Intramolecular Hydrogen Bonding in N6-Substituted 2-Chloroadenosines: Evidence from NMR Spectroscopy
Abstract
1. Introduction
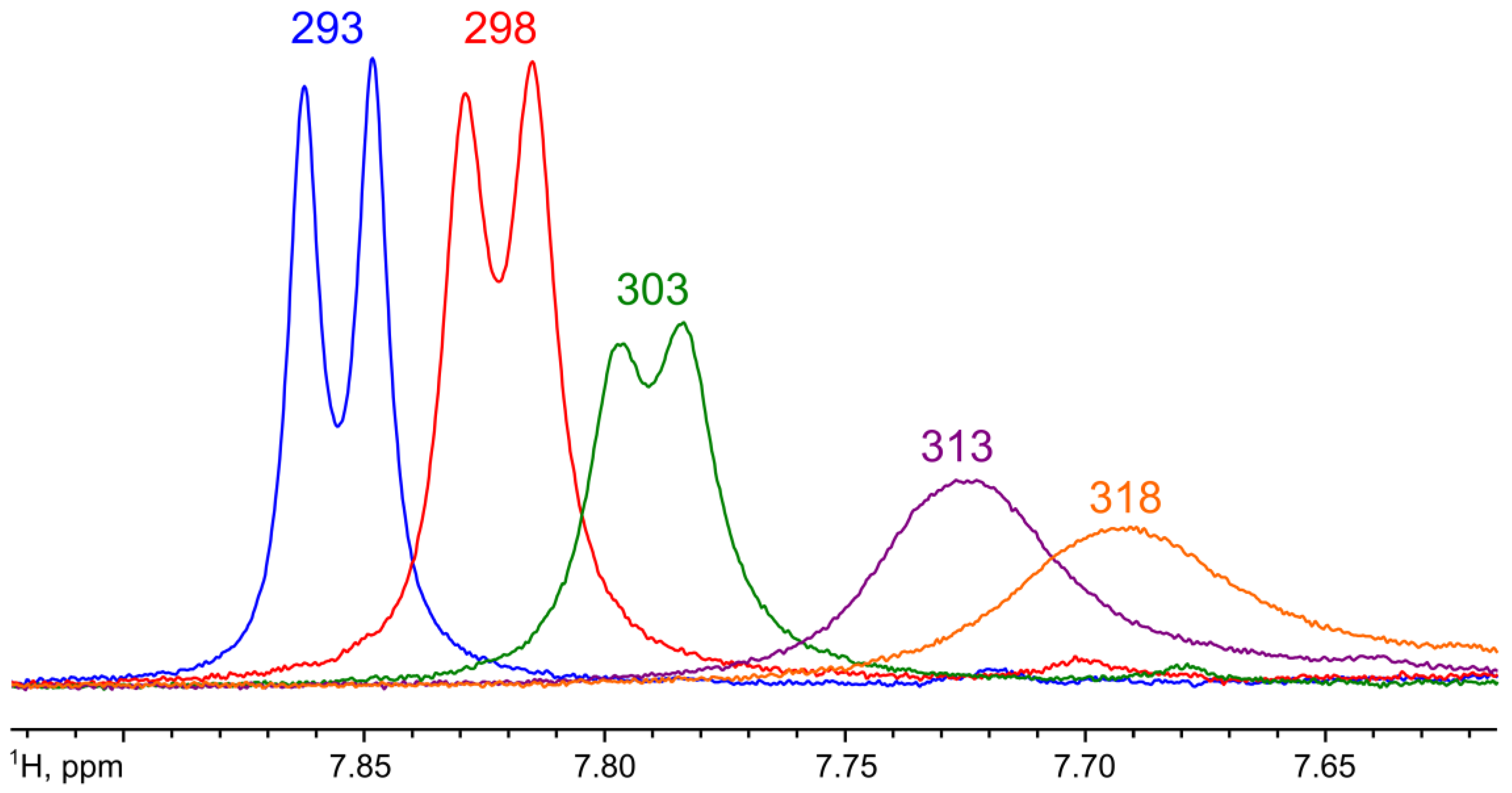
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robak, T.; Lech-Maranda, E.; Korycka, A.; Robak, E. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: Mechanism of action and clinical activity. Curr. Med. Chem. 2006, 13, 3165–3189. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Robak, T. Older and new purine nucleoside analogs for patients with acute leukemias. Cancer Treat. Rev. 2013, 39, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Aurelio, L.; Baltos, J.A.; Ford, L.; Nguyen, A.T.N.; Jorg, M.; Devine, S.M.; Valant, C.; White, P.J.; Christopoulos, A.; May, L.T.; et al. A Structure-Activity Relationship Study of Bitopic N-6-Substituted Adenosine Derivatives as Biased Adenosine A(1) Receptor Agonists. J. Med. Chem. 2018, 61, 2087–2103. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.-G.; Paoletta, S.; Kiselev, E.; Chakraborty, S.; Jayasekara, P.S.; Balasubramanian, R.; Tosh, D.K. John Daly Lecture: Structure-guided Drug Design for Adenosine and P2Y Receptors. Comput. Struct. Biotechnol. J. 2015, 13, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Berzina, M.Y.; Eletskaya, B.Z.; Kayushin, A.L.; Dorofeeva, E.V.; Lutonina, O.I.; Fateev, I.V.; Paramonov, A.S.; Kostromina, M.A.; Zayats, E.A.; Abramchik, Y.A.; et al. Synthesis of 2-chloropurine ribosides with chiral amino acid amides at C6 and their evaluation as A1 adenosine receptor agonists. Bioorg. Chem. 2022, 126, 105878. [Google Scholar] [CrossRef]
- Dračínský, M.; Pohl, R. NMR studies of purines. Annu. Rep. NMR Spectrosc. 2014, 82, 59–113. [Google Scholar] [CrossRef]
- Bakkestuen, A.K.; Gundersen, L.-L.; Petersen, D.; Utenova, B.T.; Vik, A. Synthesis and antimycobacterial activity of agelasine E and analogs. Org. Biomol. Chem. 2005, 3, 1025–1033. [Google Scholar] [CrossRef]
- Roggen, H.; Gundersen, L.L. Synthetic Studies Directed towards Agelasine Analogs–Synthesis, Tautomerism, and Alkylation of 2-Substituted N-Methoxy-9-methyl-9H-purin-6-amines. Eur. J. Org. Chem. 2008, 30, 5099–5106. [Google Scholar] [CrossRef]
- Fujii, T.; Saito, T.; Itaya, T.; Kizu, K.; Kumazawa, Y.; Nakajima, S. Purines. XXIX. Syntheses of 9-Alkyl-2-deuterio-N6-methoxyadenines and 2-Deuterio-N6, 9-dimethyladenine: Tautomerism in 9-Substituted N6-Alkoxyadenines. Chem. Pharm. Bull. 1987, 35, 4482–4493. [Google Scholar] [CrossRef]
- Morozov, Y.V.; Savin, F.; Chekhov, V.; Budowsky, E.; Yakovlev, D.Y. Photochemistry of N6-methoxyadenosine and of N4-hydroxycytidine and its methyl derivatives I: Spectroscopic and quantum chemical investigation of ionic and tautomeric forms: Syn-anti isomerization. J. Photochem. 1982, 20, 229–252. [Google Scholar] [CrossRef]
- Baranowski, D.; Framski, G.; Wyszko, E.; Ostrowski, T. Studies on structure of kinetin riboside and its analogues by variable-temperature NMR. J. Mol. Struct. 2019, 1195, 110–118. [Google Scholar] [CrossRef]
- Novotná, R.; Trávníček, Z.; Popa, I. X-ray crystallographic and NMR study of the tautomerism in kinetin, kinetin riboside and their derivatives: A comparison between the solid state and solution. J. Mol. Struct. 2010, 963, 202–210. [Google Scholar] [CrossRef]
- Martin, D.; Reese, C. Restricted rotation in methylated derivatives of adenosine and cytidine. Chem. Commun. 1967, 1275–1276. [Google Scholar] [CrossRef]
- Engel, J.D.; Von Hippel, P.H. Effects of methylation on the stability of nucleic acid conformations. Monomer level. Biochemistry 1974, 13, 4143–4158. [Google Scholar] [CrossRef]
- Dodin, G.; Dreyfus, M.; Dubois, J.-E. Restricted rotation in 6-methylaminopurine and analogues. Intramolecular hydrogen bonding. J. Chem. Soc. Perkin Trans. 2 1979, 438–442. [Google Scholar] [CrossRef]
- Vícha, J.; Malon, M.; Veselá, P.; Humpa, O.; Strnad, M.; Marek, R. 1H-, 13C-, and 15N-NMR chemical shifts for selected glucosides and ribosides of aromatic cytokinins. Magn. Reson. Chem. 2010, 48, 318–322. [Google Scholar] [CrossRef]
- Casati, S.; Manzocchi, A.; Ottria, R.; Ciuffreda, P. 1H, 13C and 15N NMR assignments for N6-isopentenyladenosine/inosine analogues. Magn. Reson. Chem. 2010, 48, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Drenichev, M.S.; Oslovsky, V.E.; Sun, L.; Tijsma, A.; Kurochkin, N.N.; Tararov, V.I.; Chizhov, A.O.; Neyts, J.; Pannecouque, C.; Leyssen, P. Modification of the length and structure of the linker of N6-benzyladenosine modulates its selective antiviral activity against enterovirus 71. Eur. J. Med. Chem. 2016, 111, 84–94. [Google Scholar] [CrossRef]
- Casati, S.; Manzocchi, A.; Ottria, R.; Ciuffreda, P. 1H, 13C and 15N NMR spectral assignments of adenosine derivatives with different amino substituents at C6-position. Magn. Reson. Chem. 2011, 49, 279–283. [Google Scholar] [CrossRef]
- Oslovsky, V.E.; Drenichev, M.S.; Alexeev, C.S.; Solyev, P.N.; Esipov, R.S.; Mikhailov, S.N. Synthesis of Cytokinins via Enzymatic Arsenolysis of Purine Nucleosides. Curr. Protoc. Nucleic Acid Chem. 2018, 75, e61. [Google Scholar] [CrossRef]
- Savelieva, E.M.; Zenchenko, A.A.; Drenichev, M.S.; Kozlova, A.A.; Kurochkin, N.N.; Arkhipov, D.V.; Chizhov, A.O.; Oslovsky, V.E.; Romanov, G.A. In Planta, In Vitro and In Silico Studies of Chiral N 6-Benzyladenine Derivatives: Discovery of Receptor-Specific S-Enantiomers with Cytokinin or Anticytokinin Activities. Int. J. Mol. Sci. 2022, 23, 11334. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Scammells, P.J. Synthesis and Utility of 2-Halo-O6-(benzotriazol-1-yl)-Functionalized Purine Nucleosides. Eur. J. Org. Chem. 2011, 6, 1092–1098. [Google Scholar] [CrossRef]
- Savelieva, E.M.; Oslovsky, V.E.; Karlov, D.S.; Kurochkin, N.N.; Getman, I.A.; Lomin, S.N.; Sidorov, G.V.; Mikhailov, S.N.; Osolodkin, D.I.; Romanov, G.A. Cytokinin activity of N6-benzyladenine derivatives assayed by interaction with the receptors in planta, in vitro, and in silico. Phytochemistry 2018, 149, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Satpati, S.; Dixit, A.; Pal, S. Novel homologated-apio adenosine derivatives as A3 adenosine receptor agonists: Design, synthesis and molecular docking studies. RSC Adv. 2016, 6, 11233–11239. [Google Scholar] [CrossRef]
- Niu, H.-Y.; Bai, S.-X.; Wu, S.; Qu, G.-R.; Guo, H.-M. Synthesis of Chiral N-(Purin-6-yl)amino Acid Derivatives by using Natural Amino Acids as Starting Materials. Asian J. Org. Chem. 2012, 1, 238–244. [Google Scholar] [CrossRef]
- Eletskaya, B.Z.; Berzina, M.Y.; Fateev, I.V.; Kayushin, A.L.; Dorofeeva, E.V.; Lutonina, O.I.; Zorina, E.A.; Antonov, K.V.; Paramonov, A.S.; Muzyka, I.S.; et al. Enzymatic Synthesis of 2-Chloropurine Arabinonucleosides with Chiral Amino Acid Amides at the C6 Position and an Evaluation of Antiproliferative Activity In Vitro. Int. J. Mol. Sci. 2023, 24, 6223. [Google Scholar] [CrossRef]
- Krasnov, V.P.; Musiyak, V.V.; Vozdvizhenskaya, O.A.; Galegov, G.A.; Andronova, V.L.; Gruzdev, D.A.; Chulakov, E.N.; Vigorov, A.Y.; Ezhikova, M.A.; Kodess, M.I. N-[ω-(Purin-6-yl) aminoalkanoyl] Derivatives of Chiral Heterocyclic Amines as Promising Anti-Herpesvirus Agents. Eur. J. Org. Chem. 2019, 2019, 4811–4821. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Smirnova, O.A.; Golubeva, N.A.; Ivanov, M.A.; Tunitskaya, V.L.; Shipitsyn, A.V.; Alexandrova, L.A. Base-Modified Ribonucleosides as Potential Anti-Hepatitis C virus Agents. Nucleic Acids Symp. Ser. 2008, 52, 619–620. [Google Scholar] [CrossRef]
- Γoлубева, Н.; Иванoв, А.; Иванoв, М.; Батюнина, О.; Шипицын, А.; Туницкая, В.; Александрoва, Л. Нoвые аналoги нуклеoзидoв и их 5′-трифoсфаты: синтез и биoлoгические свoйства. Вестник Мoскoвскoгo Университета. Серия 2 Химия 2008, 49, 112–116. [Google Scholar]
- Reitz, A.B.; Graden, D.W.; Jordan, A.D., Jr.; Maryanoff, B.E. Conformational study of N-substituted adenines by dynamic proton NMR: Relatively high barrier to rotation about C6-N6 in N3,N6-disubstituted adenines. J. Org. Chem. 1990, 55, 5761–5766. [Google Scholar] [CrossRef]
- Department of Chemistry and Biochemistry. NMR Facility. NMR Theory and Techniques. Available online: https://nmr.chem.ucsb.edu/education/part3.html (accessed on 12 April 2023).
- Dingley, A.J.; Grzesiek, S. Direct Observation of Hydrogen Bonds in Nucleic Acid Base Pairs by Internucleotide 2JNN Couplings. J. Am. Chem. Soc. 1998, 120, 8293–8297. [Google Scholar] [CrossRef]
- Meissner, A.; Sørensen, O.W. New Techniques for the Measurement of C′N and C′HNJ Coupling Constants across Hydrogen Bonds in Proteins. J. Magn. Reson. 2000, 143, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Esipov, R.S.; Gurevich, A.I.; Chuvikovsky, D.V.; Chupova, L.A.; Muravyova, T.I.; Miroshnikov, A.I. Overexpression of Escherichia coli genes encoding nucleoside phosphorylases in the pET/bl21(DE3) system yields. Act. Recomb. Enzym. Protein Expr. Purif. 2002, 24, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V.A.; Samultsev, D.O.; Rulev, A.Y.; Krivdin, L.B. Theoretical and experimental 15N NMR study of enamine–imine tautomerism of 4-trifluoromethyl[b]benzo-1,4-diazepine system. Magn. Reson. Chem. 2015, 53, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Szöllősy, Á.; Hermecz, I.; Horváth, Á.; Mészáros, Z. Tautomerism of 9-formyltetrahydro-4 H-pyrido [1, 2-a] pyrimidin-4-ones and their ring homologues: A 1H, 13C, and 15N nuclear magnetic resonance study. J. Chem. Soc. Perkin Trans. 2 1985, 12, 1881–1885. [Google Scholar] [CrossRef]
- Huang, X.S.; Liu, X.; Constantine, K.L.; Leet, J.E.; Roongta, V. Observation of O–H∙∙∙N scalar coupling across a hydrogen bond in nocathiacin I. Magn. Reson. Chem. 2007, 45, 447–450. [Google Scholar] [CrossRef]
- Jansma, A.; Zhang, Q.; Li, B.; Ding, Q.; Uno, T.; Bursulaya, B.; Liu, Y.; Furet, P.; Gray, N.S.; Geierstanger, B.H. Verification of a Designed Intramolecular Hydrogen Bond in a Drug Scaffold by Nuclear Magnetic Resonance Spectroscopy. J. Med. Chem. 2007, 50, 5875–5877. [Google Scholar] [CrossRef]
- Muller, R. Encyclopedia of Nuclear Magnetic Resonance; Wiley: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Denisov, G.S. A review with comprehensive data on experimental indirect scalar NMR spin–spin coupling constants across hydrogen bonds. Magn. Reson. Chem. 2008, 46, 599–624. [Google Scholar] [CrossRef]
- Plochocka, D.; Rabczenko, A.; Davies, D.B. Intramolecular hydrogen bonding and molecular conformations of nucleosides: N (6)-Dimethyl-2′, 3′-isopropylidene adenosine. Biochim. Biophys. Acta (BBA)-Nucleic Acids Protein Synth. 1997, 476, 1–15. [Google Scholar] [CrossRef]




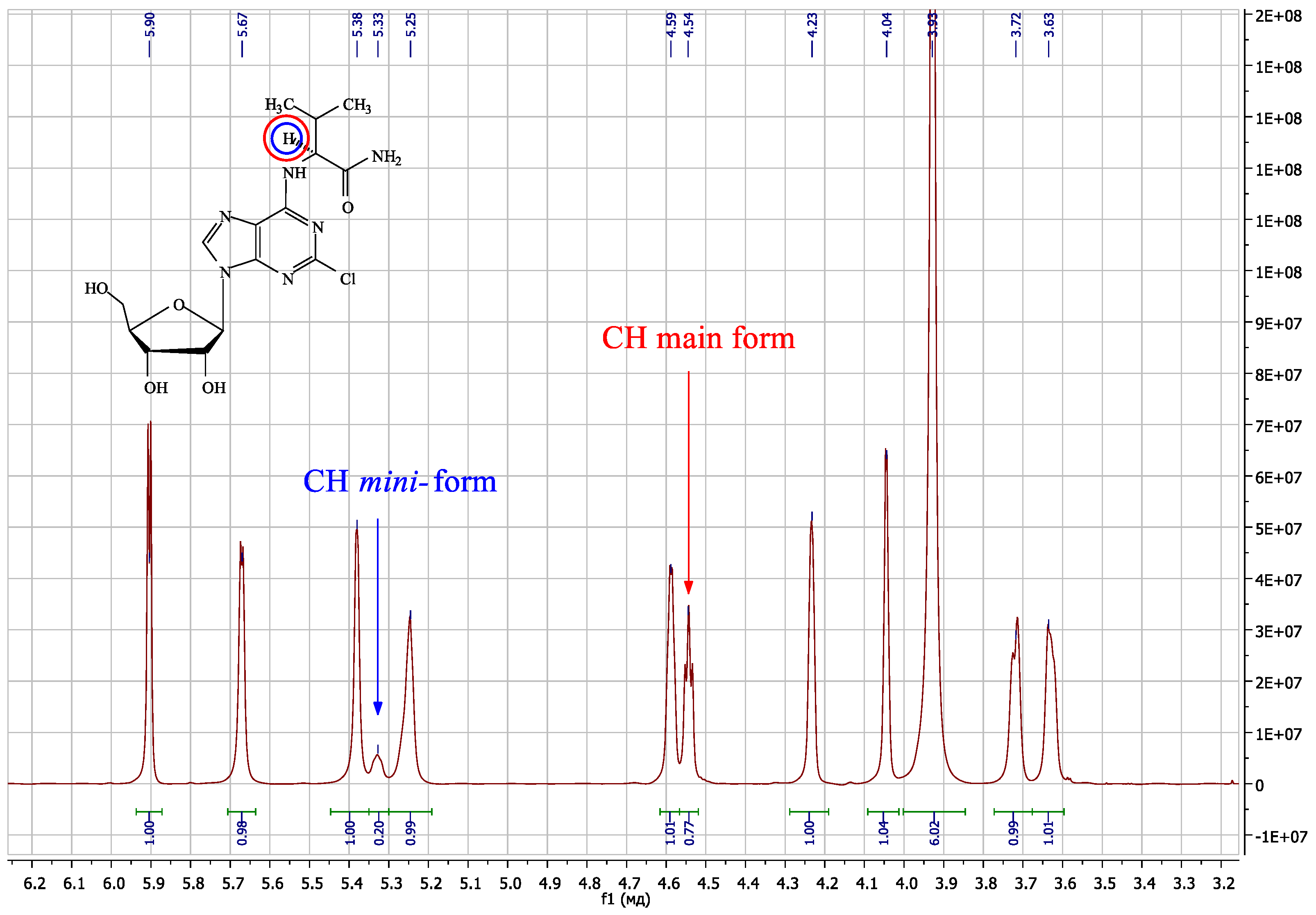
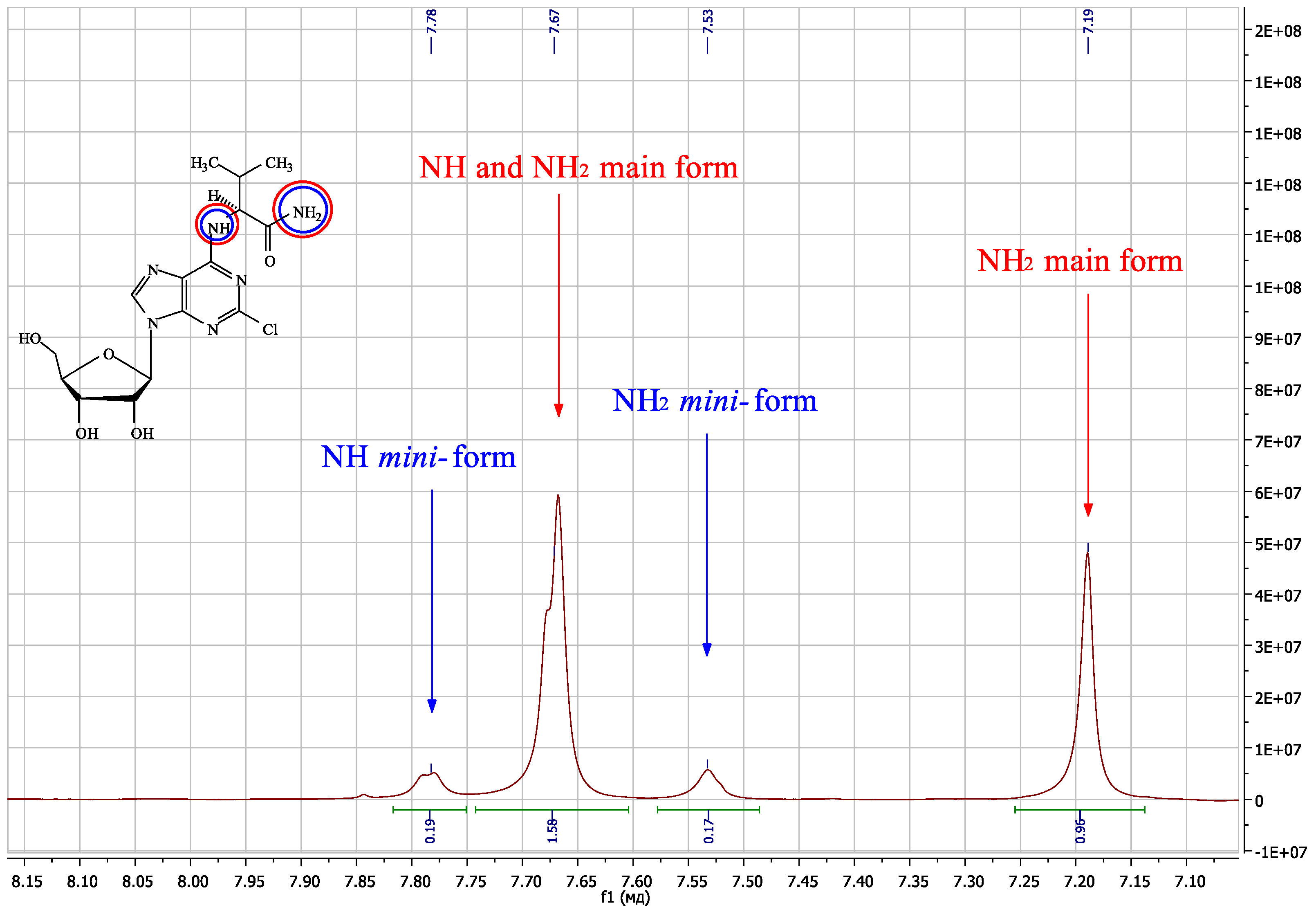
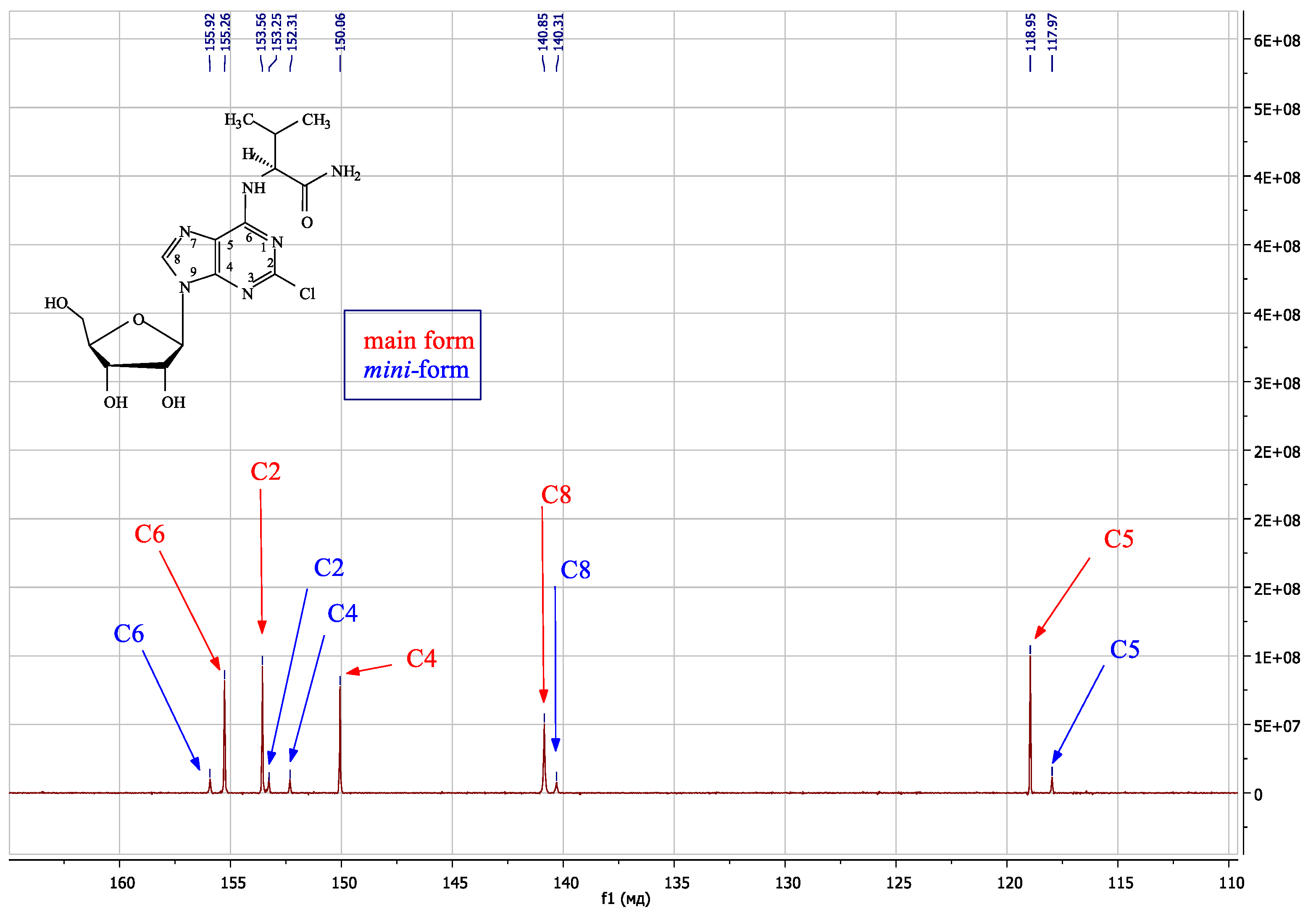

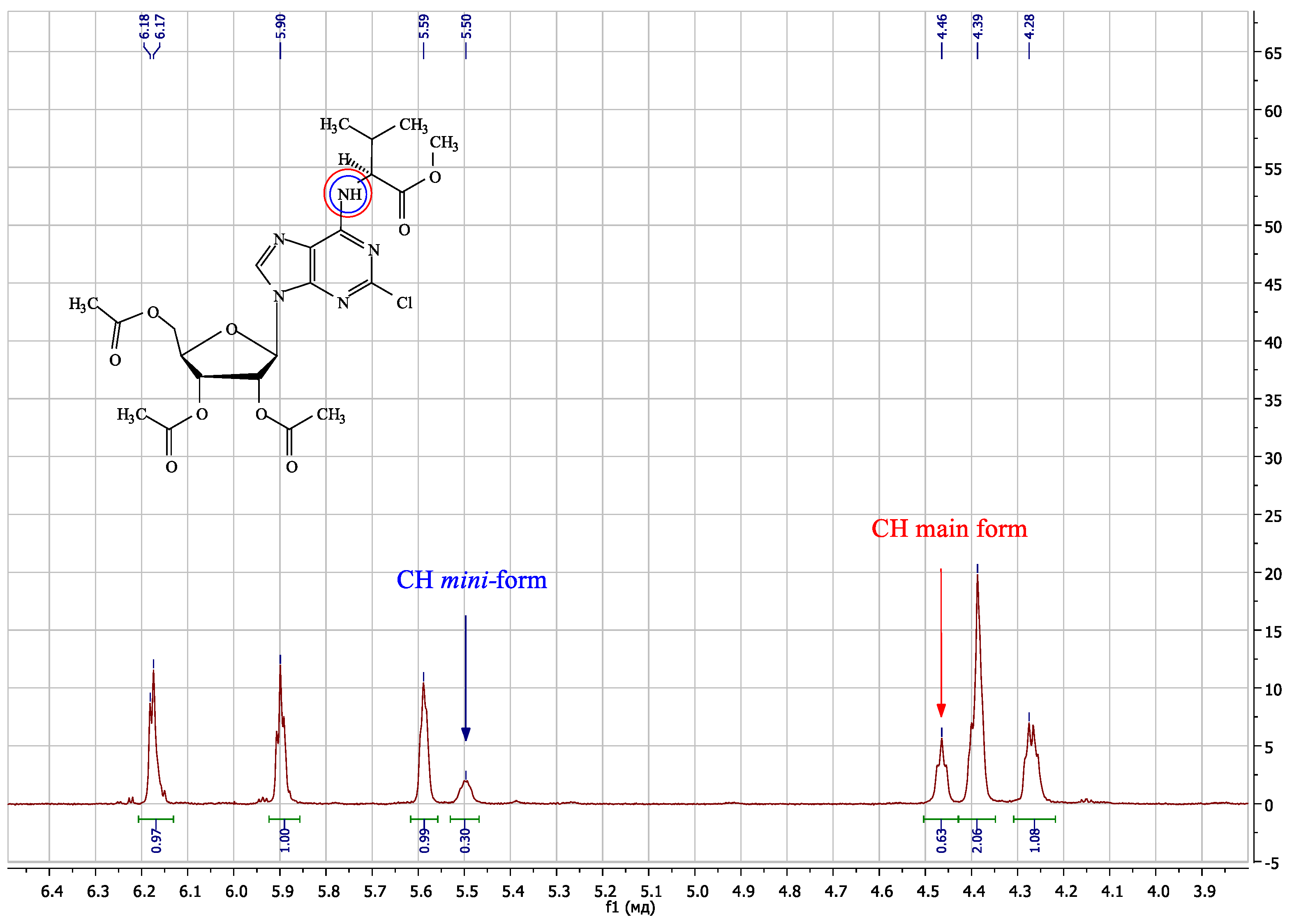








| Compound | Amino Acid Residue | Δδ N6CH, ppm | Δδ N6H, ppm | Δδ C8H, ppm | Δδ N6CH, ppm | Δδ N6H, ppm | Δδ N7, ppm | Δδ C4, ppm |
|---|---|---|---|---|---|---|---|---|
| 1a | Gly | 0.42 | −0.20 | −0.06 | 2.46 | − * | − | − |
| 2a | L-Ala | 0.72 | −0.17 | −0.07 | 2.4 | 6.6 | − | − |
| 3a | L-Val | 1.04 | −0.13 | −0.07 | 1.22 | 4.1 | − | − |
| 4a | L-Ser | 0.73 | −0.05 | −0.07 | 2.57 | 7.9 | 1.4 | 1.91 |
| 5a | D-Ser | 0.73 | −0.05 | −0.08 | 2.6 | 7.5 | 1.2 | 1.88 |
| 6a | L-Thr | 0.79 | 0.03 | −0.09 | 2.45 | 6.9 | − | 1.97 |
| 7a | L-Met | 0.72 | −0.19 | −0.07 | 2.13 | 6.4 | − | − |
| 8a | L-Tyr | 0.83 | −0.10 | −0.06 | 2.96 | 6.6 | 1.4 | 1.91 |
| 9a | L-Lys | 0.75 | −0.16 | −0.07 | 2.43 | 7.6 | − | 1.80 |
| 10a | L-S-Me-Cys | 0.86 | −0.14 | −0.04 | 2.93 | 7.1 | − | 1.83 |
| 11a | Nε-Lys | 0.40 | −0.04 | −0.08 | 2.14 | − | − | − |
| 12a | β-Ala | 0.40 | −0.04 | −0.08 | 2.14 | − | − | − |
| 1b | Gly | 0.47 | −0.39 | −0.03 | 2.54 | 20.5 | − | 2.21 |
| 2b | L-Ala | 0.85 | −0.18 | −0.03 | 1.11 | 6.5 | − | 2.28 |
| 3b | L-Val | 0.79 | 0.11 | − | 2.1 | 7.6 | 2.4 | 2.23 |
| 4b | L-Ser | 0.74 | 0.07 | −0.03 | 2.27 | 8.0 | − | 2.36 |
| 5b | D-Ser | 0.74 | 0.08 | −0.03 | 2.38 | 8.1 | 1.4 | 2.29 |
| 6b | L-Thr | 0.82 | 0.07 | −0.03 | 2.26 | 7.8 | − | 2.21 |
| 7b | L-Met | 0.71 | − | −0.01 | 9.85 | 7.5 | − | 2.28 |
| 8b | L-Tyr | 0.73 | 0.16 | − | 2.27 | 8.5 | − | 2.04 |
| 9b | L-Lys | 0.79 | −0.17 | −0.05 | 2.35 | − | − | 1.85 |
| 10b | L-S-Me-Cys | 0.78 | 0.11 | − | 2.46 | 8.8 | − | 1.97 |
| 11b | Nε-Lys | 0.72 | − | −0.02 | 2.49 | − | − | 1.85 |
| 12b | β-Ala | 0.41 | −0.09 | 0.03 | 2.55 | 7.1 | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzina, M.Y.; Eletskaya, B.Z.; Kayushin, A.L.; Dorofeeva, E.V.; Lutonina, O.I.; Fateev, I.V.; Zhavoronkova, O.N.; Bashorin, A.R.; Arnautova, A.O.; Smirnova, O.S.; et al. Intramolecular Hydrogen Bonding in N6-Substituted 2-Chloroadenosines: Evidence from NMR Spectroscopy. Int. J. Mol. Sci. 2023, 24, 9697. https://doi.org/10.3390/ijms24119697
Berzina MY, Eletskaya BZ, Kayushin AL, Dorofeeva EV, Lutonina OI, Fateev IV, Zhavoronkova ON, Bashorin AR, Arnautova AO, Smirnova OS, et al. Intramolecular Hydrogen Bonding in N6-Substituted 2-Chloroadenosines: Evidence from NMR Spectroscopy. International Journal of Molecular Sciences. 2023; 24(11):9697. https://doi.org/10.3390/ijms24119697
Chicago/Turabian StyleBerzina, Maria Ya., Barbara Z. Eletskaya, Alexei L. Kayushin, Elena V. Dorofeeva, Olga I. Lutonina, Ilya V. Fateev, Olga N. Zhavoronkova, Arthur R. Bashorin, Alexandra O. Arnautova, Olga S. Smirnova, and et al. 2023. "Intramolecular Hydrogen Bonding in N6-Substituted 2-Chloroadenosines: Evidence from NMR Spectroscopy" International Journal of Molecular Sciences 24, no. 11: 9697. https://doi.org/10.3390/ijms24119697
APA StyleBerzina, M. Y., Eletskaya, B. Z., Kayushin, A. L., Dorofeeva, E. V., Lutonina, O. I., Fateev, I. V., Zhavoronkova, O. N., Bashorin, A. R., Arnautova, A. O., Smirnova, O. S., Antonov, K. V., Paramonov, A. S., Dubinnyi, M. A., Esipov, R. S., Miroshnikov, A. I., & Konstantinova, I. D. (2023). Intramolecular Hydrogen Bonding in N6-Substituted 2-Chloroadenosines: Evidence from NMR Spectroscopy. International Journal of Molecular Sciences, 24(11), 9697. https://doi.org/10.3390/ijms24119697










