Bisphenol S Reduces Pig Spermatozoa Motility through Different Intracellular Pathways and Mechanisms than Its Analog Bisphenol A
Abstract
1. Introduction
2. Results
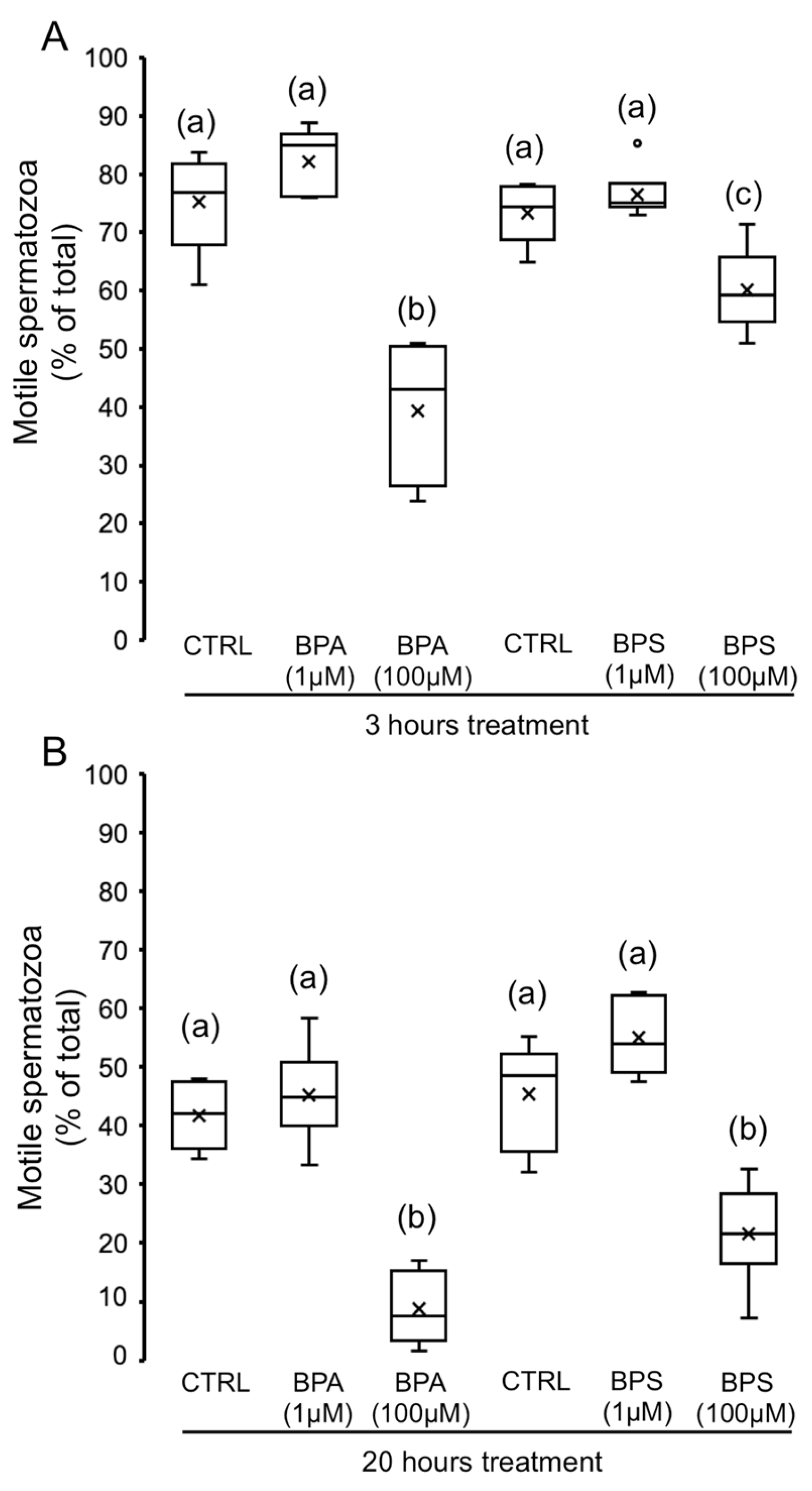
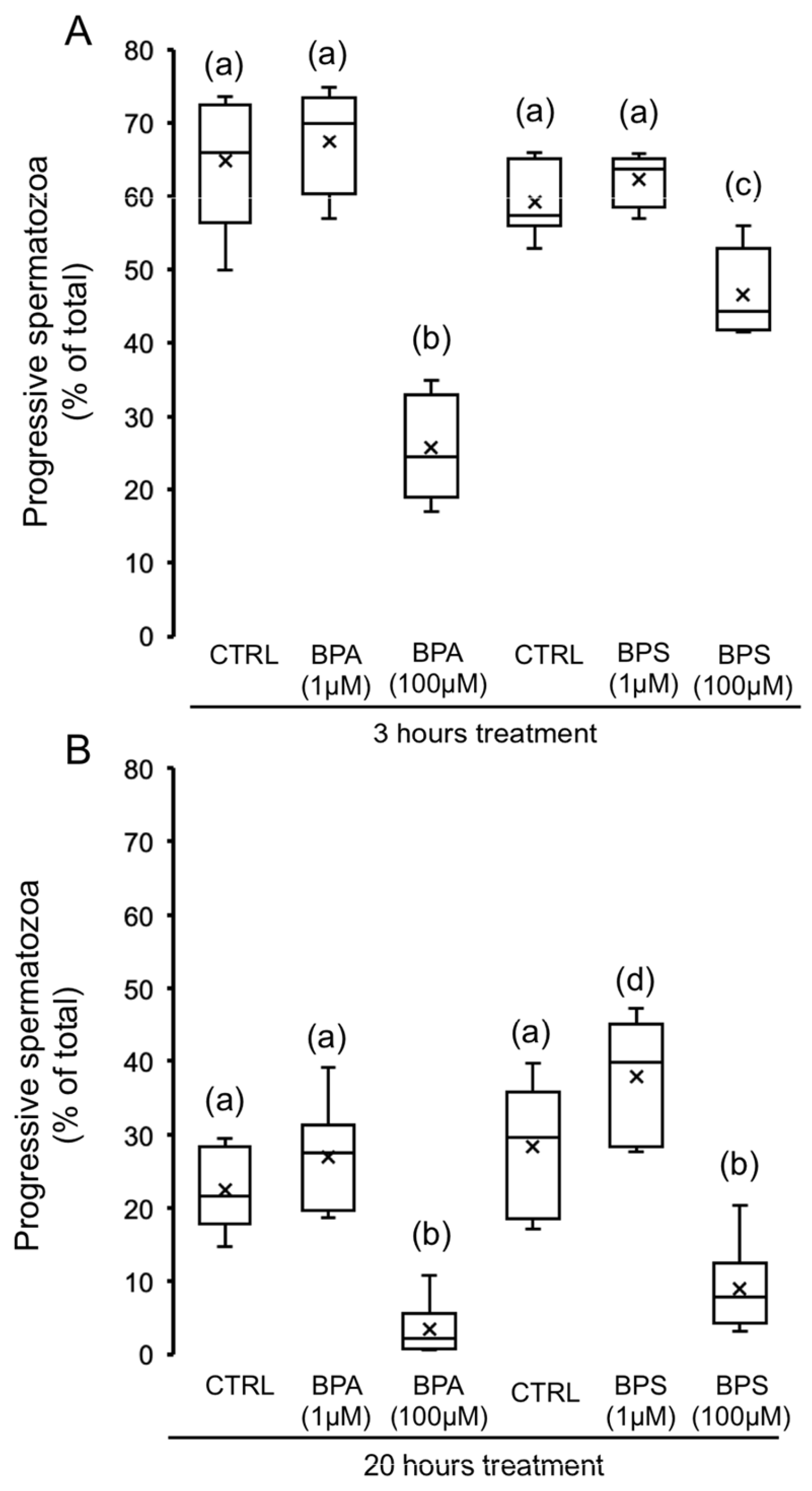
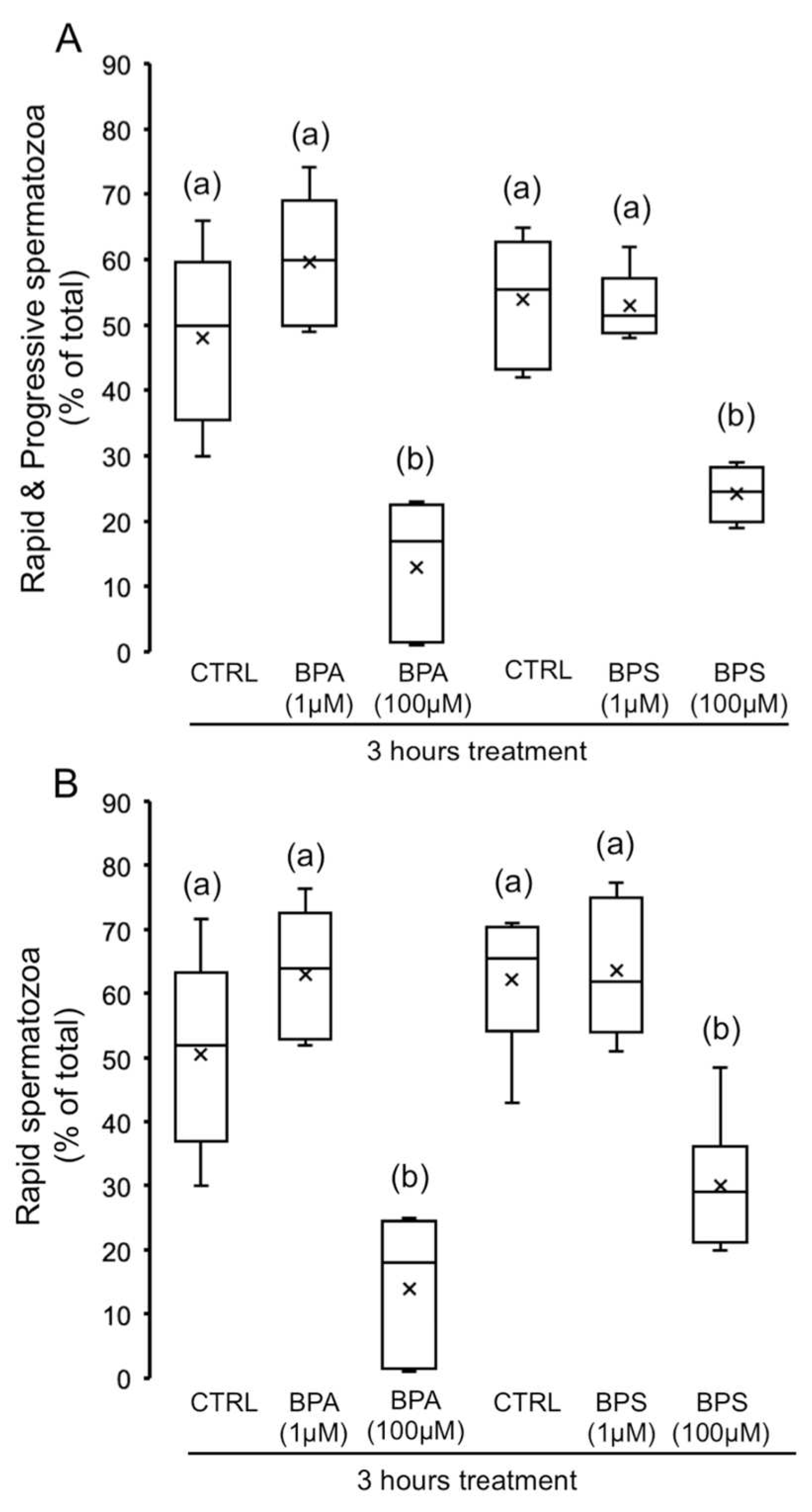
2.1. Effects of 4,4′-Sulfonyldiphenol (BPS) and Bisphenol A (BPA) on Pig Spermatozoa Motility
2.2. Effects of BPS and BPA on the Viability of Pig Spermatozoa
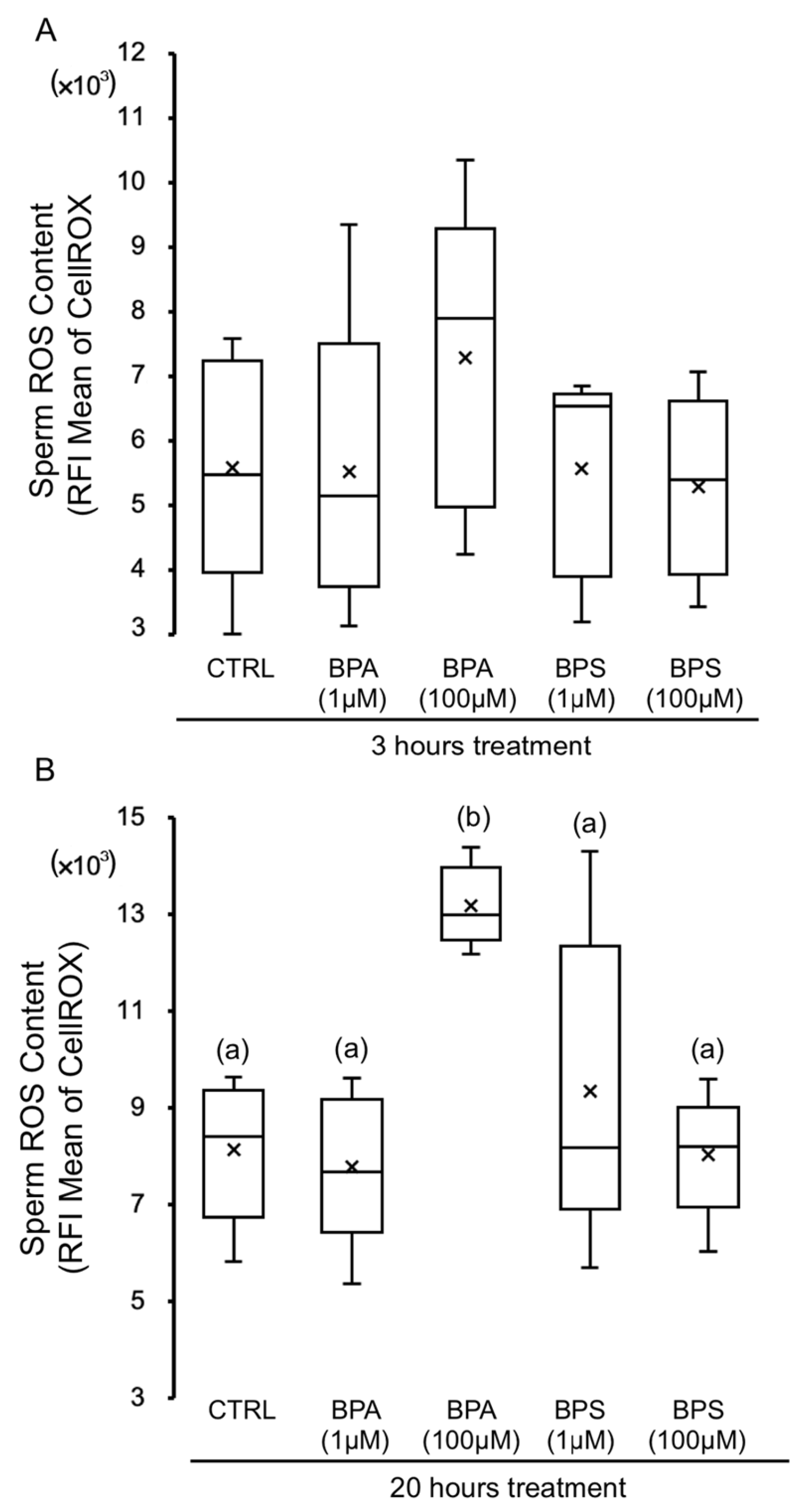
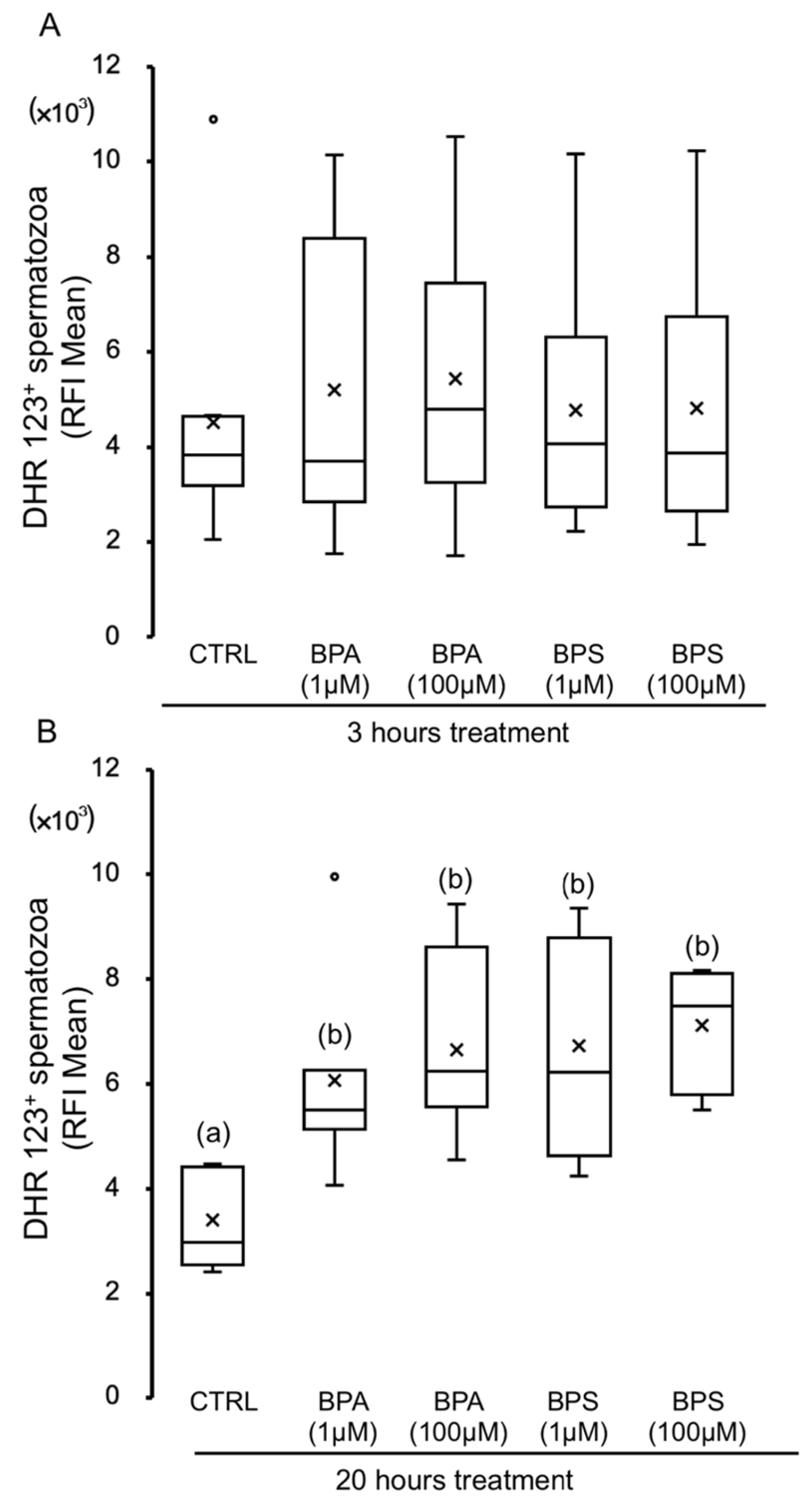
2.3. Effects of BPS and BPA on Pig Spermatozoa Reactive Oxygen Species Content
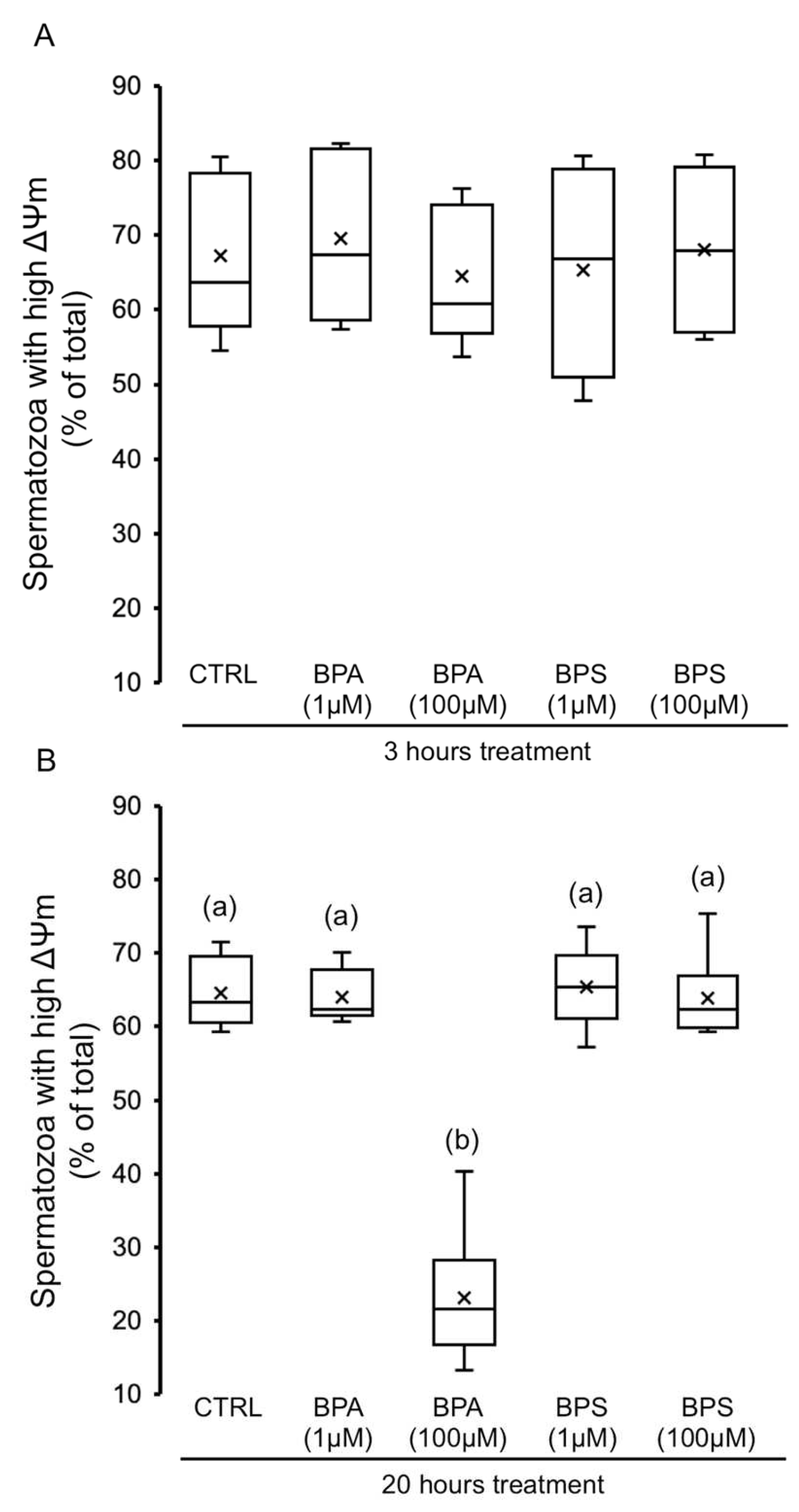
2.4. Effects of BPS and BPA on Spermatozoa Mitochondrial Membrane Potential (MMP)
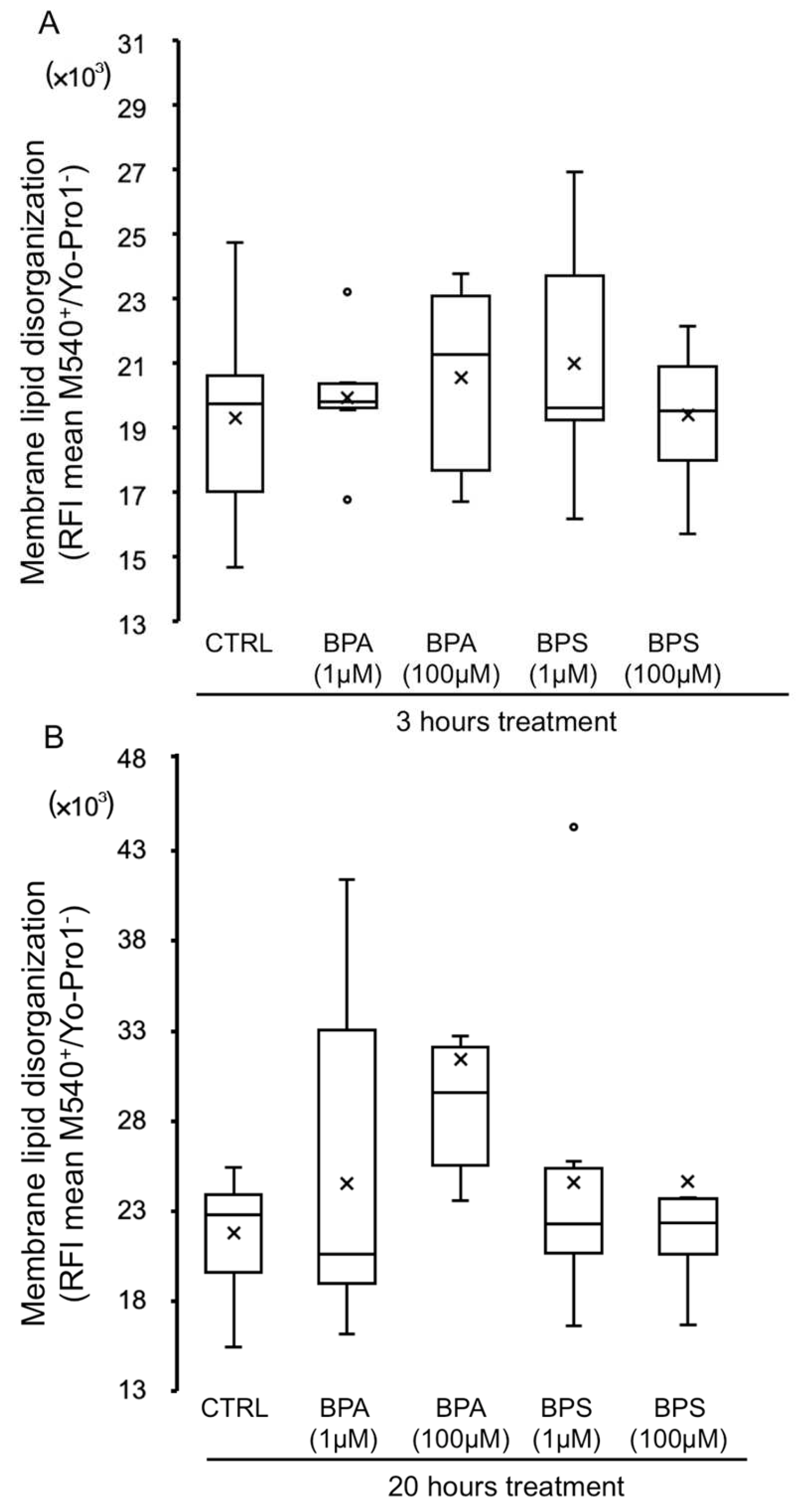
2.5. Effects of BPS and BPA on Pig Sperm Plasma Membrane Lipid Organization
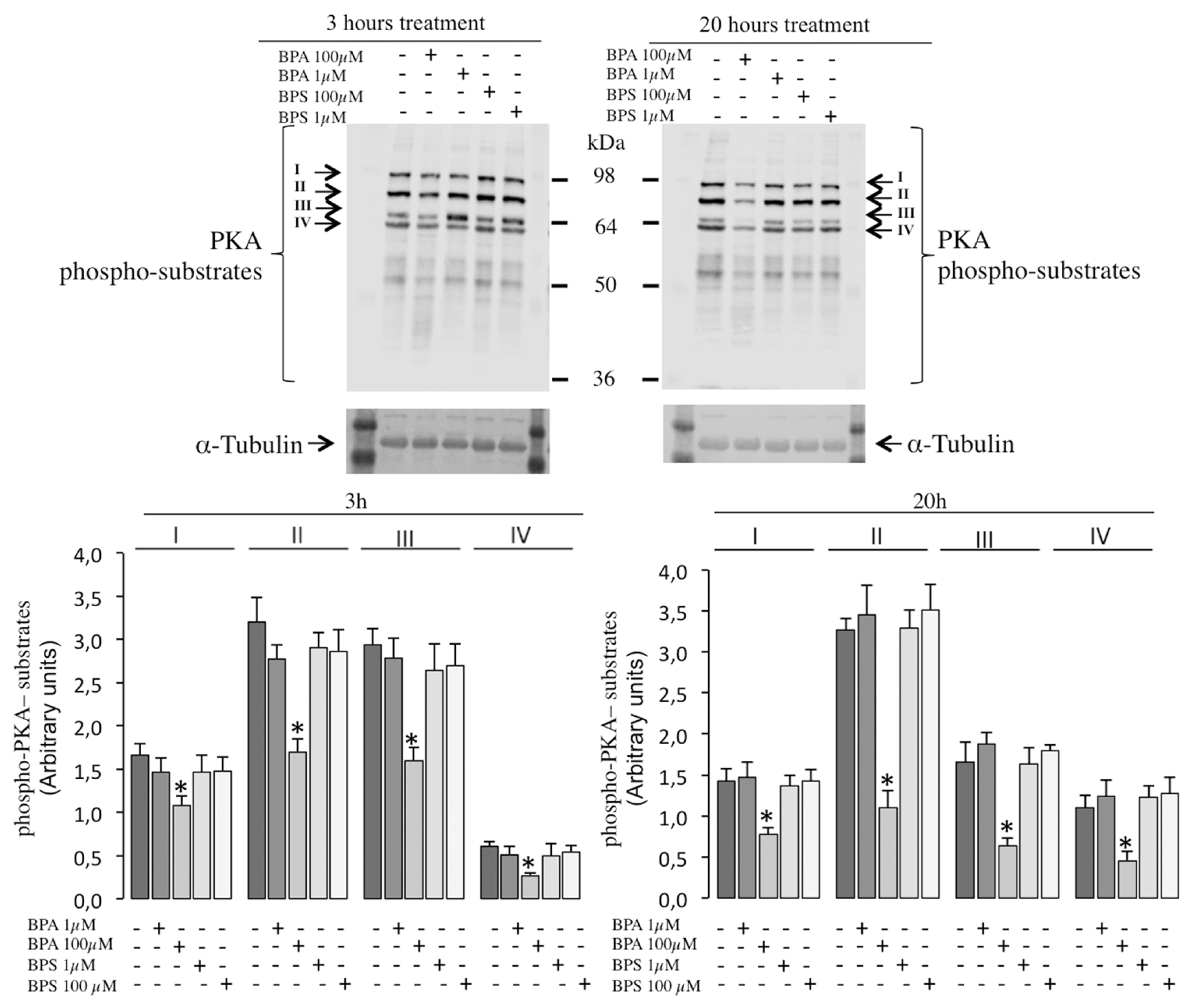
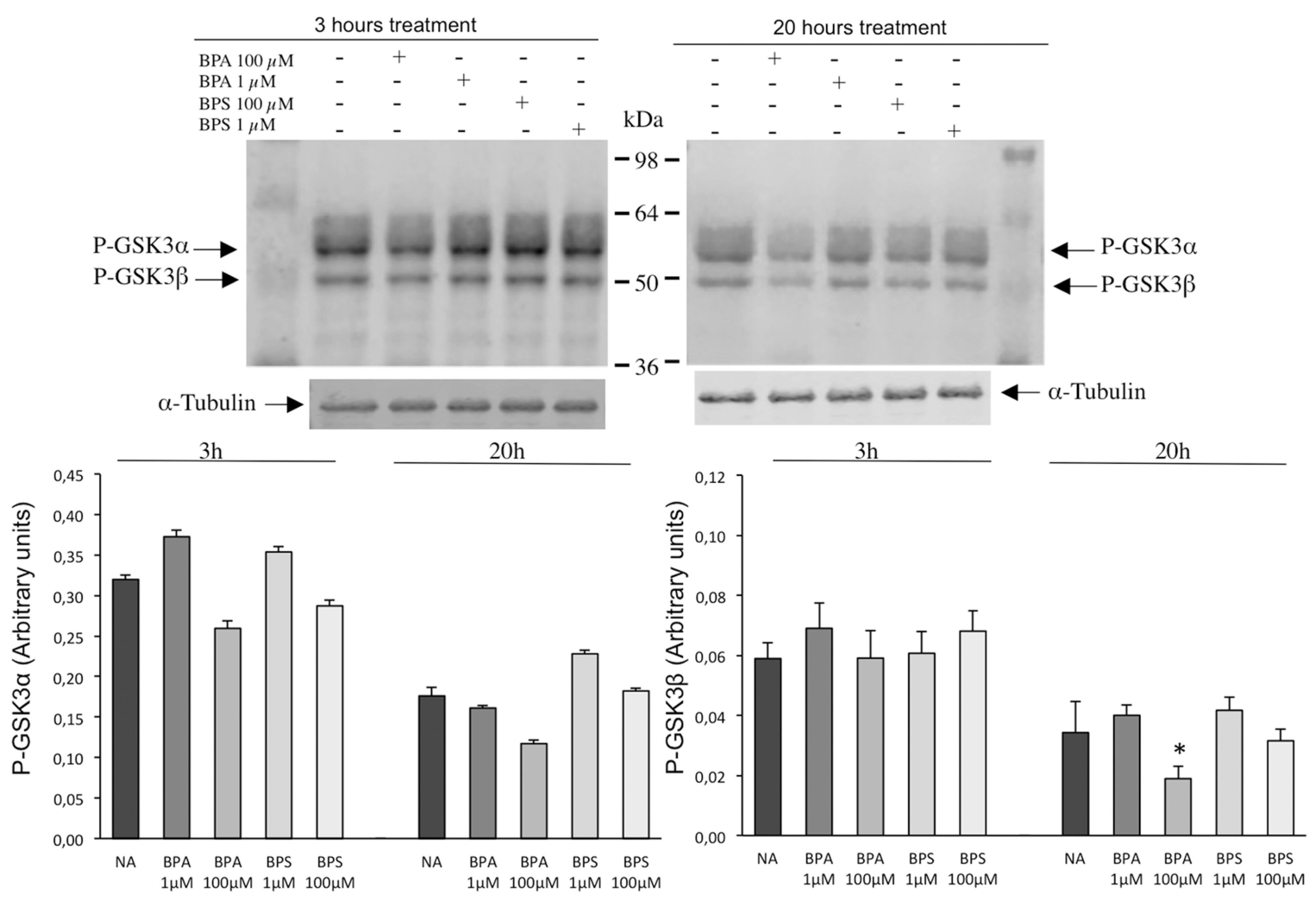
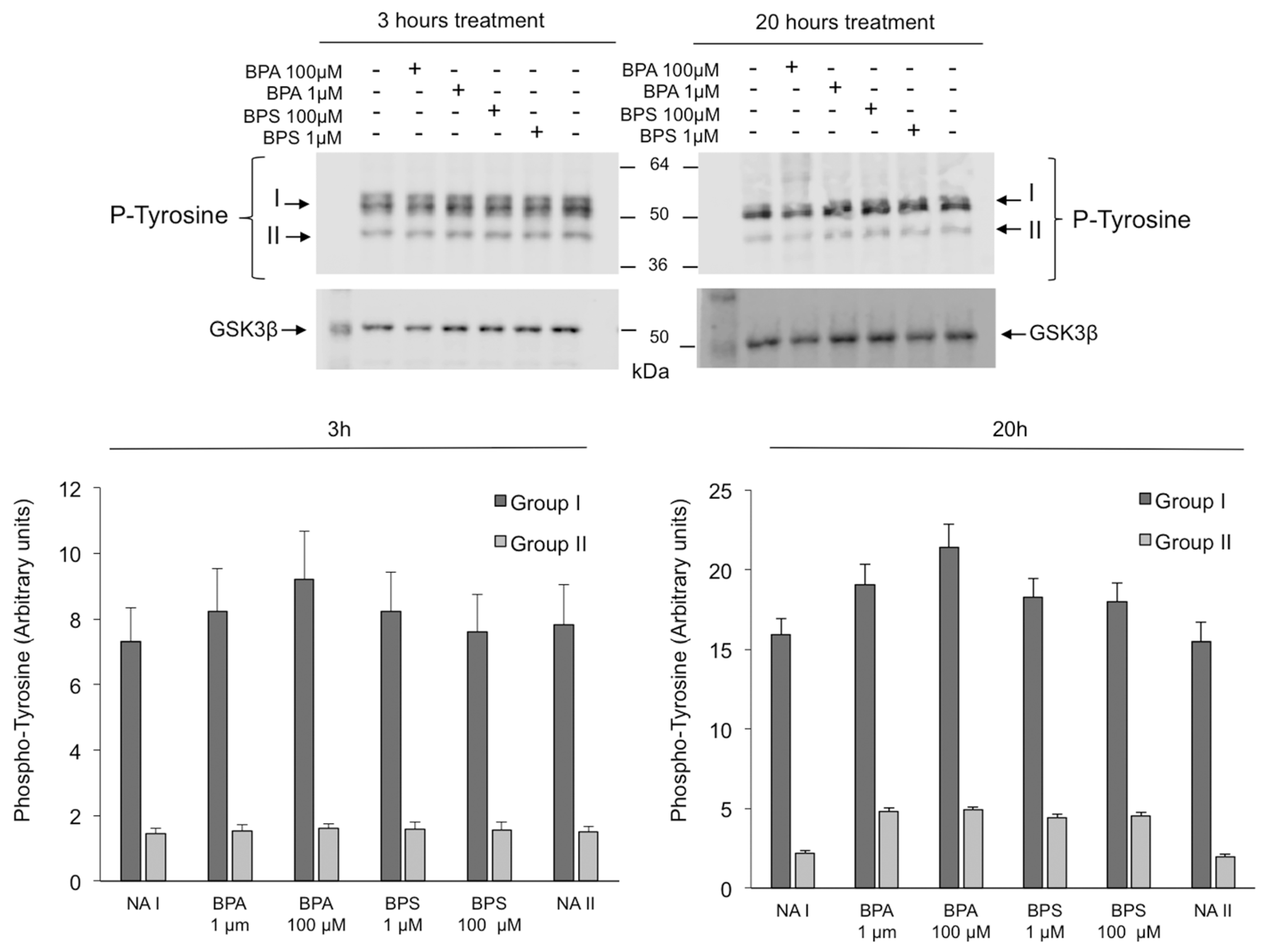
2.6. Effects of BPS and BPA on the Intracellular Signaling Pathways Mediated by PKA GSK3α/β and Tyrosine Phosphorylation in Pig Spermatozoa
3. Discussion
4. Materials and Methods
4.1. Chemical and Sources
4.2. Spermatozoa Incubation Media
4.3. Semen Samples
4.4. Boar Sperm Preparation
4.5. Evaluation of Spermatozoa Motility
4.6. Flow Cytometry Analysis
4.6.1. Analysis of Spermatozoa Viability by Flow Cytometry
4.6.2. Analysis of Sperm Mitochondrial Membrane Potential (ΔΨm) by Flow Cytometry
4.6.3. Analysis of the Degree of Sperm Plasma Membrane Lipid Organization by Flow Cytometry
4.6.4. Evaluation of Reactive Oxygen Species Production in Pig Spermatozoa
4.7. Analysis of Pig Spermatozoa Phosphorylated Proteins by Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Bonefeld-Jørgensen, E.C.; Long, M.; Hofmeister, M.V.; Vinggaard, A.M. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: New data and a brief review. Environ. Health Perspect. 2007, 115 (Suppl. 1), 69–76. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; vom Saal, F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J., Jr. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Kannan, K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, K.C.; Jung, Y.W.; Leung, P.C.; Jeung, E.B. Transfer of maternally injected endocrine disruptors through breast milk during lactation induces neonatal Calbindin-D9k in the rat model. Reprod. Toxicol. 2004, 18, 661–668. [Google Scholar] [CrossRef]
- Masuo, Y.; Ishido, M. Neurotoxicity of endocrine disruptors: Possible involvement in brain development and neurodegeneration. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 346–369. [Google Scholar] [CrossRef]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Shaheen, G.; Rehman, H.; Siddiqui, M.F.; Butt, M.A. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere 2018, 209, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Kakuta, Y.; Teramoto, T.; Koshiba, T.; Liu, X.; Okada, H.; Tokunaga, T.; Kawabata, S.; Kimura, M.; Shimohigashi, Y. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J. Biochem. 2007, 142, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol A story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Kannan, K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ. Sci. Technol. 2012, 46, 6515–6522. [Google Scholar] [CrossRef]
- Russo, G.; Barbato, F.; Mita, D.G.; Grumetto, L. Occurrence of Bisphenol A and its analogues in some foodstuff marketed in Europe. Food Chem. Toxicol. 2019, 131, 110575. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Choi, J.W.; Ahn, Y.A.; Kim, S. Pharmacokinetics of bisphenol S in humans after single oral administration. Environ. Int. 2018, 112, 127–133. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Alomirah, H.; Loi, V.D.; Mohd, M.A.; Moon, H.B.; Nakata, H.; Kannan, K. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures. Environ. Sci. Technol. 2012, 46, 6860–6866. [Google Scholar] [CrossRef]
- Ullah, H.; Jahan, S.; Ain, Q.U.; Shaheen, G.; Ahsan, N. Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere 2016, 152, 383–391. [Google Scholar] [CrossRef]
- Nevoral, J.; Kolinko, Y.; Moravec, J.; Žalmanová, T.; Hošková, K.; Prokešová, Š.; Klein, P.; Ghaibour, K.; Hošek, P.; Štiavnická, M.; et al. Long-term exposure to very low doses of bisphenol S affects female reproduction. Reproduction 2018, 156, 47–57. [Google Scholar] [CrossRef]
- Ghayda, R.A.; Williams, P.L.; Chavarro, J.E.; Ford, J.B.; Souter, I.; Calafat, A.M.; Hauser, R.; Mínguez-Alarcón, L. Urinary bisphenol S concentrations: Potential predictors of and associations with semen quality parameters among men attending a fertility center. Environ. Int. 2019, 131, 105050. [Google Scholar] [CrossRef]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Khan, M.J. Bisphenol A analogues bisphenol B, bisphenol F, and bisphenol S induce oxidative stress, disrupt daily sperm production, and damage DNA in rat spermatozoa: A comparative in vitro and in vivo study. Toxicol. Ind. Health 2019, 35, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Ru, Y.; Chu, C.; Ni, Z.; Zhou, Y.; Wang, S.; Zhou, Z.; Zhang, Y. Bisphenol A accelerates capacitation-associated protein tyrosine phosphorylation of rat sperm by activating protein kinase A. Acta Biochim. Biophys. Sin. 2016, 48, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Kang, K.H.; Arifuzzaman, S.; Pang, W.K.; Ryu, D.Y.; Song, W.H.; Park, Y.J.; Pang, M.G. Effect of antioxidants on BPA-induced stress on sperm function in a mouse model. Sci. Rep. 2019, 9, 10584. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Lee, J.S.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci. Rep. 2015, 5, 9169. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Yoon, S.J.; Park, Y.J.; Ryu, B.Y.; Pang, M.G. A novel approach to assessing bisphenol-A hazards using an in vitro model system. BMC Genom. 2016, 17, 577. [Google Scholar] [CrossRef]
- Nguyen, M.; Sabry, R.; Davis, O.S.; Favetta, L.A. Effects of BPA, BPS, and BPF on Oxidative Stress and Antioxidant Enzyme Expression in Bovine Oocytes and Spermatozoa. Genes 2022, 13, 142. [Google Scholar] [CrossRef]
- Rehfeld, A.; Andersson, A.M.; Skakkebæk, N.E. Bisphenol A Diglycidyl Ether (BADGE) and Bisphenol Analogs, but Not Bisphenol A (BPA), Activate the CatSper Ca(2+) Channel in Human Sperm. Front. Endocrinol. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Castellini, C.; Di Giammarco, N.; Santilli, G.; Francavilla, S.; Francavilla, F. In vitro exposure of human spermatozoa to bisphenol A induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod. Toxicol. 2016, 66, 61–67. [Google Scholar] [CrossRef]
- Castellini, C.; Di Giammarco, N.; D’Andrea, S.; Parisi, A.; Totaro, M.; Francavilla, S.; Francavilla, F.; Barbonetti, A. Effects of bisphenol S and bisphenol F on human spermatozoa: An in vitro study. Reprod. Toxicol. 2021, 103, 58–63. [Google Scholar] [CrossRef]
- Cassault-Meyer, E.; Gress, S.; Séralini, G.; Galeraud-Denis, I. An acute exposure to glyphosate-based herbicide alters aromatase levels in testis and sperm nuclear quality. Environ. Toxicol. Pharmacol. 2014, 38, 131–140. [Google Scholar] [CrossRef]
- Nerozzi, C.; Recuero, S.; Galeati, G.; Bucci, D.; Spinaci, M.; Yeste, M. Effects of Roundup and its main component, glyphosate, upon mammalian sperm function and survival. Sci. Rep. 2020, 10, 11026. [Google Scholar] [CrossRef]
- Vicente-Carrillo, A. The Usefulness of Sperm Kinematics in Drug-Induced Toxicity Assessment. Basic Clin. Pharmacol. Toxicol. 2018, 123, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Carrillo, A.; Edebert, I.; Garside, H.; Cotgreave, I.; Rigler, R.; Loitto, V.; Magnusson, K.E.; Rodriguez-Martinez, H. Boar spermatozoa successfully predict mitochondrial modes of toxicity: Implications for drug toxicity testing and the 3R principles. Toxicol. Vitr. 2015, 29, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Zigo, M.; Manaskova-Postlerova, P.; Zuidema, D.; Kerns, K.; Jonakova, V.; Tumova, L.; Bubenickova, F.; Sutovsky, P. Porcine model for the study of sperm capacitation, fertilization and male fertility. Cell Tissue Res. 2020, 380, 237–262. [Google Scholar] [CrossRef] [PubMed]
- Rezaee-Tazangi, F.; Zeidooni, L.; Rafiee, Z.; Fakhredini, F.; Kalantari, H.; Alidadi, H.; Khorsandi, L. Taurine effects on Bisphenol A-induced oxidative stress in the mouse testicular mitochondria and sperm motility. JBRA Assist. Reprod. 2020, 24, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Lukacova, J.; Jambor, T.; Knazicka, Z.; Tvrda, E.; Kolesarova, A.; Lukac, N. Dose- and time-dependent effects of bisphenol A on bovine spermatozoa in vitro. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 669–676. [Google Scholar] [CrossRef]
- Chitra, K.C.; Latchoumycandane, C.; Mathur, P.P. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 2003, 185, 119–127. [Google Scholar] [CrossRef]
- Darghouthi, M.; Rezg, R.; Boughmadi, O.; Mornagui, B. Low-dose bisphenol S exposure induces hypospermatogenesis and mitochondrial dysfunction in rats: A possible implication of StAR protein. Reprod. Toxicol. 2022, 107, 104–111. [Google Scholar] [CrossRef]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Thomas, B.F.; Keimowitz, A.R.; Brine, D.R.; Veselica, M.M.; Fail, P.A.; Chang, T.Y.; Seely, J.C.; et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. Off. J. Soc. Toxicol. 2002, 68, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Torres-Badia, M.; Solar-Malaga, S.; Martin-Hidalgo, D.; Hurtado de Llera, A.; Gomez-Candelo, A.; Garcia-Marin, L.J.; González-Fernández, L.; Bragado, M.J. Impaired mammalian sperm function and lower phosphorylation signaling caused by the herbicide Roundup® Ultra Plus are due to its surfactant component. Theriogenology 2021, 172, 55–66. [Google Scholar] [CrossRef]

| Time | Treatment | [] | VCL (µm s−1) | VSL (µm s−1) | VAP (µm s−1) | LIN (%) | STR (%) | WOB (%) | ALH (µm) | BCF (Hz) |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 h | BPA | Ctrl | 57.9 ± 5.9 | 44.6 ± 5.3 | 46.3 ± 5.3 | 73.8 ± 1.0 | 91.4 ± 0.6 | 78.5 ± 0.8 | 1.9 ± 0.1 | 8.5 ± 0.3 |
| 1 µM | 63.9 ± 3.1 | 49.1 ± 3.0 | 50.8 ± 2.7 | 72.9 ± 1.2 | 90.4 ± 0.9 | 77.8 ± 0.7 | 2.1 ± 0.1 | 8.8 ± 0.2 | ||
| 100 µM | 41.5 ± 4.3 * | 24.2 ± 3.0 * | 27.8 ± 3.0 * | 57.9 ± 1.4 * | 81.8 ± 1.3 * | 68.2 ± 1.5 * | 1.8 ± 0.1 | 6.6 ± 0.6 * | ||
| BPS | Ctrl | 64.4 ± 3.1 | 48.8 ± 2.9 | 51.4 ± 2.9 | 71.97 ± 0.8 | 89.2 ± 0.6 | 77.6 ± 0.6 | 2.0 ± 0.1 | 9.0 ± 0.1 | |
| 1 µM | 63.2 ± 2.3 | 47.9 ± 2.3 | 50.3 ± 2.3 | 72.8 ± 1.2 | 89.9 ± 0.6 | 78.1 ± 0.9 | 2.0 ± 0.1 | 9.1 ± 0.1 | ||
| 100 µM | 46.9 ± 2.6 * | 32.3 ± 2.1 * | 35.1 ± 2.1 * | 66.6 ± 1.1 * | 87.1 ± 0.8 | 74.4 ± 0.7 | 1.85 ± 0.1 | 7.1 ± 0.2 * | ||
| 20 h | BPA | Ctrl | 31.4 ± 1.3 | 14.4 ± 1.0 | 18.1 ± 0.8 | 47.6 ± 1.8 | 77.3 ± 1.5 | 60.3 ± 1.7 | 1.5 ± 0.0 | 6.1 ± 0.2 |
| 1 µM | 35.5 ± 1.0 | 16.1 ± 0.8 | 19.6 ± 0.7 | 46.9 ± 2.2 | 79.8 ± 1.1 | 57.6 ± 2.2 | 1.7 ± 0.0 | 6.8 ± 0.2 | ||
| 100 µM | 27.3 ± 1.5 | 11.2 ± 0.9 | 15.3 ± 0.8 | 43.0 ± 2.5 | 70.4 ± 2.6 | 58.9 ± 2.5 | 1.5 ± 0.0 | 4.2 ± 0.6 | ||
| BPS | Ctrl | 33.22 ± 1.5 | 16.5 ± 1.6 | 19.7 ± 1.5 | 50.3 ± 3.3 | 80.7 ± 1.5 | 61.0 ± 3.0 | 1.5 ± 0.0 | 6.3 ± 0.3 | |
| 1 µM | 36.9 ± 2.1 | 19.0 ± 1.6 | 22.1 ± 1.5 | 51.7 ± 2.0 | 83.1 ± 1.2 | 60.9 ± 1.8 | 1.7 ± 0.0 | 7.4 ± 0.3 | ||
| 100 µM | 28.3 ± 1.0 | 11.6 ± 0.4 | 15.6 ± 0.4 | 43.2 ± 1.9 | 73.9 ± 1.7 | 57.7 ± 1.3 | 1.4 ± 0.0 | 5.2 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Badia, M.; Martin-Hidalgo, D.; Serrano, R.; Garcia-Marin, L.J.; Bragado, M.J. Bisphenol S Reduces Pig Spermatozoa Motility through Different Intracellular Pathways and Mechanisms than Its Analog Bisphenol A. Int. J. Mol. Sci. 2023, 24, 9598. https://doi.org/10.3390/ijms24119598
Torres-Badia M, Martin-Hidalgo D, Serrano R, Garcia-Marin LJ, Bragado MJ. Bisphenol S Reduces Pig Spermatozoa Motility through Different Intracellular Pathways and Mechanisms than Its Analog Bisphenol A. International Journal of Molecular Sciences. 2023; 24(11):9598. https://doi.org/10.3390/ijms24119598
Chicago/Turabian StyleTorres-Badia, Mercedes, David Martin-Hidalgo, Rebeca Serrano, Luis J. Garcia-Marin, and Maria J. Bragado. 2023. "Bisphenol S Reduces Pig Spermatozoa Motility through Different Intracellular Pathways and Mechanisms than Its Analog Bisphenol A" International Journal of Molecular Sciences 24, no. 11: 9598. https://doi.org/10.3390/ijms24119598
APA StyleTorres-Badia, M., Martin-Hidalgo, D., Serrano, R., Garcia-Marin, L. J., & Bragado, M. J. (2023). Bisphenol S Reduces Pig Spermatozoa Motility through Different Intracellular Pathways and Mechanisms than Its Analog Bisphenol A. International Journal of Molecular Sciences, 24(11), 9598. https://doi.org/10.3390/ijms24119598







