-DNA glycosylase, the family of alkylation B (AlkB) homolog proteins, and the suicidal enzyme O6-methylguanine-DNA methyltransferase (MGMT) [32,39]. The alkylated and methylated forms of these enzymes are rapidly degraded after DNA repair. Indeed, the intact MGMT in several tissues prevents malignant transformation, and MGMT blockage is used for adjuvant chemotherapy [32] through the blockage of DNA repair in cancers that depend on the rate of MGMT re-synthesis of the malignant cells [39]. Due to several macrophage activator molecules and immune activation-induced oxidative stress in macrophages [40], DNA methylation and DNA damage in the activated cell are possible, especially after stimulation with LPS. Indeed, LPS causes DNA methylation/damage in macrophages directly [41] and indirectly through Toll-like receptor activation and reactive oxygen species (ROS), respectively [42]. Additionally, the higher levels of genomic DNA methylation patterns and hypermethylated genes associated with the pro-inflammatory pathways are demonstrated in patients with sepsis [43]. Despite a solid body of data on the role of O6MeG in malignant cells, the impact of O6MeG and MGMT on macrophages which are the cells with possibly high stress-induced DNA methylation [44] are still unclear. Because (i) sepsis and LPS induce DNA methylation, including Q6MeG [43], (ii) MGMT reduces O6MeG during the DNA repair, and (iii) an MGMT inhibitor (Lomeguatrib) enhances the death of cancer cells (DNA repair interference) [45] that might be beneficial in sepsis [46], the blockage of MGMT in macrophages might reduce macrophage activity and attenuate sepsis-related hyper-inflammation. Furthermore, epigenetic inhibitor screening has also shown that MGMT inhibitors alter the expression of inflammatory cytokine in the LPS-activated macrophages [47] and the MGMT inhibitors not only neutralize O6MeG in DNA but also link to the repair of other pathways [48]. Despite several ongoing research topics, the control of macrophage responses through their manipulations on epigenetics is interesting for controlling immune responses during sepsis [13,49].
Here, we explored the impact of mgmt on the responses to LPS, both for a single LPS activation (hyper-inflammatory responses) and double stimulation (LPS tolerance), in vitro and in vivo, using the conditional mgmt deletion mice with LysM-Cre system that selectively affects mgmt only in macrophages.
2. Results
2.1. Transcriptomic Analysis of the Influence of Epigenetic Alteration in Macrophages after Activation by Single or Double LPS Stimulation
The difference between control cells versus activated macrophages from wild-type (WT) mice using a single LPS stimulation (N/LPS) and LPS tolerance (LPS/LPS) was evaluated by RNA sequencing analysis (Figure 1A). There were 2775 and 3934 up- and down-regulated genes in the LPS-activated macrophages compared with the media control, as indicated by a Volcano plot analysis (Figure 1B). Meanwhile, there were 2115 and 2729 up- and down-regulated genes in the macrophages with LPS tolerance compared to the control (Figure 1C). The differences among the control, single LPS dose, and LPS tolerance conditions were clearly demonstrated by the heat map graphic pattern for DNA and histone modification (Figure 1D,E). Notably, only the genes with significant differences or a tendency of difference among these groups were included. For DNA modification, the genes between control and LPS stimulation, dnmt1 (DNA methyltransferases) and mgmt (O6-methylguanine-DNA methyltransferase), were lower, while mbd2 (methyl-cytosine binding domain2), sap30 (Shrimp alkaline phosphatase30), mecp2 (methyl-CpG binding protein2), and hdac1 (histone deacetylase) were higher compared with the control (Figure 1D). Additionally, hdac1 was categorized in both DNA and histone modification due to the collaboration of hdac1 with dnmt1 to form a complex [50]. Interestingly, the expression of these DNA modification genes in macrophages between single LPS and LPS tolerance was opposite, as indicated by the heat map analysis pattern (Figure 1D). For the histone modification genes between control and LPS stimulation, ezh1 (histone-lysine N-methyltransferase1) and aurkb (aurora kinase B) were lower than control (Figure 1E), while sirt6 (sirtuin6), kmt5a (lysine methyltransferase 5a), rnf2 (ring finger protein 2), hdac1, and kdm6b (lysine demethylase 6b) were higher than the control (Figure 1E). In addition, the expression of these histone modification genes in macrophages between single LPS and LPS tolerance was also the opposite, as indicated by the heat map analysis, except for sirt6 and kmt5a (Figure 1E). Hence, these data supported the idea of an epigenetic alteration in the macrophage responses against LPS, which might be different between single and double LPS stimulations.
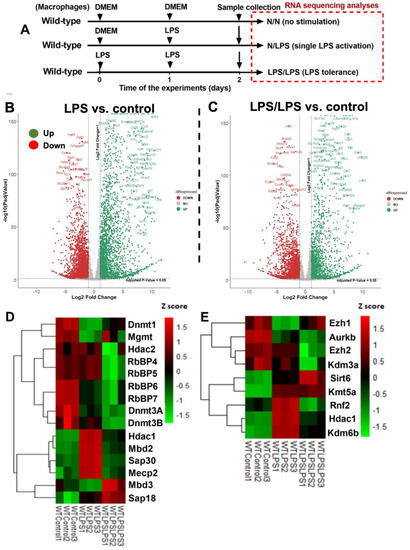
Figure 1.
The transcriptome profiles and the log2 of the transcript count per million (TPM) of genes in bone marrow-derived macrophages from wild-type mice activated by lipopolysaccharide (LPS) in a single protocol (N/LPS), which started with the culture media followed by LPS 24 h later or LPS tolerance (LPS/LPS) by the two sequential LPS stimulations, or control (N/N; control) using the culture media incubation only (A), as indicated by the Volcano plot analysis (B,C), the heatmap of the genes of epigenetic changes in DNA methylation or acetylation (D), and the histone modification (E) with difference (or tendency of difference) to the control. Macrophages were isolated from three different mice. List of abbreviations: Dnmt (DNA methyltransferases), Mgmt (O6-methylguanine-DNA methyltransferase), Hdac (histone deacetylase), RbBP (Retinoblastoma-Binding Protein), Mbd (methyl-cytosine binding domain), Sap (Shrimp alkaline phosphatase), Mecp (methyl-CpG binding protein), Ezh (histone-lysine N-methyltransferase), Aurkb (aurora kinase B), Kdm (lysine demethylase), Sirt (sirtuin), Kmt (lysine methyltransferase), and Rnf2 (ring finger protein).
2.2. Reduction in Macrophage Pro-Inflammation with mgmt Interferences after Either a Single LPS or Two Sequential LPS Stimulations: The Beneficial Effect in Hyper-Inflammatory Sepsis, but Not LPS Tolerance
The relationship between mgmt and the LPS responses was initially explored through silencing of the mgmt gene by siRNA (mgmt siRNA) in the macrophage cell line (RAW264.7) (Figure 2A). The mgmt silencing in either LPS-stimulated macrophages (N/LPS) or LPS tolerance (LPS/LPS) reduced the secretion of pro-inflammatory cytokines (TNF-α and IL-6, but not IL-10) and M1 pro-inflammatory polarization, as indicated by inducible nitric oxide synthase (iNOS) and interleukin-1β (IL-1β) (Figure 2B–F), the upregulated M2 anti-inflammatory polarization marker arginase-1 (Arg-1), and the transforming growth factor-β (TGF-β), but not resistin-like molecule-1 (Fizz-1), compared with the littermate control cells (mgmt control) (Figure 2G–I). Additionally, there was a reduction in secreted cytokines (TNF-α and IL-6) in the LPS/LPS macrophages with both mgmt non-siRNA control and mgmt siRNA when compared to a single LPS stimulation (N/LPS), supporting the main characteristics of LPS tolerance as previously described [24,51,52], with the higher level of these cytokines in mgmt non-siRNA over the mgmt siRNA cells (Figure 2B,C). These data imply reduced macrophage cytokine production, perhaps due to the methylation of the DNA that is responsible for these cytokines, or due to a lack of MGMT to repair the DNA [44]. Moreover, mgmt silencing also reduced the pro-inflammatory M1 polarization (iNOS and IL-1β) and enhanced the M2 anti-inflammatory polarization (Arg-1 and TGF-β) of macrophages compared with the non-silencing cells (Figure 2E–H). Notably, prominent M1 polarization (iNOS and IL-1β) with lower M2 polarization (Arg-1 and TGF-β) in single LPS stimulation over LPS tolerance, and profound M2 polarization (Arg-1) with low M1 polarization (iNOS and IL-1β) in LPS tolerance over single LPS activation, in either non-siRNA or mgmt siRNA groups, were also demonstrated (Figure 2E–H). To further investigate the mgmt impacts on LPS stimulation, macrophages from the mice with the conditional mgmt deletion (the LysM-Cre system) were then used (Figure 3A). Similar to the mgmt-silencing siRNA, the mgmt null macrophages (mgmtfl/fl; LysM-Crecre/−) displayed lower supernatant cytokines (TNF-α, IL-6, and IL-10), down-regulated cytokine genes, and up-regulated M1 polarization (iNOS and IL-1β), without the alteration of M2 polarization genes, when compared with the littermate cells (mgmtfl/fl; LysM-Cre−/−) (mgmt control) (Figure 3B–G). Additionally, lower cytokine production in LPS/LPS compared with N/LPS (the characteristics of LPS tolerance) was evident in macrophages from both mouse strains (mgmt control and mgmt null) (Figure 3B–G). However, most of the cytokines (supernatant and gene expression) in LPS/LPS mgmt null macrophages were lower than the LPS/LPS in cells from littermate mice (mgmt control) (Figure 3B–G). In addition, there were less prominent M1 pro-inflammatory polarization genes (iNOS and IL-1β) with higher M2 polarization (Arg-1 but not TGF-β and Fizz) (Figure 3H–L), implying a possible more severe LPS tolerance (low cytokine production and high M2 anti-inflammatory direction that might be inadequate for the microbial control) in mgmt null macrophages than in the control. These data suggest that MGMT blockage might be beneficial for the anti-inflammation but can worsen LPS tolerance through the overwhelming anti-inflammatory response direction of the macrophages.
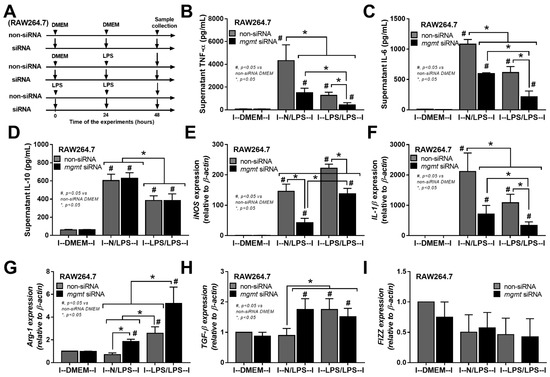
Figure 2.
The schema of the experiments in a murine macrophage cell line (RAW264.7) with the silencing of mgmt gene using small interfering RNA (mgmt siRNA) or the control siRNA (non-targeting pool siRNA; non-siRNA) after activation by lipopolysaccharide (LPS) in a single protocol (N/LPS), which started with the culture media followed by LPS 24 h later or LPS tolerance (LPS/LPS) by the two sequential LPS stimulations, or control (N/N), using the culture media incubation only (A). The characteristics of macrophages under these protocols as indicated by secreted cytokines (TNF-α, IL-6, and IL-10) (B–D), the expression of pro-inflammatory genes of M1 polarization (iNOS and IL-1β) (E,F), and the anti-inflammatory genes of M2 polarization (Arg-1, TGF-β, and Fizz-1) (G–I). Triplicate independent experiments were performed. Mean ± SEM with one-way ANOVA followed by Tukey’s analysis was used. #, p ˂ 0.05 mgmt vs. control DMEM; *, p ˂ 0.05 between the indicated groups.
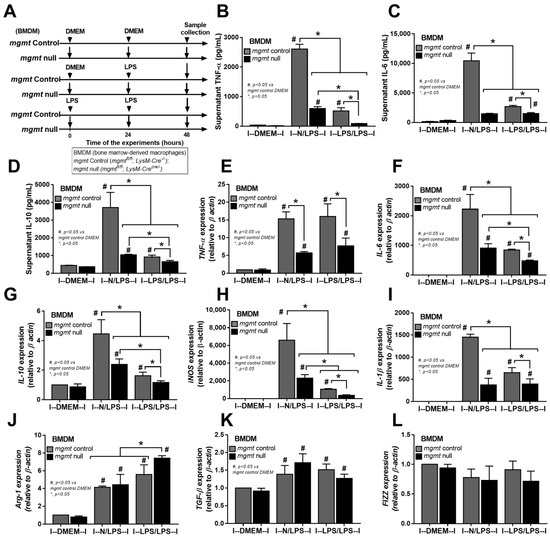
Figure 3.
The schema of the experiments in bone marrow-derived macrophages from mgmt control (mgmtfl/fl; LysM-Cre−/−) and mgmt null (mgmtfl/fl; LysM-Crecre/−) mice after activation by lipopolysaccharide (LPS) in a single protocol (N/LPS), which started with the culture media followed by LPS 24 h later or LPS tolerance (LPS/LPS) by the two sequential LPS stimulations, or control (N/N), using the culture media incubation only (A). The characteristics of macrophages under these protocols, as indicated by supernatant cytokines (TNF-α, IL-6, and IL-10) (B–D), the gene expression of cytokines (TNF-α, IL-6, and IL-10) (E–G), M1 macrophage polarization (iNOS and IL-1β) (H,I), and the M2 macrophage polarization (Arg-1, TGF-β, and Fizz-1) (J–L), are also demonstrated. Triplicate independent experiments were performed. Mean ± SEM with one-way ANOVA followed by Tukey’s analysis was used. #, p ˂ 0.05 mgmt vs. control DMEM; *, p ˂ 0.05 between the indicated groups.
Despite the down-regulation of mgmt in 24 h LPS-stimulated macrophages in the RNA sequencing analysis (Figure 1D), macrophages from mgmt null mice were further tested (Figure 4A) and the mgmt expression in single LPS (N/LPS) was similar to the control (Figure 4B). Meanwhile, mgmt was upregulated in LPS/LPS, compared with the control groups, in both the RNA sequencing (Figure 1D) and PCR analyses (Figure 4B). These data imply the obvious need for MGMT enzyme as a DNA repair factor in LPS tolerance, and possibly less need for it in single LPS activation. Because reactive oxygen species (ROS) are natural products during regular processes of the cells, especially by mitochondrial activation, and ROS-induced DNA methylation is known, the non-difference mgmt expression in macrophages between control and 24 h LPS might have been due to the similar MGMT levels in both conditions, and the reduced MGMT might have affected the macrophages. Indeed, the mgmt null macrophages were more vulnerable to LPS-induced injury, as demonstrated by the reduction of cell viability (MTT assay) and the increased ROS of mgmt null cells in either single or double LPS stimulation compared with the mgmt control cells (Figure 4C,D), despite the neutral mgmt expression of LPS-stimulated cells versus control. Interestingly, the ROS in the mgmt null cells with N/LPS was higher than in the LPS/LPS cells (Figure 4D), perhaps due to the inadequate MGMT in N/LPS compared with the LPS/LPS group. In parallel, there was an increase in cell proliferation after LPS stimulation with either the single or double LPS protocols (more prominent in LPS tolerance) in mgmt control cells but not in mgmt null macrophages (Figure 4B). Meanwhile, the ROS level after single LPS stimulation was not different from the LPS tolerance in both strains of macrophages (Figure 4D), implying a lack of difference in ROS production between the single versus twice LPS stimulations. Additionally, the possible cell damage after LPS activation and LPS tolerance was demonstrated by an elevation of supernatant cell-free DNA compared with the control group, which was similar between N/LPS and LPS/LPS (Figure 4E). Additionally, there was a DNA break, as was indicated by the immunofluorescent staining of phosphohistone H2A.X after the single and twice LPS activations in both mgmt control and mgmt null macrophages with a similar intensity between groups (Figure 4F,G). However, the highest intensity of phosphohistone H2A.X in the nuclei of the mgmt null cells after LPS/LPS activation highlighted the positive DNA damage in the mgmt null macrophages with LPS tolerance (Figure 4G). Meanwhile, the positive green color in the other groups was not in the nuclei, which, for at least some of them, might have been a false positive result (Figure 4G). Due to the association between the cell energy status versus cell activities [23,53,54,55,56], the extracellular flux analysis between mgmt null and the control was examined (Figure 4H–K). There was a reduction in maximal respiratory capacity (mitochondrial activity), without glycolysis alteration, similarly in both strains of macrophages after activation by either one or two doses of LPS (Figure 4H–K). Thus, the mgmt gene seemed to have less impact on the cell energy status, despite the evident impact on cytokine production.
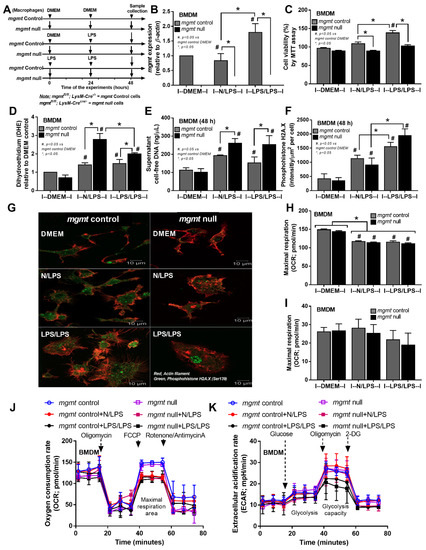
Figure 4.
The schema of the experiments in bone marrow-derived macrophages from mgmt control (mgmtfl/fl; LysM-Cre−/− and mgmt null (mgmtfl/fl; LysM-Crecre/−) mice after activation by lipopolysaccharide (LPS) in a single protocol (N/LPS), which started with the culture media followed by LPS 24 h later or LPS tolerance (LPS/LPS) by the two sequential LPS stimulations, or control (N/N), using the culture media incubation only (A). The characteristics of macrophages under these protocols, as indicated by the expression of mgmt (B), cell viability using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (C), reactive oxygen species with dihydroethidium stain (DHE) (D), supernatant cell-free DNA (E), the DNA damage score with the representative immunofluorescent pictures of phosphohistone H2A.X (a DNA break biomarker) (F,G), and the energy status of cells (extracellular flux analysis) (H–K). Independent triplicated experiments were performed. Mean ± SEM with one-way ANOVA followed by Tukey’s analysis was used. #, p ˂ 0.05 mgmt vs. control DMEM; *, p ˂ 0.05 between the indicated groups.
2.3. The mgmt Null Mice Demonstrated Less Pro-Inflammatory Cytokine Production in Both Single LPS Injection and LPS Tolerance
Because of the characteristics of mgmt-manipulated macrophages (Figure 3 and Figure 4), further experiments with mgmt littermate control (mgmtfl/fl; LysM-Cre−/−) and mgmt null mice (mgmtfl/fl; LysM-Crecre/−) were performed using a single LPS injection (N/LPS) and LPS tolerance (LPS/LPS) (Figure 5A). After a single LPS injection, serum cytokines (TNF-α, IL-6, but not IL-10) in the mgmt null mice were lower than in the LPS-administered mgmt control (Figure 5B–D), similar to the lower secreted cytokines in LPS-activated mgmt null macrophages compared to the control cells (Figure 3B–G). In LPS tolerance, the characteristics of lower serum cytokines in double LPS stimulation compared with only one LPS injection were demonstrated by all of these cytokines in the mgmt littermate control mice (open circles versus open square in Figure 5B–D). Meanwhile, this feature was demonstrated only by serum TNF-α (1 h of the protocol) and IL-10 (1 and 3 h of the protocol) in the mgmt null mice (blue circle versus red square in Figure 5B–D). Within the LPS/LPS stimulation (LPS tolerance), only serum IL-10 was lower in the mgmt null mice, with similar levels of other cytokines (TNF-α and IL-6) compared with the LPS/LPS mgmt control mice (open square versus red square in Figure 5B–D), suggesting a similar feature of LPS tolerance between these mouse strains.
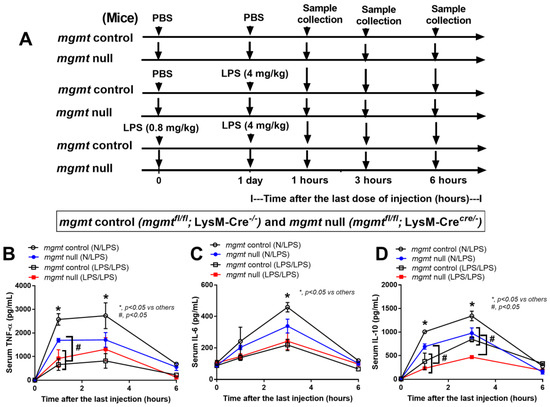
Figure 5.
Schematic workflow (A) demonstrates the experimental groups, including lipopolysaccharide (LPS) tolerance, started with LPS intraperitoneal (ip) injection (0.8 mg/kg) followed by LPS (4 mg/kg) (LPS/LPS); a single LPS stimulation, started with phosphate buffer solution (PBS) followed by LPS (4 mg/kg) (N/LPS), in mgmt control (mgmtfl/fl; LysM-Cre−/−) and Mgmt null (mgmtfl/fl; LysM-Crecre/−) mice as indicated by serum cytokines (TNF-α, IL-6, and IL-10) (B–D), are demonstrated (n = 5–7/group and time-point). Mean ± SEM with one-way ANOVA followed by Tukey’s analysis was used. *, p ˂ 0.05 between mgmt control versus others; #, p ˂ 0.05 between the indicated groups.
3. Discussion
3.1. Epigenetic Regulation of Macrophage Responses to LPS Is an Interesting Strategy in Immune Response Manipulation for Sepsis
Endotoxemia, the presence of lipopolysaccharide (LPS) in blood, can be found in several conditions, including sepsis, uremia, and obesity [57,58,59], mainly because of gut barrier damage [1,22,24,60] and Gram-negative bacteremia [1,61,62]. As one of the pathogen-associated molecular patterns (PAMPs), LPS stimulates all cells in the body, including macrophages, which are the major innate immune cells responsible for the recognition of foreign molecules [53,63]. In the macrophages, the responses to LPS with both a single LPS stimulation and LPS tolerance induced both DNA and histone modifications, as indicated by the RNA sequencing analysis (Figure 1D,E). Indeed, DNA methylation and acetylation at either the cytosine-phosphate-guanine (CpG) or non-CpG sites, and the histone alteration at the N-terminal tails, were critical regulators of gene expression through the chromatin structures [64]. Several key enzymes of epigenetic-induced DNA modification (methylation and acetylation) at the DNA promoter regions controlled the chromatin accessibility, which is well-known in cancer [65]; however, fewer data exist for sepsis [66]. Generally, DNA methylation is mostly the transfer of a methyl group to the C-5 position of the cytosine ring of DNA by DNA methyltransferase (DNMT) onto any cytosines of the genome, especially at the CpG regions [67]. Indeed, DNMT1 induces DNA methylation in macrophages, enhancing pro-inflammatory direction as the deletion of dnmt1 enhances anti-inflammatory macrophages [44]. Hence, the depletion of dnmt1 and methyl-binding domain3 (mbd3; the linkage of histone methylation to the regions of DNA methylation) [68] in the LPS-stimulated macrophages compared with the control might have been an adaptation to reduce the LPS-induced hyper-inflammation. In contrast, the enhanced dnmt1 in the LPS/LPS macrophages compared with the LPS macrophages (Figure 1D) might have served to increase the pro-inflammatory direction during the too-low cytokine production of LPS tolerance. Although methylation at the cytosine of CpG regions is common [69], methylation of guanine at the N-7 and O-6 positions of guanine, referred to as N7MeG (or 7-MG) and O6MeG, respectively, that are controlled by mettl1 (Methyltransferase 1, tRNA methyl-guanosine) and mgmt (O6-methylguanine-DNA methyltransferase), respectively, have also been described, especially for cancers and cell viability [70,71]. While mettl1 was not in the significant genes list from macrophages in this study, alteration of mgmt was observed in both the single and double LPS stimulations (Figure 1D). Despite very scarce data on MGMT in macrophages, MGMT in cancer cells has been characterized as an enzyme that removes the methyl group from O6MeG, which enhances the cell viability, and too little MGMT might cause cell injury from the blockage of DNA translation by O6MeG on the DNA [72]. Similar to dnmt1, the low mgmt at 24 h compared with the control (Figure 1D) might have been a self-adaptation of the LPS-activated macrophages to reduce pro-inflammatory cytokines through sustained DNA damage. However, DNA damage after LPS stimulation is possibly insufficient to reduce cytokine production. Meanwhile, increased mgmt in LPS/LPS macrophages compared with the LPS alone (Figure 1D) might have been aimed at neutralizing DNA damage to increase cytokine production, thus counteracting LPS tolerance. Similarly, increased mgmt in LPS tolerance seemed to be inadequate as cytokine production was still low, despite the possible reduction of DNA damage. Notably, mgmt was the only enzyme that was responsible for guanine methylation from the list of RNA sequencing analyses here. For histone modification in LPS-activated macrophages [73], some of the enzymes were different among the single LPS, LPS tolerance, and control groups. For example, ezh1 (Enhancer of zeste homolog1), an enzyme for the methylation of lysine on histones (H3K27Me) that inhibits the DNA reading [74], was down-regulated only in the single LPS stimulations, perhaps causing the hyper-responsiveness against LPS. In addition, rnf2 (Ring finger 2; the core subunit of polycomb repressor complex 1) was up-regulated only in LPS alone, perhaps to control hyper-inflammation [75], and the hdac1 (histone deacetylases1) [76] and kdm6b (lysine demethylase 6B) [77] enzymes responsible for the removal of acetyl and methyl groups from lysine, respectively, were up-regulated both in LPS alone and LPS tolerance (Figure 1E).
These data support the effect of DNA and histone modification in macrophages during LPS activation [78]. Because of (i) previous reports of DNA methylation (dnmt) and histone modification (ezh) on sepsis [43,73,79,80], (ii) possible enhanced cell injury from the presence of O6MeG due to the loss of mgmt for DNA repair [72], (iii) the availability of the MGMT inhibitor for oncotherapy [81] and its possible use in some chemotherapeutic strategies as a sepsis immune modulator [82], and (iv) epigenetic changes in LPS-activated macrophages and the reversal of LPS tolerance by the mgmt inhibitors [47,83], further tests on mgmt will be interesting. Theoretically, MGMT can cause less DNA methylation, leading to better cytokine production, which might correlate with more severe inflammation in single LPS and be beneficial in LPS tolerance. In contrast, MGMT blockage might reduce cytokines through enhanced DNA damage, which might be beneficial in hyper-inflammatory sepsis but harmful to LPS tolerance. Indeed, too few cytokines might be inadequate for proper inflammation for the microbial control process, thus leading to secondary infection [84,85,86].
3.2. Influence of mgmt Enzyme on Sepsis-Related Hyper-Inflammation and Immune Exhaustion
To test the effects of mgmt in sepsis, mgmt silencing by siRNA in RAW264.7 cells (a macrophage cell line) and bone marrow-derived macrophages from mgmt null mice (mgmtfl/fl; LysM-Crecre/−) were used. Both siRNA-deleted mgmt and mgmt null macrophages displayed anti-inflammatory effects as supernatant cytokines (TNF-α and IL-6), and genes of M1 pro-inflammatory macrophages (iNOS and IL-1β) in both mgmt-deleted cells with either a single LPS or LPS tolerance were similarly lower than the control (Figure 2 and Figure 3). There was a prominent anti-inflammatory state of LPS tolerance over the LPS alone in the control macrophages, as indicated by the lower cytokine responses (TNF-α and IL-6) and higher Arg-1 (an M2 macrophage polarization gene). However, mgmt deletion further directed LPS tolerance macrophages into a more prominent anti-inflammatory state as there were even lower inflammatory cytokines (TNF-α) (Figure 2B and Figure 3B) and higher Arg-1 (Figure 2G and Figure 3J) compared with LPS tolerance in control cells. These data suggest a possible anti-inflammatory effect of mgmt blockage; however, this might be harmful in LPS tolerance or sepsis-induced immune exhaustion. In mice, the characteristics of LPS tolerance, as indicated by lower serum cytokines in the double LPS injection compared with a single LPS administration, were observed in both littermate control (mgmtfl/fl; LysM-Cre−/−) and mgmt null (mgmtfl/fl; LysM-Crecre/−) mice.
Due to the influence of mgmt on DNA repair through the removal of O6MeG (methyl group at the sixth oxygen molecule on guanine) of DNA, the effects of mgmt deletion in both macrophages and mice, together with the altered mgmt in RNA sequencing analysis, indicate the possible DNA methylation in activated macrophages (Figure 1). Additionally, macrophage injury, including increased reactive oxygen species (ROS) and cell death after LPS-induced activation, might be partly due to DNA damage [87,88]. Mice with acute endotoxemia [21,89] or with chronic LPS elevation (a possible LPS tolerance) displayed increased spleen apoptosis [56] similar to the in vitro apoptosis of immune cells after LPS stimulation [90]. Although dihydroethidium (DHE; a representative ROS) and mitochondrial injury (reduced maximal respiratory capacity) was worse in the mgmt null and control macrophages after both types of activation (single and double LPS), DHE was more profound in the mgmt null groups, especially with LPS tolerance (Figure 4). Additionally, the possible higher cell injury in the mgmt null macrophages over the control also manifested through lower cell viability (MTT assay) in the mgmt null macrophages after both the single and double LPS stimulations. From the MTT assay, both LPS and LPS tolerance stimulated macrophages and increased cell proliferation in the control macrophages (more prominent in LPS tolerance) but not in the mgmt null macrophages, possibly due to the higher cell injury. Additionally, the higher supernatant cell-free DNA in activated mgmt null macrophages than the control, in either single or twice LPS stimulation, supported a possible susceptibility to LPS-induced cell injury in macrophages without MGMT enzyme. Because phosphorylation of the histone variant H2A.X is a key factor for DNA damage response to assembly of the DNA repair proteins at the chromatin damaged sites, immunofluorescence staining of phosphohistone H2A.X is frequently used to detect DNA damage [91]. Despite the detectable phosphohistone H2A.X in all groups of macrophages (control, N/LPS, and LPS/LPS), the intensity of the damage in N/LPS and LPS/LPS was higher than the control, indicating possible LPS-induced DNA damages that were similar in the mgmt null and control cells (Figure 4F,G). Although the loss of MGMT enzyme did not induce higher DNA damage, as determined by the intensity of the fluorescent color per cell, the high color intensity that was clearly located in the nuclei (the actual site of DNA repair) was demonstrated only in LPS/LPS mgmt null macrophages (Figure 4G). Because the positive phosphohistone H2A.X staining outside the nuclei might have been false positive fluorescence, other methods for DNA damage detection are needed for a solid conclusion.
Nevertheless, the maintenance of cell viability through DNA methylation at guanine seems to be important in the activated macrophages, despite more common methylation at the cytosine residues [67]. Although direct evaluation of O6MeG in macrophages after LPS or LPS tolerance was not performed here, the differences between mgmt null macrophages versus control after activation indirectly support the presence of O6MeG in macrophages. It is interesting to note that the standard method for the direct detection of O6MeG is still unclear [92,93], and the detection of a DNA break in immune cells might be different from the well-known protocol for DNA break detection of the parenchymal cells that is mostly studied in cancer topics [94]. In comparison with the control, the difference in cell viability, increased ROS, and elevated cell-free DNA, together with similar reduced cytokine production in stimulated macrophages with a lack of MGMT using mgmt null cells, indirectly support an influence of MGMT in LPS-activated macrophages. Although more experiments are required for an in-depth understanding of the mechanisms involved, our initial results support a possible extended use of an MGMT inhibitor (Lomeguatrib), a chemotherapeutic agent [95], on the attenuation of sepsis hyper-inflammation.
Furthermore, our data also support the idea that serum cytokines, in response to LPS injection (both a single or a double injection), are mainly produced from macrophages (Figure 5B–D), as previously reported [96], and that the signaling blockage only in macrophages, but not in other cells, might be an effective treatment with limited side effects. Due to lower levels of pro-inflammatory cytokines after the first dose of LPS injection, the severity of LPS tolerance, as indicated by the difference between the first and second dose of LPS in mgmt null mice, was lower than in the control mice. While the lower serum TNF-α was very obvious in the control mice with LPS/LPS compared with N/LPS, as expected from LPS tolerance, serum TNF-α in LPS/LPS mgmt null mice were not different from mgmt null mice with LPS alone. Although the mgmt depletion in the macrophages protected the mice from too high pro-inflammatory septic shock, the loss of mgmt induced too little pro-inflammatory cytokines with more prominent LPS tolerance, which could correlate with the enhanced susceptibility to secondary infection [97].
3.3. Clinical Aspects and Future Experiments
We hypothesized that activation by a single LPS dose or LPS tolerance would induce injury in macrophages, partly through DNA methylation and acetylation, which need enzymes, such as MGMT, to remove the methyl or acetyl groups in order to revitalize macrophages and maintain the regular cell functions (Figure 6). To continuously produce cytokines, the DNA methylation needed to be repaired and the failure of DNA repair caused a reduction in macrophage function, especially cytokine production (Figure 6). Thus, MGMT inhibition might be useful to attenuate sepsis-related hyperinflammation. Currently, the treatment of some cancers starts with an alkylating agent to induce DNA methylation at guanine (O6MeG), especially Temozolomide, and the combination with Lomeguatrib (an MGMT inhibitor) results in more cancer cell death, taking advantage of the high mutagenicity of O6MeG [95]. In sepsis, MGMT inhibitors might induce injury in the regular cells and short-term administration, and/or the MGMT blockage specifically focused on macrophages might be theoretically better. Notably, good microbial control, especially by effective antibiotics, is necessary for all of the immune-mediated adjunctive therapies in sepsis, and determination of the immune directions is possibly needed. Theoretically, sepsis immune responses are crudely divided into hyper-inflammation and immune exhaustion by several biomarkers. For example, high serum IL-6 and IL-1 might be biomarkers for sepsis-related hyper-inflammation [98,99], while down-regulated HLA-DR and viral reactivation (cytomegalovirus; the common dormant virus in the human host) possibly indicate sepsis-related immune exhaustion [100,101]. Thus, immune monitoring in sepsis is needed for the use of MGMT inhibitors, and down-regulated HLA-DR and lower inflammatory cytokines might be a contraindication because the overwhelming inhibition might escalate infection susceptibility. Because LPS response and LPS tolerance are only a subset of sepsis-related immune responses [20], further evaluations of the effect of MGMT in sepsis are warranted.
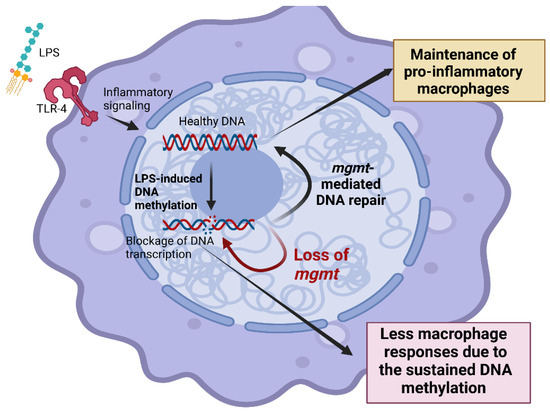
Figure 6.
The proposed working hypothesis demonstrated the impact of O6-methylguanine-DNA methyltransferase (MGMT) in responses against lipopolysaccharide (LPS) of macrophages. LPS activates inflammatory responses through Toll-like receptor 4 (TLR-4), which causes methylation in several areas of DNA, including O6-methylguanine (O6MeG). DNA methylation impairs DNA transcription and induces programmed cell death, especially apoptosis [100]. The MGMT enzyme, referred to as “a DNA suicide repair enzyme”, transfers the methyl group at the O6 site of guanine to the cysteine residues of MGMT, allowing macrophages to maintain their functions (yellow-colored box). Without MGMT or using an MGMT inhibitor, there might be an impairment of macrophage cytokine production (red-colored box) that is beneficial in hyper-inflammatory sepsis. This figure was created by BioRender.com.
Finally, there are several limitations in our study that should be mentioned. First, supportive information on the transcriptome results, especially from the Western blot analysis, was not performed. Second, the impacts of the MGMT overexpression in macrophages with LPS stimulation were not examined. Third, the direct detection of Q6MeG on macrophages was not conducted here, partly due to the unclear standard proper methods of O6MeG detection [92,93]. Fourth, additional DNA damage detection methods are necessary for the determination of DNA damage. Nevertheless, an initial proof of concept on the impacts of MGMT enzyme in LPS-stimulated macrophages is presented here, which indicates several more interesting experiments on the topic. Overall, we conclude that MGMT inhibitors, an available adjunctive therapy for malignancy, might be beneficial in some situations of sepsis. More studies are warranted.
4. Materials and Methods
4.1. Small Interfering RNA (siRNA) in the Macrophage Cell Line
Murine macrophage-like cells (RAW264.7; TIB-71) (American Type Culture Collection; ATCC, Manassas, VA, USA) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) in a humidified incubator at 37 °C with 5% CO2. The small interfering RNA (siRNA) silencing of mgmt was then performed using RAW264.7 at 106 cells/mL seeded into 6-well plates with the siRNA for mgmt (DharmaconTM AccellTM, Horizon Discovery, Watwebeach, UK) in siRNA buffer, following a previous publication [73]. Briefly, the siRNA at 1 μM per well was incubated at 37 °C with 5% CO2 for 48 h. The non-targeting pool siRNA (DharmaconTM) was used as a control. Macrophages with mgmt-siRNA and non-siRNA (non-targeting pool siRNA) were activated by three different protocols for a proper comparison. First, the single lipopolysaccharide (LPS) stimulation protocol began with DMEM, followed by LPS (Escherichia coli 026: B6; Sigma-Aldrich, Waltham, MA, USA) (100 ng/mL) 24 h later (N/LPS). Second, the LPS tolerance protocol used two sequential stimulations of 100 ng/mL LPS at 24 h and 48 h (LPS/LPS) in the experiments. Third, the control protocol (N/N) was performed by the incubation in twice-changed DMEM only. After another 24 h of each protocol, the sample collection (supernatant and cells) was then performed and supernatant cytokines (TNF-α, IL-6, and IL-10) were evaluated by ELISA (Invitrogen, Carlsbad, CA, USA). Meanwhile, the gene expression was evaluated by quantitative real-time polymerase chain reaction (PCR), as previously described [102,103,104,105,106]. In brief, the PCR started with RNA extraction from the cells with TRIzol Reagent (Invitrogen), together with RNeasy Mini Kit (Qiagen, Hilden, Germany); then 1 mg of total RNA was used for cDNA synthesis with iScript reverse transcription super-mix (Bio-Rad, Hercules, CA, USA). Quantitative real-time PCR was performed on a QuantStudio 6 real-time PCR system (Thermo Fisher Scientific, San Jose, CA, USA) using SsoAdvance Universal SYBR Green Super-mix (Bio-Rad). The gene expression was normalized to beta-actin (β-actin; an endogenous housekeeping gene) and the fold change was calculated by the ∆∆Ct method. The primers used in this study are listed in Table 1.

Table 1.
Lists of primers used in the study.
4.2. The Transcriptome Analysis
The RNA sequencing analysis in WT macrophages was performed to determine epigenetic alterations in macrophages with LPS stimulations (a single LPS stimulation and LPS tolerance). Bone marrow-derived macrophages (BMDM) were prepared from the femurs of mice using supplemented Dulbecco’s Modified Eagle’s Medium (DMEM) with a 20% conditioned medium of the L929 cells (ATCC CCL-1), as previously described [53,54,56,106]. Macrophages at 5 × 104 cells/well in supplemented DMEM (Thermo Fisher Scientific) were incubated in 5% carbon dioxide (CO2) at 37 °C for 24 h before being treated by 3 experimental protocols, as mentioned above. The RNA from macrophages extracted by a RNeasy mini kit (Qiagen) was then processed with the RNA sequencing (BGISEQ-50) platform, as previously published [107]. The mRNA analysis was conducted based on triplicate macrophage samples. The FastQC was used to determine the sequencing quality. The raw sequencing reads were mapped and aligned against Mus musculus reference genome GRCm39 using STAR [108], followed by gene quantification against the reference mouse transcriptome by Kallisto [109]. Read counts were normalized and analyzed (differentially expressed genes; DEGs) using the edgeR [110] and limma-voom packages [111,112]. Genes were considered significant differences (p-value < 0.05) when the log2 value of fold change level was less than −2 or greater than 2, indicating down- or up-regulation, respectively. The DEGs clustering was performed based on Euclidean distance and the Ward.D2 method with the ComplexHeatmap package [113], and the log2 expression (TPM; transcript count per million) of selected epigenetic-related genes [114] was compared to determine statistical significance using the Wilcoxon test in the ggpubr package [115]. A p-value less than 0.05 indicated statistical significance.
4.3. The Intro Experiments
Bone marrow-derived macrophage from mgmt control (mgmtfl/fl; LysM-Cre−/−) or mgmt null (mgmtfl/fl; LysM-Crecre/−) mice were extracted from mouse femurs before activation by several protocols (N/LPS, LPS/LPS, or N/N). The supernatant cytokines (TNF-α, IL-6, and IL-10) and gene expression were measured by ELISA and PCR, as mentioned above. Because of the influence of cell viability and reactive oxygen species (ROS) in cell injury, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay and dihydroethidium (DHE) were measured according to the published protocols [51,58,116]. For MTT, the activated cells were incubated with 0.5 mg/mL of MTT solution (Thermo Fisher Scientific) for 2 h at 37 °C in the dark, before MTT removal with the dilution with dimethyl sulfoxide (DMSO), and measured with a Varioskan Flash microplate reader at an absorbance of optical density at 570 nm. Additionally, DHE (Sigma-Aldrich) at 20 µM was incubated in the activated macrophages for 20 min at 37 °C before DHE measurement, and the fluorescence readings were analyzed at 520 nm by a Varioskan Flash microplate reader and presented by the fluorescence arbitrary unit. Supernatant cell-free DNA was detected by Quanti PicoGreen assay (Sigma-Aldrich). For DNA break determination, macrophages at 3 × 106 cells were seeded on glass-bottomed 6-well plates before activation (N/LPS, LPS/LPS, or N/N). The cells were then fixed with 4% paraformaldehyde in Tris Buffered Saline (TBS) for 15 min, permeabilized with 0.1% triton X-100, and subsequently washed three times in 1X TBS with 0.05% Tween-20. The fixed samples were blocked with 2% bovine serum albumin in 1X TBS for 1 h at room temperature and then incubated overnight at 4 °C with phospho-histone H2A.X (Ser139) (20E3) rabbit mAb (Cell signaling). Proteins were visualized using goat anti-mouse IgG H&L tagged Alexa Flour 488 (Abcam; ab150113) (green) and actin filaments were labeled with DY-554 phalloidin (red); the fluorescent intensity per cell was evaluated by confocal laser scanning microscope with the ZEN 3.0 software (CLSM, Zeiss, Germany) at 630× magnification in 10 randomly selected fields per slide.
4.4. Extracellular Flux Analysis
For the cell energy status (extracellular flux analysis), Seahorse XFp Analyzers (Agilent, Santa Clara, CA, USA) were used. As such, the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were used to represent mitochondrial function (respiration) and glycolysis activity, respectively, following previous publications [23,55,107,117,118]. In brief, the activated macrophages (1 × 105 cells/well) were incubated in the Seahorse media (DMEM complemented with glucose, pyruvate, and l-glutamine) (Agilent, 103575–100) before activation by different metabolic interference compounds of the protocol, including oligomycin, carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP), and rotenone/antimycin A for OCR evaluation, or glucose, oligomycin, and 2-Deoxy-d-glucose (2-DG) for ECAR measurement. The maximal respiration and maximal glycolysis capacity were calculated by Seahorse Wave 2.6 software.
4.5. Animal and Animal Model
Protocol No. 017/2562 was approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, according to National Institutes of Health (NIH) criteria. For the transcriptomic analysis, macrophages were prepared from the long bone (femurs and tibias) of 8-week-old male wild-type (WT) C57BL/6 mice purchased from Nomura Siam (Pathumwan, Bangkok, Thailand). In parallel, mgmtflox/flox and LyM-CreCre/Cre mice were purchased from RIKEN BRC Experimental Animal Division (Ibaraki, Japan) and cross-bred to produce mgmt littermate control (mgmtfl/fl; LysM-Cre−/−) or mgmt null (mgmtfl/fl; LysM-Crecre/−) mice in F3 of the breeding protocol. As such, the mgmtflox/flox mice with the loxP sites were bred with LysM-CreCre/Cre mice. The mice with a Cre recombinase under the control of lysozyme M were used to target mgmt for mgmt deletion only in the myeloid cells, including macrophages and neutrophils. The offspring were either mgmtflox/flox with no LysM-Cre (mgmtfl/fl; LysM-Cre−/−), which were categorized as the littermate controls or mgmt control. Meanwhile, the mice that were positive for the Cre driver were mgmt null (mgmtfl/fl; LysM-Crecre/−) mice with a lack of MGMT enzyme. The conditional targeted Cre positive mice (mgmt null) were age- and gender-matched with the floxed/floxed littermate controls (mgmt control) using the male mice aged 8–10 weeks old. To genotype these mice on the loxP sites insertion, the following primers were used: (i) LysM-cre primer; F: 5′-GAACGCACTGATTTCGACCA-3′, R: 5′-GCTAACCAGCGTTTTCGTTC-3′, (ii) mgmt-loxP primer F; 5′-TGGGCTTCAAATCAAGGAACAGAA-3′, R: 5′-AACTATCCTGCTCACTCTCTGTAG-3′, and (iii) Cre recombination (for Cre activity); F: 5′-GGTGTGGATCCCAAGAAATTGAAG-3′, R: 5′-TGTTCAAGAGTGACACACAGTCA-3′ [73]. The mice homozygous for the flox were selected and genotyped for the expression of LysM-Cre using the primers: F: 5′-CTTGGGCTGCCAGAATTCTC-3′, R: 5′-CCCAGAAATGCCAGATTACG-3′. The mice were divided into 3 protocols similar to the in vitro experiments. As such, the LPS tolerance (LPS/LPS) was performed by an intraperitoneal injection of 0.8 mg/kg LPS (Escherichia coli 026:B6) (Sigma-Aldrich, St. Louis, MO, USA), with an additional dose of 4 mg/kg LPS 24 h later. In parallel, the single LPS stimulation was conducted by an intraperitoneal injection of phosphate buffer solution (PBS), followed by LPS (4 mg/kg) 24 h later. For the control (N/N) protocol, twice intraperitoneal PBS injection with a 24 h duration between the doses was performed. Following these protocols, blood was collected through tail vein nicking at 1 and 3 h afterward and mice were sacrificed with cardiac puncture under isoflurane anesthesia with blood collection at 6 h after the last injection. Serum cytokines were evaluated by ELISA (Invitrogen). The mouse genotype data are demonstrated in Figure 7.
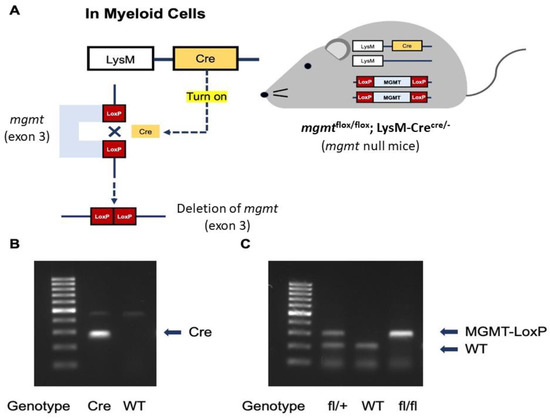
Figure 7.
The diagram demonstrates the necessary of Cre to activate LoxP for the deletion of mgmt gene (exon 3) only in the myeloid cells of mgmt null mice (A), and the representative genotype to identify mgmt null mice (mgmtflox/flox; LysM-Crecre/−) with the identified bands of Cre and MGMT-LoxP for the flox/flox (fl/fl) group (B,C) are demonstrated.
4.6. Statistical Analysis
All the data were analyzed with GraphPad Prism6 and shown as mean ± S.E.M (standard error). One-way analysis of variance (ANOVA) with Tukey’s comparison test was used and a p-value less than 0.05 was considered significant.
5. Conclusions
The alteration of several enzymes of epigenetic processes for DNA and histone modifications after single LPS activation and LPS tolerance was demonstrated in wild-type macrophages. Reduced pro-inflammatory cytokines with a single LPS stimulation and more severe LPS tolerance (lower supernatant cytokines with a second dose of LPS) in mgmt null (mgmtfl/fl; LysM-Crecre/−) macrophages and mice compared with the control groups (mgmtfl/fl; LysM-Cre−/−) supported the conclusion regarding the possible benefits and limitations of MGMT blockage in hyper-inflammation and LPS tolerance, respectively. Hence, the use of MGMT blockage which is an available drug in cancer therapy is proposed to attenuate severe inflammation in sepsis. More studies are warranted.
Author Contributions
Conceptualization, A.L. and T.P.; methodology, W.S. and A.L.; formal analysis, W.S., P.P. and P.V.; investigation, J.I.-A., J.M., A.B., K.S.-k. and S.B.; resources, A.L., T.P. and A.N.-L.; data curation W.S., A.N.-L. and A.L.; writing—original draft preparation, W.S. and A.L.; writing—review and editing T.P. and A.L.; supervision, A.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded the Thai government and Chulalongkorn University through the Program Management Unit for Human Resources, Institutional Development, Research, and Innovation (B16F640175) with the Rachadapisek Sompote Matching Fund (RA-MF-22/65 and RA-MF-13/66), the Thailand Science Research and Innovation Fund, Chulalongkorn University (HEA663000017 and CU_FRB65_hea (62)_125_23_55), and the National Research Council of Thailand (NRCT-N41A640076 and NRCT-N34A660583). This research has been supported in part by the Intramural Research Program of NIAID, NIH. TP was funded by the National Research Council of Thailand (811/2563). JM was supported by the Second Century Fund (C2F) for postdoctoral fellowship, Chulalongkorn University. WS was supported by the Second Century Fund (C2F) for high-efficiency Ph.D. candidates, Chulalongkorn University.
Institutional Review Board Statement
The study was conducted in accordance with the National Institutes of Health (NIH) criteria, and the animal study protocol was approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (CU-ACUP No. 021/2562) and Chulalongkorn University Laboratory Animal Center (CU-LAC) with the approving protocol No. 2073016.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amornphimoltham, P.; Yuen, P.S.T.; Star, R.A.S.; Leelahavanichkul, A. Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis. Dig. Dis. Sci. 2019, 64, 2416–2428. [Google Scholar] [CrossRef]
- Lacal, I.; Ventura, R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Jia, L.; Lv, W.; Wang, L.; Cui, W. Epigenetic Enzyme Mutations: Role in Tumorigenesis and Molecular Inhibitors. Front. Oncol. 2019, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef]
- Liu, D.; Huang, S.-Y.; Sun, J.-H.; Zhang, H.-C.; Cai, Q.-L.; Gao, C.; Li, L.; Cao, J.; Xu, F.; Zhou, Y.; et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Ward, P.A. Sepsis-Induced Immune Dysfunction: Can Immune Therapies Reduce Mortality? J. Clin. Investig. 2016, 126, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.; Horie, S.; Laffey, J.G. Role of the Adaptive Immune Response in Sepsis. Intensive Care Med. Exp. 2020, 8 (Suppl. S1), 20. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Yasuda, H.; Doi, K.; Hu, X.; Zhou, H.; Yuen, P.S.; Star, R.A. Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am. J. Physiol. Renal Physiol. 2008, 295, F1825–F1835. [Google Scholar] [CrossRef] [PubMed]
- Taratummarat, S.; Sangphech, N.; Vu, C.T.B.; Palaga, T.; Ondee, T.; Surawut, S.; Sereemaspun, A.; Ritprajak, P.; Leelahavanichkul, A. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018, 18, 85. [Google Scholar] [CrossRef]
- Panpetch, W.; Chancharoenthana, W.; Bootdee, K.; Nilgate, S.; Finkelman, M.; Tumwasorn, S.; Leelahavanichkul, A. Lactobacillus rhamnosus L34 Attenuates Gut Translocation-Induced Bacterial Sepsis in Murine Models of Leaky Gut. Infect. Immun. 2018, 86, e00700-17. [Google Scholar] [CrossRef] [PubMed]
- Issara-Amphorn, J.; Chancharoenthana, W.; Visitchanakun, P.; Leelahavanichkul, A. Syk Inhibitor Attenuates Polymicrobial Sepsis in FcgRIIb-Deficient Lupus Mouse Model, the Impact of Lupus Characteristics in Sepsis. J. Innate Immun. 2020, 12, 461–479. [Google Scholar] [CrossRef]
- Dang, C.P.; Leelahavanichkul, A. Over-expression of miR-223 induces M2 macrophage through glycolysis alteration and attenuates LPS-induced sepsis mouse model, the cell-based therapy in sepsis. PLoS ONE 2020, 15, e0236038. [Google Scholar] [CrossRef] [PubMed]
- Chancharoenthana, W.; Udompronpitak, K.; Manochantr, Y.; Kantagowit, P.; Kaewkanha, P.; Issara-Amphorn, J.; Leelahavanichkul, A. Repurposing of High-Dose Erythropoietin as a Potential Drug Attenuates Sepsis in Preconditioning Renal Injury. Cells 2021, 10, 3133. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.P.; Issara-Amphorn, J.; Charoensappakit, A.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Saisorn, W.; Sae-Khow, K.; Leelahavanichkul, A. BAM15, a Mitochondrial Uncoupling Agent, Attenuates Inflammation in the LPS Injection Mouse Model: An Adjunctive Anti-Inflammation on Macrophages and Hepatocytes. J. Innate Immun. 2021, 13, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Perner, A.; Rhodes, A.; Venkatesh, B.; Angus, D.C.; Martin-Loeches, I.; Preiser, J.C.; Vincent, J.L.; Marshall, J.; Reinhart, K.; Joannidis, M.; et al. Sepsis: Frontiers in supportive care, organisation and research. Intensive Care Med. 2017, 43, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Mithal, L.B.; Arshad, M.; Swigart, L.R.; Khanolkar, A.; Ahmed, A.; Coates, B.M. Mechanisms and modulation of sepsis-induced immune dysfunction in children. Pediatr. Res. 2022, 91, 447–453. [Google Scholar] [CrossRef]
- Schrijver, I.T.; Théroude, C.; Roger, T. Myeloid-Derived Suppressor Cells in Sepsis. Front. Immunol. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Ma, T.; Chai, Y.F.; Shou, S.T. The role of regulatory T cells in immune dysfunction during sepsis. World J. Emerg. Med. 2015, 6, 5–9. [Google Scholar] [CrossRef]
- Vergadi, E.; Vaporidi, K.; Tsatsanis, C. Regulation of Endotoxin Tolerance and Compensatory Anti-inflammatory Response Syndrome by Non-coding RNAs. Front. Immunol. 2018, 9, 2705. [Google Scholar] [CrossRef] [PubMed]
- Chancharoenthana, W.; Sutnu, N.; Visitchanakun, P.; Sawaswong, V.; Chitcharoen, S.; Payungporn, S.; Schuetz, A.; Schultz, M.J.; Leelahavanichkul, A. Critical roles of sepsis-reshaped fecal virota in attenuating sepsis severity. Front. Immunol. 2022, 13, 940935. [Google Scholar] [CrossRef]
- Chancharoenthana, W.; Kamolratanakul, S.; Ariyanon, W.; Thanachartwet, V.; Phumratanaprapin, W.; Wilairatana, P.; Leelahavanichkul, A. Abnormal Blood Bacteriome, Gut Dysbiosis, and Progression to Severe Dengue Disease. Front. Cell Infect. Microbiol. 2022, 12, 890817. [Google Scholar] [CrossRef] [PubMed]
- Hiengrach, P.; Visitchanakun, P.; Tongchairawewat, P.; Tangsirisatian, P.; Jungteerapanich, T.; Ritprajak, P.; Wannigama, D.L.; Tangtanatakul, P.; Leelahavanichkul, A. Sepsis Encephalopathy Is Partly Mediated by miR370-3p-Induced Mitochondrial Injury but Attenuated by BAM15 in Cecal Ligation and Puncture Sepsis Male Mice. Int. J. Mol. Sci. 2022, 23, 5445. [Google Scholar] [CrossRef] [PubMed]
- Thim-Uam, A.; Makjaroen, J.; Issara-Amphorn, J.; Saisorn, W.; Wannigama, D.L.; Chancharoenthana, W.; Leelahavanichkul, A. Enhanced Bacteremia in Dextran Sulfate-Induced Colitis in Splenectomy Mice Correlates with Gut Dysbiosis and LPS Tolerance. Int. J. Mol. Sci. 2022, 23, 1676. [Google Scholar] [CrossRef]
- Ondee, T.; Surawut, S.; Taratummarat, S.; Hirankarn, N.; Palaga, T.; Pisitkun, P.; Pisitkun, T.; Leelahavanichkul, A. Fc Gamma Receptor IIB Deficient Mice: A Lupus Model with Increased Endotoxin Tolerance-Related Sepsis Susceptibility. Shock 2017, 47, 743–752. [Google Scholar] [CrossRef]
- Seeley, J.J.; Ghosh, S. Molecular Mechanisms of Innate Memory and Tolerance to Lps. J. Leukoc. Biol. 2017, 101, 107–119. [Google Scholar] [CrossRef]
- Gillen, J.; Ondee, T.; Gurusamy, D.; Issara-Amphorn, J.; Manes, N.P.; Yoon, S.H.; Leelahavanichkul, A.; Nita-Lazar, A. Lps Tolerance Inhibits Cellular Respiration and Induces Global Changes in the Macrophage Secretome. Biomolecules 2021, 11, 164. [Google Scholar] [CrossRef]
- López-Collazo, E.; del Fresno, C. Pathophysiology of Endotoxin Tolerance: Mechanisms and Clinical Consequences. Crit. Care 2013, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Naler, L.B.; Hsieh, Y.P.; Geng, S.; Zhou, Z.; Li, L.; Lu, C. Epigenomic and Transcriptomic Analyses Reveal Differences between Low-Grade Inflammation and Severe Exhaustion in Lps-Challenged Murine Monocytes. Commun. Biol. 2022, 5, 102. [Google Scholar] [CrossRef]
- Koos, B.; Moderegger, E.L.; Rump, K.; Nowak, H.; Willemsen, K.; Holtkamp, C.; Thon, P.; Adamzik, M.; Rahmel, T. LPS-Induced Endotoxemia Evokes Epigenetic Alterations in Mitochondrial DNA that Impacts Inflammatory Response. Cells 2020, 9, 2282. [Google Scholar] [CrossRef] [PubMed]
- Casorelli, I.; Russo, M.T.; Bignami, M. Role of Mismatch Repair and Mgmt in Response to Anticancer Therapies. Anticancer Agents Med. Chem. 2008, 8, 368–380. [Google Scholar] [CrossRef]
- Sharma, S.; Salehi, F.; Scheithauer, B.W.; Rotondo, F.; Syro, L.V.; Kovacs, K. Role of Mgmt in Tumor Development, Progression, Diagnosis, Treatment and Prognosis. Anticancer Res. 2009, 29, 3759–3768. [Google Scholar]
- Rye, P.T.; Delaney, J.C.; Netirojjanakul, C.; Sun, D.X.; Liu, J.Z.; Essigmann, J.M. Mismatch Repair Proteins Collaborate with Methyltransferases in the Repair of O(6)-Methylguanine. DNA Repair 2008, 7, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Drablos, F.; Feyzi, E.; Aas, P.A.; Vaagbo, C.B.; Kavli, B.; Bratlie, M.S.; Pena-Diaz, J.; Otterlei, M.; Slupphaug, G.; Krokan, H.E. Alkylation Damage in DNA and Rna—Repair Mechanisms and Medical Significance. DNA Repair 2004, 3, 1389–1407. [Google Scholar] [CrossRef] [PubMed]
- Klapacz, J.; Pottenger, L.H.; Engelward, B.P.; Heinen, C.D.; Johnson, G.E.; Clewell, R.A.; Carmichael, P.L.; Adeleye, Y.; Andersen, M.E. Contributions of DNA Repair and Damage Response Pathways to the Non-Linear Genotoxic Responses of Alkylating Agents. Mutat. Res. Rev. Mutat. Res. 2016, 767, 77–91. [Google Scholar] [CrossRef]
- Warren, J.J.; Forsberg, L.J.; Beese, L.S. The Structural Basis for the Mutagenicity of O(6)-Methyl-Guanine Lesions. Proc. Natl. Acad. Sci. USA 2006, 103, 19701–19706. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-Induced DNA Damage, Mutations and Cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef]
- Ezerskyte, M.; Paredes, J.A.; Malvezzi, S.; Burns, J.A.; Margison, G.P.; Olsson, M.; Scicchitano, D.A.; Dreij, K. O(6)-Methylguanine-Induced Transcriptional Mutagenesis Reduces P53 Tumor-Suppressor Function. Proc. Natl. Acad. Sci. USA 2018, 115, 4731–4736. [Google Scholar] [CrossRef] [PubMed]
- Christmann, M.; Verbeek, B.; Roos, W.P.; Kaina, B. O(6)-Methylguanine-DNA Methyltransferase (Mgmt) in Normal Tissues and Tumors: Enzyme Activity, Promoter Methylation and Immunohistochemistry. Biochim. Biophys. Acta 2011, 1816, 179–190. [Google Scholar] [CrossRef]
- Kirkham, P. Oxidative Stress and Macrophage Function: A Failure to Resolve the Inflammatory Response. Biochem. Soc. Trans. 2007, 35, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Wei, W.; Liu, L. Regulation of DNA Repair by S-Nitrosylation. Biochim. Biophys. Acta 2012, 1820, 730–735. [Google Scholar] [CrossRef]
- Colonna, M. DNA Damage Response Impacts Macrophage Functions. Blood 2015, 126, 2440–2442. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, T.; Lang, X.; Jia, S.; Yang, Y.; Zhu, C.; Wang, Y.; Feng, S.; Wang, C.; Zhang, P.; et al. Inhibiting DNA Methylation Improves Survival in Severe Sepsis by Regulating Nf-Kappab Pathway. Front. Immunol. 2020, 11, 1360. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Q.; Yu, L.; Shi, H.; Xue, B.; Shi, H. Epigenetic Regulation of Macrophage Polarization and Inflammation by DNA Methylation in Obesity. JCI Insight 2016, 1, e87748. [Google Scholar] [CrossRef]
- Khan, O.A.; Ranson, M.; Michael, M.; Olver, I.; Levitt, N.C.; Mortimer, P.; Watson, A.J.; Margison, G.P.; Midgley, R.; Middleton, M.R. A Phase II Trial of Lomeguatrib and Temozolomide in Metastatic Colorectal Cancer. Br. J. Cancer 2008, 98, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Rumienczyk, I.; Kulecka, M.; Statkiewicz, M.; Ostrowski, J.; Mikula, M. Oncology Drug Repurposing for Sepsis Treatment. Biomedicines 2022, 10, 921. [Google Scholar] [CrossRef]
- Benjaskulluecha, S.; Boonmee, A.; Pattarakankul, T.; Wongprom, B.; Klomsing, J.; Palaga, T. Screening of compounds to identify novel epigenetic regulatory factors that affect innate immune memory in macrophages. Sci. Rep. 2022, 12, 1912. [Google Scholar] [CrossRef]
- Pegg, A.E. Multifaceted Roles of Alkyltransferase and Related Proteins in DNA Repair, DNA Damage, Resistance to Chemotherapy, and Research Tools. Chem. Res. Toxicol. 2011, 24, 618–639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef]
- Robertson, K.D.; Ait-Si-Ali, S.; Yokochi, T.; Wade, P.A.; Jones, P.L.; Wolffe, A.P. Dnmt1 Forms a Complex with Rb, E2f1 and Hdac1 and Represses Transcription from E2f-Responsive Promoters. Nat. Genet. 2000, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Jaroonwitchawan, T.; Visitchanakun, P.; Dang, P.C.; Ritprajak, P.; Palaga, T.; Leelahavanichkul, A. Dysregulation of Lipid Metabolism in Macrophages Is Responsible for Severe Endotoxin Tolerance in FcgRIIB-Deficient Lupus Mice. Front. Immunol. 2020, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Ondee, T.; Jaroonwitchawan, T.; Pisitkun, T.; Gillen, J.; Nita-Lazar, A.; Leelahavanichkul, A.; Somparn, P. Decreased Protein Kinase C-beta Type II Associated with the Prominent Endotoxin Exhaustion in the Macrophage of FcGRIIb−/− Lupus Prone Mice is Revealed by Phosphoproteomic Analysis. Int. J. Mol. Sci. 2019, 20, 1354. [Google Scholar] [CrossRef]
- Binmama, S.; Dang, C.P.; Visitchanakun, P.; Hiengrach, P.; Somboonna, N.; Cheibchalard, T.; Pisitkun, P.; Chindamporn, A.; Leelahavanichkul, A. Beta-Glucan from S. cerevisiae Protected AOM-Induced Colon Cancer in cGAS-Deficient Mice Partly through Dectin-1-Manipulated Macrophage Cell Energy. Int. J. Mol. Sci. 2022, 23, 10951. [Google Scholar] [CrossRef]
- Hiengrach, P.; Visitchanakun, P.; Finkelman, M.A.; Chancharoenthana, W.; Leelahavanichkul, A. More Prominent Inflammatory Response to Pachyman than to Whole-Glucan Particle and Oat-β-Glucans in Dextran Sulfate-Induced Mucositis Mice and Mouse Injection through Proinflammatory Macrophages. Int. J. Mol. Sci. 2022, 23, 4026. [Google Scholar] [CrossRef]
- Makjaroen, J.; Thim-Uam, A.; Dang, C.P.; Pisitkun, T.; Somparn, P.; Leelahavanichkul, A. A Comparison Between 1 Day versus 7 Days of Sepsis in Mice with the Experiments on LPS-Activated Macrophages Support the Use of Intravenous Immunoglobulin for Sepsis Attenuation. J. Inflamm. Res. 2021, 14, 7243–7263. [Google Scholar] [CrossRef]
- Udompornpitak, K.; Charoensappakit, A.; Sae-Khow, K.; Bhunyakarnjanarat, T.; Dang, C.P.; Saisorn, W.; Visitchanakun, P.; Phuengmaung, P.; Palaga, T.; Ritprajak, P.; et al. Obesity Exacerbates Lupus Activity in Fc Gamma Receptor IIb Deficient Lupus Mice Partly through Saturated Fatty Acid-Induced Gut Barrier Defect and Systemic Inflammation. J. Innate Immun. 2022, 15, 240–261. [Google Scholar] [CrossRef]
- Tungsanga, S.; Panpetch, W.; Bhunyakarnjanarat, T.; Udompornpitak, K.; Katavetin, P.; Chancharoenthana, W.; Chatthanathon, P.; Somboonna, N.; Tungsanga, K.; Tumwasorn, S.; et al. Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and β-d-Glucan, but Is Attenuated by Lacticaseibacillus rhamnosus L34. Int. J. Mol. Sci. 2022, 23, 2511. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Panpetch, W.; Saisorn, W.; Chatthanathon, P.; Wannigama Dhammika, L.; Thim-uam, A.; Svasti, S.; Fucharoen, S.; Somboonna, N.; Leelahavanichkul, A. Increased susceptibility to dextran sulfate-induced mucositis of iron-overload β-thalassemia mice, another endogenous cause of septicemia in thalassemia. Clin. Sci. 2021, 135, 1467–1486. [Google Scholar] [CrossRef] [PubMed]
- Boonhai, S.; Bootdee, K.; Saisorn, W.; Takkavatakarn, K.; Sitticharoenchai, P.; Tungsanga, S.; Tiranathanagul, K.; Leelahavanichkul, A. TMAO reductase, a biomarker for gut permeability defect induced inflammation, in mouse model of chronic kidney disease and dextran sulfate solution-induced mucositis. Asian Pac. J. Allergy Immunol. 2021. [Google Scholar] [CrossRef]
- Hiengrach, P.; Panpetch, W.; Chindamporn, A.; Leelahavanichkul, A. Macrophage depletion alters bacterial gut microbiota partly through fungal overgrowth in feces that worsens cecal ligation and puncture sepsis mice. Sci. Rep. 2022, 12, 9345. [Google Scholar] [CrossRef] [PubMed]
- Sae-Khow, K.; Charoensappakit, A.; Chiewchengchol, D.; Leelahavanichkul, A. High-Dose Intravenous Ascorbate in Sepsis, a Pro-Oxidant Enhanced Microbicidal Activity and the Effect on Neutrophil Functions. Biomedicines 2022, 11, 51. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef] [PubMed]
- Charoensappakit, A.; Sae-Khow, K.; Leelahavanichkul, A. Gut Barrier Damage and Gut Translocation of Pathogen Molecules in Lupus, an Impact of Innate Immunity (Macrophages and Neutrophils) in Autoimmune Disease. Int. J. Mol. Sci. 2022, 23, 8223. [Google Scholar] [CrossRef] [PubMed]
- Ezponda, T.; Licht, J.D. Molecular Pathways: Deregulation of Histone H3 Lysine 27 Methylation in Cancer-Different Paths, Same Destination. Clin. Cancer Res. 2014, 20, 5001–5008. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.N.; Dupere-Richer, D.; Ezponda, T.; Licht, J.D.; Miller, W.H., Jr. H3k27 Methylation: A Focal Point of Epigenetic Deregulation in Cancer. Adv. Cancer Res. 2016, 131, 59–95. [Google Scholar] [CrossRef]
- Yue, D.; Wang, Z.; Yang, Y.; Hu, Z.; Luo, G.; Wang, F. EZH2 inhibitor GSK343 inhibits sepsis-induced intestinal disorders. Exp. Ther. Med. 2021, 21, 437. [Google Scholar] [CrossRef]
- Jin, B.; Li, Y.; Robertson, K.D. DNA Methylation: Superior or Subordinate in the Epigenetic Hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef]
- Du, Q.; Luu, P.L.; Stirzaker, C.; Clark, S.J. Methyl-Cpg-Binding Domain Proteins: Readers of the Epigenome. Epigenomics 2015, 7, 1051–1073. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Thomas, B.; Matson, S.; Chopra, V.; Sun, L.; Sharma, S.; Hersch, S.; Rosas, H.D.; Scherzer, C.; Ferrante, R.; Matson, W. A Novel Method for Detecting 7-Methyl Guanine Reveals Aberrant Methylation Levels in Huntington Disease. Anal. Biochem. 2013, 436, 112–120. [Google Scholar] [CrossRef]
- Fan, C.H.; Liu, W.L.; Cao, H.; Wen, C.; Chen, L.; Jiang, G. O6-Methylguanine DNA Methyltransferase as a Promising Target for the Treatment of Temozolomide-Resistant Gliomas. Cell Death Dis. 2013, 4, e876. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, L.; Wei, Q.; Shao, A. O(6)-Methylguanine-DNA Methyltransferase (Mgmt): Challenges and New Opportunities in Glioma Chemotherapy. Front. Oncol. 2019, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Kunanopparat, A.; Leelahavanichkul, A.; Visitchanakun, P.; Kueanjinda, P.; Phuengmaung, P.; Sae-Khow, K.; Boonmee, A.; Benjaskulluecha, S.; Palaga, T.; Hirankarn, N. The Regulatory Roles of Ezh2 in Response to Lipopolysaccharide (LPS) in Macrophages and Mice with Conditional Ezh2 Deletion with LysM-Cre System. Int. J. Mol. Sci. 2023, 24, 5363. [Google Scholar] [CrossRef] [PubMed]
- Volkel, P.; Bary, A.; Raby, L.; Chapart, A.; Dupret, B.; Le Bourhis, X.; Angrand, P.O. Ezh1 Arises from Ezh2 Gene Duplication but Its Function Is Not Required for Zebrafish Development. Sci. Rep. 2019, 9, 4319. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, L.; Xing, C.; Chen, Y.; Xu, P.; Li, M.; Zeng, L.; Li, C.; Ghosh, S.; Manna, D.M.; et al. Rnf2 Ablation Reprograms the Tumor-Immune Microenvironment and Stimulates Durable Nk and Cd4(+) T-Cell-Dependent Antitumor Immunity. Nat. Cancer 2021, 2, 1018–1038. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Wang, W.; Cho, H.; Lee, J.W.; Lee, S.K. The Histone Demethylase Kdm6b Regulates Subtype Diversification of Mouse Spinal Motor Neurons During Development. Nat. Commun. 2022, 13, 958. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Wei, Y.; Wei, X. Epigenetic Regulation of Macrophages: From Homeostasis Maintenance to Host Defense. Cell Mol. Immunol. 2020, 17, 36–49. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, H.; Zhuang, S.; Liu, N.; Bao, X.; Liu, X.; Ren, H.; Lv, D.; Li, Z.; Bai, J.; et al. Novel Pharmacological Inhibition of Ezh2 Attenuates Septic Shock by Altering Innate Inflammatory Responses to Sepsis. Int. Immunopharmacol. 2019, 76, 105899. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Z.; Liu, X.; Liu, N.; Bao, X.; Sun, H.; Meng, Q.; Ren, H.; Bai, J.; Zhou, X.; et al. Lymphocyte Expression of Ezh2 Is Associated with Mortality and Secondary Infectious Complications in Sepsis. Int. Immunopharmacol. 2020, 89, 107042. [Google Scholar] [CrossRef]
- Das, A.; Henderson, F.C., Jr.; Alshareef, M.; Porto, G.B.F.; Kanginakudru, I.; Infinger, L.K.; Vandergrift, W.A., III; Lindhorst, S.M.; Varma, A.K.; Patel, S.J.; et al. Mgmt-Inhibitor in Combination with Tgf-Betari Inhibitor or Cdk 4/6 Inhibitor Increases Temozolomide Sensitivity in Temozolomide-Resistant Glioblastoma Cells. Clin. Transl. Oncol. 2021, 23, 612–619. [Google Scholar] [CrossRef]
- Davies, R.; O’Dea, K.; Gordon, A. Immune Therapy in Sepsis: Are We Ready to Try Again? J. Intensive Care Soc. 2018, 19, 326–344. [Google Scholar] [CrossRef]
- Ruenjaiman, V.; Butta, P.; Leu, Y.W.; Pongpanich, M.; Leelahavanichkul, A.; Kueanjinda, P.; Palaga, T. Profile of Histone H3 Lysine 4 Trimethylation and the Effect of Lipopolysaccharide/Immune Complex-Activated Macrophages on Endotoxemia. Front. Immunol. 2019, 10, 2956. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, A.; Somparn, P.; Bootprapan, T.; Tu, H.; Tangtanatakul, P.; Nuengjumnong, R.; Worasilchai, N.; Tiranathanagul, K.; Eiam-ong, S.; Levine, M.; et al. High-dose ascorbate with low-dose amphotericin B attenuates severity of disease in a model of the reappearance of candidemia during sepsis in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R223–R234. [Google Scholar] [CrossRef]
- Vu, C.T.B.; Thammahong, A.; Leelahavanichkul, A.; Ritprajak, P. Alteration of macrophage immune phenotype in a murine sepsis model is associated with susceptibility to secondary fungal infection. Asian Pac. J. Allergy Immunol. 2022, 40, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.T.B.; Thammahong, A.; Yagita, H.; Azuma, M.; Hirankarn, N.; Ritprajak, P.; Leelahavanichkul, A. Blockade Of PD-1 Attenuated Postsepsis Aspergillosis Via The Activation of IFN-gamma and The Dampening of IL-10. Shock 2020, 53, 514–524. [Google Scholar] [CrossRef]
- Xaus, J.; Comalada, M.; Valledor, A.F.; Lloberas, J.; Lopez-Soriano, F.; Argiles, J.M.; Bogdan, C.; Celada, A. LPS Induces Apoptosis in Macrophages Mostly through the Autocrine Production of TNF-Alpha. Blood 2000, 95, 3823–3831. [Google Scholar] [CrossRef]
- Canton, M.; Sanchez-Rodriguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef] [PubMed]
- Tongthong, T.; Kaewduangduen, W.; Phuengmaung, P.; Chancharoenthana, W.; Leelahavanichkul, A. Lacticaseibacillus Rhamnosus Dfa1 Attenuate Cecal Ligation-Induced Systemic Inflammation through the Interference in Gut Dysbiosis, Leaky Gut, and Enterocytic Cell Energy. Int. J. Mol. Sci. 2023, 24, 3756. [Google Scholar] [CrossRef] [PubMed]
- Thim-Uam, A.; Surawut, S.; Issara-Amphorn, J.; Jaroonwitchawan, T.; Hiengrach, P.; Chatthanathon, P.; Wilantho, A.; Somboonna, N.; Palaga, T.; Pisitkun, P.; et al. Leaky-Gut Enhanced Lupus Progression in the Fc Gamma Receptor-IIb Deficient and Pristane-Induced Mouse Models of Lupus. Sci. Rep. 2020, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010, 920161. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; McKeague, M.; Seiwert, N.; Nagel, G.; Geisen, S.M.; Ziegler, N.; Trantakis, I.A.; Kaina, B.; Thomas, A.D.; Sturla, S.J.; et al. Immunological and mass spectrometry-based approaches to determine thresholds of the mutagenic DNA adduct O(6)-methylguanine in vivo. Arch. Toxicol. 2019, 93, 559–572. [Google Scholar] [CrossRef]
- Aloisi, C.M.N.; Sturla, S.J.; Gahlon, H.L. A gene-targeted polymerase-mediated strategy to identify O(6)-methylguanine damage. Chem. Commun. 2019, 55, 3895–3898. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.D.; Pittman, D.L. Methylating agents and DNA repair responses: Methylated bases and sources of strand breaks. Chem. Res. Toxicol. 2006, 19, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Ranson, M.; Hersey, P.; Thompson, D.; Beith, J.; McArthur, G.A.; Haydon, A.; Davis, I.D.; Kefford, R.F.; Mortimer, P.; Harris, P.A.; et al. Randomized Trial of the Combination of Lomeguatrib and Temozolomide Compared with Temozolomide Alone in Chemotherapy Naive Patients with Metastatic Cutaneous Melanoma. J. Clin. Oncol. 2007, 25, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Park, J.H.; Seok, H.; Choi, W.S. Diagnostic and Prognostic Value of Interleukin-6, Pentraxin 3, and Procalcitonin Levels among Sepsis and Septic Shock Patients: A Prospective Controlled Study According to the Sepsis-3 Definitions. BMC Infect. Dis. 2019, 19, 968. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Wang, D. Inflammatory Cytokine Expression in Patients with Sepsis at an Intensive Care Unit. Exp. Ther. Med. 2018, 16, 2126–2131. [Google Scholar] [CrossRef]
- Greco, M.; Mazzei, A.; Suppressa, S.; Palumbo, C.; Verri, T.; Lobreglio, G. Human Leukocyte Antigen-Dr Isotype Expression in Monocytes and T Cells Interferon-Gamma Release Assay in Septic Patients and Correlation with Clinical Outcome. J. Clin. Med. Res. 2021, 13, 293–303. [Google Scholar] [CrossRef]
- Mansfield, S.; Griessl, M.; Gutknecht, M.; Cook, C.H. Sepsis and Cytomegalovirus: Foes or Conspirators? Med. Microbiol. Immunol. 2015, 204, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gopisetty, G.; Ramachandran, K.; Singal, R. DNA Methylation and Apoptosis. Mol. Immunol. 2006, 43, 1729–1740. [Google Scholar] [CrossRef]
- Hiengrach, P.; Panpetch, W.; Chindamporn, A.; Leelahavanichkul, A. Helicobacter pylori, Protected from Antibiotics and Stresses Inside Candida albicans Vacuoles, Cause Gastritis in Mice. Int. J. Mol. Sci. 2022, 23, 8568. [Google Scholar] [CrossRef] [PubMed]
- Panpetch, W.; Visitchanakun, P.; Saisorn, W.; Sawatpanich, A.; Chatthanathon, P.; Somboonna, N.; Tumwasorn, S.; Leelahavanichkul, A. Lactobacillus rhamnosus attenuates Thai chili extracts induced gut inflammation and dysbiosis despite capsaicin bactericidal effect against the probiotics, a possible toxicity of high dose capsaicin. PLoS ONE 2021, 16, e0261189. [Google Scholar] [CrossRef]
- Phuengmaung, P.; Mekjaroen, J.; Saisorn, W.; Chatsuwan, T.; Somparn, P.; Leelahavanichkul, A. Rapid Synergistic Biofilm Production of Pseudomonas and Candida on the Pulmonary Cell Surface and in Mice, a Possible Cause of Chronic Mixed Organismal Lung Lesions. Int. J. Mol. Sci. 2022, 23, 9202. [Google Scholar] [CrossRef] [PubMed]
- Singkham-In, U.; Phuengmaung, P.; Makjaroen, J.; Saisorn, W.; Bhunyakarnjanarat, T.; Chatsuwan, T.; Chirathaworn, C.; Chancharoenthana, W.; Leelahavanichkul, A. Chlorhexidine Promotes Psl Expression in Pseudomonas aeruginosa That Enhances Cell Aggregation with Preserved Pathogenicity Demonstrates an Adaptation against Antiseptic. Int. J. Mol. Sci. 2022, 23, 8308. [Google Scholar] [CrossRef]
- Issara-Amphorn, J.; Dang, C.P.; Saisorn, W.; Limbutara, K.; Leelahavanichkul, A. Candida Administration in Bilateral Nephrectomy Mice Elevates Serum (1→3)-Β-D-Glucan That Enhances Systemic Inflammation through Energy Augmentation in Macrophages. Int. J. Mol. Sci. 2021, 22, 5031. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. Star: Ultrafast Universal Rna-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-Optimal Probabilistic Rna-Seq Quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. Edger: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for Rna-Seq Read Counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.G.; Mandloi, T.; Kunte, P.; Natu, A.; Rashid, M.; Reddy, D.; Gadewal, N.; Gupta, S. Histome2: A Database of Histone Proteins, Modifiers for Multiple Organisms and Epidrugs. Epigenetics Chromatin 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves Using ‘Ggplot2’; R Package Version 0.3 1; 2017. Available online: https://cran.r-project.org (accessed on 15 April 2023).
- Ondee, T.; Gillen, J.; Visitchanakun, P.; Somparn, P.; Issara-Amphorn, J.; Dang Phi, C.; Chancharoenthana, W.; Gurusamy, D.; Nita-Lazar, A.; Leelahavanichkul, A. Lipocalin-2 (Lcn-2) Attenuates Polymicrobial Sepsis with LPS Preconditioning (LPS Tolerance) in FcGRIIb Deficient Lupus Mice. Cells 2019, 8, 1064. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Kaewduangduen, W.; Chareonsappakit, A.; Susantitaphong, P.; Pisitkun, P.; Ritprajak, P.; Townamchai, N.; Leelahavanichkul, A. Interference on Cytosolic DNA Activation Attenuates Sepsis Severity: Experiments on Cyclic Gmp–Amp Synthase (Cgas) Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11450. [Google Scholar] [CrossRef]
- Saisorn, W.; Saithong, S.; Phuengmaung, P.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Visitchanakun, P.; Chareonsappakit, A.; Pisitkun, P.; Chiewchengchol, D.; Leelahavanichkul, A. Acute Kidney Injury Induced Lupus Exacerbation through the Enhanced Neutrophil Extracellular Traps (and Apoptosis) in Fcgr2b Deficient Lupus Mice with Renal Ischemia Reperfusion Injury. Front. Immunol. 2021, 12, 669162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).