Lipid Membrane Remodeling by the Micellar Aggregation of Long-Chain Unsaturated Fatty Acids for Sustainable Antimicrobial Strategies
Abstract
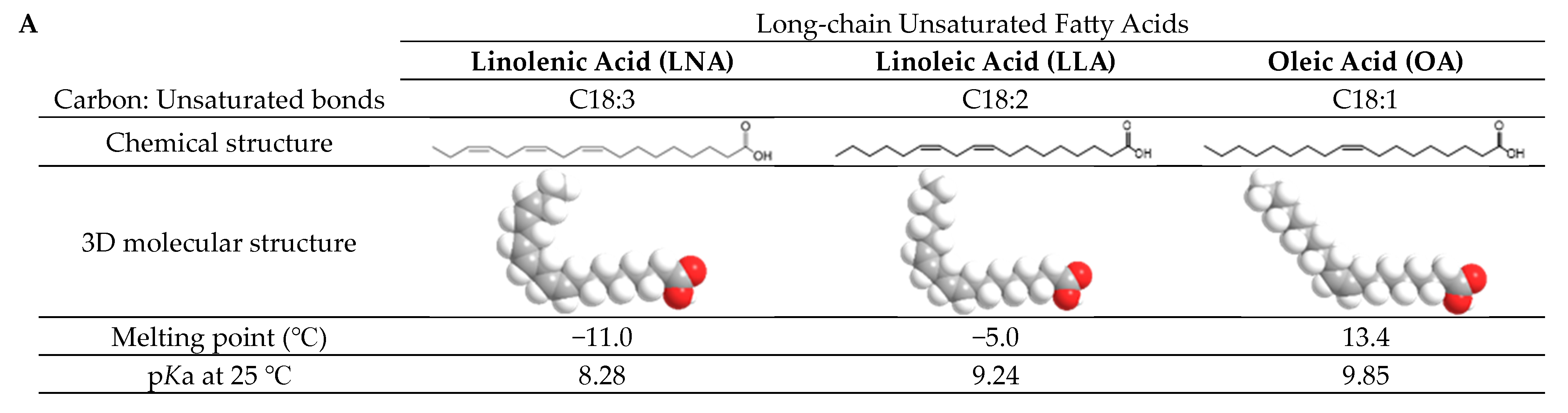
1. Introduction
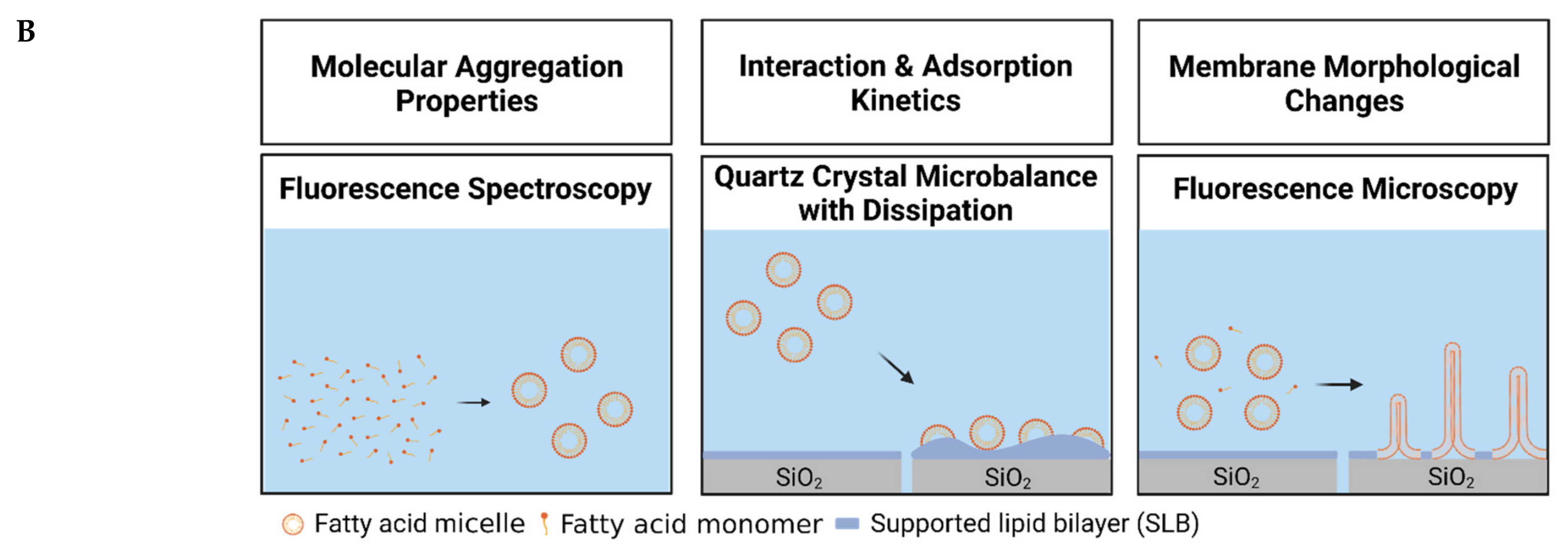
2. Results and Discussion
2.1. Determination of the Critical Micelle Concentration
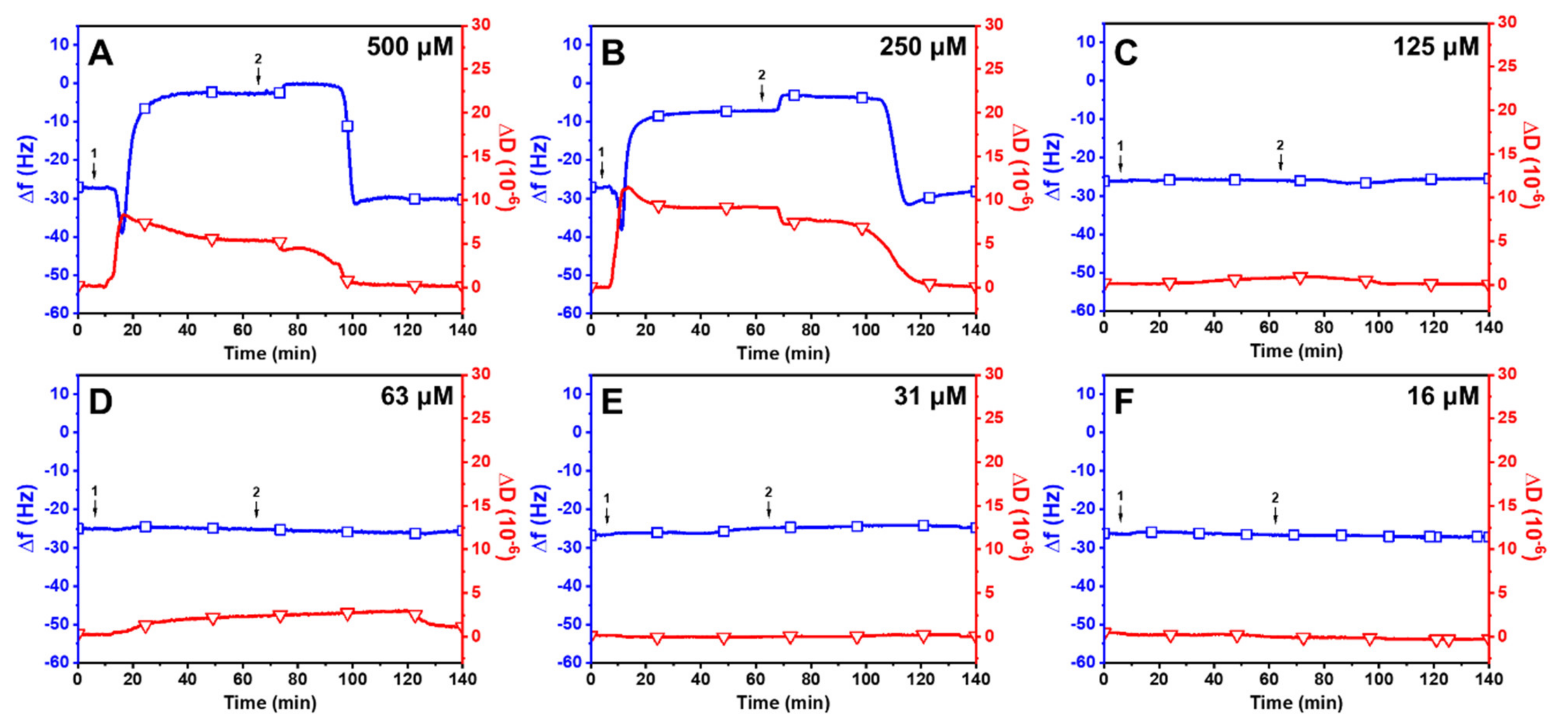
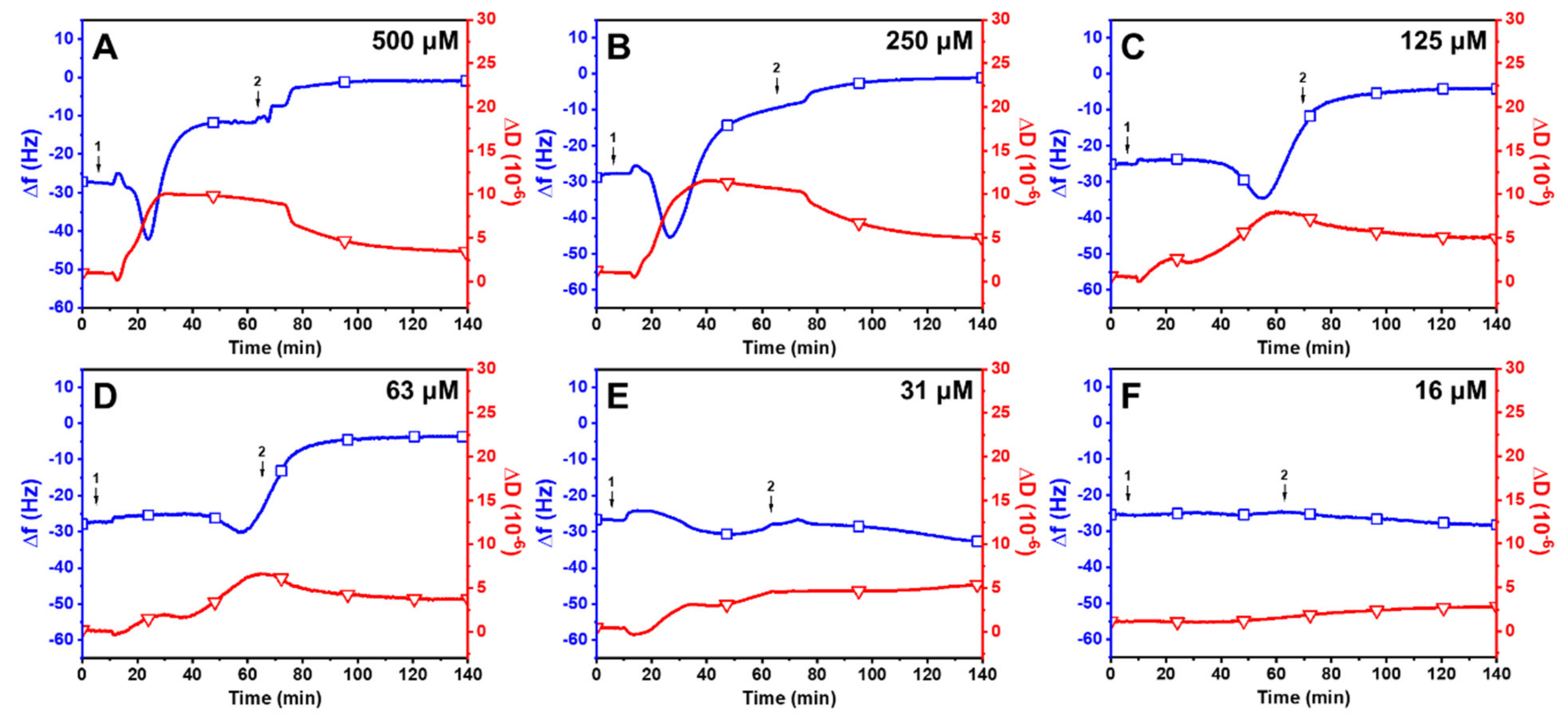
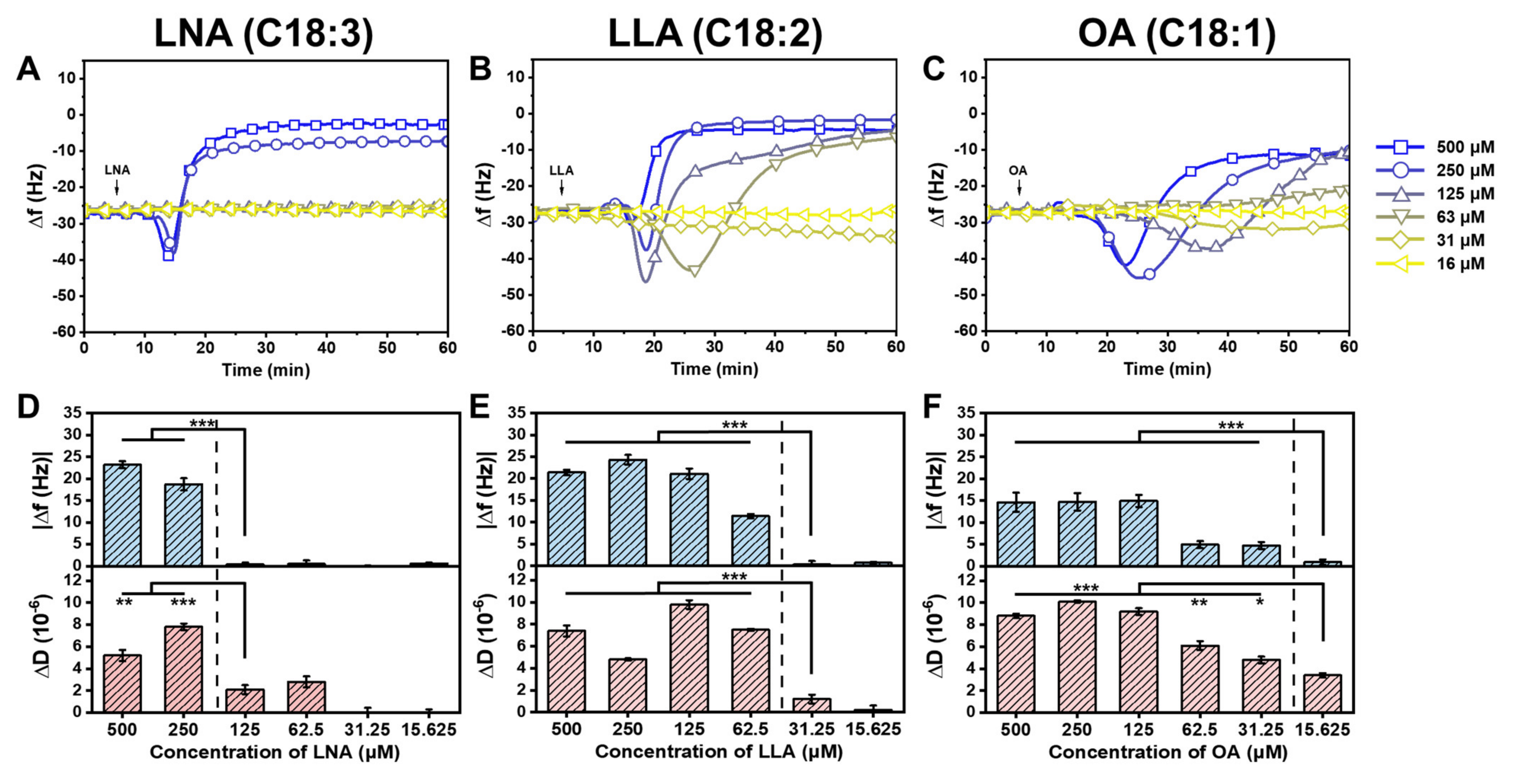
2.2. Interaction between Long-Chain Unsaturated Fatty Acids and Supported Lipid Bilayers
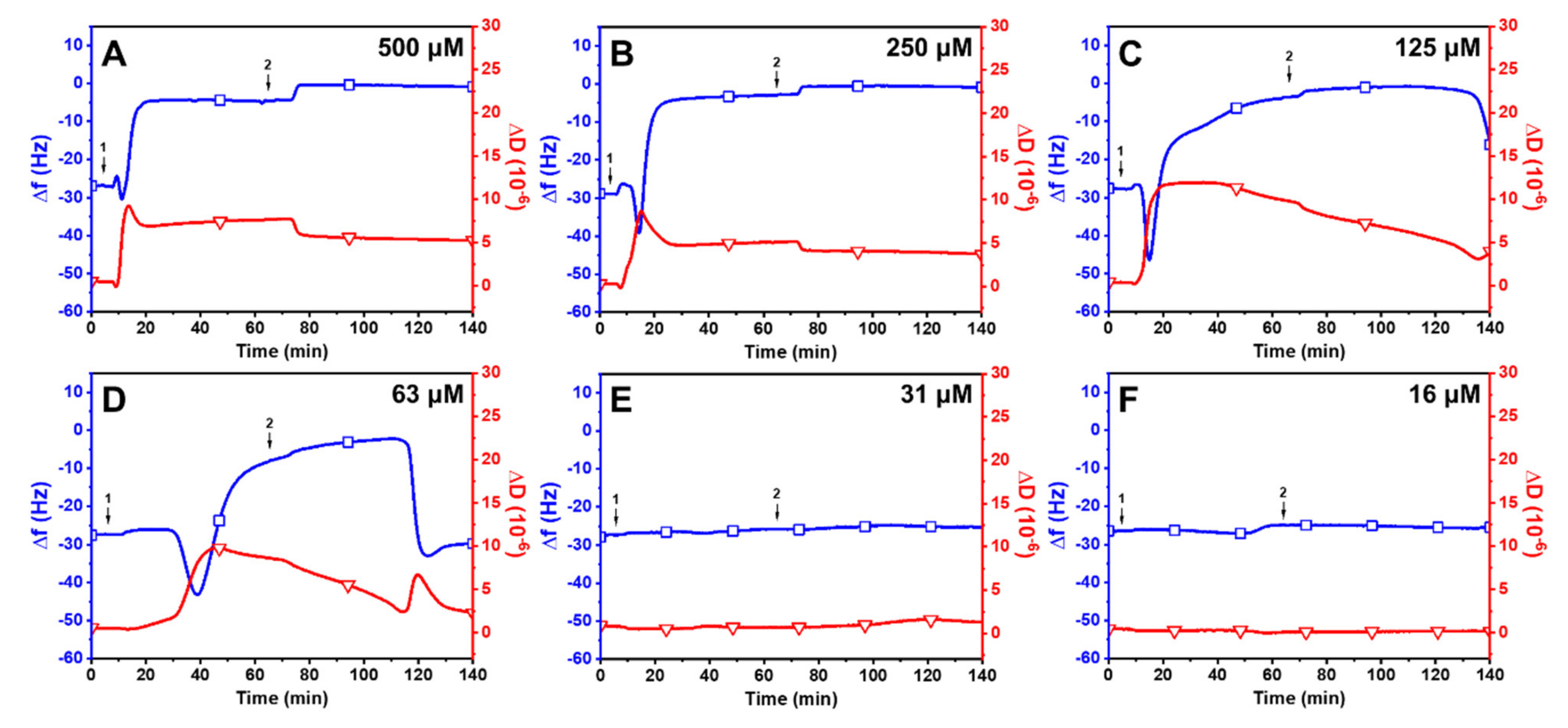
2.3. Trend in the Interaction Kinetics of Long-Chain Unsaturated Fatty Acids on Supported Lipid Bilayers
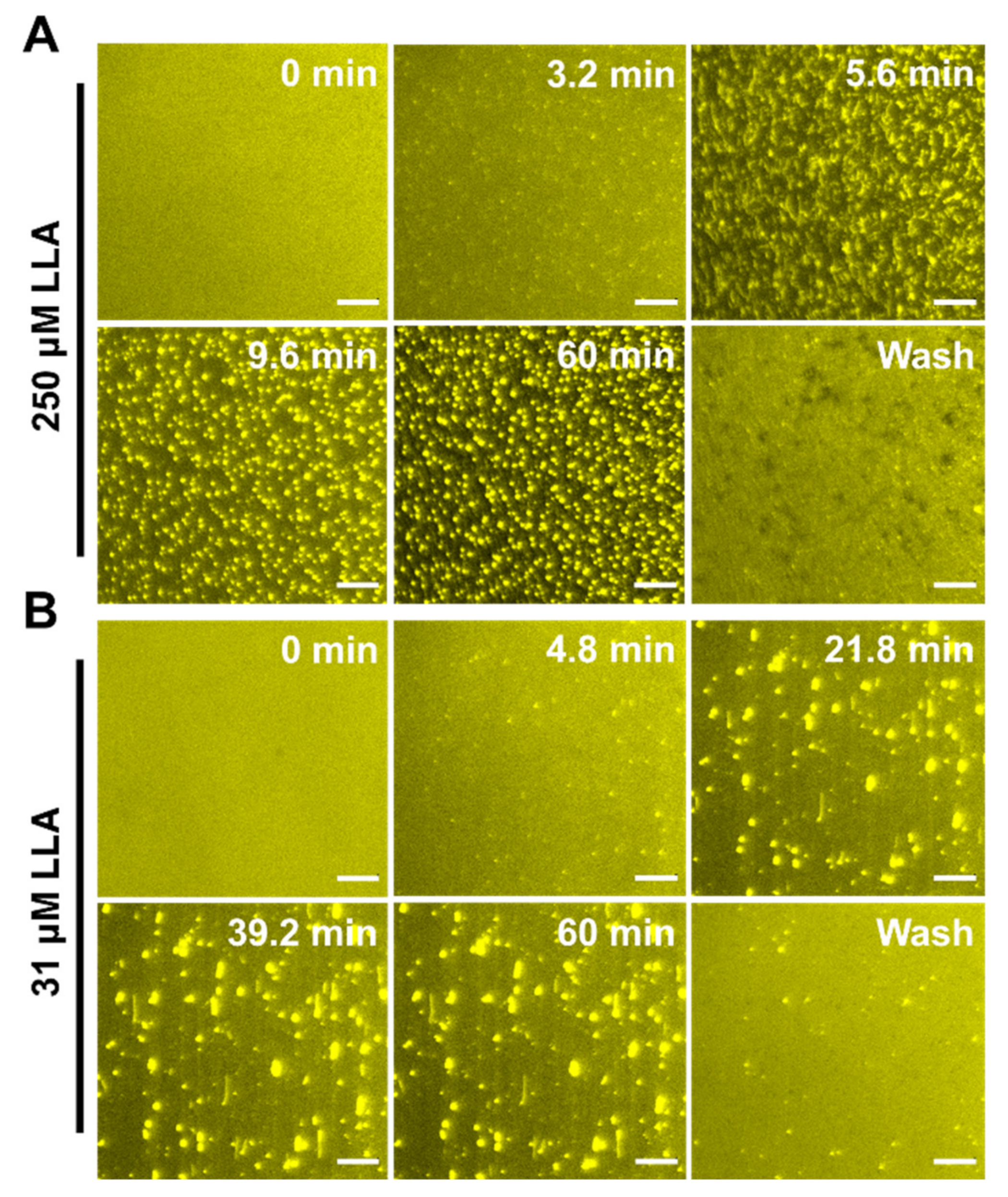
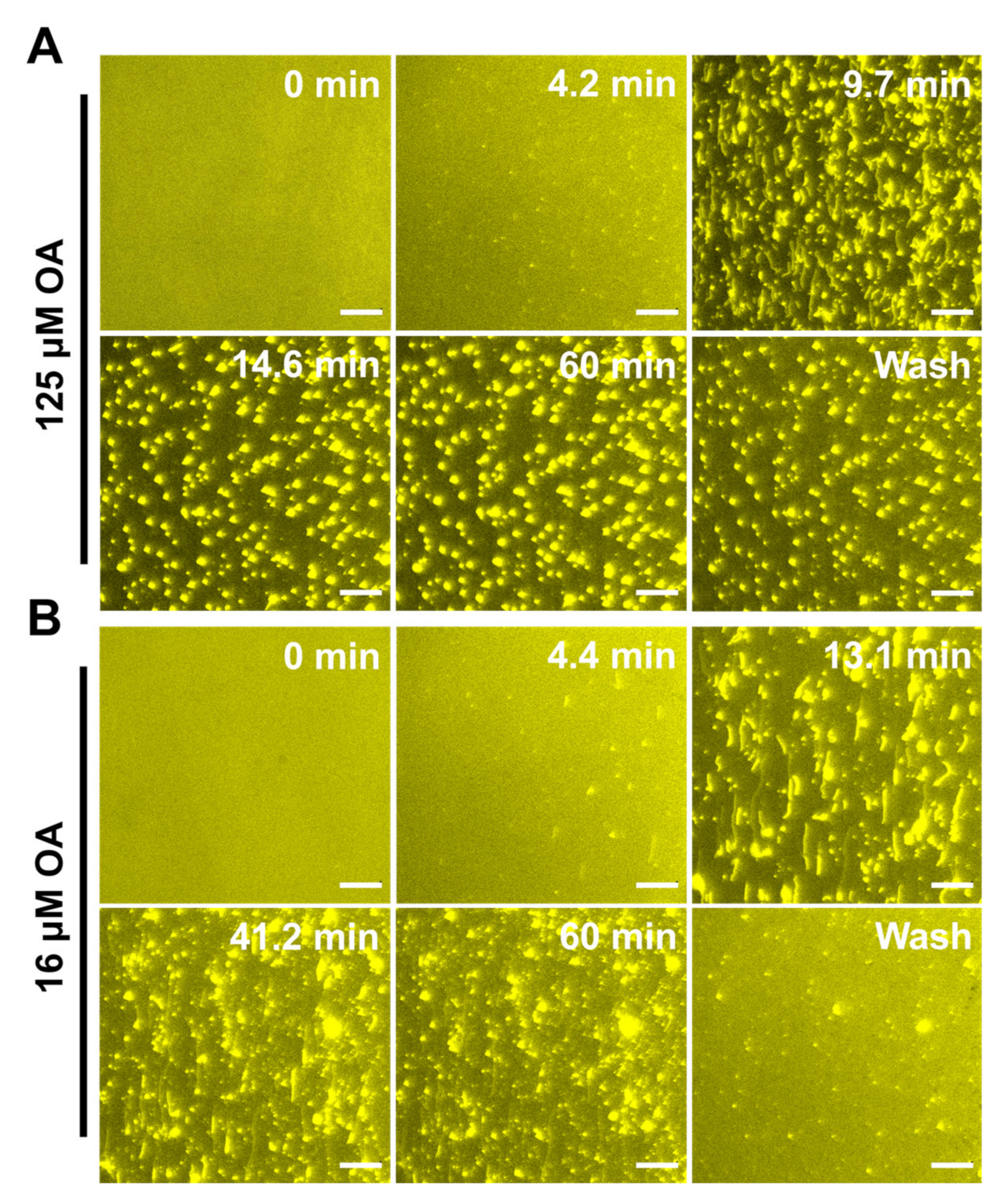
2.4. Observation of Membrane Morphological Responses in Supported Lipid Bilayers
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fatty Acid Solutions
3.3. Fluorescence Spectroscopy
3.4. Quartz Crystal Microbalance–Dissipation (QCM-D)
3.5. Time-Lapse Fluorescence Microscopy
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Desbois, A.P. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat. Ant-Infect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.D.C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Glycolipid biosurfactants: Main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 2016, 96, 4310–4320. [Google Scholar] [CrossRef]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef]
- Nitschke, M.; Marangon, C.A. Microbial surfactants in nanotechnology: Recent trends and applications. Crit. Rev. Biotechnol. 2022, 42, 294–310. [Google Scholar] [CrossRef]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Bhadani, A.; Iwabata, K.; Sakai, K.; Koura, S.; Sakai, H.; Abe, M. Sustainable oleic and stearic acid based biodegradable surfactants. RSC Adv. 2017, 7, 10433–10442. [Google Scholar] [CrossRef]
- Bhadani, A.; Kafle, A.; Ogura, T.; Akamatsu, M.; Sakai, K.; Sakai, H.; Abe, M. Current perspective of sustainable surfactants based on renewable building blocks. Curr. Opin. Colloid Interface Sci. 2020, 45, 124–135. [Google Scholar] [CrossRef]
- Verma, C.; Hussain, C.M.; Quraishi, M.A.; Alfantazi, A. Green surfactants for corrosion control: Design, performance and applications. Adv. Colloid Interface Sci. 2023, 311, 102822. [Google Scholar] [CrossRef]
- Halliday, H.L. Surfactants: Past, present and future. J. Perinatol. 2008, 28, S47–S56. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Natural surfactants used in cosmetics: Glycolipids. Int. J. Cosmet. Sci. 2009, 31, 255–261. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Q.; Li, X.; Chen, Q. Adsorption Behavior and Wettability of Rhodochrosite Surface: Effect of C18 Fatty Acid Unsaturation. Minerals 2020, 10, 905. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog Lipid Res 2021, 82, 101093. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Thormar, H. Antibacterial Effects of Lipids: Historical Review (1881 to 1960). In Lipids and Essential Oils as Antimicrobial Agents; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 25–45. [Google Scholar] [CrossRef]
- Thormar, H.; Hilmarsson, H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem. Phys. Lipids 2007, 150, 1–11. [Google Scholar] [CrossRef]
- FDA.gov. GRAS Substances (SCOGS) Database. 2022. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 10 April 2023).
- Huang, T.-H.; Wang, P.-W.; Yang, S.-C.; Chou, W.-L.; Fang, J.-Y. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef]
- Kelm, G.R.; Wickett, R.R. Chapter 12—The Role of Fatty Acids in Cosmetic Technology. In Fatty Acids; Ahmad, M.U., Ed.; AOCS Press: Amsterdam, The Netherlands, 2017; pp. 385–404. [Google Scholar] [CrossRef]
- Vecino, X.; Barbosa-Pereira, L.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Optimization of extraction conditions and fatty acid characterization of Lactobacillus pentosus cell-bound biosurfactant/bioemulsifier. J. Sci. Food Agric. 2015, 95, 313–320. [Google Scholar] [CrossRef]
- Lykke, A.M.; Gregersen, S.B.; Padonou, E.A.; Bassolé, I.; Dalsgaard, T.K. Potential of Unconventional Seed Oils and Fats from West African Trees: A Review of Fatty Acid Composition and Perspectives. Lipids 2021, 56, 357–390. [Google Scholar] [CrossRef]
- Desbois, A.P.; Lebl, T.; Yan, L.; Smith, V.J. Isolation and structural characterisation of two antibacterial free fatty acids from the marine diatom, Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2008, 81, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, H.; Miller, T.B.; Paton, A.M.; Thompson, J.K. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J. Appl. Bacteriol. 1971, 34, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; O’Connor, C.J.; Roberton, A.M. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2003, 36, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zotos, A.; Bampidis, V.A. Milk fat quality of Greek buffalo (Bubalus bubalis). J. Food Compos. Anal. 2014, 33, 181–186. [Google Scholar] [CrossRef]
- Yang, J.; Gan, K.; Li, S.; Gai, Y.; Zhang, Z.; Yan, S.; Chen, Y.; Zhang, X.; Lu, Y.; Xu, J. Temperature induced gelation of non–aqueous alumina suspension using oleic acid as dispersant. Ceram. Int. 2017, 43, 11361–11366. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of Degree, Type, and Position of Unsaturation on the pKa of Long-Chain Fatty Acids. J. Colloid Interface Sci. 2002, 256, 201–207. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Thomas, J.K. Photophysics of polycyclic aromatic molecules on semiconductor powders. J. Am. Chem. Soc. 1983, 105, 6383–6389. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Shen, Q.; Shen, J.; Han, Y.; Zhang, H. Investigation on the self-assembled behaviors of C18 unsaturated fatty acids in arginine aqueous solution. RSC Adv. 2017, 7, 41561–41572. [Google Scholar] [CrossRef]
- Tang, K.S. Protective effect of arachidonic acid and linoleic acid on 1-methyl-4-phenylpyridinium-induced toxicity in PC12 cells. Lipids Health Dis. 2014, 13, 197. [Google Scholar] [CrossRef]
- Kawakami, L.M.; Yoon, B.K.; Jackman, J.A.; Knoll, W.; Weiss, P.S.; Cho, N.-J. Understanding How Sterols Regulate Membrane Remodeling in Supported Lipid Bilayers. Langmuir 2017, 33, 14756–14765. [Google Scholar] [CrossRef]
- Thid, D.; Benkoski, J.J.; Svedhem, S.; Kasemo, B.; Gold, J. DHA-induced changes of supported lipid membrane morphology. Langmuir 2007, 23, 5878–5881. [Google Scholar] [CrossRef]
- Valle-González, E.R.; Jackman, J.A.; Yoon, B.K.; Park, S.; Sut, T.N.; Cho, N.-J. Characterizing How Acidic pH Conditions Affect the Membrane-Disruptive Activities of Lauric Acid and Glycerol Monolaurate. Langmuir 2018, 34, 13745–13753. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Cho, N.J. Spectrum of Membrane Morphological Responses to Antibacterial Fatty Acids and Related Surfactants. Langmuir 2015, 31, 10223–10232. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Sut, T.N.; Cho, N.-J. Correlating Membrane Morphological Responses with Micellar Aggregation Behavior of Capric Acid and Monocaprin. Langmuir 2017, 33, 2750–2759. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Tabaei, S.R.; Choi, J.H.; Haw Zan, G.; Zhdanov, V.P.; Cho, N.J. Solvent-assisted lipid bilayer formation on silicon dioxide and gold. Langmuir 2014, 30, 10363–10373. [Google Scholar] [CrossRef]
- Cho, N.J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef]
- Keller, C.A.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef]
- Ikawa, M.; Sasner, J.J.; Haney, J.F. Inhibition of Chlorella growth by degradation and related products of linoleic and linolenic acids and the possible significance of polyunsaturated fatty acids in phytoplankton ecology. Hydrobiologia 1997, 356, 143–148. [Google Scholar] [CrossRef]
- Greenway, D.L.A.; Dyke, K.G.H. Mechanism of the Inhibitory Action of Linoleic Acid on the Growth of Staphylococcus aureus. J. Gen. Microbiol. 1979, 115, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Ash, M.E. Lipid replacement therapy: A natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2014, 1838, 1657–1679. [Google Scholar] [CrossRef] [PubMed]
- Tyler, A.I.I.; Greenfield, J.L.; Seddon, J.M.; Brooks, N.J.; Purushothaman, S. Coupling Phase Behavior of Fatty Acid Containing Membranes to Membrane Bio-Mechanics. Front. Cell Dev. Biol. 2019, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Rohde, M.; Molinari, G.; Stadler, M.; Abraham, W.-R. Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria. Antibiotics 2020, 9, 788. [Google Scholar] [CrossRef]
- Eltigani, S.A.; Eltayeb, M.M.; Ishihara, A.; Arima, J. Isolates from Monechma ciliatum seeds’ extract hampered Porphyromonas gingivalis hemagglutinins. J. Food Biochem. 2019, 43, e13029. [Google Scholar] [CrossRef]
- Waters, J.C. Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol. 2009, 185, 1135–1148. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P.; Harvey, D.J.; Watts, A.; Newsholme, E.A. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem. J. 1994, 300 Pt 2, 509–518. [Google Scholar] [CrossRef]
- Leekumjorn, S.; Cho, H.J.; Wu, Y.; Wright, N.T.; Sum, A.K.; Chan, C. The role of fatty acid unsaturation in minimizing biophysical changes on the structure and local effects of bilayer membranes. Biochim. Biophys. Acta 2009, 1788, 1508–1516. [Google Scholar] [CrossRef]
- Ashaduzzaman, M.; Kunitake, M. Aqueous Dispersion and Temperature Induced Reversible Fluorescence Properties of 1-Pyrenecarboxaldehyde. Int. Lett. Chem. Phys. Astron. 2013, 1, 55–62. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Fredriksson, C.; Keller, C.A.; Krozer, A.; Brzezinski, P.; Voinova, M.; Kasemo, B. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 1997, 107, 229–246. [Google Scholar] [CrossRef]
- Rodahl, M.; Kasemo, B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev. Sci. Instrum. 1996, 67, 3238–3241. [Google Scholar] [CrossRef]
- Glasmästar, K.; Larsson, C.; Höök, F.; Kasemo, B. Protein Adsorption on Supported Phospholipid Bilayers. J. Colloid Interface Sci. 2002, 246, 40–47. [Google Scholar] [CrossRef]
- Jackman, J.A.; Zhao, Z.; Zhdanov, V.P.; Frank, C.W.; Cho, N.J. Vesicle adhesion and rupture on silicon oxide: Influence of freeze-thaw pretreatment. Langmuir 2014, 30, 2152–2160. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Yoon, B.K.; Park, S.; Sut, T.N.; Chin, H.; Park, J.H.; Jackman, J.A.; Cho, N.J. Solvent-assisted preparation of supported lipid bilayers. Nat. Protoc. 2019, 14, 2091–2118. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Tae, H.; Park, S.; Cho, N.-J. Lipid Membrane Remodeling by the Micellar Aggregation of Long-Chain Unsaturated Fatty Acids for Sustainable Antimicrobial Strategies. Int. J. Mol. Sci. 2023, 24, 9639. https://doi.org/10.3390/ijms24119639
Shin S, Tae H, Park S, Cho N-J. Lipid Membrane Remodeling by the Micellar Aggregation of Long-Chain Unsaturated Fatty Acids for Sustainable Antimicrobial Strategies. International Journal of Molecular Sciences. 2023; 24(11):9639. https://doi.org/10.3390/ijms24119639
Chicago/Turabian StyleShin, Sungmin, Hyunhyuk Tae, Soohyun Park, and Nam-Joon Cho. 2023. "Lipid Membrane Remodeling by the Micellar Aggregation of Long-Chain Unsaturated Fatty Acids for Sustainable Antimicrobial Strategies" International Journal of Molecular Sciences 24, no. 11: 9639. https://doi.org/10.3390/ijms24119639
APA StyleShin, S., Tae, H., Park, S., & Cho, N.-J. (2023). Lipid Membrane Remodeling by the Micellar Aggregation of Long-Chain Unsaturated Fatty Acids for Sustainable Antimicrobial Strategies. International Journal of Molecular Sciences, 24(11), 9639. https://doi.org/10.3390/ijms24119639





