Effects of Different Nitrogen Levels on Lignocellulolytic Enzyme Production and Gene Expression under Straw-State Cultivation in Stropharia rugosoannulata
Abstract
1. Introduction
2. Results
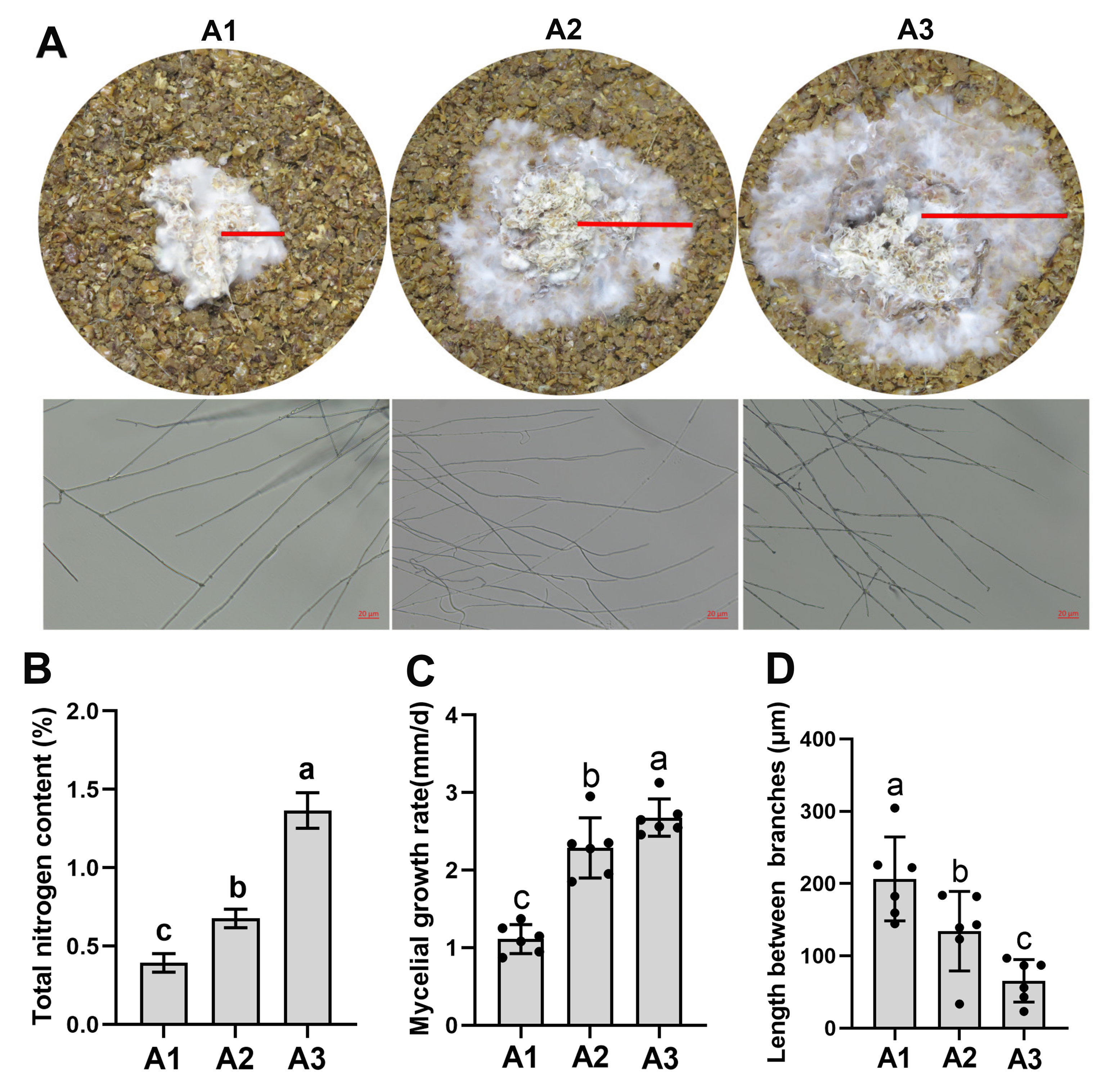
2.1. Nitrogen Levels Regulated the Mycelial Growth of S. rugosoannulata
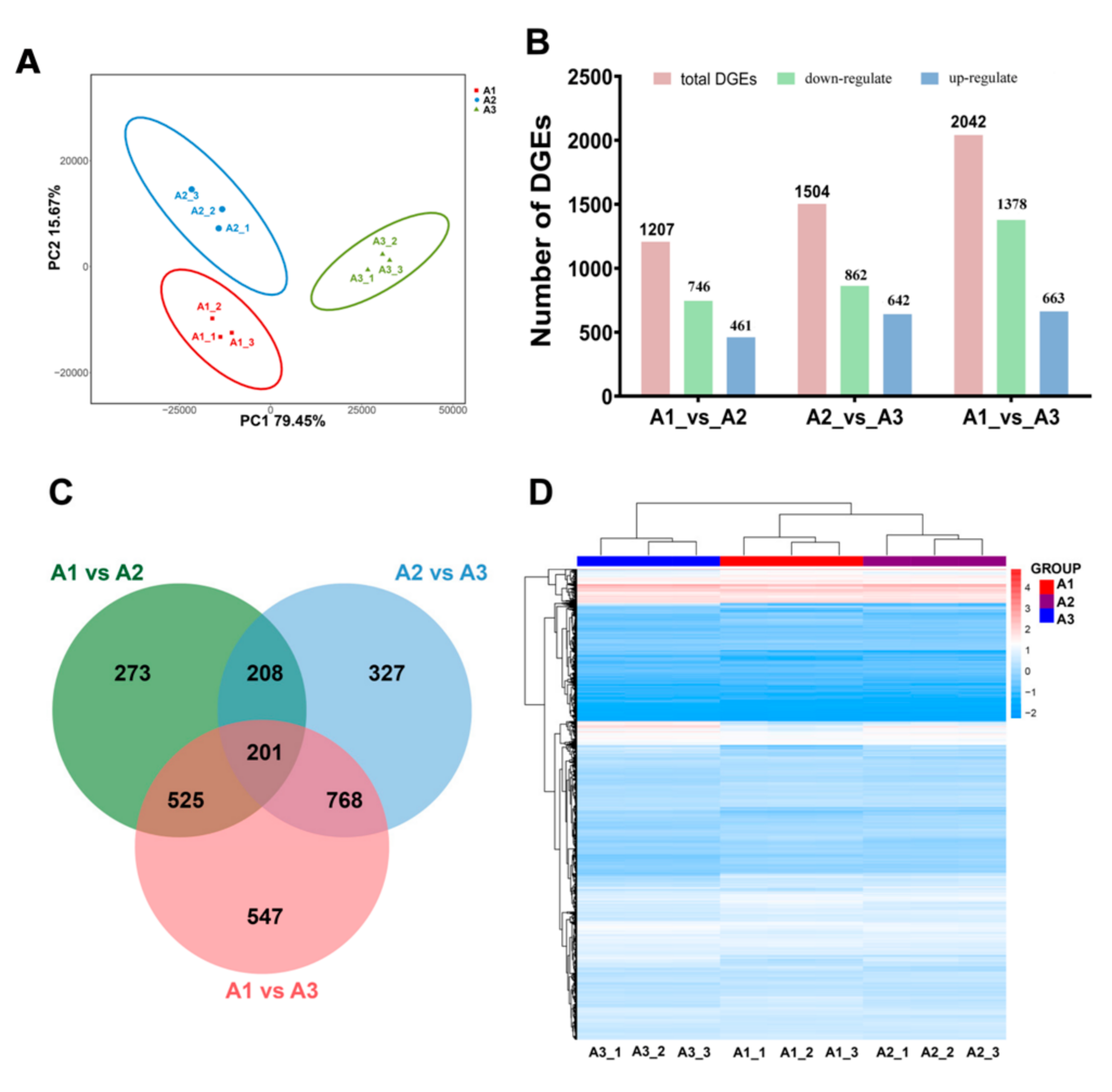
2.2. Global Transcriptomic Analysis of S. rugosoannulata and Identification of Differentially Expressed Genes (DEGs)
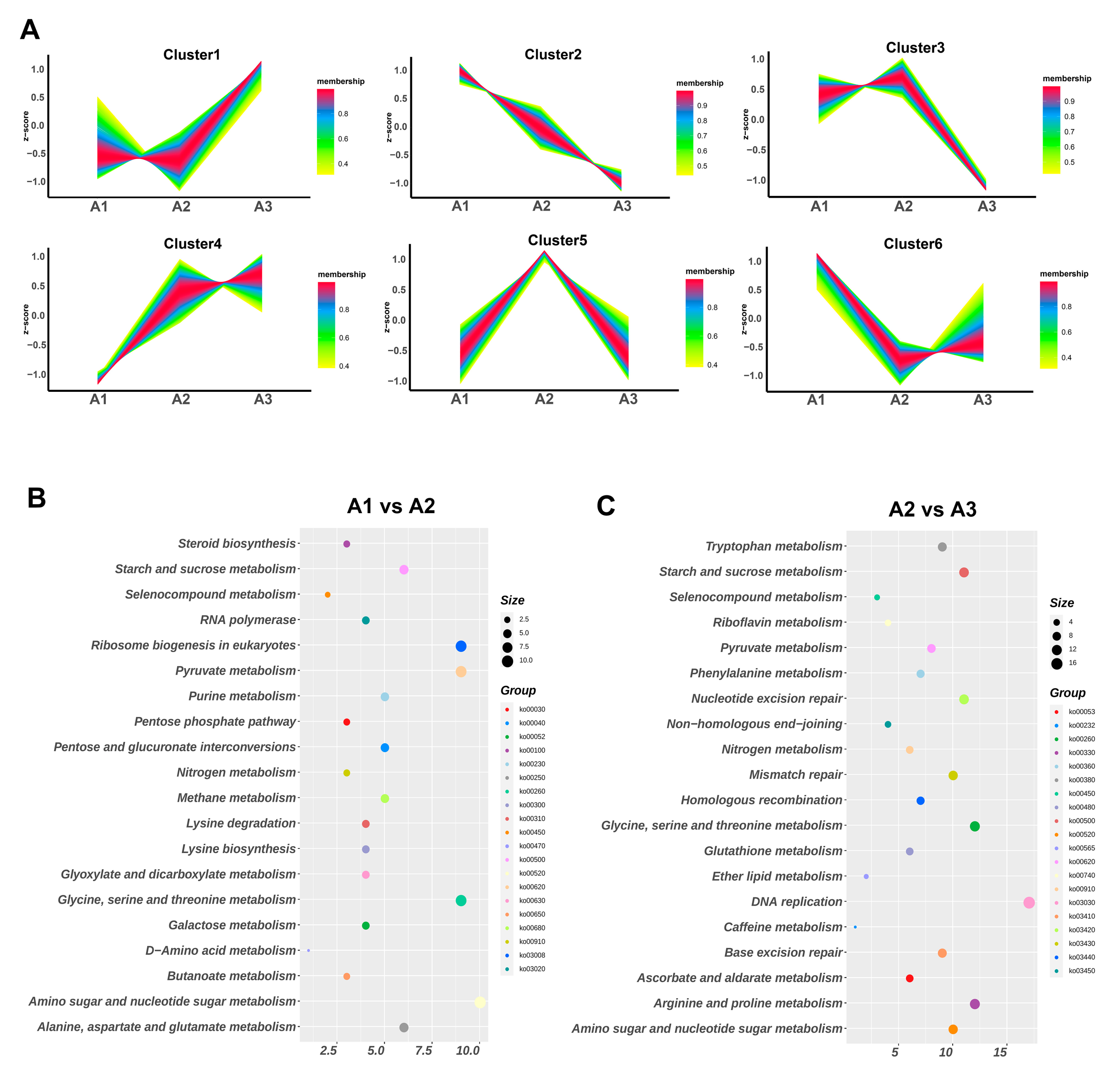
2.3. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway and Gene Ontology (GO) Enrichment Analyses of the Differentially Expressed Genes Were Used
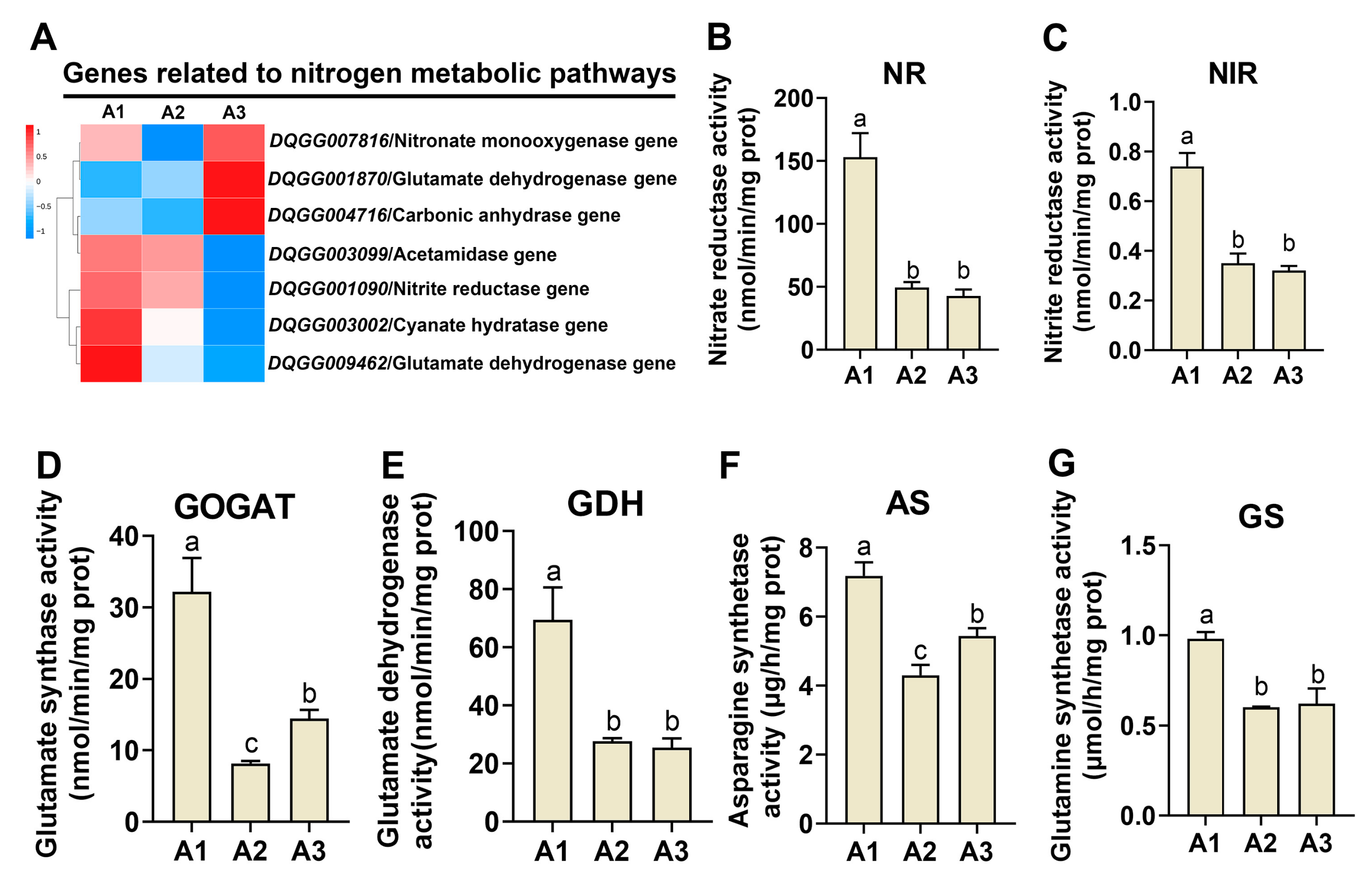
2.4. Analysis of Nitrogen Metabolism Genes at the Three Nitrogen Levels
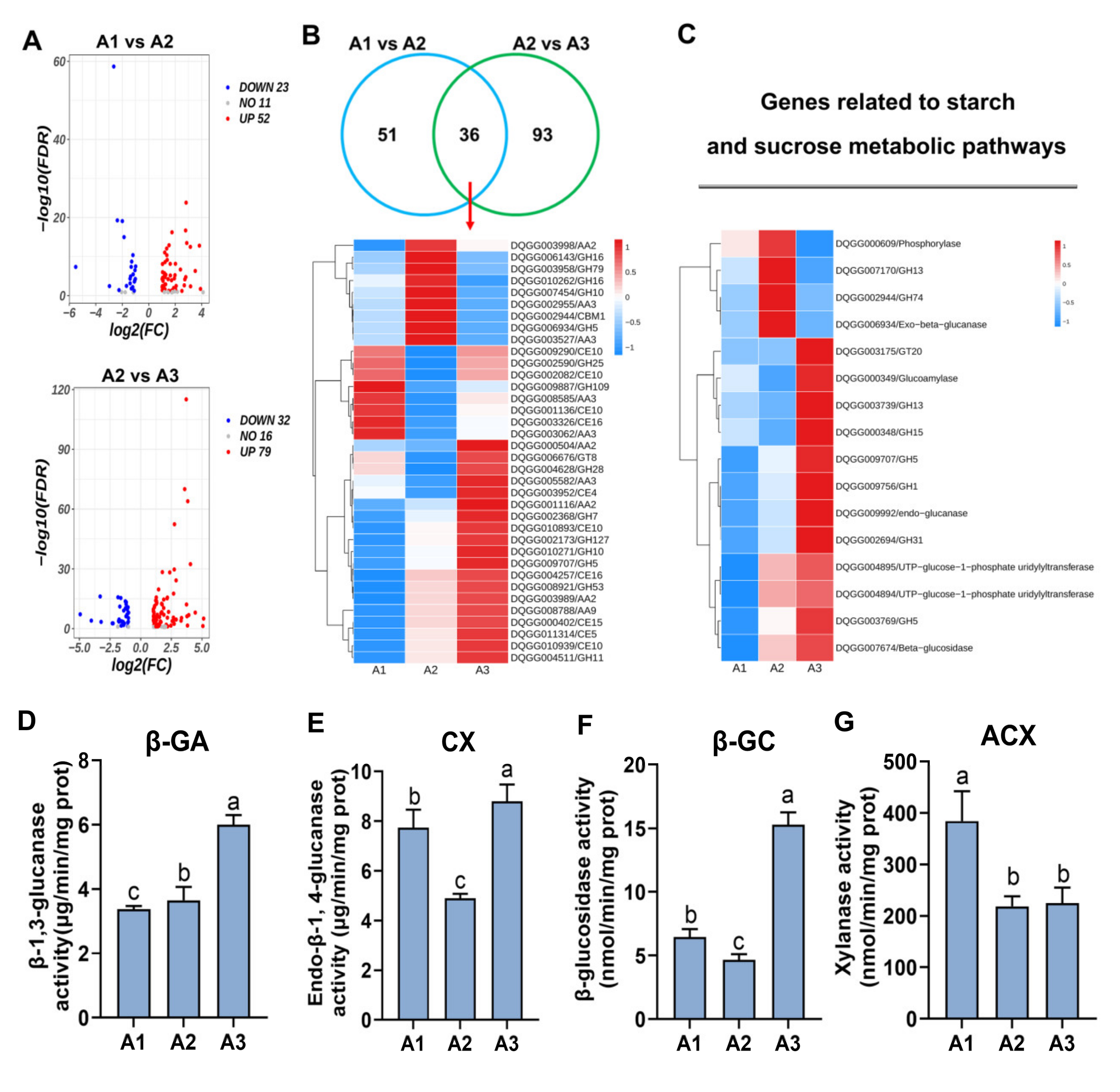
2.5. Analysis of Carbon Metabolism Genes at the Three Nitrogen Levels
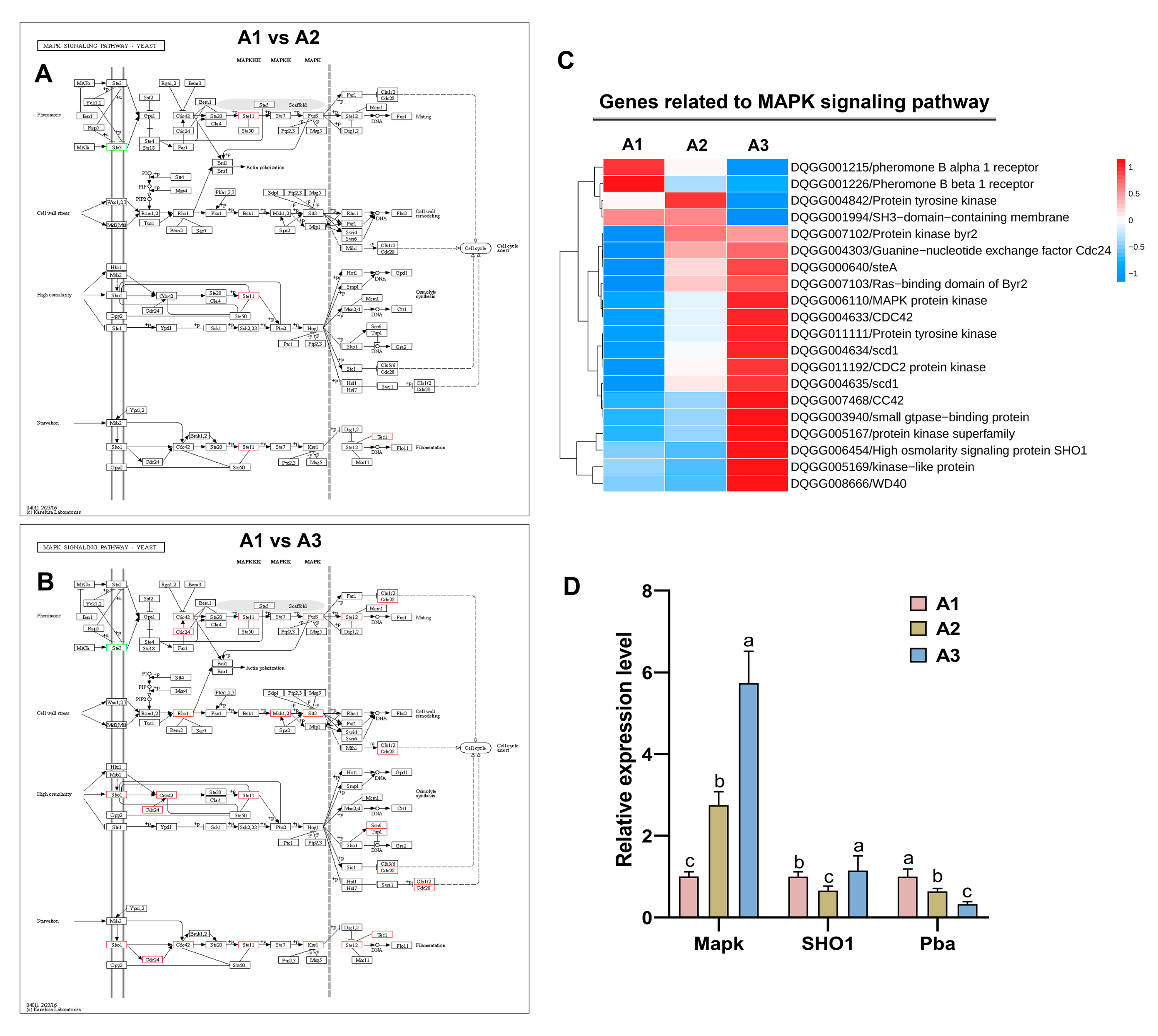
2.6. Analysis of the Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway at the Three Nitrogen Levels
2.7. Validation of Transcriptomic Data by qRT–PCR
3. Discussion
4. Materials and Methods
4.1. Collection of Stropharia rugosoannulata Materials at Different Nitrogen Levels
4.2. Quantification of the Distance between Hyphal Branches
4.3. Total RNA Isolation, cDNA Library Preparation and Illumina Sequencing
4.4. Read Quality Control and Mapping
4.5. Differential Expression and Functional Enrichment Analyses
4.6. Determination of Cellulase and Hemicellulase Enzymes Activity
4.7. Determination of Nitrogen Metabolism Enzyme Activity
4.8. Gene Expression Levels as Determined via qRT–PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Chen, Y.Q.; Xiao, X.W.; Zhong, R.T.; Yang, C.F.; Liu, B.; Zhao, C. Nutrient properties and nuclear magnetic resonancebased metabonomic analysis of macrofungi. Foods 2019, 8, 397. [Google Scholar] [CrossRef]
- Gong, S.; Chen, C.; Zhu, J.X.; Qi, G.G.; Jinag, S.X. Effects of wine-cap Stropharia cultivation on soil nutrients and bacterial communities in forestlands of northern China. Peer J. 2018, 6, e5741. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L.; Wang, X.D.; Lee, D.J. Proteomic researches for lignocellulose-degrading enzymes: A mini-review. Bioresour. Technol. 2018, 265, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.X.; Wang, M.M.; Ma, L.; Li, T.; Huang, Q.W.; Liu, D.Y.; Shen, Q.R. Effects of amino acids on the lignocellulose degradation by Aspergillus fumigatus Z5: Insights into performance transcriptional and proteomic profiles. Biotechnol. Biofuels 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, G.; Zhou, J.; Chen, J. Regulation of sensing, transportation, and catabolism of nitrogen sources in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2018, 82, e00040-17. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Pan, Y.Y.; Liu, G. Disruption of the nitrogen regulatory gene AcareA in Acremonium chrysogenum leads to reduction of cephalosporin production and repression of nitrogen metabolism. Fungal Genet. Biol. 2013, 61, 69–79. [Google Scholar] [CrossRef]
- Liu, X.J.; Hu, B.; Chu, C.C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, K.; Shen, Q.J.; Zhao, S.; Liu, J.L. Filamentation of asparagine synthetase in Saccharomyces cerevisiae. PLoS Genet. 2018, 14, e1007737. [Google Scholar] [CrossRef]
- Sieg, A.G.; Trotter, P.J. Differential contribution of the proline and glutamine pathways to glutamate biosynthesis and nitrogen assimilation in yeast lacking glutamate dehydrogenase. Microbiol. Res. 2014, 169, 709–716. [Google Scholar] [CrossRef]
- Mara, P.; Fragiadakis, G.S.; Gkountromichos, F.; Alexandraki, D. The pleiotropic effects of the glutamate dehydrogenase (GDH) pathway in Saccharomyces cerevisiae. Microb. Cell Fact. 2018, 17, 170. [Google Scholar] [CrossRef]
- Dong, C.D.; Patel, A.K.; Madhavan, A.; Chen, C.W.; Singhania, R.R. Significance of glycans in cellulolytic enzymes for lignocellulosic biorefinery. Bioresour. Technol. 2023, 379, 128992. [Google Scholar] [CrossRef] [PubMed]
- Werghemmi, W.; Abou Fayssal, S.; Mazouz, H.; Hajjaj, H.; Hajji, L. Olive and green tea leaves extract in Pleurotus ostreatus var. florida culture media: Effect on mycelial linear growth rate, diameter and growth induction index. IOP Conf. Ser. Earth Environ. Sci. 2022, 1090, 012020. [Google Scholar] [CrossRef]
- Sista Kameshwar, A.K.; Qin, W. Comparative study of genome-wide plant biomass-degrading CAZymes in white rot brown rot and soft rot fungi. Mycology 2018, 9, 93–105. [Google Scholar] [CrossRef]
- Park, Y.J.; Jeong, Y.N.; Kong, W.S. Genome sequencing and carbohydrate-active enzyme (CAZyme) repertoire of the white rot fungus Flammulina elastica. Int. J. Mol. Sci. 2018, 19, 2379. [Google Scholar] [CrossRef]
- Cristica, M.; Barbaneagra, T.; Todirascu-Ciornea, E.; Manoliu, A. Influence of some amino acids on the activity of cellulolytic and xylanolytic enzymes in the fungus Trichoderma reesei QM-9414. Lucrări Ştiinţifice seria. Agronomie 2012, 55, 321–325. [Google Scholar]
- Elbagory, M.; El-Nahrawy, S.; Omara, A.E.; Eid, E.M.; Bachheti, A.; Kumar, P.; Fayssal, S.A.; Adelodun, B.; Bachheti, R.K.; Kumar, P.; et al. Sustainable bioconversion of wetland plant biomass for Pleurotus ostreatus var. florida cultivation: Studies on proximate and biochemical characterization. Agriculture 2022, 12, 2095. [Google Scholar] [CrossRef]
- Colla, I.M.; de Filho, O.B.Q.; Bertéli, M.B.D.; de Freitas, J.D.S.; Avelino, K.V.; Ruiz, S.P.; do Valle, J.S.; Linde, G.A.; Colauto, N.B. Carbon-to-nitrogen ratios on laccase and mushroom production of Lentinus crinitus. Int. J. Environ. Sci. Tehnol. 2023, 20, 3941–3952. [Google Scholar] [CrossRef]
- Chmelová, D.; Ondrejovič, M.; Ondáš, V.; Šturdík, E. Influence of cultivation conditions on production of lignocellulolytic enzymes by Ceriporiopsis subvermispora. Biologia 2011, 66, 748–754. [Google Scholar] [CrossRef]
- Reddy, M.S.; Kanwal, H.K. Influence of carbon, nitrogen sources, inducers, and substrates on lignocellulolytic enzyme activities of Morchella spongiola. J. Agric. Food Res. 2022, 7, 100271. [Google Scholar] [CrossRef]
- England, J.C.; Perchuk, B.S.; Laub, M.T.; Gober, J.W. Global regulation of gene expression and cell differentiation in Caulobacter crescentus in response to nutrient availability. J. Bacteriol. 2010, 192, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.F.; Wang, Z.X.; Xia, Y.X.; Zheng, F.L.; Kang, F.S.; Meng, X.F.; Zhang, W.X. Role of the nitrogen metabolism regulator TAM1 in regulation of cellulase gene expression in Trichoderma reesei. Appl. Environ. Microbiol. 2023, 89, e0142122. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Rinne-Garmston (Rinne), K.T.; Penttil, R.; Vadeboncoeur, M.A.; Chen, J.; Makipa, R. Carbon and nitrogen acquisition strategies by wood decay fungi influence their isotopic signatures in Picea abies forests. Fungal Ecol. 2021, 52, 101069. [Google Scholar] [CrossRef]
- Okal, E.J.; Aslam, M.M.; Karanja, J.K.; Witness, J. Advances in understanding regulation of cellulase enzyme in white-rot basidiomycetes. Microb. Pathog. 2020, 147, 104410. [Google Scholar] [CrossRef]
- Qian, Y.H.; Sun, Y.; Zhong, L.X.; Sun, N.N.; Sheng, Y.F.; Qu, Y.B.; Zhong, Y.H. The GATA-type transcriptional factor are1 modulates the expression of extracellular proteases and cellulases in Trichoderma reesei. Int. J. Mol. Sci. 2019, 20, 4100. [Google Scholar] [CrossRef]
- Edwards, I.P.; Zak, D.R.; Kellner, H.; Eisenlord, S.D.; Pregitzer, K.S. Simulated atmospheric N deposition alters fungal community composition and suppresses ligninolytic gene expression in a northern hardwood forest. PLoS ONE 2011, 6, e20421. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.Q.; Xie, X.Y.; Zhang, R.J.; Wei, Z.M. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef]
- Pang, A.P.; Zhang, F.N.; Hu, X.; Luo, Y.S.; Wang, H.Y.; Samran, D.; Wu, F.G.; Li, B.Z.; Zhou, Z.H.; Rytioja, J.; et al. Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiol. Mol. Biol. Rev. 2014, 78, 614–649. [Google Scholar]
- Xiong, Y.; Wu, V.W.; Lubbe, A.; Qin, L.; Deng, S.; Kennedy, M.A. Fungal transcription factor essential for starch degradation affects integration of carbon and nitrogen metabolism. PLoS Genet. 2017, 13, e1006737. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.H.; Li, L.; Liu, X.; Zhong, X.; Wang, H.Z.; Zhou, S.; Gu, L. Time-scale dynamics of proteome predicts the central carbon metabolism involved in triterpenoid accumulation responsive to nitrogen limitation in Ganoderma lucidum. Fungal Biol. 2021, 125, 294–304. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhang, R.J.; Yang, Q.Y.; Li, X.Z.; Xiang, Q.J.; Zhao, K.; Ma, M.J.; Yu, X.M.; Chen, Q.; Zeng, X.F.; et al. Cultivating Lentinula edodes on substrate containing composted sawdust affects the expression of carbohydrate and aromatic amino acid metabolism-related genes. Msystems 2022, 7, e00827-21. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Freund, H.L.; Kasanjian, J.; Berlemont, R. Function, distribution and annotation of characterized cellulases xylanases and chitinases from CAZy. Appl. Microbiol. Biotechnol. 2018, 102, 1629–1637. [Google Scholar] [CrossRef]
- Medie, F.M.; Davies, G.J.; Drancourt, M.; Henrissat, B. Genome analyses highlight the different biological roles of cellulases. Nat. Rev. Microbiol. 2012, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Daou, M.; Farfan Soto, C.; Majira, A.; Cézard, L.; Cottyn, B.; Pion, F.; Navarro, D.; Oliveira Correia, L.; Drula, E.; Record, E.; et al. Fungal treatment for the valorization of technical soda lignin. J. Fungi 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, Y.; Cui, F.; Wang, Y.; Sun, L.; Zan, X.; Sun, W. UDP-glycosyltransferases in edible fungi: Function structure and catalytic mechanism. Fermentation 2023, 9, 164. [Google Scholar] [CrossRef]
- Xu, F.; Wang, P.; Zhang, Y.Z.; Chen, X.L. Diversity of three-dimensional structures and catalytic mechanisms of alginate lyases. Appl. Environ. Microbiol. 2018, 84, e02040-17. [Google Scholar] [CrossRef]
- Rafeeq, H.; Hussain, A.; Shabbir, S.; Ali, S.; Bilal, M.; Sher, F.; Iqbal, H.M.N. Esterases as emerging biocatalysts: Mechanistic insights, genomic and metagenomic, immobilization, and biotechnological applications. Biotechnol. Appl. Biochem. 2021, 69, 2176–2194. [Google Scholar] [CrossRef]
- De Vries, S.; de Vries, J. A global survey of carbohydrate esterase families 1 and 10 in Oomycetes. Front. Genet. 2020, 11, 756. [Google Scholar] [CrossRef]
- Cong, J.; Xiao, K.Q.; Jiao, W.L.; Zhang, C.; Zhang, X.H.; Liu, J.L.; Zhang, Y.H.; Pan, H.Y. The Coupling Between Cell Wall Integrity Mediated by MAPK Kinases and SsFkh1 Is Involved in Sclerotia Formation and Pathogenicity of Sclerotinia sclerotiorum. Front Microbiol. 2022, 13, 816091. [Google Scholar] [CrossRef]
- Marjatta, R.; Erika, K. Basidiomycete mating type genes and pheromone signaling. Eukaryot. Cell 2010, 9, 847–859. [Google Scholar]
- Carmen, H.D.; Elvira, R.; Jesús, P.; Rebeca, A.M. Hog controls lipids homeostasis upon osmotic stress in Candida albicans. J. Fungi 2020, 6, 355. [Google Scholar]
- Mu, D.; Li, C.; Zhang, X.; Li, X.; Shi, L.; Ren, A.; Zhao, M. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: An essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, and oxidative-stress resistance. Environ. Microbiol. 2014, 16, 1709–1728. [Google Scholar] [CrossRef]
- Ziv, C.; Feldman, D.; Aharoni-Kats, L.; Chen, S.; Liu, Y.; Yarden, O. The N-terminal region of the Neurospora NDR kinase COT1 regulates morphology via its interactions with MOB2A/B. Mol. Microbiol. 2013, 90, 383–399. [Google Scholar] [PubMed]
- Hao, H.H.; Zhang, J.J.; Wang, Q.; Huang, J.C.; Juan, J.J.; Kuai, B.K.; Feng, Z.Y.; Chen, H. Transcriptome and Differentially Expressed Gene Profiles in Mycelium, Primordium and Fruiting Body Development in Stropharia rugosoannulata. Genes 2022, 13, 1080. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| GeneID | baseMean-A1 | baseMean-A2 | baseMean-A3 | Pval | Gene Function |
|---|---|---|---|---|---|
| DQGG009599 | 533.06 | 550.04 | 145.56 | 8.44 × 10−26 | glyoxylate reductase |
| DQGG008189 | 249.17 | 280.02 | 113.21 | 1.74 × 10−6 | amine oxidase |
| DQGG003527 | 443.70 | 1328.30 | 440.56 | 3.0 × 10−11 | glucose methanol choline oxidoreductase |
| DQGG005387 | 53.63 | 36.96 | 236.78 | 5.95 × 10−27 | GMC oxidoreductase |
| DQGG003665 | 2045.36 | 2586.69 | 29347.66 | 2.8 × 10−119 | GMC oxidoreductase |
| DQGG008582 | 1528.91 | 1674.92 | 20710.85 | 1.33 × 10−67 | GMC oxidoreductase |
| DQGG001973 | 9854.39 | 7970.89 | 4091.93 | 1.96 × 10−7 | amine oxidase |
| DQGG008585 | 338.21 | 99.35 | 248.68 | 9.11 × 10−8 | GMC oxidoreductase |
| DQGG010007 | 2985.59 | 744.15 | 9090.15 | 1.07 × 10−84 | cystathionine gamma lyase |
| DQGG003537 | 3070.79 | 1684.82 | 3702.9 | 0.0169 | GMC oxidoreductase |
| DQGG005389 | 274.17 | 116.56 | 342.15 | 6.49 × 10−7 | GMC oxidoreductase |
| DQGG005390 | 117.85 | 52.48 | 156.73 | 1.85 × 10−5 | GMC oxidoreductase |
| DQGG008219 | 159.11 | 27.57 | 177.07 | 2.2 × 10−6 | GMC oxidoreductase |
| DQGG000370 | 3016.03 | 2606.26 | 1380.65 | 2.25 × 10−6 | amine oxidase |
| DQGG006088 | 2300.694 | 1127.07 | 816.63 | 0.1015 | phosphoglycerate mutase |
| DQGG011096 | 5562.959 | 7671.32 | 13109.48 | 1.97 × 10−11 | aryl-alcohol oxidase |
| DQGG001126 | 12,550.27 | 21,874.07 | 30,393.51 | 4.65 × 10−6 | 5-aminolevulinate synthase |
| DQGG004458 | 0.50 | 1.49 | 6.17 | 0.1174 | pyranose dehydrogenase |
| DQGG008578 | 423.43 | 1536.30 | 1623.87 | 0.2570 | GMC oxidoreductase |
| DQGG004502 | 437.49 | 1165.83 | 1718.57 | 0.0805 | pyranose dehydrogenase |
| DQGG010568 | 463.16 | 1096.68 | 626.52 | 0.0001 | GMC oxidoreductase |
| DQGG005392 | 1120.01 | 528.09 | 766.45 | 0.0065 | GMC oxidoreductase |
| DQGG005391 | 312.05 | 153.79 | 179.9 | 0.0662 | GMC oxidoreductase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhuo, X.; Wang, Q.; Ji, H.; Chen, H.; Hao, H. Effects of Different Nitrogen Levels on Lignocellulolytic Enzyme Production and Gene Expression under Straw-State Cultivation in Stropharia rugosoannulata. Int. J. Mol. Sci. 2023, 24, 10089. https://doi.org/10.3390/ijms241210089
Zhang J, Zhuo X, Wang Q, Ji H, Chen H, Hao H. Effects of Different Nitrogen Levels on Lignocellulolytic Enzyme Production and Gene Expression under Straw-State Cultivation in Stropharia rugosoannulata. International Journal of Molecular Sciences. 2023; 24(12):10089. https://doi.org/10.3390/ijms241210089
Chicago/Turabian StyleZhang, Jinjing, Xinyi Zhuo, Qian Wang, Hao Ji, Hui Chen, and Haibo Hao. 2023. "Effects of Different Nitrogen Levels on Lignocellulolytic Enzyme Production and Gene Expression under Straw-State Cultivation in Stropharia rugosoannulata" International Journal of Molecular Sciences 24, no. 12: 10089. https://doi.org/10.3390/ijms241210089
APA StyleZhang, J., Zhuo, X., Wang, Q., Ji, H., Chen, H., & Hao, H. (2023). Effects of Different Nitrogen Levels on Lignocellulolytic Enzyme Production and Gene Expression under Straw-State Cultivation in Stropharia rugosoannulata. International Journal of Molecular Sciences, 24(12), 10089. https://doi.org/10.3390/ijms241210089





