Inhibition of Gap Junction Formation Prior to Implantation of Bone Marrow-Derived Mesenchymal Cells Improves Function in the Ischemic Myocardium
Abstract
1. Introduction
2. Results
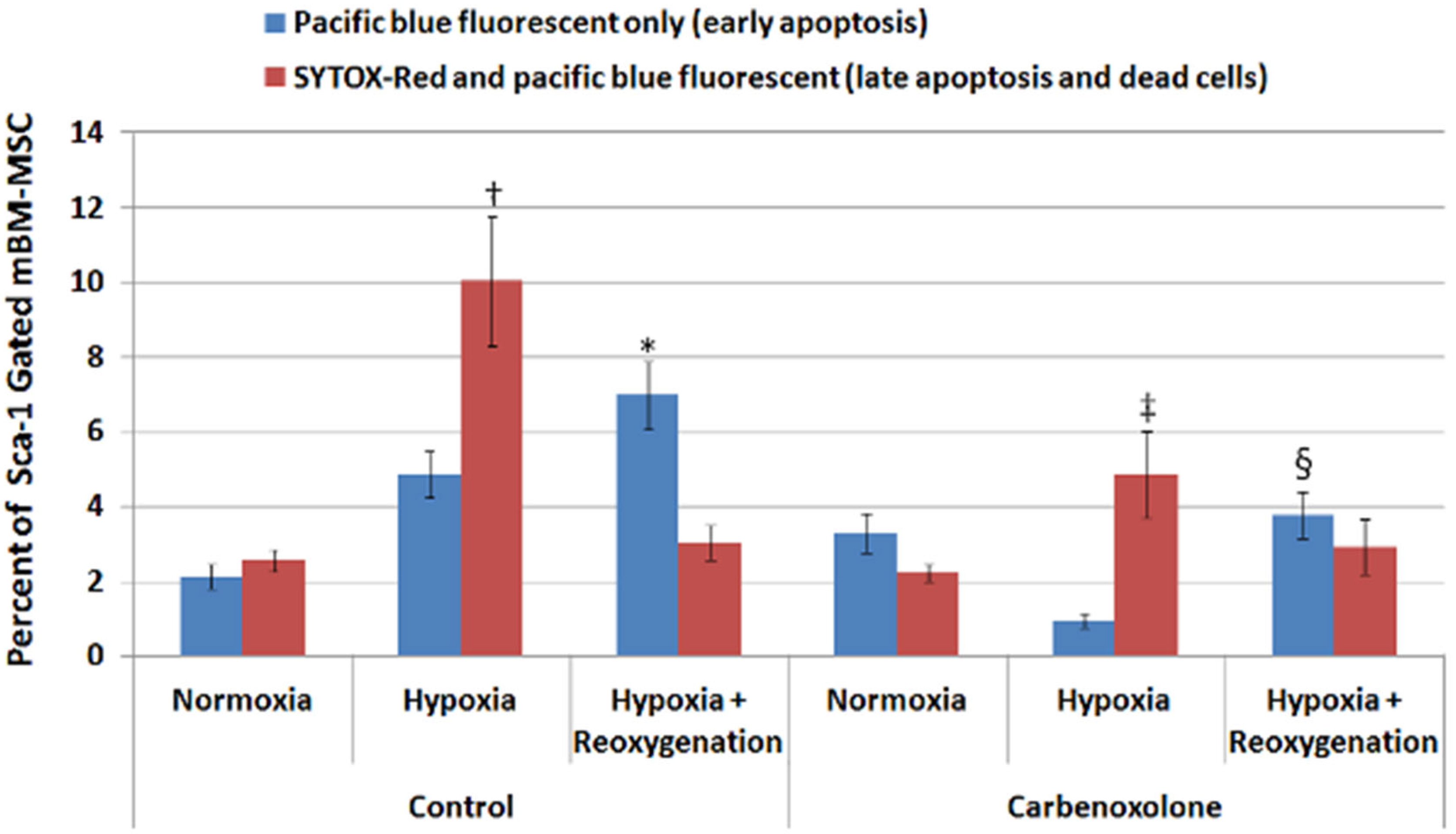
2.1. Hypoxic Myocytes Induced Apoptosis and Cell Death in Co-Cultured BM-MSCs Which Could Be Attenuated by Carbenoxolone (CBX)
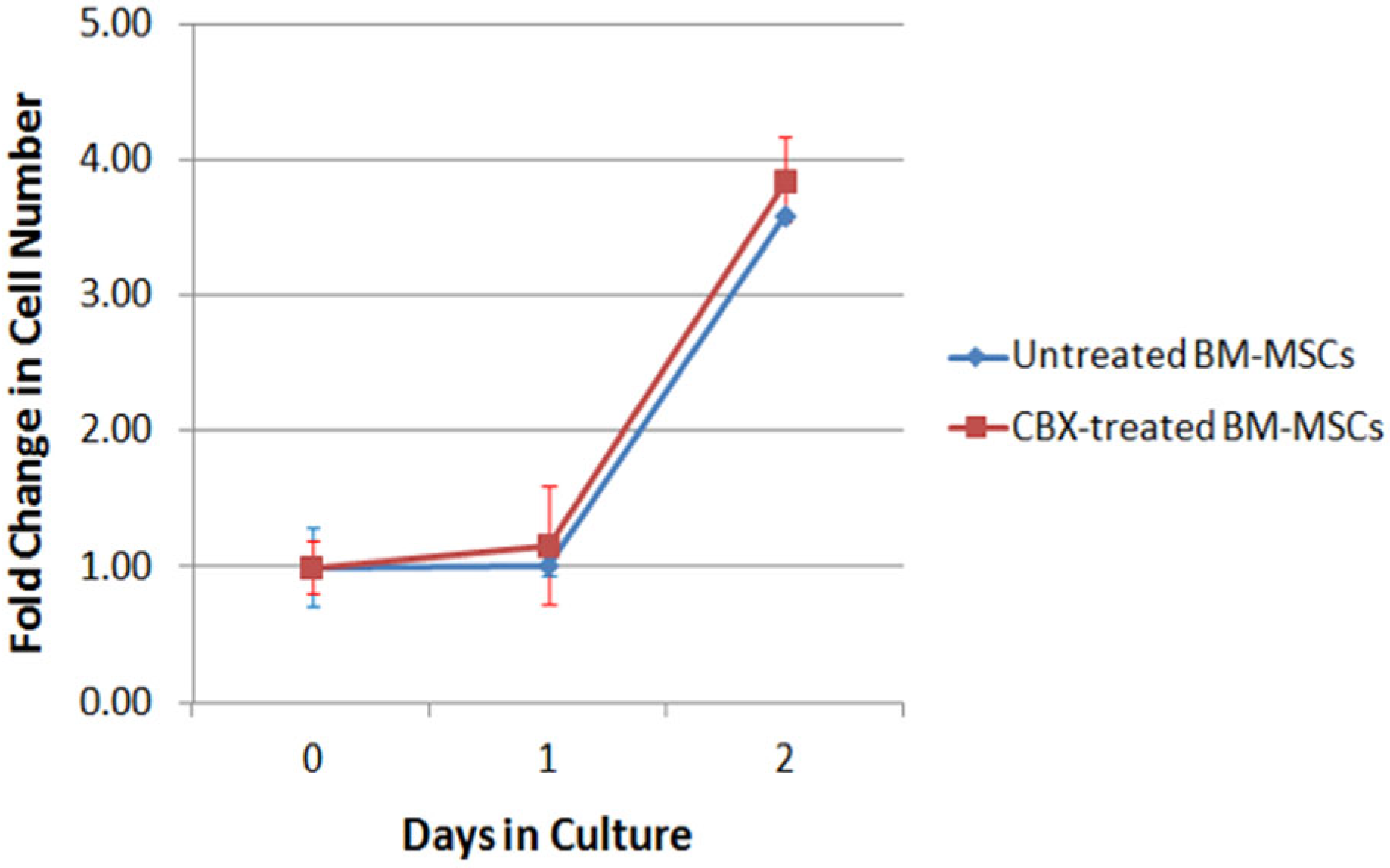
2.2. CBX Treatment Did Not Affect BM-MSC Proliferation
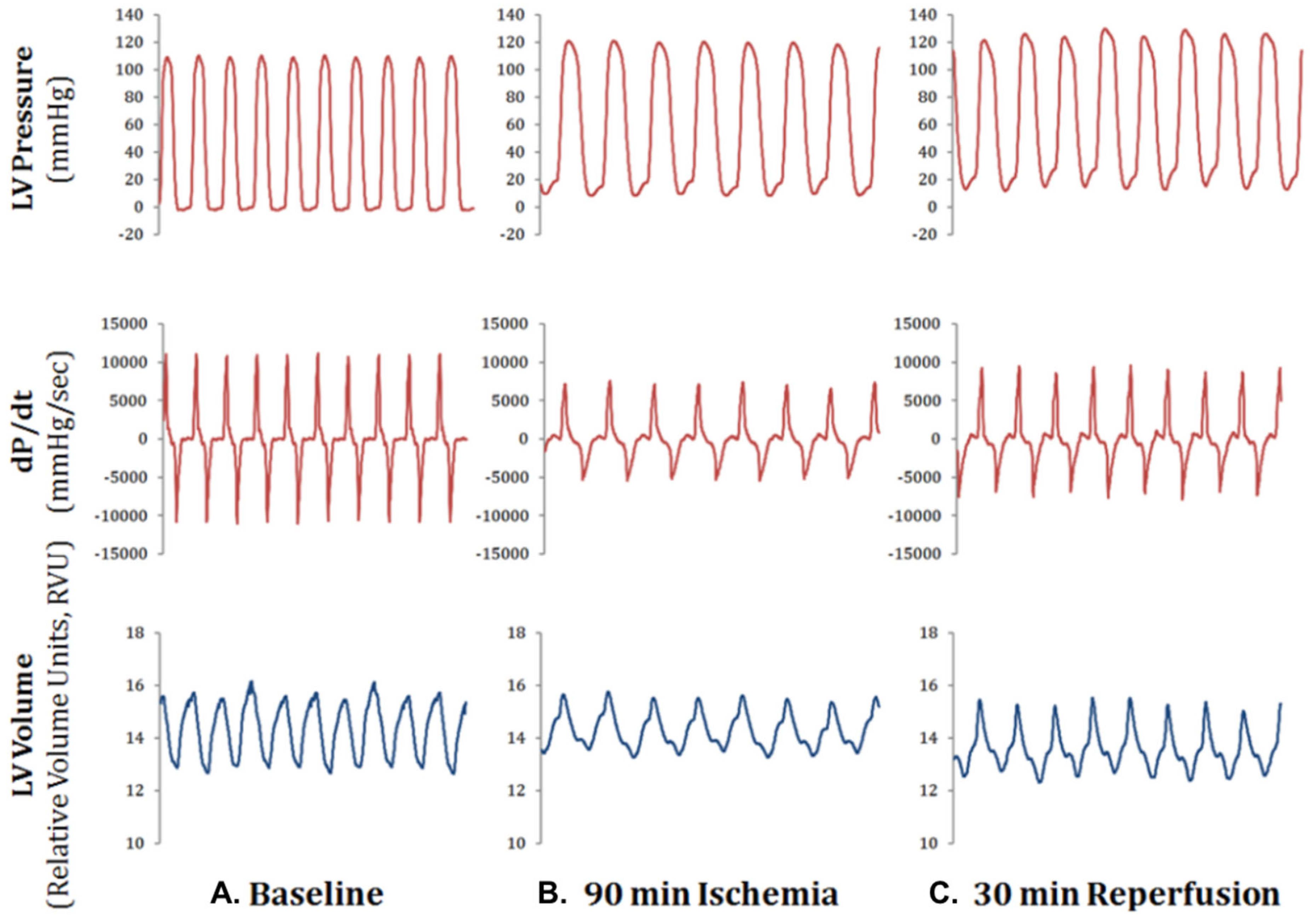
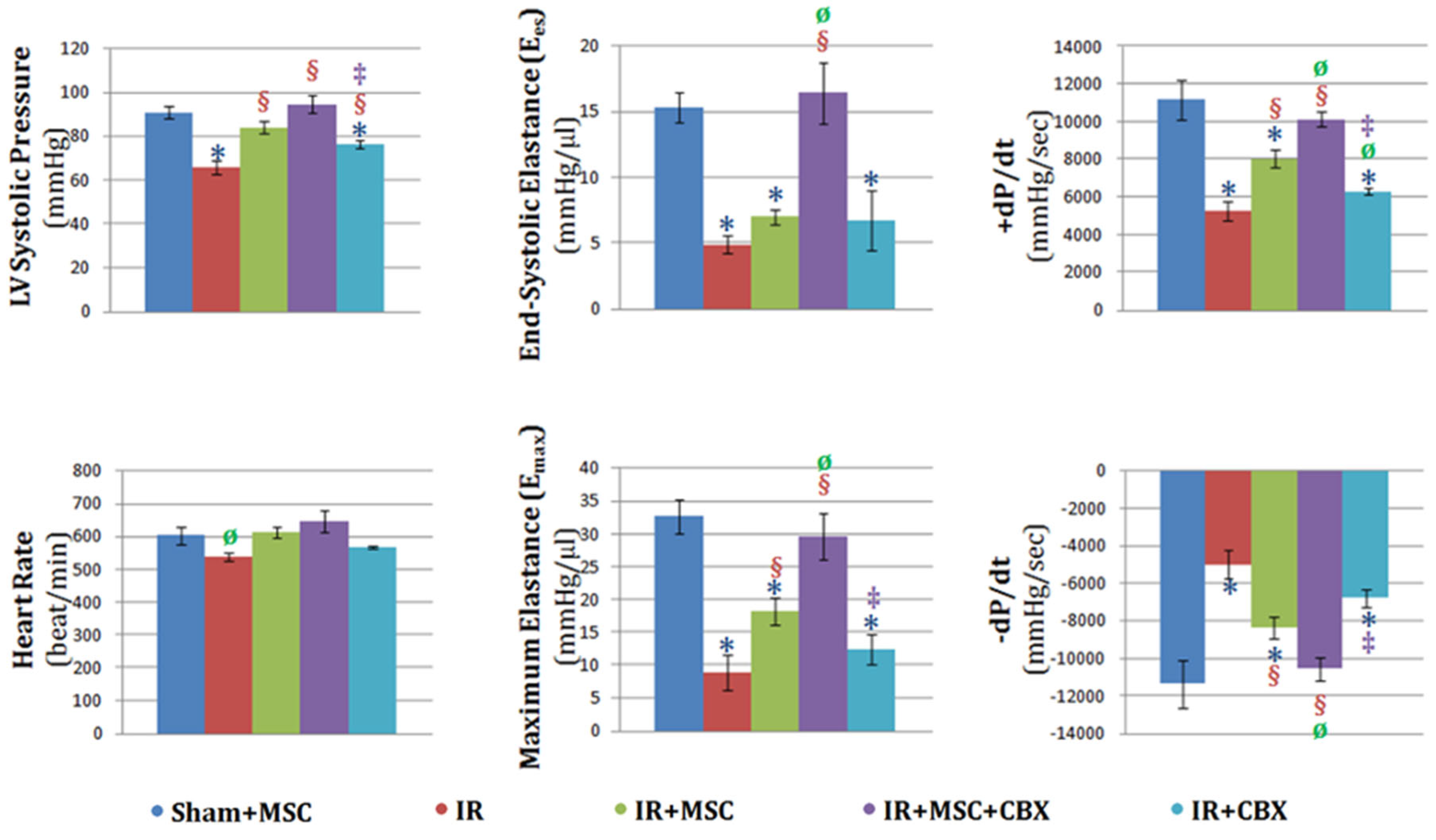
2.3. CBX-Treated BM-MSCs Improved Cardiac Function after Ischemia-Reperfusion Injury
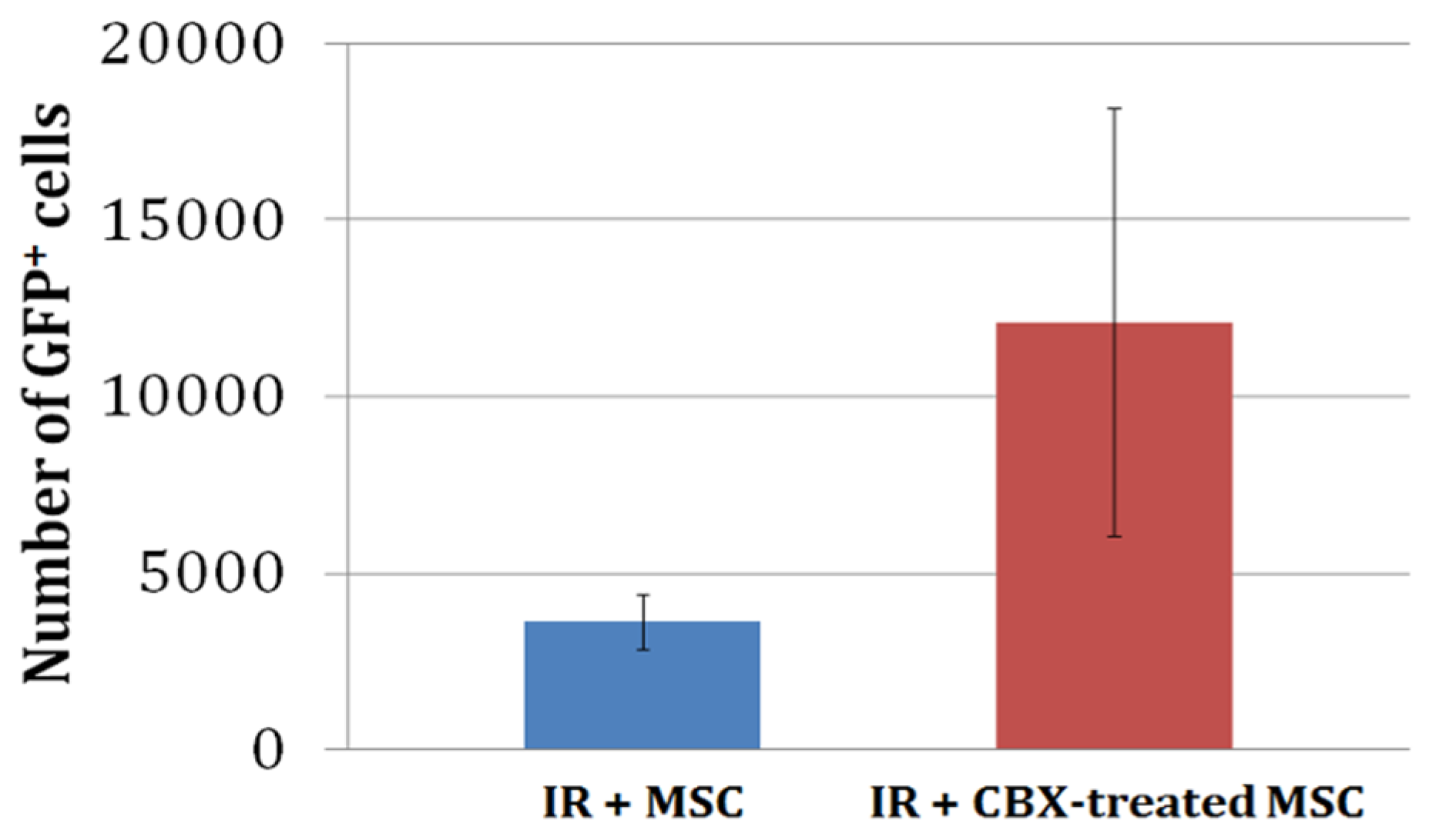
2.4. CBX-Treated mBM-MSC Retention in the Heart 24 h after Ischemia-Reperfusion Injury
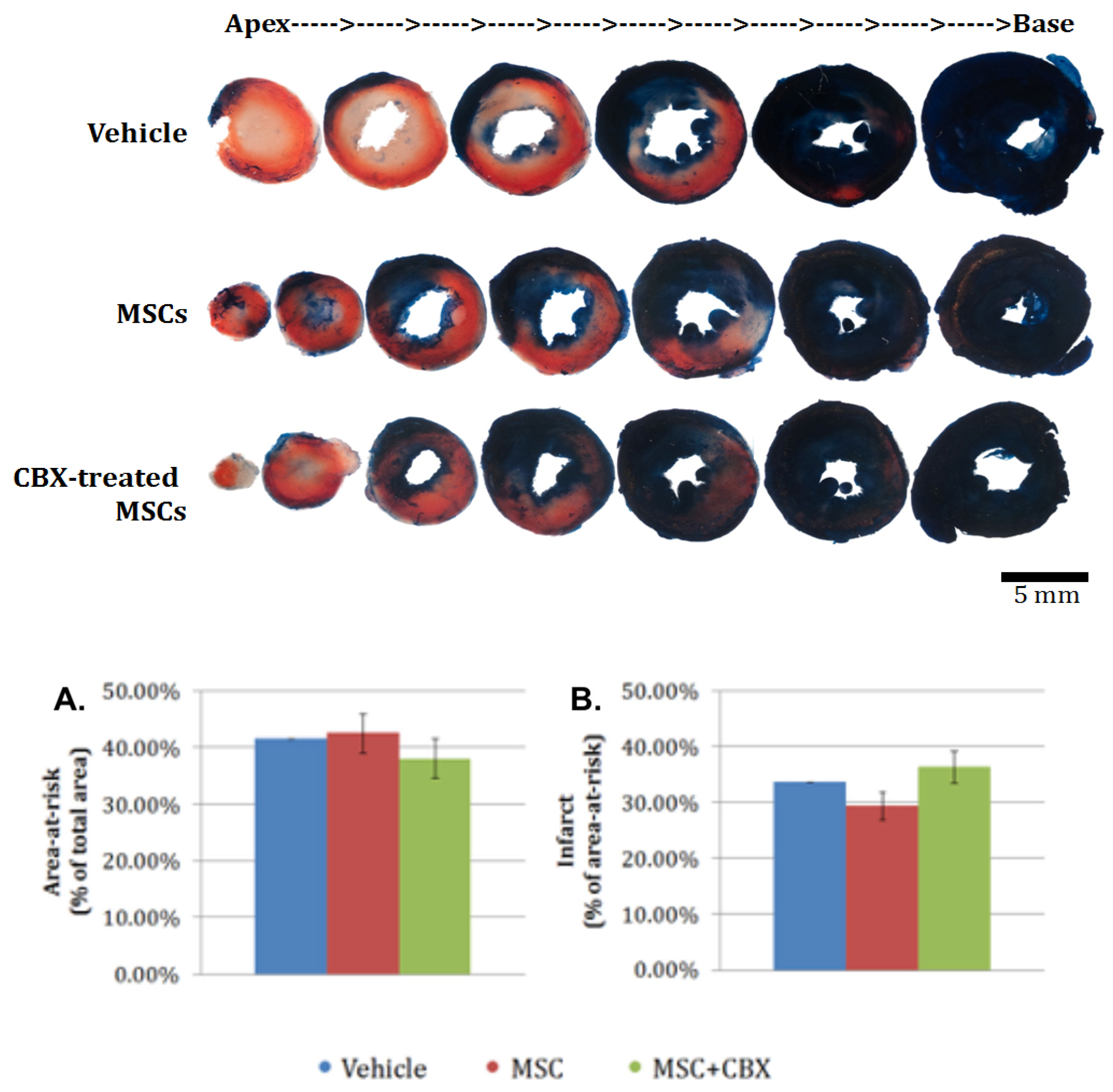
2.5. CBX-Treated mBM-MSC Did Not Alter Area-at-Risk or Infarct Size at 24 h
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Bone Marrow Mesenchymal Stem Cell Harvest
4.3. Adenoviral-Mediated Fluorescent Labeling of Mesenchymal Stem Cells
4.4. Flow Cytometry Analysis and Fluorescent-Activated Cell Sorting
4.5. Co-Culture Experiments
4.6. Coronary Ligation and Cell Injection
4.7. Hemodynamic Measurements
4.8. Whole Heart Digests
4.9. Infarct Size Measurement by Tetrazolium Staining
4.10. Murine Bone Marrow Mesenchymal Stem Cell Proliferation Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; Thygesen, K.; Alpert, J.S.; White, H.D.; et al. Third Universal Definition of Myocardial Infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion--from Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Bradfute, S.B.; Gallardo, T.D.; Nakamura, T.; Gaussin, V.; Mishina, Y.; Pocius, J.; Michael, L.H.; Behringer, R.R.; Garry, D.J.; et al. Cardiac Progenitor Cells from Adult Myocardium: Homing, Differentiation, and Fusion after Infarction. Proc. Natl. Acad. Sci. USA 2003, 100, 12313–12318. [Google Scholar] [CrossRef] [PubMed]
- Laugwitz, K.-L.; Moretti, A.; Lam, J.; Gruber, P.; Chen, Y.; Woodard, S.; Lin, L.-Z.; Cai, C.-L.; Lu, M.M.; Reth, M.; et al. Postnatal Isl1+ Cardioblasts Enter Fully Differentiated Cardiomyocyte Lineages. Nature 2005, 433, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.M.; Meeson, A.P.; Robertson, S.M.; Hawke, T.J.; Richardson, J.A.; Bates, S.; Goetsch, S.C.; Gallardo, T.D.; Garry, D.J. Persistent Expression of the ATP-Binding Cassette Transporter, Abcg2, Identifies Cardiac SP Cells in the Developing and Adult Heart. Dev. Biol. 2004, 265, 262–275. [Google Scholar] [CrossRef]
- Glimm, H.; Oh, I.H.; Eaves, C.J. Human Hematopoietic Stem Cells Stimulated to Proliferate in Vitro Lose Engraftment Potential during Their S/G(2)/M Transit and Do Not Reenter G(0). Blood 2000, 96, 4185–4193. [Google Scholar] [CrossRef]
- Muller-Borer, B.J.; Cascio, W.E.; Anderson, P.A.W.; Snowwaert, J.N.; Frye, J.R.; Desai, N.; Esch, G.L.; Brackham, J.A.; Bagnell, C.R.; Coleman, W.B.; et al. Adult-Derived Liver Stem Cells Acquire a Cardiomyocyte Structural and Functional Phenotype Ex Vivo. Am. J. Pathol. 2004, 165, 135–145. [Google Scholar] [CrossRef]
- Wobus, A.M.; Kaomei, G.; Shan, J.; Wellner, M.C.; Rohwedel, J.; Guanju, J.; Fleischmann, B.; Katus, H.A.; Hescheler, J.; Franz, W.M. Retinoic Acid Accelerates Embryonic Stem Cell-Derived Cardiac Differentiation and Enhances Development of Ventricular Cardiomyocytes. J. Mol. Cell. Cardiol. 1997, 29, 1525–1539. [Google Scholar] [CrossRef]
- Clifford, D.M.; Fisher, S.A.; Brunskill, S.J.; Doree, C.; Mathur, A.; Watt, S.; Martin-Rendon, E. Stem Cell Treatment for Acute Myocardial Infarction. Cochrane Database Syst. Rev. 2012, CD006536. [Google Scholar] [CrossRef]
- Boomsma, R.A.; Swaminathan, P.D.; Geenen, D.L. Intravenously Injected Mesenchymal Stem Cells Home to Viable Myocardium after Coronary Occlusion and Preserve Systolic Function without Altering Infarct Size. Int. J. Cardiol. 2007, 122, 17–28. [Google Scholar] [CrossRef]
- Angelini, A.; Castellani, C.; Tona, F.; Gambino, A.; Caforio, A.P.; Feltrin, G.; Della Barbera, M.; Valente, M.; Gerosa, G.; Thiene, G. Continuous Engraftment and Differentiation of Male Recipient Y-Chromosome-Positive Cardiomyocytes in Donor Female Human Heart Transplants. J. Heart Lung Transpl. Off. Publ. Int. Soc. Heart Transpl. 2007, 26, 1110–1118. [Google Scholar] [CrossRef]
- Quaini, F.; Urbanek, K.; Beltrami, A.P.; Finato, N.; Beltrami, C.A.; Nadal-Ginard, B.; Kajstura, J.; Leri, A.; Anversa, P. Chimerism of the Transplanted Heart. N. Engl. J. Med. 2002, 346, 5–15. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Myerson, D.; Saffitz, J.E.; Murry, C.E. Evidence for Cardiomyocyte Repopulation by Extracardiac Progenitors in Transplanted Human Hearts. Circ. Res. 2002, 90, 634–640. [Google Scholar] [CrossRef]
- Müller, P.; Pfeiffer, P.; Koglin, J.; Schäfers, H.-J.; Seeland, U.; Janzen, I.; Urbschat, S.; Böhm, M. Cardiomyocytes of Noncardiac Origin in Myocardial Biopsies of Human Transplanted Hearts. Circulation 2002, 106, 31–35. [Google Scholar] [CrossRef]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal Stem Cells: Mechanisms of Immunomodulation and Homing. Cell Transpl. 2010, 19, 667–679. [Google Scholar] [CrossRef]
- Chedrawy, E.G.; Wang, J.-S.; Nguyen, D.M.; Shum-Tim, D.; Chiu, R.C.J. Incorporation and Integration of Implanted Myogenic and Stem Cells into Native Myocardial Fibers: Anatomic Basis for Functional Improvements. J. Thorac. Cardiovasc. Surg. 2002, 124, 584–590. [Google Scholar] [CrossRef]
- Pillekamp, F.; Halbach, M.; Reppel, M.; Pfannkuche, K.; Nazzal, R.; Nguemo, F.; Matzkies, M.; Rubenchyk, O.; Hannes, T.; Khalil, M.; et al. Physiological Differences between Transplanted and Host Tissue Cause Functional Decoupling after in Vitro Transplantation of Human Embryonic Stem Cell-Derived Cardiomyocytes. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2009, 23, 65–74. [Google Scholar] [CrossRef]
- Rota, M.; Kajstura, J.; Hosoda, T.; Bearzi, C.; Vitale, S.; Esposito, G.; Iaffaldano, G.; Padin-Iruegas, M.E.; Gonzalez, A.; Rizzi, R.; et al. Bone Marrow Cells Adopt the Cardiomyogenic Fate in Vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 17783–17788. [Google Scholar] [CrossRef]
- Xue, T.; Cho, H.C.; Akar, F.G.; Tsang, S.-Y.; Jones, S.P.; Marbán, E.; Tomaselli, G.F.; Li, R.A. Functional Integration of Electrically Active Cardiac Derivatives from Genetically Engineered Human Embryonic Stem Cells with Quiescent Recipient Ventricular Cardiomyocytes: Insights into the Development of Cell-Based Pacemakers. Circulation 2005, 111, 11–20. [Google Scholar] [CrossRef]
- Dbouk, H.A.; Mroue, R.M.; El-Sabban, M.E.; Talhouk, R.S. Connexins: A Myriad of Functions Extending beyond Assembly of Gap Junction Channels. Cell Commun. Signal. CCS 2009, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Weigel, H.; Cotrina, M.L.; Liu, S.; Bueno, E.; Hansen, A.J.; Hansen, T.W.; Goldman, S.; Nedergaard, M. Gap-Junction-Mediated Propagation and Amplification of Cell Injury. Nat. Neurosci. 1998, 1, 494–500. [Google Scholar] [CrossRef]
- Peixoto, P.M.; Ryu, S.-Y.; Pruzansky, D.P.; Kuriakose, M.; Gilmore, A.; Kinnally, K.W. Mitochondrial Apoptosis Is Amplified through Gap Junctions. Biochem. Biophys. Res. Commun. 2009, 390, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Xie, L.-H.; John, S.A.; Subramaniam, S.; Lal, R. A Novel Role for Connexin Hemichannel in Oxidative Stress and Smoking-Induced Cell Injury. PLoS ONE 2007, 2, e712. [Google Scholar] [CrossRef] [PubMed]
- Cusato, K.; Bosco, A.; Rozental, R.; Guimarães, C.A.; Reese, B.E.; Linden, R.; Spray, D.C. Gap Junctions Mediate Bystander Cell Death in Developing Retina. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 6413–6422. [Google Scholar] [CrossRef]
- Maass, K.; Chase, S.E.; Lin, X.; Delmar, M. Cx43 CT Domain Influences Infarct Size and Susceptibility to Ventricular Tachyarrhythmias in Acute Myocardial Infarction. Cardiovasc. Res. 2009, 84, 361–367. [Google Scholar] [CrossRef]
- Qiao, H.; Surti, S.; Choi, S.R.; Raju, K.; Zhang, H.; Ponde, D.E.; Kung, H.F.; Karp, J.; Zhou, R. Death and Proliferation Time Course of Stem Cells Transplanted in the Myocardium. Mol. Imaging Biol. 2009, 11, 408. [Google Scholar] [CrossRef]
- Sjostedt, S.; Bezak, E. Experimental Investigation of the Cytotoxicity of Medium-Borne Signals in Human Prostate Cancer Cell Line. Acta Oncol. 2012, 51, 1086–1094. [Google Scholar] [CrossRef]
- Mureli, S.; Gans, C.P.; Bare, D.J.; Geenen, D.L.; Kumar, N.M.; Banach, K. Mesenchymal Stem Cells Improve Cardiac Conduction by Upregulation of Connexin 43 through Paracrine Signaling. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H600–H609. [Google Scholar] [CrossRef]
- Boomsma, R.A.; Geenen, D.L. Mesenchymal Stem Cells Secrete Multiple Cytokines That Promote Angiogenesis and Have Contrasting Effects on Chemotaxis and Apoptosis. PLoS ONE 2012, 7, e35685. [Google Scholar] [CrossRef]
- Kunze, A.; Congreso, M.R.; Hartmann, C.; Wallraff-Beck, A.; Hüttmann, K.; Bedner, P.; Requardt, R.; Seifert, G.; Redecker, C.; Willecke, K.; et al. Connexin Expression by Radial Glia-like Cells Is Required for Neurogenesis in the Adult Dentate Gyrus. Proc. Natl. Acad. Sci. USA 2009, 106, 11336–11341. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.S.Y.; Unsicker, K.; Reuss, B. Expression and Developmental Regulation of Gap Junction Connexins Cx26, Cx32, Cx43 and Cx45 in the Rat Midbrain-Floor. Int. J. Dev. Neurosci. 2002, 20, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.A.; Lüneborg, N.L.; Becker, D.L.; Mobbs, P. Gap Junctions Modulate Interkinetic Nuclear Movement in Retinal Progenitor Cells. J. Neurosci. 2005, 25, 10803–10814. [Google Scholar] [CrossRef]
- Reaume, A.G.; Sousa, P.d.; Kulkarni, S.; Langille, B.L.; Zhu, D.; Davies, T.C.; Juneja, S.C.; Kidder, G.M.; Rossant, J. Cardiac Malformation in Neonatal Mice Lacking Connexin43. Science 1995, 267, 1831–1834. [Google Scholar] [CrossRef] [PubMed]
- Rozental, R.; Morales, M.; Mehler, M.F.; Urban, M.; Kremer, M.; Dermietzel, R.; Kessler, J.A.; Spray, D.C. Changes in the Properties of Gap Junctions during Neuronal Differentiation of Hippocampal Progenitor Cells. J. Neurosci. 1998, 18, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Villars, F.; Guillotin, B.; Amédée, T.; Dutoya, S.; Bordenave, L.; Bareille, R.; Amédée, J. Effect of HUVEC on Human Osteoprogenitor Cell Differentiation Needs Heterotypic Gap Junction Communication. Am. J. Physiol.-Cell Physiol. 2002, 282, C775–C785. [Google Scholar] [CrossRef]
- Rose, R.A.; Jiang, H.; Wang, X.; Helke, S.; Tsoporis, J.N.; Gong, N.; Keating, S.C.J.; Parker, T.G.; Backx, P.H.; Keating, A. Bone Marrow-Derived Mesenchymal Stromal Cells Express Cardiac-Specific Markers, Retain the Stromal Phenotype, and Do Not Become Functional Cardiomyocytes In Vitro. Stem Cells 2008, 26, 2884–2892. [Google Scholar] [CrossRef]
- Dürig, J.; Rosenthal, C.; Halfmeyer, K.; Wiemann, M.; Novotny, J.; Bingmann, D.; Dührsen, U.; Schirrmacher, K. Intercellular Communication between Bone Marrow Stromal Cells and CD34+ Haematopoietic Progenitor Cells Is Mediated by Connexin 43-Type Gap Junctions. Br. J. Haematol. 2000, 111, 416–425. [Google Scholar] [CrossRef]
- Nikjoo, H.; Khvostunov, I.K. Biophysical Model of the Radiation-Induced Bystander Effect. Int. J. Radiat. Biol. 2003, 79, 43–52. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Baars, T.; Heusch, G. Calcium Antagonists in Myocardial Ischemia/Reperfusion—Update 2012. Wien. Med. Wochenschr. 2012, 162, 302–310. [Google Scholar] [CrossRef]
- Muñoz-Pinedo, C. Signaling Pathways That Regulate Life and Cell Death: Evolution of Apoptosis in the Context of Self-Defense. Adv. Exp. Med. Biol. 2012, 738, 124–143. [Google Scholar] [CrossRef]
- Goldspink, P.H.; Montgomery, D.E.; Walker, L.A.; Urboniene, D.; McKinney, R.D.; Geenen, D.L.; Solaro, R.J.; Buttrick, P.M. Protein Kinase Cepsilon Overexpression Alters Myofilament Properties and Composition during the Progression of Heart Failure. Circ. Res. 2004, 95, 424–432. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Rodrigo, M.C.; Simpson, P.C. Isolation and Culture of Adult Mouse Cardiac Myocytes. Cardiovasc. Proteom. Methods Protoc. 2007, 357, 271–296. [Google Scholar] [CrossRef]
- Redel, A.; Jazbutyte, V.; Smul, T.M.; Lange, M.; Eckle, T.; Eltzschig, H.; Roewer, N.; Kehl, F. Impact of Ischemia and Reperfusion Times on Myocardial Infarct Size in Mice in Vivo. Exp. Biol. Med. 2008, 233, 84–93. [Google Scholar] [CrossRef]
- Downey, J.M. Measuring Infarct Size by the Tetrazolium Method. Available online: https://www.southalabama.edu/ishr/help/ttc/ (accessed on 7 April 2023).
- Ramkisoensing, A.A.; Pijnappels, D.A.; Swildens, J.; Goumans, M.J.; Fibbe, W.E.; Schalij, M.J.; de Vries, A.A.F.; Atsma, D.E. Gap Junctional Coupling with Cardiomyocytes Is Necessary but Not Sufficient for Cardiomyogenic Differentiation of Cocultured Human Mesenchymal Stem Cells. Stem Cells Dayt. Ohio 2012, 30, 1236–1245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatchavalvanich, S.; Boomsma, R.A.; Tietema, J.M.; Geenen, D.L. Inhibition of Gap Junction Formation Prior to Implantation of Bone Marrow-Derived Mesenchymal Cells Improves Function in the Ischemic Myocardium. Int. J. Mol. Sci. 2023, 24, 9653. https://doi.org/10.3390/ijms24119653
Chatchavalvanich S, Boomsma RA, Tietema JM, Geenen DL. Inhibition of Gap Junction Formation Prior to Implantation of Bone Marrow-Derived Mesenchymal Cells Improves Function in the Ischemic Myocardium. International Journal of Molecular Sciences. 2023; 24(11):9653. https://doi.org/10.3390/ijms24119653
Chicago/Turabian StyleChatchavalvanich, Santipongse, Robert A. Boomsma, Jack M. Tietema, and David L. Geenen. 2023. "Inhibition of Gap Junction Formation Prior to Implantation of Bone Marrow-Derived Mesenchymal Cells Improves Function in the Ischemic Myocardium" International Journal of Molecular Sciences 24, no. 11: 9653. https://doi.org/10.3390/ijms24119653
APA StyleChatchavalvanich, S., Boomsma, R. A., Tietema, J. M., & Geenen, D. L. (2023). Inhibition of Gap Junction Formation Prior to Implantation of Bone Marrow-Derived Mesenchymal Cells Improves Function in the Ischemic Myocardium. International Journal of Molecular Sciences, 24(11), 9653. https://doi.org/10.3390/ijms24119653





