Hyaluronan with Different Molecular Weights Can Affect the Gut Microbiota and Pathogenetic Progression of Post-Intensive Care Syndrome Mice in Different Ways
Abstract
1. Introduction
2. Results
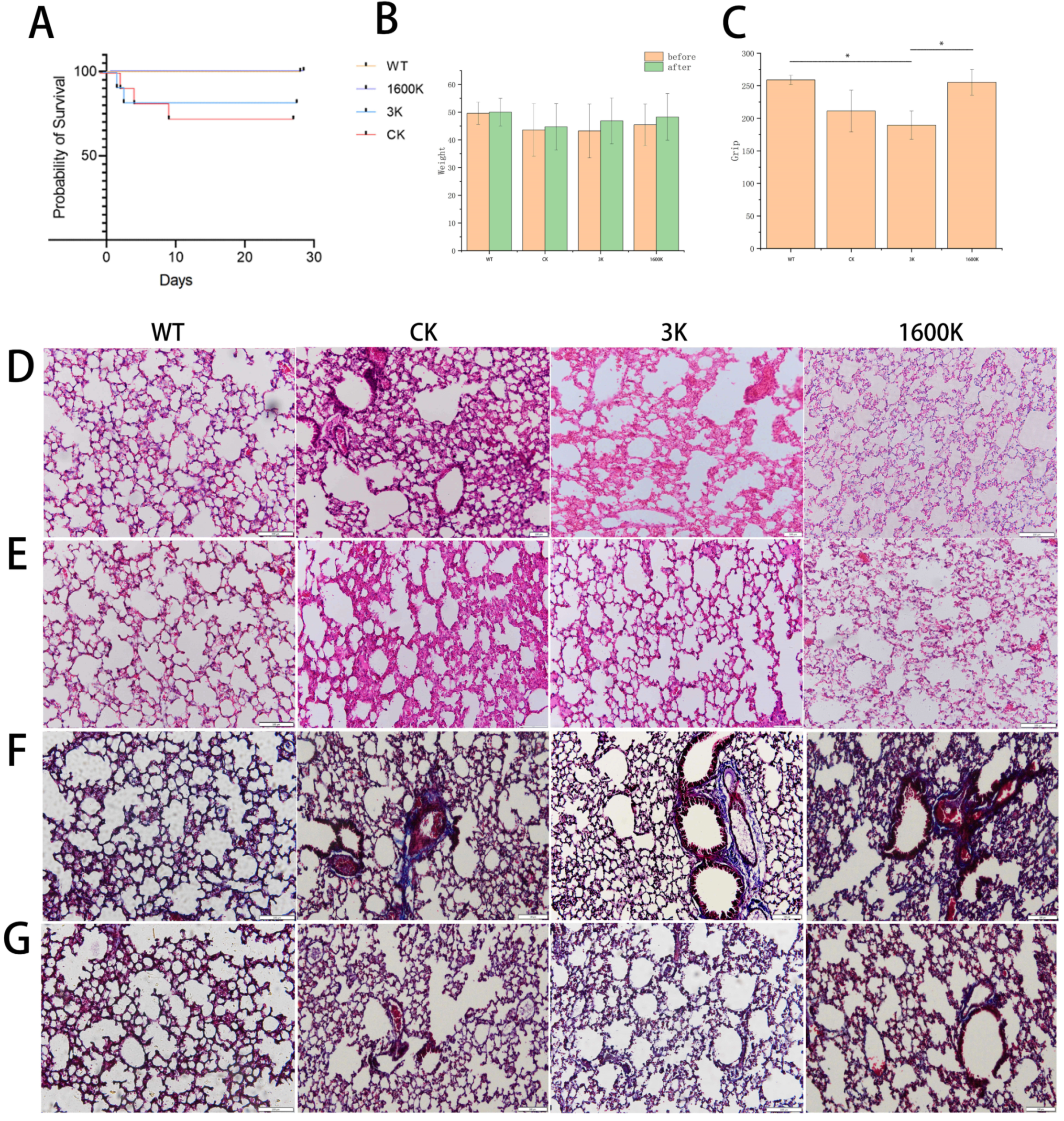
2.1. The Effects of Oral Administration of 3 kDa-HA and 1600 kDa-HA on the Survival Rate, Body Weight, and Grip Strength of PICS Mice
2.2. Effect of Oral Administration of 3 kDa-HA and 1600 kDa-HA on Lung Pathological Changes and Fibrosis Degree in PICS Mice
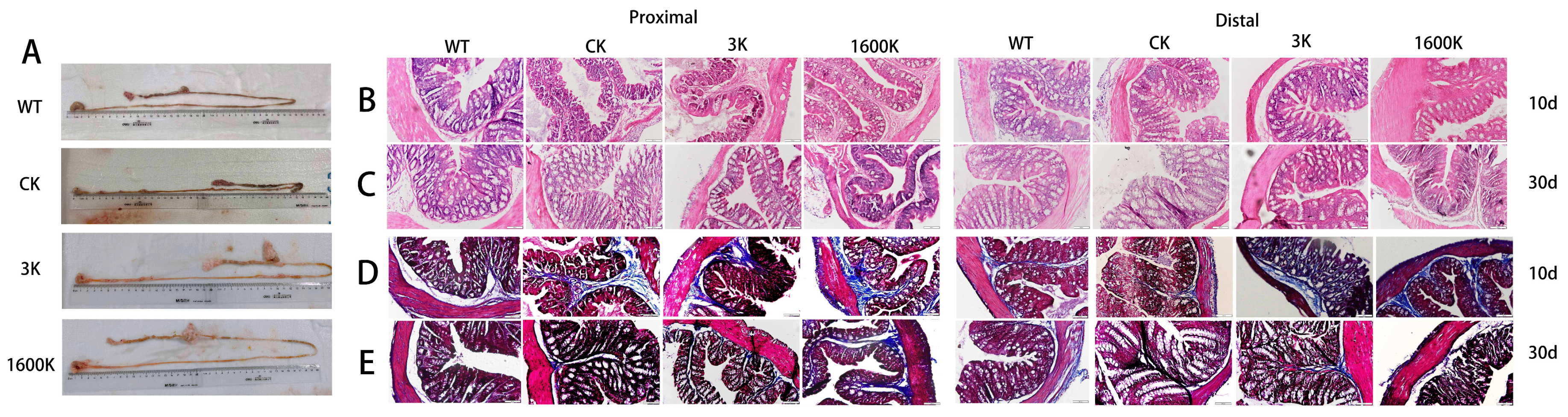
2.3. Morphology and Pathological Changes of Intestinal Tissues in Each Group of Mice
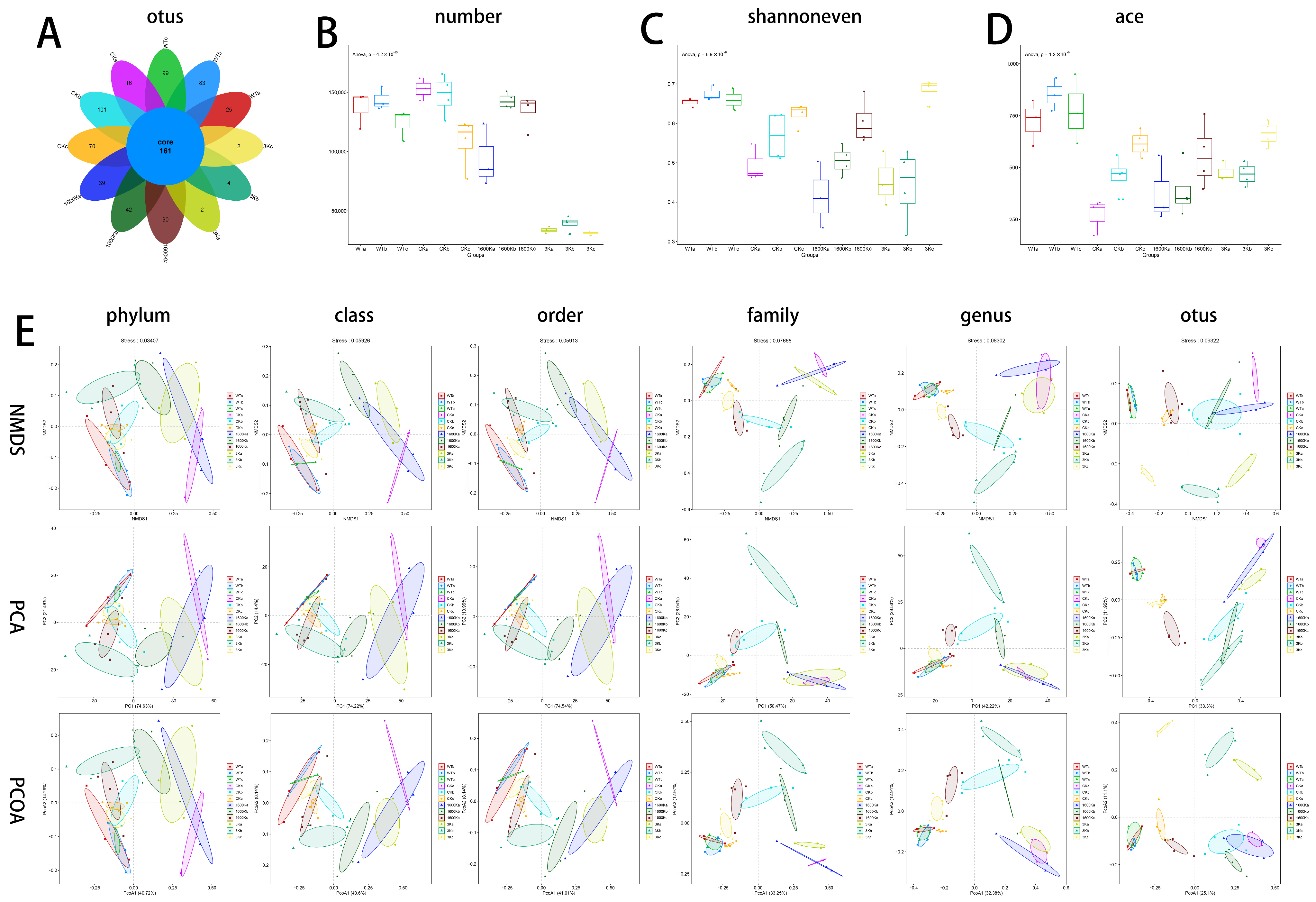
2.4. Number of Mice Gut Microbiota and Community Diversity
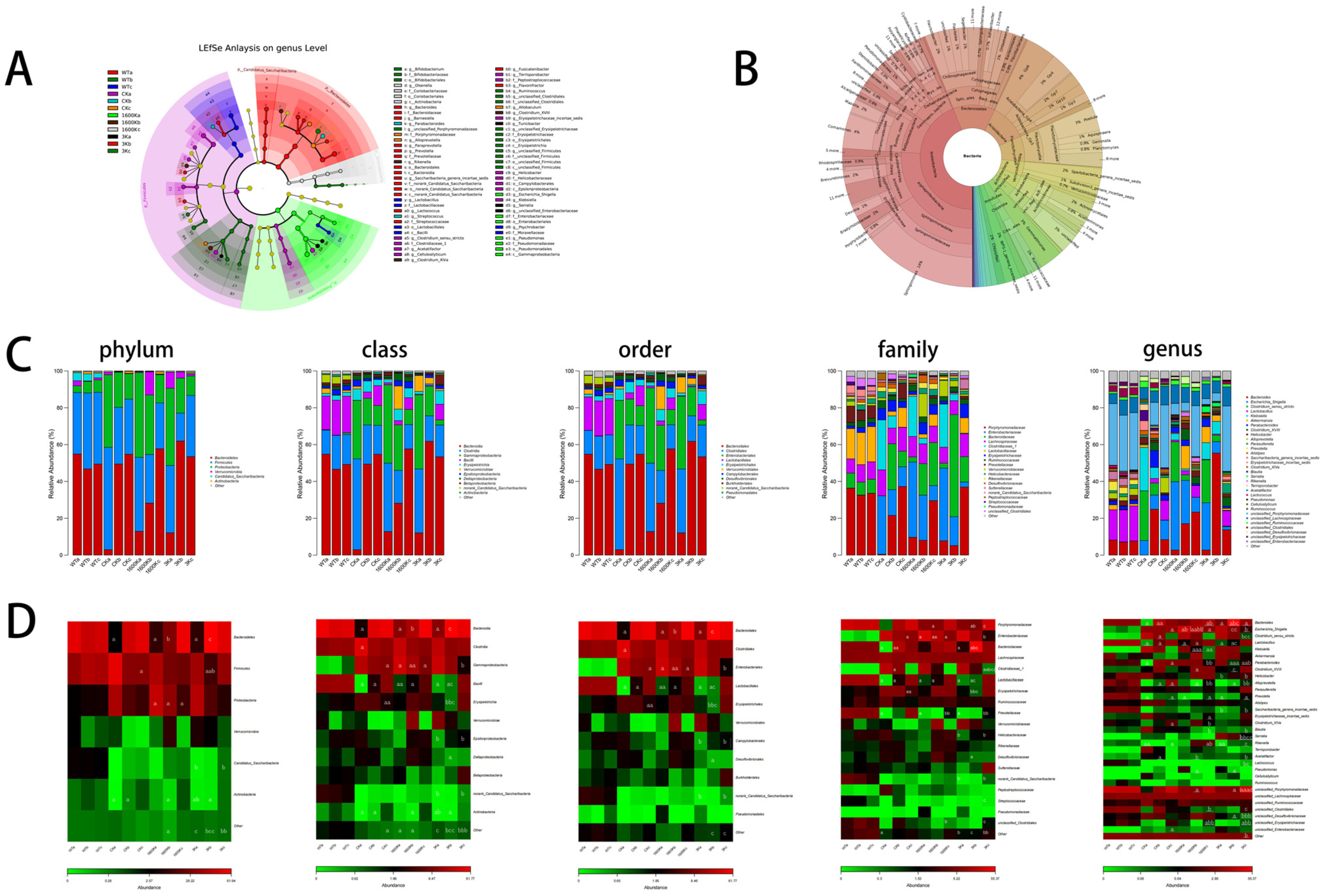
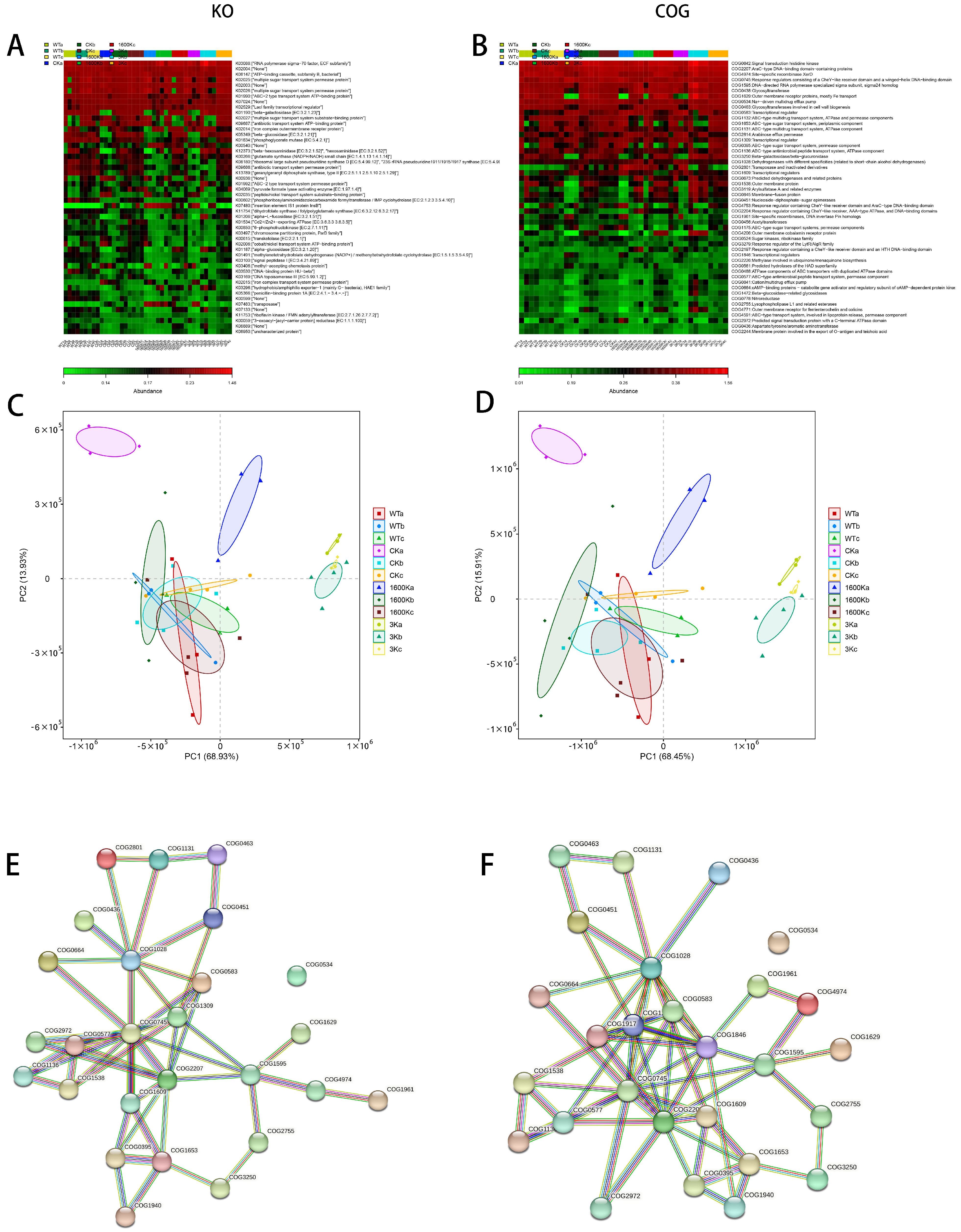
2.5. Structure and Abundance of Mouse Gut Microbiota
2.6. Mice Intestinal Microbiota Functional Analysis
3. Discussion
4. Materials and Methods
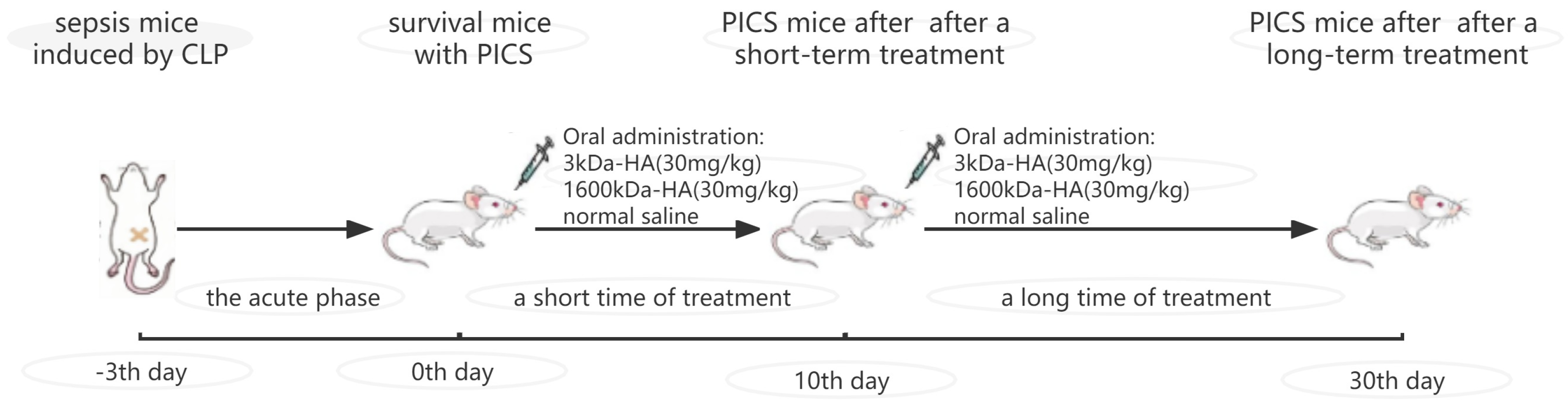
4.1. Animal Experiment
4.2. Monitoring of Mouse Health Status during Treatment
4.3. Collection of Mouse Organs and Histopathological Examination
4.4. Collection and Analysis of Intestinal Microbiota
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosinski, S.; Mohammad, R.A.; Pitcher, M.; Haezebrouck, E.; Coe, A.B.; Costa, D.K.; Prescott, H.C.; Iwashyna, T.J.; McSparron, J.I. What Is Post-Intensive Care Syndrome (PICS)? Am. J. Respir. Crit. Care Med. 2020, 201, P15–P16. [Google Scholar] [CrossRef]
- Guirgis, F.W.; Brakenridge, S.; Sutchu, S.; Khadpe, J.D.; Robinson, T.; Westenbarger, R.; Topp, S.T.; Kalynych, C.J.; Reynolds, J.; Dodani, S.; et al. The long-term burden of severe sepsis and septic shock: Sepsis recidivism and organ dysfunction. J. Trauma. Acute Care Surg. 2016, 81, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Stortz, J.A.; Mira, J.C.; Raymond, S.L.; Loftus, T.J.; Ozrazgat-Baslanti, T.; Wang, Z.; Ghita, G.L.; Leeuwenburgh, C.; Segal, M.S.; Bihorac, A.; et al. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J. Trauma. Acute Care Surg. 2018, 84, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Liu, K.; Kawakami, D.; Kawai, Y.; Morisawa, T.; Nishida, T.; Sumita, H.; Unoki, T.; Hifumi, T.; Iida, Y.; et al. Post-Intensive Care Syndrome and Its New Challenges in Coronavirus Disease 2019 (COVID-19) Pandemic: A Review of Recent Advances and Perspectives. J. Clin. Med. 2021, 10, 3870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, W.; Miao, C.; Zhong, J. Hypercatabolism and Anti-catabolic Therapies in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Nutr. 2022, 9, 941097. [Google Scholar] [CrossRef]
- Haak, B.W.; Wiersinga, W.J. The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2017, 2, 135–143. [Google Scholar] [CrossRef]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Hyaluronan as a Prominent Biomolecule with Numerous Applications in Medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef]
- Lee, Y.; Sugihara, K.; Gillilland, M.G.; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef]
- Asari, A.; Kanemitsu, T.; Kurihara, H. Oral administration of high molecular weight hyaluronan (900 kDa) controls immune system via Toll-like receptor 4 in the intestinal epithelium. J. Biol. Chem. 2010, 285, 24751–24758. [Google Scholar] [CrossRef]
- Kang, Y.; Sun, W.; Li, S.; Li, M.; Fan, J.; Du, J.; Liang, X.J.; Peng, X. Oligo Hyaluronan-Coated Silica/Hydroxyapatite Degradable Nanoparticles for Targeted Cancer Treatment. Adv. Sci. 2019, 6, 1900716. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, Y.K.; Kim, G.B.; Nam, G.H.; Kim, S.A.; Park, Y.; Yang, Y.; Kim, I.S. Degradation of tumour stromal hyaluronan by small extracellular vesicle-PH20 stimulates CD103+ dendritic cells and in combination with PD-L1 blockade boosts anti-tumour immunity. J. Extracell. Vesicles 2019, 8, 1670893. [Google Scholar] [CrossRef]
- Chang, C.C.; Hsieh, M.S.; Liao, S.T.; Chen, Y.H.; Cheng, C.W.; Huang, P.T.; Lin, Y.F.; Chen, C.H. Hyaluronan regulates PPARγ and inflammatory responses in IL-1β-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr. Polym. 2012, 90, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Pugh, A.M.; Auteri, N.J.; Goetzman, H.S.; Caldwell, C.C.; Nomellini, V. A Murine Model of Persistent Inflammation, Immune Suppression, and Catabolism Syndrome. Int. J. Mol. Sci. 2017, 18, 1741. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kawasaki, A.; Suzuki, N.; Hosoi, S.; Fujita, T.; Hachisu, S.; Nakano, H.; Naraba, H.; Mochizuki, M.; Takahashi, Y. Grip Strength Correlates with Mental Health and Quality of Life after Critical Care: A Retrospective Study in a Post-Intensive Care Syndrome Clinic. J. Clin. Med. 2021, 10, 3044. [Google Scholar] [CrossRef]
- Pitcher, M.C.; Cummings, J.H. Hydrogen sulphide: A bacterial toxin in ulcerative colitis? Gut 1996, 39, 1–4. [Google Scholar] [CrossRef]
- Ramnarain, D.; Aupers, E.; Oudsten, B.; Oldenbeuving, A.; Vries, J.; Pouwels, S. Post Intensive Care Syndrome (PICS): An overview of the definition, etiology, risk factors, and possible counseling and treatment strategies. Expert. Rev. Neurother. 2021, 21, 1159–1177. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery From Sepsis: A Review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.C.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Rubenfeld, G.D. Understanding Long-Term Outcomes Following Sepsis: Implications and Challenges. Curr. Infect. Dis. Rep. 2016, 18, 37. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Verma, A.K.; Ahuja, V.; Paul, J. Real-time analysis of mucosal flora in patients with inflammatory bowel disease in India. J. Clin. Microbiol. 2010, 48, 4279–4282. [Google Scholar] [CrossRef]
- Willing, B.; Halfvarson, J.; Dicksved, J.; Rosenquist, M.; Järnerot, G.; Engstrand, L.; Tysk, C.; Jansson, J.K. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 653–660. [Google Scholar] [CrossRef]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef]
- Dong, N.; Yang, X.; Chan, E.W.; Zhang, R.; Chen, S. Klebsiella species: Taxonomy, hypervirulence and multidrug resistance. EBioMedicine. 2022, 79, 103998. [Google Scholar] [CrossRef]
- Martin, H.M.; Campbell, B.J.; Hart, C.A.; Mpofu, C.; Nayar, M.; Singh, R.; Englyst, H.; Williams, H.F.; Rhodes, J.M. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology 2004, 127, 80–93. [Google Scholar] [CrossRef]
- Sepehri, S.; Khafipour, E.; Bernstein, C.N.; Coombes, B.K.; Pilar, A.V.; Karmali, M.; Ziebell, K.; Krause, D.O. Characterization of Escherichia coli isolated from gut biopsies of newly diagnosed patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 1451–1463. [Google Scholar] [CrossRef]
- Samuelsson, A.; Isaksson, B.; Hanberger, H.; Olhager, E. Late-onset neonatal sepsis, risk factors and interventions: An analysis of recurrent outbreaks of Serratia marcescens, 2006–2011. J. Hosp. Infect. 2014, 86, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Khaitlina, S.; Bozhokina, E.; Tsaplina, O.; Efremova, T. Bacterial Actin-Specific Endoproteases Grimelysin and Protealysin as Virulence Factors Contributing to the Invasive Activities of Serratia. Int. J. Mol. Sci. 2020, 21, 4025. [Google Scholar] [CrossRef] [PubMed]
- Mooij, C.E.M.; Groningen, L.F.J.; Molendijk, E.B.D.; Wardill, H.R.; Velden, W.J.F.M.; Blijlevens, N.M.A. Blautia Abundance and Mucosal Barrier Injury: A Complex Play of Cause and Effect. Clin. Infect. Dis. 2023, 76, 1152–1153. [Google Scholar] [CrossRef] [PubMed]
- Dürre, P. Physiology and Sporulation in Clostridium. Microbiol. Spectr. 2014, 2, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Tet, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680.e7. [Google Scholar] [CrossRef]
- Satchell, K.J.F. Engineered Bacteria for Cholera Prophylaxis. Cell Host Microbe 2018, 24, 192–194. [Google Scholar] [CrossRef]
- Ruggirello, M.; Cocolin, L.; Dolci, P. Fate of Lactococcus lactis starter cultures during late ripening in cheese models. Food Microbiol. 2016, 59, 112–118. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Dupont, H.L.; Jiang, Z.D.; Dupont, A.W.; Utay, N.S. The Intestinal Microbiome in Human Health and Disease. Trans. Am. Clin. Clim. Assoc. 2020, 131, 178–197. [Google Scholar]
- Mizrahy, S.; Raz, S.R.; Hasgaard, M.; Liu, H.; Soffer-Tsur, N.; Cohen, K.; Dvash, R.; Landsman-Milo, D.; Bremer, M.G.; Moghimi, S.M.; et al. Hyaluronan-coated nanoparticles: The influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J. Control Release 2011, 156, 231–238. [Google Scholar] [CrossRef]
- Chaaban, H.; Burge, K.; Eckert, J.; Trammell, M.; Dyer, D.; Keshari, R.S.; Silasi, R.; Regmi, G.; Lupu, C.; Good, M.; et al. Acceleration of Small Intestine Development and Remodeling of the Microbiome Following Hyaluronan 35 kDa Treatment in Neonatal Mice. Nutrients 2021, 13, 2030. [Google Scholar] [CrossRef]
- Lee, C.H.; Chiang, C.F.; Kuo, F.C.; Su, S.C.; Huang, C.L.; Liu, J.S.; Lu, C.H.; Hsieh, C.H.; Wang, C.C.; Lee, C.H.; et al. High-Molecular-Weight Hyaluronic Acid Inhibits IL-1β-Induced Synovial Inflammation and Macrophage Polarization through the GRP78-NF-κB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 11917. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Su, C.W.; Ji, Q.; Chen, C.Y.; Wang, R.; Vijaya Kumar, D.; Lan, J.; Jiao, L.; Shi, H.N. Hyaluronan-induced alterations of the gut microbiome protects mice against Citrobacter rodentium infection and intestinal inflammation. Gut Microbes 2021, 13, 1972757. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, G.; Shang, Q.; Chen, X.; Liu, W.; Pi, X.; Zhu, L.; Yin, Y.; Yu, G.; Wang, X. In vitro fermentation of alginate and its derivatives by human gut microbiota. Anaerobe 2016, 39, 19–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Jiang, Y.; Zhu, Q.; Liu, D.; Chang, M.; Wang, Y.; Xi, R.; Wang, W. Hyaluronan with Different Molecular Weights Can Affect the Gut Microbiota and Pathogenetic Progression of Post-Intensive Care Syndrome Mice in Different Ways. Int. J. Mol. Sci. 2023, 24, 9757. https://doi.org/10.3390/ijms24119757
Li L, Jiang Y, Zhu Q, Liu D, Chang M, Wang Y, Xi R, Wang W. Hyaluronan with Different Molecular Weights Can Affect the Gut Microbiota and Pathogenetic Progression of Post-Intensive Care Syndrome Mice in Different Ways. International Journal of Molecular Sciences. 2023; 24(11):9757. https://doi.org/10.3390/ijms24119757
Chicago/Turabian StyleLi, Lu, Yuanyuan Jiang, Qianqian Zhu, Dawei Liu, Mingkai Chang, Yongzhe Wang, Ruitong Xi, and Wenfei Wang. 2023. "Hyaluronan with Different Molecular Weights Can Affect the Gut Microbiota and Pathogenetic Progression of Post-Intensive Care Syndrome Mice in Different Ways" International Journal of Molecular Sciences 24, no. 11: 9757. https://doi.org/10.3390/ijms24119757
APA StyleLi, L., Jiang, Y., Zhu, Q., Liu, D., Chang, M., Wang, Y., Xi, R., & Wang, W. (2023). Hyaluronan with Different Molecular Weights Can Affect the Gut Microbiota and Pathogenetic Progression of Post-Intensive Care Syndrome Mice in Different Ways. International Journal of Molecular Sciences, 24(11), 9757. https://doi.org/10.3390/ijms24119757





