Abstract
Well-differentiated/de-differentiated liposarcoma (WDLPS/DDLPS) is one of the most common histologic subtypes of soft tissue sarcoma (STS); however, treatment options remain limited. WDLPS and DDLPS both exhibit the characteristic amplification of chromosome region 12q13-15, which contains the genes CDK4 and MDM2. DDLPS exhibits higher amplification ratios of these two and carries additional genomic lesions, including the amplification of chromosome region 1p32 and chromosome region 6q23, which may explain the more aggressive biology of DDLPS. WDLPS does not respond to systemic chemotherapy and is primarily managed with local therapy, including multiple resections and debulking procedures whenever clinically feasible. In contrast, DDLPS can respond to chemotherapy drugs and drug combinations, including doxorubicin (or doxorubicin in combination with ifosfamide), gemcitabine (or gemcitabine in combination with docetaxel), trabectedin, eribulin, and pazopanib. However, the response rate is generally low, and the response duration is usually short. This review highlights the clinical trials with developmental therapeutics that have been completed or are ongoing, including CDK4/6 inhibitors, MDM2 inhibitors, and immune checkpoint inhibitors. This review will also discuss the current landscape in assessing biomarkers for identifying tumors sensitive to immune checkpoint inhibitors.
1. Introduction
Liposarcoma (LPS) is the most common soft tissue sarcoma (STS) subtype in adults, accounting for up to 25% of all adult sarcomas [1,2,3]. Liposarcomas can be broadly categorized into three histologic subtypes: well-differentiated (WDLPS) and de-differentiated LPS (DDLPS), myxoid/round cell LPS, and pleomorphic LPS. WDLPS and DDLPS represent over 60% of all LPSs; however, treatment options remain limited [4]. A number of clinical trials targeting MDM2 are ongoing for patients with advanced de-differentiated liposarcoma. Here, we will review the clinical and genomic characteristics and novel therapeutic strategies involving immune checkpoint inhibitors and CDK4/6 and MDM2 inhibitors, and discuss several studies that explored potential biomarkers for predicting response to immune checkpoint inhibitors.
2. Clinical Characteristics of WDLPS/DDLPS
WDLPS is primarily a loco-regional disease without metastatic potential. Local recurrences can occur in 30–50% of patients. In 10% of patients, especially those of retroperitoneum, mediastinum, and spermatic cord primary, WDLPS can de-differentiate into DDLPS [5,6]. WDLPS is sometimes also called an atypical lipomatous tumor (ALT) when they occur in the extremities and have a better prognosis [6,7].
DDLPS occurs primarily in adults over 40 years old, with equal gender distribution, and with retroperitoneum as the most common primary site. Extremities, paratesticular space, mediastinum, and head and neck are less common primary sites [6]. DDLPS accounts for more than 50% of all primary retroperitoneal sarcomas, and approximately 80–90% of them occur de novo [8,9]. The rest of DDLPS derives from the recurrence of WDLPS after an average of approximately 8 years from the onset of the diagnosis [6]. DDLPS can behave as an intermediate- and high-grade sarcoma, with approximately a 40% risk of local relapse and a 30% risk of distant metastasis. Biologically, it appears to be less aggressive compared to other pleomorphic sarcomas, such as undifferentiated pleomorphic sarcoma (UPS) [6,8,10].
Morphologically, WDLPS recapitulates the histologic characteristics of mature fat. Classically, WDLPS appears as lobulated, circumscribed, pale, soft, yellowish fat masses. The most predominant histological subtype of WDLPS is adipocytic (lipoma-like). Less common subtypes include sclerosing and inflammatory variants. Sclerosing WDLPS is the next most common WDLPS subtype and is frequently found in retroperitoneal and paratesticular sites. Sclerosing WDLPS shows scattered atypical stromal cells within the prominent collagenous stroma. Inflammatory WDLPS is a rare variant frequently found in the retroperitoneum and shows a chronic inflammatory infiltrate comprised of lymphocytes and plasma cells with lymphoid follicles.
DDLPS displays more heterogeneous morphologies with non-lipogenic components that are often characteristic of pleomorphic or unspecified spindle cell sarcoma [6,7,11,12,13]. DDLPS are often large, multinodular tumors that typically show moderate or high cellularity. Cytomorphology is variable, although significant nuclear pleomorphism is often observed. The stroma can be collagenous, myxocollagenous or myxoid, and even appear similar to high-grade myxofibrosarcoma. DDLPS can rarely also appear morphologically low-grade with bland, non-pleomorphic cytologic features, resembling desmoid fibromatosis. Despite their low-grade histological appearance, these DDLPS appear to have a similar ability as conventional DDLPS to metastasize.
WDLPS does not respond to systemic chemotherapy and is primarily managed with local therapy, including multiple resections and/or debulking procedures whenever clinically feasible [14]. Unlike WDLPS, DDLPS can respond to chemotherapy drugs and drug combinations, including Doxorubicin (or Doxorubicin in combination with ifosfamide), gemcitabine (or gemcitabine in combination with docetaxel), trabectedin, eribulin, and pazopanib [1,2]. However, the response rates are generally low with short duration [15,16,17].
3. Genomic Characteristics of WDLPS/DDLPS
WDLPS and DDLPS both exhibit the characteristic amplification of chromosome region 12q13-15. This region encompasses several genes, including CDK4 and MDM2, HMGA2 at 12q14.3, CPM at 12q15, SAS/TSPAN31 at 12q14.1, and YEATS4 at 12q15. CDK4 (12q14.1) is located on a different amplicon from MDM2 (12q15), which may explain why the two genes are not always co-expressed. CDK4 is frequently, though not always, amplified in WDLPS/DDLPS [6]. MDM2 amplification frequently includes DDIT3, which can sometimes cause confusion with myxoid liposarcoma [18,19]. MDM2 is consistently amplified and overexpressed in WDLPS/DDLPS (nearly 100%) and is considered to be the main driver gene within the 12q amplicon. HMGA2 is often coamplified with MDM2 in WDLPS and DDLPS, but more commonly altered in benign lipomas [20,21,22,23,24]. MDM2 amplification is not present in benign lipomas and is considered the molecular marker that distinguishes between benign lipomas and WDLPS [25,26]
DDLPS appears to exhibit higher amplification profiles of genes in the 12q13-15 region compared to that WDLPS [27]. There is evidence that DDLPS contained a higher ratio of MDM2 amplification compared to WDLPS [28]. In addition, DDLPS contains additional genomic lesions, including the amplification of chromosome region 1p32, where the gene JUN is located, and chromosome region 6q23, where the activating kinase of JUN (ASK1) is located [29]. Amplifications of JUN and ASK1 are thought to be mutually exclusive and found predominantly in DDLPS. Preclinical studies have shown that JUN amplification and overexpression block adipocytic differentiation in sarcomas, which may provide the pathologic basis of progression from WDLPS to DDLPS. These and other molecular lesions are likely responsible for the more aggressive behaviors of DDLPS compared to WDLPS [30,31]. A study showed that high JUN amplification (more than 16 copies) correlated with decreased DFS in DDLPS [32].
MDM2 is an E3 ubiquitin ligase that regulates the protein level and activities of p53 [33]. It acts as an oncogene by suppressing the p53 function, but in certain contexts, it has been found to act as a tumor suppressor [33,34]. In some retrospective studies, the degree of MDM2 amplification in de-differentiated liposarcoma was found to be associated with poor overall survival (OS) and reduced interval from the time of resection to the time of recurrence [35,36]. MDM2 amplification results in overexpression of MDM2 protein and its increased binding to p53, inhibiting its function and causing p53 degradation [33,34,37]. MDM2 can bind directly to the N-terminus of p53 and inhibit its transcriptional activation function. MDM2 prevents p53 from interacting with transcriptional co-activators and recruits transcriptional co-repressors to p53 (Figure 1). The C-terminal RING finger domain of MDM2 possesses E3 ubiquitin ligase activity that targets p53 for modification and subsequent degradation through the 26S proteasome [33,38]. p53 regulates MDM2 expression by binding to its promoter, creating an autoregulatory feedback loop [33,39]. Several MDM2 inhibitors have been identified, and some of them have entered clinical trials [37,40].
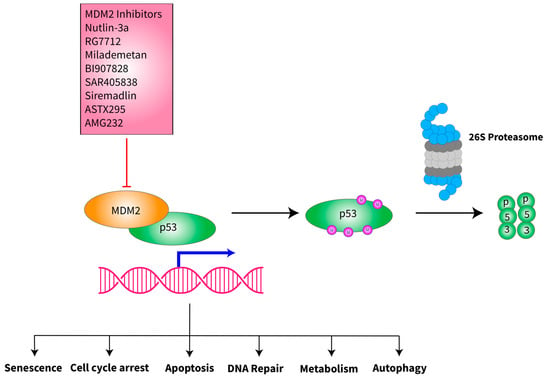
Figure 1.
Schematic of MDM2 interaction with p53 and inhibition of MDM2 by its inhibitors. MDM2 is an E3 ubiquitin ligase that binds directly to the N-terminus of p53, bringing p53 to 26S proteasome for degradation, preventing the tumor suppressive function of p53, which regulates numerous cellular functions to safeguard the cells from oncogenesis. A number of MDM2 inhibitors have been synthesized and tested in vitro, in vivo and in early-stage clinical trials (see ongoing clinical trials section below). Many of these inhibitors bind to the MDM2-p53 interface and prevent the binding of MDM2 to p53, therefore stabilizing the protein levels of the wild-type p53 protein. The clinical trials with MDM2 inhibitors are therefore focused on malignancies that retain wild-type p53.
CDK4, a cell cycle regulator, suppresses the retinoblastoma protein RB1 to stimulate cell cycle progression and the expression of a battery of genes, including MDM2. The CDK4 gene encodes a 33 kD protein that forms molecular complexes with members of the cyclin D family, including cyclin D1, D2 and D3. The CDK4-CCND1 complex phosphorylates RB1 protein, which releases the E2F transcription factor, and in turn, up-regulates gene expression required for progression through the S-, G2-, and M-phases [41,42,43]. Amplification of CDK4 and its cyclin partner CCND1, as well as the deletion of CDKN2A (an inhibitor of CDK4/6), is associated with worse survival in patients with advanced soft tissue sarcomas (Figure 2) [44,45]. A high copy number of MDM2 and CDK4 was shown to correlate with decreased disease-free survival (DFS) and disease-specific survival in DDLPS [32]. TP53 mutations are also common in some soft tissue sarcomas and likely confer aggressive biological behaviors [45,46].

Figure 2.
Schematic of CDK4 in cell cycle progression. CDK4 forms molecular complexes with members of the cyclin D family, including cyclin D1, D2 and D3 and becomes activated. The CDK4-CCND complex phosphorylates the retinoblastoma protein RB1 that binds to E2F to inhibit cell cycle progression. Phosphorylation of the Rb protein releases the E2F transcription factor, which subsequently stimulates the expression of genes that regulate the G1/S and G2/M checkpoint, leading to cell cycle progression.
4. Novel Therapeutic Regimens in WDLPS/DDLPS
In Table 1, we have outlined some of the important clinical trials involving DDLPS that have been completed, including the trials that tested CDK4/6 inhibitors and immune checkpoint inhibitors.

Table 1.
Select published trials.
5. CDK4/6 Inhibitors
Three CDK4/6 inhibitors (abemaciclib, palbociclib and ribociclib) have shown clinical benefit in patients with advanced hormone receptor (HR)-positive/HER2-negative breast cancer when given in combination with endocrine therapy [60,61,62,63,64,65,66]. abemaciclib plus endocrine therapy has also demonstrated superior DFS over endocrine therapy alone in adjuvant settings in patients with high-risk early-stage breast cancer [63]. As monotherapy, only abemaciclib produced a meaningful overall response rate (ORR), while palbociclib and ribociclib resulted in primarily stable disease (SD) [60,67]. The ORR was 24.1% with abemaciclib at 150 mg twice daily and 32.5% with 200 mg twice daily in a phase II trial in patients with advanced breast cancer [68]. Despite the fact that CDK4/6 alterations are very common in many types of malignancies and that numerous preclinical studies on these agents have shown tumor suppressive effect, significant clinical benefits have only been demonstrated primarily in breast cancer and to a lesser extent, WDLPS/DDLPS [69,70,71]. Studies both in vitro and in vivo have shown that downregulation of CDK4/6 expression can inhibit the proliferation of liposarcoma cells by preventing the phosphorylation of Rb protein [72,73]. In addition, palbociclib can arrest soft tissue sarcoma cells in the G1 phase by inhibiting Wee1 kinase and also induce apoptosis and senescence [74,75,76].
In a phase I trial with palbociclib (PD 0332991), Schwartz et al. treated 33 patients with Rb-positive solid tumors or refractory non-Hodgkin’s lymphoma and found durable SD in 4 out of 7 patients with liposarcoma, subtype unspecified [47]. This led to the subsequent phase II trial by Dickson et al., which enrolled 30 patients with advanced WDLPS or DDLPS with CDK4 amplification by fluorescence in situ hybridization and RB expression by immunohistochemistry (≥1+) [48]. The majority of these patients had retroperitoneal DDLPS [48]. The median progression-free survival (PFS) was 17.9 weeks, and the estimated 12-week progression-free survival (PFS) was 66%. One patient (3%) had a partial response (PR), and 30% had some degree of tumor shrinkage [48]. The dosing of palbociclib in this trial was 200 mg for 14 days in a 21-day cycle [48]. This trial was extended with an expansion cohort that enrolled an additional 30 patients (90% were DDLPS), and the dosing was changed to 125 mg for 21 days in a 28-day cycle which is the standard dosing for patients with advanced breast cancer [49,66,77]. The median PFS for this cohort of 60 patients was 18 weeks, and the 12-week PFS was 57%. There was one complete response (CR). Nine patients from the expansion cohort had paired tumor tissue biopsies performed, which revealed that downregulation of MDM2 expression mediated through ATRX was associated with clinical benefit [78]. Preliminary results from the TAPUR basket trial demonstrated the anti-tumor activity of palbociclib monotherapy in 29 patients with advanced STS with CDK4 amplification and no RB1 mutations (histologic subtype breakdown not reported). Patients were treated with palbociclib 125 mg for 21 days in a 28-day cycle. The median PFS was 16 weeks, and one patient had a PR [57]. Based on these data, National Comprehensive Cancer Network (NCCN) has recommended palbociclib as a treatment option for WDLPS and DDLPS [79].
A phase II study of abemaciclib 200 mg twice a day in 30 patients with DDLPS [53] reported a 12-week PFS of 76% and a median PFS of 30 weeks. In addition, three patients had more than 10% shrinkage of the tumor. These results appear favorable compared to the results from the palbociclib trials [48,49]. Based on these data, SARC041 is currently enrolling patients with advanced DDLPS for a placebo-controlled randomized trial to test the efficacy of abemaciclib in this patient population. Other trials that involved liposarcoma include a phase I trial with ribociclib in which six patients with liposarcoma had SD for more than 6 months; however, it was not clear if these six patients had WDLPS/DDLPS or other types of liposarcoma (pleomorphic or myxoid liposarcoma) [50].
The toxicities of palbociclib and abemaciclib in the phase II trials with WDLPS and DDLPS patients are similar to that of phase II and III trials in advanced breast cancer, including mild to moderate neutropenia and gastrointestinal toxicities [60,64,65,66,80,81,82]. Both agents are generally well tolerated. Abemaciclib appears to induce a lower degree of neutropenia compared to palbociclib [69].
A phase IB study testing the combination of CDK4/6 inhibitor ribociclib and MDM2 inhibitor Siremadlin in patients with WDLPS and patients with DDLPS showed limited activities, with 3 PRs out of 74 patients. In addition, there were ten patients who reached dose-limiting toxicities primarily associated with hematologic events [59]. This type of combination will require further exploration with different doses and particular types of molecules.
6. Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) have also been studied in WDLPS and DDLPS [83,84,85]. The SARC028 trial enrolled patients with advanced soft tissue and bone sarcoma who had progressed after at least one line of therapy with 86 patients enrolled and 80 patients evaluable, including ten patients each with UPS, DDLPS, synovial sarcoma and leiomyosarcoma, and 40 patients with osteosarcoma, Ewing sarcoma and de-differentiated chondrosarcoma [51]. Patients were given Pembrolizumab 200 mg intravenously every 3 weeks. The ORR for the entire cohort was 18%. Two out of 10 patients with DDLPS had a PR [51]. In the SARC028 expansion cohort [54], an additional 30 patients with UPS and 30 patients with DDLPS were enrolled for a total of 40 UPS and 40 LPS patients. The ORR for DDLPS was 10%, the median PFS was 2 months, and the 12-week PFS was 44%. In the Alliance trial A091401 [52], 96 patients with metastatic sarcoma were enrolled. Of the 85 eligible patients, 43 were randomized to single-agent Nivolumab and 42 to Nivolumab plus Ipilimumab. The ORR was 5% for Nivolumab and 16% for Nivolumab plus Ipilimumab. No response was seen in five patients with DDLPS. A meta-analysis including 27 trials with a total of 1012 patients treated with ICIs showed an 11% ORR for patients with liposarcoma and approximately 14% for all sarcomas [85]. Other meta-analyses and retrospective studies have shown similar ORR for advanced sarcoma [86,87,88,89].
The combination of Doxorubicin and Pembrolizumab has also been evaluated in phase I/II trials with 37 sarcoma patients. The primary endpoint was ORR by RECIST 1.1, with a 2-stage study design to rule out ORR of 15% or less with 85% power if the true ORR was 35%, using a 1-sided 5% level test. Unfortunately, accrual was closed at 31 of 35 planned patients because of an insufficient number of second-stage PRs, indicating that the study would not achieve the primary endpoint of ORR. ORR was 13% for phase 2 patients (19% overall). Two of 4 patients with DDLPS had durable PRs [55]. A similar single institution phase II trial with 30 patients treated with Doxorubicin and Pembrolizumab showed an ORR of 37% including 1 CR and 1 PR of 7 patients with liposarcoma [58]. The combination of Pembrolizumab with cryotherapy has also been studied in STS, including DDLPS [90]. A study using the combination of Pembrolizumab and Talimogene Laherparepvec in 20 patients with advanced sarcoma showed an ORR of 35% [56].
As illustrated above, CDK4/6 inhibitors and ICIs as monotherapy have demonstrated activity in DDLPS. However, it is not clear if the combination of these two classes of agents has synergistic activity. Several in vitro studies have shown that CDK4/6 inhibition modulates immune responses and may cooperate with ICIs to suppress tumor growth [91]. Preclinical studies have shown that abemaciclib activates the expression of endogenous retroviral elements in tumor cells and increases the intracellular level of double-stranded RNA, which stimulates the expression of type-III interferon and increased antigen presentation in the tumor microenvironment [92]. abemaciclib also increases tumor T lymphocyte infiltration, creating an inflamed T cell tumor microenvironment, and when combined with an ICI, leads to complete regression of tumors [93]. CDK4/6 inhibition by palbociclib or Trilaciclib enhances T cell activation by de-repressing NFAT signaling, leading to increased tumor T lymphocyte infiltration and activation of effector T cells. In addition, CDK4/6 inhibition up-regulates PD-L1 expression through suppression of the NF-kB pathway [94] and degradation of SPOP protein. CDK4/6 inhibition with anti-PD-1 immunotherapy enhances tumor regression and improves survival rate in a mouse model [95]. CDK4/6 inhibition suppresses CD4+ Tregs more than CD8+ cytotoxic T cells, increases the CD8+ T cells/Tregs ratio in the tumor microenvironment, and, when combined with an ICI, leads to the clearance of tumor cells by cytotoxic T cells [96]. These studies suggest the potential synergistic or enhanced effect of a CDK4/6 inhibitor and an ICI combination that could be evaluated prospectively in a clinical trial.
7. ICI Biomarker Studies in Sarcoma
The search for biomarkers predictive of the benefit of ICIs has been an intense research effort [97]. Tumor mutation burden, PD-L1 expression of tumor cells, peripheral blood lymphocyte counts, and others have been associated with higher response rates and clinical benefits from ICIs [98,99,100,101]. However, in soft tissue and bone sarcomas, PD-L1 expression does not correlate well with a response or clinical benefit [86]. Several studies attempted to analyze the tumor microenvironment (TME) of sarcomas, including the composition of the tumor infiltrating CD4+ and CD8+ T cells, tumor-associated macrophages (TAM), and dendritic cells. Unfortunately, many of these studies were affected by a limited number of cases and histologic heterogeneity [102,103,104,105,106]. The complexity of TME is being increasingly revealed by ever more sophisticated techniques (e.g., single-cell RNA sequencing etc.) and well-designed studies in different malignancies.
The gene expression profiles of TME of 608 sarcoma specimens with a wide range of histology types were reported by Petitprez. In this analysis, five different phenotypes were described based on the immune infiltrates within the TME [107]. These five phenotypes included immune-low (A and B), immune-high (D and E), and highly vascularized (C). In addition, Group E showed a better response to Pembrolizumab and better survival compared to the other groups and was associated with the presence of tertiary lymphoid structures and enrichment of B cells within the tumors [107].
The immunostaining of 1072 sarcoma specimens showed that sarcomas with complex genomic lesions (mutations, copy number alterations) often contained a higher density of tumor infiltrating lymphocytes compared to fusion-driven sarcomas [108]. DDLPS and UPS were found to be the two top sarcomas that contained a higher density of tumor infiltrating lymphocytes among all the sarcomas [108]. In addition, this study showed that approximately 10–22% of all sarcomas had detectable PD-L1 or PD1 and that expression of PD-L1 and CD56 were associated with worse survival [108].
In a separate study, DDLPS was shown to contain higher density of CD68+ M1 macrophages and CD163+ M2 macrophages compared to fusion-driven sarcomas [109]. The study by Pollack et al. on 81 patients with sarcoma showed that tumor T-cell infiltration and clonality correlated with PD-1 and PD-L1 expression [110]. A phase II trial combining Pembrolizumab and metronomic Cyclophosphamide in 50 patients with advanced sarcoma showed little activity but found infiltration of M2 macrophages expressing indoleamine 2,3-dioxygenase (IDO) in the TME [111]. The phase I/II trial with Doxorubicin and Pembrolizumab showed that the presence of tumor infiltrating lymphocytes (TILs) is associated with worse OS in sarcoma [55]. Sarcomas can still respond to immunotherapy even without detectable PD-L1 expression [112].
The biomarker study of SARC028 tumor specimens pre- and post-treatment with Pembrolizumab suggested a correlation between the higher density of CD8+CD3+PD-L1+ activated T cells and response. Pre-treatment tumors with higher baseline density of effector memory CD8+ T cells and regulatory T cells (Tregs) showed a better response and better PFS [113]. These results are consistent with the results of the other studies [114]. It remains important and useful to investigate the mechanisms of response or the lack of response to ICIs in sarcoma through clinical trials as well as laboratory studies which could facilitate the development of novel therapeutics.
8. Ongoing Clinical Trials
Several clinical trials for WDLPS and DDLPS are ongoing (Table 2). The most anticipated clinical trials for DDLPS currently are in the arena of targeting MDM2 with a newer generation of inhibitors. Nutlin-3a was the first specific small molecule MDM2 inhibitor developed that displaced MDM2 from p53 using its cis-imidazoline core structure [115,116,117,118]. The newer generation of MDM2 inhibitors has shown improved specificity and likely better activities in tumor inhibition [40,119]. Many of them are being tested for other diseases as well, including myelofibrosis and acute myeloid leukemia, etc. [120,121,122,123,124]. We will only discuss the clinical trials involving liposarcoma in this review. The “Treatment of Milademetan Versus Trabectedin in Patients With De-differentiated Liposarcoma (MANTRA)” study was just closed after completing enrollment. This trial enrolled 160 patients whose DDLPS had progressed on one or more lines of systemic therapy (at least one line of systemic therapy containing doxorubicin) and compared Milademetan versus Trabectedin with the primary endpoint of PFS. In the phase I trial, Milademetan produced a disease control rate of 58.5% and PFS of 7.2 months in the subgroup of patients with DDLPS in a phase I trial [125]. The clinical trial “Brightline-1: A Study to Compare BI 907828 With Doxorubicin in People With a Type of Cancer Called De-differentiated Liposarcoma” is testing another MDM2 inhibitor versus doxorubicin chemotherapy. This is a first-line trial that does not require previous exposure to chemotherapy. The trial plans to enroll 390 patients with PFS as the primary endpoint [126]. In a phase IA/IB dose escalation trial BI907828 appeared to show encouraging activities in patients with MDM2-amplified biliary tract cancer [127]. In preclinical studies, BI907828 showed excellent activities in de-differentiated liposarcoma xenografts carrying MDM2 amplification [128]. Some of the other MDM2 inhibitors have been studied in early-stage trials with limited activities, including SAR405838 and Siremadlin [129,130,131]. Another MDM2 inhibitor, ASTX295, is currently being tested in a phase I trial in patients with advanced de-differentiated liposarcoma. The clinical trial “Open-Label Study of the CDK4/6 Inhibitor SPH4336 in Subjects With Locally Advanced or Metastatic Liposarcomas” is testing a different CDK4/6 inhibitor for patients with WDLPS and DDLPS who have received no more than three previous lines of therapy with PFS as the primary endpoint. The clinical trial “palbociclib and INCMGA00012 (Retifanlimab) in People With Advanced Liposarcoma” testing the combination of palbociclib and a PD-1 inhibitor INCMGA00012 in patients with advanced WDLPS or DDLPS. Retifanlimab appears to have a similar toxicity profile with the other known anti-PD1 checkpoint inhibitor [132]. The clinical trial “ATX-101 in Advanced De-differentiated Liposarcoma and Leiomyosarcoma (ATX-101)” recruits patients with advanced DDLPS and LMS whose tumor had progressed on one line of therapy. ATX-101 is a small molecule peptide made of a novel human proliferating cell nuclear antigen (PCNA) interacting motif called APIM that is coupled to cellular and nuclear delivery domains and aims to target PCNA for cytotoxicity. In a phase I trial, no response was obtained, but 70% of patients (n = 20) had stable disease [133,134,135]. A dose escalation trial combining Vimseltinib and Avelumab is ongoing for advanced sarcomas, including DDLPS [136]. Vimseltinib is a CSF1R inhibitor initially developed for patients with recurrent tenosynovial giant cell tumor (TGCT) [137,138].

Table 2.
Select ongoing trials in LPS.
9. Conclusions
WDLPS and DDLPS are among the most common histological subtypes of STS. The standard systemic treatment options are limited to chemotherapeutic drugs. Recent data shows promising activity with CDK4/6 and immune checkpoint inhibitors in DDLPS. In addition, a number of clinical trials are evaluating the activity of small molecules targeting MDM2. Combination with a checkpoint inhibitor remains to be tested for safety and preliminary efficacy. Results from these trials shall be eagerly anticipated.
Author Contributions
Conceptualization, M.P.; writing—original draft preparation, M.P. and M.Y.Z.; writing—review and editing, M.Y.Z., N.Q.B., G.W.C., K.N.G. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Livingston, J.A.; Bugano, D.; Barbo, A.; Lin, H.; Madewell, J.E.; Wang, W.L.; Lazar, A.J.; Tseng, W.W.; Roland, C.L.; Feig, B.W.; et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: Defining the benefit and challenges of the standard. Sci. Rep. 2017, 7, 11836. [Google Scholar] [CrossRef] [PubMed]
- Seto, T.; Song, M.-N.; Trieu, M.; Yu, J.; Sidhu, M.; Liu, C.-M.; Sam, D.; Pan, M. Real-World Experiences with Pazopanib in Patients with Advanced Soft Tissue and Bone Sarcoma in Northern California. Med. Sci. 2019, 7, 48. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Tos, A.P.D. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Conyers, R.; Young, S.; Thomas, D.M. Liposarcoma: Molecular Genetics and Therapeutics. Sarcoma 2011, 2011, 483154. [Google Scholar] [CrossRef]
- Lucas, D.R.; Nascimento, A.G.; Sanjay, B.K.S.; Rock, M.G. Well-differentiated Liposarcoma: The Mayo Clinic Experience with 58 Cases. Am. J. Clin. Pathol. 1994, 102, 677–683. [Google Scholar] [CrossRef]
- Thway, K. Well-differentiated liposarcoma and dedifferentiated liposarcoma: An updated review. Semin. Diagn. Pathol. 2019, 36, 112–121. [Google Scholar] [CrossRef]
- Lee, A.T.J.; Thway, K.; Huang, P.; Jones, R.L. Clinical and Molecular Spectrum of Liposarcoma. J. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- Henricks, W.H.; Chu, Y.C.; Goldblum, J.R.; Weiss, S.W. Dedifferentiated Liposarcoma. Am. J. Surg. Pathol. 1997, 21, 271–281. [Google Scholar] [CrossRef]
- Fabre-Guillevin, E.; Coindre, J.M.; Somerhausen, N.S.; Bonichon, F.; Stoeckle, E.; Bui, N.B. Retroperitoneal liposarcomas: Follow-up analysis of dedifferentiation after clinicopathologic reexamination of 86 liposarcomas and malignant fibrous histiocytomas. Cancer 2006, 106, 2725–2733. [Google Scholar] [CrossRef]
- McCormick, D.; Mentzel, T.; Beham, A.; Fletcher, C.D. Dedifferentiated Liposarcoma Clinicopathologic Analysis of 32 Cases Suggesting a Better Prognostic Subgroup Among Pleomorphic Sarcomas. Am. J. Surg. Pathol. 1994, 18, 1213–1223. [Google Scholar] [CrossRef]
- Thway, K.; Jones, R.L.; Noujaim, J.; Zaidi, S.; Miah, A.B.; Fisher, C. Dedifferentiated Liposarcoma: Updates on Morphology, Genetics, and Therapeutic Strategies. Adv. Anat. Pathol. 2016, 23, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Thway, K.; Robertson, D.; Thway, Y.; Fisher, C. Dedifferentiated Liposarcoma With Meningothelial-like Whorls, Metaplastic Bone Formation, and CDK4, MDM2, and p16 Expression: A morphologic and immunohistochemical study. Am. J. Surg. Pathol. 2011, 35, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Seki, K.; Hasegawa, F.; Matsuno, Y.; Shimoda, T.; Hirose, T.; Sano, T.; Hirohashi, S. Dedifferentiated liposarcoma ofretroperitoneum and mesentery: Varied growth patterns and histological grades—A clinicopathologic study of 32 cases. Hum. Pathol. 2000, 31, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Vullo, S.L.; Fiore, M.; Mussi, C.; Stacchiotti, S.; Collini, P.; Lozza, L.; Pennacchioli, E.; Mariani, L.; Casali, P.G. Aggressive Surgical Policies in a Retrospectively Reviewed Single-Institution Case Series of Retroperitoneal Soft Tissue Sarcoma Patients. J. Clin. Oncol. 2009, 27, 24–30. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Schöffski, P.; Grignani, G.; Blay, J.-Y.; Maki, R.G.; Van Tine, B.A.; Alcindor, T.; Jones, R.L.; D’adamo, D.R.; Guo, M.; et al. Activity of Eribulin in Patients With Advanced Liposarcoma Demonstrated in a Subgroup Analysis From a Randomized Phase III Study of Eribulin Versus Dacarbazine. J. Clin. Oncol. 2017, 35, 3433–3439. [Google Scholar] [CrossRef]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Kuczkiewicz-Siemion, O.; Wiśniewski, P.; Dansonka-Mieszkowska, A.; Grabowska-Kierył, M.; Olszewska, K.; Goryń, T.; Prochorec-Sobieszek, M.; Rutkowski, P.; Szumera-Ciećkiewicz, A. The utility of fluorescence in situ hybridization (FISH) in determining DNA damage-inducible transcript 3 (DDIT3) amplification in dedifferentiated liposarcomas—An important diagnostic pitfall. Pathol.-Res. Pract. 2021, 225, 153555. [Google Scholar] [CrossRef]
- Matthyssens, L.E.; Creytens, D.; Ceelen, W.P. Retroperitoneal Liposarcoma: Current Insights in Diagnosis and Treatment. Front. Surg. 2015, 2, 4. [Google Scholar] [CrossRef]
- Wang, X.; Hulshizer, R.L.; Erickson-Johnson, M.R.; Flynn, H.C.; Jenkins, R.B.; Lloyd, R.V.; Oliveira, A.M. Identification of novelHMGA2fusion sequences in lipoma: Evidence that deletion of let-7 miRNA consensus binding site 1 in theHMGA23′ UTR is not critical forHMGA2transcriptional upregulation. Genes Chromosom. Cancer 2009, 48, 673–678. [Google Scholar] [CrossRef]
- Panagopoulos, I.; Gorunova, L.; Bjerkehagen, B.; Lobmaier, I.; Heim, S. The recurrent chromosomal translocation t(12;18) (q14~15;q12~21) causes the fusion gene HMGA2-SETBP1 and HMGA2 expression in lipoma and osteochondrolipoma. Int. J. Oncol. 2015, 47, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Panagopoulos, I.; Mertens, F.; Mandahl, N. Fusion of the HMGA2 and NFIB genes in lipoma. Virchows Arch. 2005, 447, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Fernandez, C.; Saada, E.; Keslair, F.; Hery, G.; Zattara, H.; Pedeutour, F. HMGA2–NFIB fusion in a pediatric intramuscular lipoma: A novel case of NFIB alteration in a large deep-seated adipocytic tumor. Cancer Genet. Cytogenet. 2009, 195, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Bianchini, L.; Keslair, F.; Bonnafous, S.; Cardot-Leccia, N.; Coindre, J.-M.; Dumollard, J.-M.; Hofman, P.; Leroux, A.; Mainguené, C.; et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int. J. Cancer 2008, 122, 2233–2241. [Google Scholar] [CrossRef]
- Sciot, R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics 2021, 11, 496. [Google Scholar] [CrossRef]
- Boltze, C.; Schneider-Stock, R.; Jäger, V.; Roessner, A. Distinction Between Lipoma and Liposarcoma by MDM2 Alterations: A case report of simultaneously occurring tumors and review of the literature. Pathol.-Res. Pract. 2001, 197, 563–568. [Google Scholar] [CrossRef]
- Creytens, D.; Van Gorp, J.; Speel, E.-J.; Ferdinande, L. Characterization of the 12q amplicons in lipomatous soft tissue tumors by multiplex ligation-dependent probe amplification-based copy number analysis. Anticancer Res. 2015, 35, 1835–1842. [Google Scholar]
- Ware, P.L.; Snow, A.N.; Gvalani, M.; Pettenati, M.J.; Qasem, S.A. MDM2 Copy Numbers in Well-Differentiated and Dedifferentiated Liposarcoma: Characterizing progression to high-grade tumors. Am. J. Clin. Pathol. 2014, 141, 334–341. [Google Scholar] [CrossRef]
- Tap, W.D.; Eilber, F.C.; Ginther, C.; Dry, S.M.; Reese, N.; Barzan-Smith, K.; Chen, H.-W.; Wu, H.; Eilber, F.R.; Slamon, D.J.; et al. Evaluation of well-differentiated/de-differentiated liposarcomas by high-resolution oligonucleotide array-based comparative genomic hybridization. Genes Chromosom. Cancer 2011, 50, 95–112. [Google Scholar] [CrossRef]
- Snyder, E.L.; Sandstrom, D.J.; Law, K.; Fiore, C.; Sicinska, E.; Brito, J.; Bailey, D.; A Fletcher, J.; Loda, M.; Rodig, S.J.; et al. c-Jun amplification and overexpression are oncogenic in liposarcoma but not always sufficient to inhibit the adipocytic differentiation programme. J. Pathol. 2009, 218, 292–300. [Google Scholar] [CrossRef]
- Mariani, O.; Brennetot, C.; Coindre, J.-M.; Gruel, N.; Ganem, C.; Delattre, O.; Stern, M.-H.; Aurias, A. JUN Oncogene Amplification and Overexpression Block Adipocytic Differentiation in Highly Aggressive Sarcomas. Cancer Cell 2007, 11, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, R.W.; Baraff, A.J.; Jour, G.; Kyriss, M.; Wu, Y.; Liu, Y.; Li, S.-C.; Hoch, B.; Liu, Y.J. High amplification levels of MDM2 and CDK4 correlate with poor outcome in patients with dedifferentiated liposarcoma: A cytogenomic microarray analysis of 47 cases. Cancer Genet. 2017, 218–219, 69–80. [Google Scholar] [CrossRef]
- Manfredi, J.J. The Mdm2–p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev. 2010, 24, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Hamard, P.-J.; Manfredi, J.J. Mdm2’s Dilemma: To Degrade or to Translate p53? Cancer Cell 2012, 21, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Bill, K.L.J.; Seligson, N.D.; Hays, J.L.; Awasthi, A.; Demoret, B.; Stets, C.W.; Duggan, M.C.; Bupathi, M.; Brock, G.N.; Millis, S.Z.; et al. Degree of MDM2 Amplification Affects Clinical Outcomes in Dedifferentiated Liposarcoma. Oncologist 2019, 24, 989–996. [Google Scholar] [CrossRef]
- Yamashita, K.; Kohashi, K.; Yamada, Y.; Akatsuka, S.; Ikuta, K.; Nishida, Y.; Toyokuni, S.; Oda, Y. Prognostic significance of the MDM2 / HMGA2 ratio and histological tumor grade in dedifferentiated liposarcoma. Genes Chromosom. Cancer 2021, 60, 26–37. [Google Scholar] [CrossRef]
- Bohlman, S.; Manfredi, J.J. Mdm2-RNA Interactions as a Target for Cancer Therapy: It’s Not All About p53. Cancer Cell 2016, 30, 513–514. [Google Scholar] [CrossRef]
- Poyurovsky, M.V.; Katz, C.; Laptenko, O.; Beckerman, R.; Lokshin, M.; Ahn, J.; Byeon, I.-J.L.; Gabizon, R.; Mattia, M.; Zupnick, A.; et al. The C terminus of p53 binds the N-terminal domain of MDM2. Nat. Struct. Mol. Biol. 2010, 17, 982–989. [Google Scholar] [CrossRef]
- Chène, P. Inhibiting the p53–MDM2 interaction: An important target for cancer therapy. Nat. Rev. Cancer 2003, 3, 102–109. [Google Scholar] [CrossRef]
- Konopleva, M.; Martinelli, G.; Daver, N.; Papayannidis, C.; Wei, A.; Higgins, B.; Ott, M.; Mascarenhas, J.; Andreeff, M. MDM2 inhibition: An important step forward in cancer therapy. Leukemia 2020, 34, 2858–2874. [Google Scholar] [CrossRef]
- Sherr, C.J. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2001, 2, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Levine, A. p53 Research: The Past Thirty Years and the Next Thirty Years. Cold Spring Harb. Perspect. Biol. 2010, 2, a000893. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef]
- Bui, N.Q.; Przybyl, J.; Trabucco, S.E.; Frampton, G.; Hastie, T.; Van De Rijn, M.; Ganjoo, K.N. A clinico-genomic analysis of soft tissue sarcoma patients reveals CDKN2A deletion as a biomarker for poor prognosis. Clin. Sarcoma Res. 2019, 9, 12. [Google Scholar] [CrossRef]
- Pan, M.; Trieu, M.K.; Sidhu, M.; Yu, J.; Seto, T.; Ganjoo, K. Fourteen-Day Gemcitabine-Docetaxel Chemotherapy Is Effective and Safer Compared to 21-Day Regimen in Patients with Advanced Soft Tissue and Bone Sarcoma. Cancers 2021, 13, 1983. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Jiang, C.; Tse, P.; Achacoso, N.; Alexeeff, S.; Solorzano, A.V.; Chung, E.; Hu, W.; Truong, T.-G.; Arora, A.; et al. TP53 Gain-of-Function and Non–Gain-of-Function Mutations Are Differentially Associated With Sidedness-Dependent Prognosis in Metastatic Colorectal Cancer. J. Clin. Oncol. 2022, 40, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.K.; LoRusso, P.M.; A Dickson, M.; Randolph, S.S.; Shaik, M.N.; Wilner, K.D.; Courtney, R.; O’Dwyer, P.J. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br. J. Cancer 2011, 104, 1862–1868. [Google Scholar] [CrossRef]
- Dickson, M.A.; Tap, W.D.; Keohan, M.L.; D’Angelo, S.P.; Gounder, M.M.; Antonescu, C.R.; Landa, J.; Qin, L.-X.; Rathbone, D.D.; Condy, M.M.; et al. Phase II Trial of the CDK4 Inhibitor PD0332991 in Patients With Advanced CDK4-Amplified Well-Differentiated or Dedifferentiated Liposarcoma. J. Clin. Oncol. 2013, 31, 2024–2028. [Google Scholar] [CrossRef]
- Dickson, M.A.; Schwartz, G.K.; Keohan, M.L.; D’angelo, S.P.; Gounder, M.M.; Chi, P.; Antonescu, C.R.; Landa, J.; Qin, L.-X.; Crago, A.M.; et al. Progression-Free Survival Among Patients With Well-Differentiated or Dedifferentiated Liposarcoma Treated WithCDK4Inhibitor Palbociclib: A Phase 2 Clinical Trial. JAMA Oncol. 2016, 2, 937–940. [Google Scholar] [CrossRef]
- Infante, J.R.; Cassier, P.A.; Gerecitano, J.F.; Witteveen, P.O.; Chugh, R.; Ribrag, V.; Chakraborty, A.; Matano, A.; Dobson, J.R.; Crystal, A.S.; et al. A Phase I Study of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) in Patients with Advanced Solid Tumors and Lymphomas. Clin. Cancer Res. 2016, 22, 5696–5705. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Koff, A.; D’Angelo, S.P.; Gounder, M.M.; Keohan, M.L.; Kelly, C.M.; Chi, P.; Antonescu, C.R.; Landa, J.; Qin, L.-X.; et al. Phase 2 study of the CDK4 inhibitor abemaciclib in dedifferentiated liposarcoma. J. Clin. Oncol. 2019, 37 (Suppl. 15), 11004. [Google Scholar] [CrossRef]
- Burgess, M.A.; Bolejack, V.; Schuetze, S.; Van Tine, B.A.; Attia, S.; Riedel, R.F.; Hu, J.S.; Davis, L.E.; Okuno, S.H.; Priebat, D.A.; et al. Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): Final results of SARC028 expansion cohorts. J. Clin. Oncol. 2019, 37 (Suppl. 15), 11015. [Google Scholar] [CrossRef]
- Pollack, S.M.; Redman, M.W.; Baker, K.K.; Wagner, M.J.; Schroeder, B.A.; Loggers, E.T.; Trieselmann, K.; Copeland, V.C.; Zhang, S.; Black, G.; et al. Assessment of Doxorubicin and Pembrolizumab in Patients With Advanced Anthracycline-Naive Sarcoma: A Phase 1/2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1778. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.M.; Antonescu, C.R.; Bowler, T.; Munhoz, R.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; Movva, S.; Dholakia, R.; et al. Objective Response Rate Among Patients With Locally Advanced or Metastatic Sarcoma Treated With Talimogene Laherparepvec in Combination With Pembrolizumab: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, S.; Rothe, M.; Mangat, P.K.; Garrett-Mayer, L.; Meric-Bernstam, F.; Farrington, L.C.; Calfa, C.; D’Andre, S.D.; Livingston, M.B.; Thota, R.; et al. Palbociclib (P) in patients (pts) with soft tissue sarcoma (STS) with CDK4 amplification: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study. J. Clin. Oncol. 2021, 39, 11565. [Google Scholar] [CrossRef]
- Livingston, M.B.; Jagosky, M.H.; Robinson, M.M.; Ahrens, W.A.; Benbow, J.H.; Farhangfar, C.J.; Foureau, D.M.; Maxwell, D.M.; Baldrige, E.A.; Begic, X.; et al. Phase II Study of Pembrolizumab in Combination with Doxorubicin in Metastatic and Unresectable Soft-Tissue Sarcoma. Clin. Cancer Res. 2021, 27, 6424–6431. [Google Scholar] [CrossRef]
- Razak, A.R.A.; Bauer, S.; Suarez, C.; Lin, C.-C.; Quek, R.; Hütter-Krönke, M.L.; Cubedo, R.; Ferretti, S.; Guerreiro, N.; Jullion, A.; et al. Co-Targeting of MDM2 and CDK4/6 with Siremadlin and Ribociclib for the Treatment of Patients with Well-Differentiated or Dedifferentiated Liposarcoma: Results from a Proof-of-Concept, Phase Ib Study. Clin. Cancer Res. 2022, 28, 1087–1097. [Google Scholar] [CrossRef]
- Messina, C.; Cattrini, C.; Buzzatti, G.; Cerbone, L.; Zanardi, E.; Messina, M.; Boccardo, F. CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: A systematic review and meta-analysis of randomized trials. Breast Cancer Res. Treat. 2018, 172, 9–21. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Harbeck, N.; Rastogi, P.; Martin, M.; Tolaney, S.; Shao, Z.; Fasching, P.; Huang, C.; Jaliffe, G.; Tryakin, A.; Goetz, M.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021, 32, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Bartlett, C.H.; Zhang, K.; et al. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2− Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef]
- Hamilton, E.; Cortes, J.; Ozyilkan, O.; Chen, S.-C.; Petrakova, K.; Manikhas, A.; Jerusalem, G.; Hegg, R.; Huober, J.; Chapman, S.C.; et al. nextMONARCH: Abemaciclib Monotherapy or Combined With Tamoxifen for Metastatic Breast Cancer. Clin. Breast Cancer 2021, 21, 181–190.e2. [Google Scholar] [CrossRef]
- O’Leary, B.; Finn, R.S.; Turner, B.O.N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef]
- Du, Q.; Guo, X.; Wang, M.; Li, Y.; Sun, X.; Li, Q. The application and prospect of CDK4/6 inhibitors in malignant solid tumors. J. Hematol. Oncol. 2020, 13, 41. [Google Scholar] [CrossRef]
- Schettini, F.; De Santo, I.; Rea, C.G.; De Placido, P.; Formisano, L.; Giuliano, M.; Arpino, G.; De Laurentiis, M.; Puglisi, F.; De Placido, S.; et al. CDK 4/6 Inhibitors as Single Agent in Advanced Solid Tumors. Front. Oncol. 2018, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Taylor, B.S.; Banerji, S.; Ramos, A.H.; Lagos-Quintana, M.; DeCarolis, P.L.; Shah, K.; Socci, N.D.; Weir, B.A.; Ho, A.; et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat. Genet. 2010, 42, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Sicinska, E.; Czaplinski, J.T.; Remillard, S.P.; Moss, S.; Wang, Y.; Brain, C.; Loo, A.; Snyder, E.L.; Demetri, G.D.; et al. Antiproliferative Effects of CDK4/6 Inhibition in CDK4-Amplified Human Liposarcoma In Vitro and In Vivo. Mol. Cancer Ther. 2014, 13, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.M.; Alexander, A.; Liu, Y.; Vijayaraghavan, S.; Low, K.H.; Yang, D.; Bui, T.; Somaiah, N.; Ravi, V.; Keyomarsi, K.; et al. CDK4/6 Inhibitors Sensitize Rb-positive Sarcoma Cells to Wee1 Kinase Inhibition through Reversible Cell-Cycle Arrest. Mol. Cancer Ther. 2017, 16, 1751–1764. [Google Scholar] [CrossRef]
- Li, X.; Seebacher, N.A.; Garbutt, C.; Ma, H.; Gao, P.; Xiao, T.; Hornicek, F.J.; Duan, Z. Inhibition of cyclin-dependent kinase 4 as a potential therapeutic strategy for treatment of synovial sarcoma. Cell Death Dis. 2018, 9, 446. [Google Scholar] [CrossRef]
- Perez, M.; Muñoz-Galván, S.; Jiménez-García, M.P.; Marín, J.J.; Carnero, A. Efficacy of CDK4 inhibition against sarcomas depends on their levels of CDK4 and p16ink4 mRNA. Oncotarget 2015, 6, 40557–40574. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Kovatcheva, M.; Liu, D.D.; Dickson, M.A.; Klein, M.E.; O’connor, R.; Wilder, F.O.; Socci, N.D.; Tap, W.D.; Schwartz, G.K.; Singer, S.; et al. MDM2 turnover and expression of ATRX determine the choice between quiescence and senescence in response to CDK4 inhibition. Oncotarget 2015, 6, 8226–8243. [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Bui, M.M.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J. Natl. Compr. Cancer Netw. 2020, 18, 1604–1612. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.; Stemmer, S.; Burris, H.; Yap, Y.; Sonke, G.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.; Winer, E.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2019, 30, 1842. [Google Scholar] [CrossRef]
- Wisdom, A.J.; Mowery, Y.M.; Riedel, R.F.; Kirsch, D.G. Rationale and emerging strategies for immune checkpoint blockade in soft tissue sarcoma. Cancer 2018, 124, 3819–3829. [Google Scholar] [CrossRef] [PubMed]
- Siozopoulou, V.; Domen, A.; Zwaenepoel, K.; Van Beeck, A.; Smits, E.; Pauwels, P.; Marcq, E. Immune Checkpoint Inhibitory Therapy in Sarcomas: Is There Light at the End of the Tunnel? Cancers 2021, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Saerens, M.; Brusselaers, N.; Rottey, S.; Decruyenaere, A.; Creytens, D.; Lapeire, L. Immune checkpoint inhibitors in treatment of soft-tissue sarcoma: A systematic review and meta-analysis. Eur. J. Cancer 2021, 152, 165–182. [Google Scholar] [CrossRef]
- Italiano, A.; Bellera, C.; D’angelo, S. PD1/PD-L1 targeting in advanced soft-tissue sarcomas: A pooled analysis of phase II trials. J. Hematol. Oncol. 2020, 13, 55. [Google Scholar] [CrossRef]
- Quiroga, D.; Liebner, D.A.; Philippon, J.S.; Hoffman, S.; Tan, Y.; Chen, J.L.; Lenobel, S.; Wakely, P.E.; Pollock, R.; Tinoco, G. Activity of PD1 inhibitor therapy in advanced sarcoma: A single-center retrospective analysis. BMC Cancer 2020, 20, 527. [Google Scholar] [CrossRef]
- Ayodele, O.; Razak, A.A. Immunotherapy in Soft-Tissue Sarcoma. Curr. Oncol. 2020, 27 (Suppl. 1), 17–23. [Google Scholar] [CrossRef]
- Klemen, N.D.; Hwang, S.; Bradic, M.; Rosenbaum, E.; Dickson, M.A.; Gounder, M.M.; Kelly, C.M.; Keohan, M.L.; Movva, S.; Thornton, K.A.; et al. Long-term Follow-up and Patterns of Response, Progression, and Hyperprogression in Patients after PD-1 Blockade in Advanced Sarcoma. Clin. Cancer Res. 2021, 28, 939–947. [Google Scholar] [CrossRef]
- Doshi, A.; Zhou, M.; Bui, N.; Wang, D.S.; Ganjoo, K.; Hwang, G.L. Safety and Feasibility of Cryoablation during Immunotherapy in Patients with Metastatic Soft Tissue Sarcoma. J. Vasc. Interv. Radiol. 2021, 32, 1688–1694. [Google Scholar] [CrossRef]
- Fassl, A.; Geng, Y.; Sicinski, P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science 2022, 375, eabc1495. [Google Scholar] [CrossRef]
- Deng, J.; Wang, E.S.; Jenkins, R.W.; Li, S.; Dries, R.; Yates, K.; Chhabra, S.; Huang, W.; Liu, H.; Aref, A.R.; et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018, 8, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.A.; Beckmann, R.P.; Dempsey, J.A.; Huber, L.; Forest, A.; Amaladas, N.; Li, Y.; Wang, Y.C.; Rasmussen, E.R.; Chin, D.; et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell Rep. 2018, 22, 2978–2994. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ding, D.; Yan, Y.; Li, H.; Wang, B.; Ma, L.; Ye, Z.; Ma, T.; Wu, Q.; Rodrigues, D.N.; et al. Phosphorylated RB Promotes Cancer Immunity by Inhibiting NF-κB Activation and PD-L1 Expression. Mol. Cell 2019, 73, 22–35.e6. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, X.; Wang, H.; Zhu, Y.; Geng, Y.; Nihira, N.T.; Tan, Y.; Ci, Y.; Wu, F.; Dai, X.; et al. Cyclin D–CDK4 kinase destabilizes PD-L1 via cullin 3–SPOP to control cancer immune surveillance. Nature 2018, 553, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef]
- Alban, T.J.; Chan, T.A. Immunotherapy biomarkers: The long and winding road. Nat. Rev. Clin. Oncol. 2021, 18, 323–324. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune checkpoint therapy: Forging ahead. Sci. Transl. Med. 2022, 14, eadf2947. [Google Scholar] [CrossRef]
- Mckean, W.B.; Moser, J.C.; Rimm, D.; Hu-Lieskovan, S. Biomarkers in Precision Cancer Immunotherapy: Promise and Challenges. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e275–e291. [Google Scholar] [CrossRef]
- Seto, T.; Sam, D.; Pan, M. Mechanisms of Primary and Secondary Resistance to Immune Checkpoint Inhibitors in Cancer. Med. Sci. 2019, 7, 14. [Google Scholar] [CrossRef]
- Pan, M.; Alavi, M.; Herrinton, L.J. Association of Inflammatory Markers with Disease Progression in Patients with Metastatic Melanoma Treated with Immune Checkpoint Inhibitors. Perm. J. 2018, 22, 17–149. [Google Scholar] [CrossRef] [PubMed]
- D’angelo, S.P.; Shoushtari, A.N.; Agaram, N.P.; Kuk, D.; Qin, L.-X.; Carvajal, R.D.; Dickson, M.A.; Gounder, M.; Keohan, M.L.; Schwartz, G.K.; et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2015, 46, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.W.; Malu, S.; Zhang, M.; Chen, J.; Sim, G.C.; Wei, W.; Ingram, D.; Somaiah, N.; Lev, D.C.; Pollock, R.; et al. Analysis of the Intratumoral Adaptive Immune Response in Well Differentiated and Dedifferentiated Retroperitoneal Liposarcoma. Sarcoma 2015, 2015, 547460. [Google Scholar] [CrossRef]
- Kostine, M.; Cleven, A.H.; de Miranda, N.F.C.C.; Italiano, A.; Cleton-Jansen, A.-M.; Bovée, J.V.M.G. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod. Pathol. 2016, 29, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.M.; Shenasa, E.; Nielsen, T.O. Sarcomas: Immune biomarker expression and checkpoint inhibitor trials. Cancer Treat. Rev. 2020, 91, 102115. [Google Scholar] [CrossRef]
- Kim, J.R.; Moon, Y.J.; Kwon, K.S.; Bae, J.S.; Wagle, S.; Kim, K.M.; Park, H.S.; Lee, H.; Moon, W.S.; Chung, M.J.; et al. Tumor Infiltrating PD1-Positive Lymphocytes and the Expression of PD-L1 Predict Poor Prognosis of Soft Tissue Sarcomas. PLoS ONE 2013, 8, e82870. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Dancsok, A.R.; Setsu, N.; Gao, D.; Blay, J.-Y.; Thomas, D.; Maki, R.G.; Nielsen, T.O.; Demicco, E.G. Expression of lymphocyte immunoregulatory biomarkers in bone and soft-tissue sarcomas. Mod. Pathol. 2019, 32, 1772–1785. [Google Scholar] [CrossRef]
- Dancsok, A.R.; Gao, D.; Lee, A.F.; Steigen, S.E.; Blay, J.-Y.; Thomas, D.M.; Maki, R.G.; Nielsen, T.O.; Demicco, E.G. Tumor-associated macrophages and macrophage-related immune checkpoint expression in sarcomas. Oncoimmunology 2020, 9, 1747340. [Google Scholar] [CrossRef]
- Pollack, S.M.; He, Q.; Yearley, J.H.; Emerson, R.; Vignali, M.; Zhang, Y.; Redman, M.W.; Baker, K.K.; Cooper, S.; Donahue, B.; et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer 2017, 123, 3291–3304. [Google Scholar] [CrossRef]
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.-Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Grellety, T.; et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bui, N.; Lohman, M.; van de Rjin, M.; Hwang, G.; Ganjoo, K. Long-Term Remission with Ipilimumab/Nivolumab in Two Patients with Different Soft Tissue Sarcoma Subtypes and No PD-L1 Expression. Case Rep. Oncol. 2021, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Burgess, M.; Salazar, R.; Parra, E.R.; Rodrigues-Canales, J.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Attia, S.; Riedel, R.F.; et al. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin. Cancer Res. 2020, 26, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Birdi, H.K.; Jirovec, A.; Cortés-Kaplan, S.; Werier, J.; Nessim, C.; Diallo, J.-S.; Ardolino, M. Immunotherapy for sarcomas: New frontiers and unveiled opportunities. J. Immunother. Cancer 2021, 9, e001580. [Google Scholar] [CrossRef]
- Carvajal, D.; Tovar, C.; Yang, H.; Vu, B.T.; Heimbrook, D.C.; Vassilev, L.T. Activation of p53 by MDM2 Antagonists Can Protect Proliferating Cells from Mitotic Inhibitors. Cancer Res 2005, 65, 1918–1924. [Google Scholar] [CrossRef]
- Tovar, C.; Rosinski, J.; Filipovic, Z.; Higgins, B.; Kolinsky, K.; Hilton, H.; Zhao, X.; Vu, B.T.; Qing, W.; Packman, K.; et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: Implications for therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 1888–1893. [Google Scholar] [CrossRef]
- Vu, B.T.; Vassilev, L. Small-Molecule Inhibitors of the p53-MDM2 Interaction. Curr. Top Microbiol. Immunol. 2010, 348, 151–172. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, H.; Ji, Y.; Zhou, Q.; Du, Z.; Tian, L.; Jiang, Y.; Yao, K.; Zhou, Z. Targeting p53–MDM2 interaction by small-molecule inhibitors: Learning from MDM2 inhibitors in clinical trials. J. Hematol. Oncol. 2022, 15, 91. [Google Scholar] [CrossRef]
- Erba, H.P.; Becker, P.S.; Shami, P.J.; Grunwald, M.R.; Flesher, D.L.; Zhu, M.; Rasmussen, E.; Henary, H.A.; Anderson, A.A.; Wang, E.S. Phase 1b study of the MDM2 inhibitor AMG 232 with or without trametinib in relapsed/refractory acute myeloid leukemia. Blood Adv. 2019, 3, 1939–1949. [Google Scholar] [CrossRef]
- Verstovsek, S.; Al-Ali, H.K.; Mascarenhas, J.; Mead, A.J.; Perkins, A.; Vannucchi, A.M.; Uyei, A.; Rothbaum, W.P.; McGreivy, J.; Kiladjian, J.-J. BOREAS: A global phase 3 study of KRT-232, a first-in-class murine double minute 2 (MDM2) inhibitor in TP53WT relapsed/refractory (R/R) myelofibrosis (MF). J. Clin. Oncol. 2021, 39 (Suppl. 15), TPS7057. [Google Scholar] [CrossRef]
- Hassin, O.; Oren, M. Drugging p53 in cancer: One protein, many targets. Nat. Rev. Drug Discov. 2023, 22, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Singh, M.; Santos, G.S.; Guerlavais, V.; Carvajal, L.A.; Aivado, M.; Zhan, Y.; Oliveira, M.M.; Westerberg, L.S.; Annis, D.A.; et al. Pharmacologic Activation of p53 Triggers Viral Mimicry Response Thereby Abolishing Tumor Immune Evasion and Promoting Antitumor Immunity. Cancer Discov. 2021, 11, 3090–3105. [Google Scholar] [CrossRef] [PubMed]
- Sanz, G.; Singh, M.; Peuget, S.; Selivanova, G. Inhibition of p53 inhibitors: Progress, challenges and perspectives. J. Mol. Cell Biol. 2019, 11, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Bauer, T.M.; Schwartz, G.K.; Weise, A.M.; LoRusso, P.; Kumar, P.; Tao, B.; Hong, Y.; Patel, P.; Lu, Y.; et al. A First-in-Human Phase I Study of Milademetan, an MDM2 Inhibitor, in Patients With Advanced Liposarcoma, Solid Tumors, or Lymphomas. J. Clin. Oncol. 2023, 41, 1714–1724. [Google Scholar] [CrossRef]
- Schöffski, P.; Lahmar, M.; Lucarelli, A.; Maki, R.G. Brightline-1: Phase II/III trial of the MDM2–p53 antagonist BI 907828 versus doxorubicin in patients with advanced DDLPS. Futur. Oncol. 2023, 19, 621–629. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tolcher, A.W.; Hafez, N.; Lugowska, I.; Ramlau, R.; Gounder, M.M.; Geng, J.; Li, J.; Teufel, M.; Maerten, A.; et al. Efficacy and safety of the MDM2–p53 antagonist BI 907828 in patients with advanced biliary tract cancer: Data from two phase Ia/Ib dose-escalation/expansion trials. J. Clin. Oncol. 2023, 41 (Suppl. 4), 543. [Google Scholar] [CrossRef]
- Cornillie, J.; Wozniak, A.; Li, H.; Gebreyohannes, Y.K.; Wellens, J.; Hompes, D.; Debiec-Rychter, M.; Sciot, R.; Schöffski, P. Anti-tumor activity of the MDM2-TP53 inhibitor BI-907828 in dedifferentiated liposarcoma patient-derived xenograft models harboring MDM2 amplification. Clin. Transl. Oncol. 2020, 22, 546–554. [Google Scholar] [CrossRef]
- Stein, E.M.; DeAngelo, D.J.; Chromik, J.; Chatterjee, M.; Bauer, S.; Lin, C.-C.; Suarez, C.; de Vos, F.; Steeghs, N.; Cassier, P.A.; et al. Results from a First-in-Human Phase I Study of Siremadlin (HDM201) in Patients with Advanced Wild-Type TP53 Solid Tumors and Acute Leukemia. Clin. Cancer Res. 2022, 28, 870–881. [Google Scholar] [CrossRef]
- de Jonge, M.; de Weger, V.A.; Dickson, M.A.; Langenberg, M.; Le Cesne, A.; Wagner, A.J.; Hsu, K.; Zheng, W.; Macé, S.; Tuffal, G.; et al. A phase I study of SAR405838, a novel human double minute 2 (HDM2) antagonist, in patients with solid tumours. Eur. J. Cancer 2017, 76, 144–151. [Google Scholar] [CrossRef]
- De Weger, V.A.; De Jonge, M.; Langenberg, M.H.G.; Schellens, J.H.M.; Lolkema, M.; Varga, A.; Demers, B.; Thomas, K.; Hsu, K.; Tuffal, G.; et al. A phase I study of the HDM2 antagonist SAR405838 combined with the MEK inhibitor pimasertib in patients with advanced solid tumours. Br. J. Cancer 2019, 120, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Schenker, M.; Medioni, J.; Mandziuk, S.; Majem, M.; Gravis, G.; Cornfeld, M.J.; Ranganathan, S.; Yao, Y.; Yeh, H.S.-H.; et al. Phase 2 study of retifanlimab (INCMGA00012) in patients (pts) with selected solid tumors (POD1UM-203). J. Clin. Oncol. 2021, 39 (Suppl. 15), 2571. [Google Scholar] [CrossRef]
- Bose, S.; Ingham, M.; Singh-Kandah, S.V.; Magana, W.; Schwartz, G.K. A phase II study, with a safety lead-in, to evaluate ATX-101, a peptide drug targeting PCNA, in advanced dedifferentiated liposarcoma and leiomyosarcoma. J. Clin. Oncol. 2022, 40 (Suppl. 16), TPS11587. [Google Scholar] [CrossRef]
- Lemech, C.R.; Kichenadasse, G.; Marschner, J.-P.; Alevizopoulos, K.; Otterlei, M.; Millward, M. ATX-101, a cell-penetrating protein targeting PCNA, can be safely administered as intravenous infusion in patients and shows clinical activity in a Phase 1 study. Oncogene 2023, 42, 541–544. [Google Scholar] [CrossRef]
- Gravina, G.L.; Colapietro, A.; Mancini, A.; Rossetti, A.; Martellucci, S.; Ventura, L.; Di Franco, M.; Marampon, F.; Mattei, V.; Biordi, L.A.; et al. ATX-101, a Peptide Targeting PCNA, Has Antitumor Efficacy Alone or in Combination with Radiotherapy in Murine Models of Human Glioblastoma. Cancers 2022, 14, 289. [Google Scholar] [CrossRef]
- Rosenbaum, E.; Movva, S.; Kelly, C.M.; Dickson, M.A.; Keohan, M.L.; Gounder, M.M.; Thornton, K.A.; Chi, P.; Chan, J.E.; Nacev, B.; et al. A phase 1b study of avelumab plus DCC-3014, a potent and selective inhibitor of colony stimulating factor 1 receptor (CSF1R), in patients with advanced high-grade sarcoma. J. Clin. Oncol. 2021, 39 (Suppl. 15), 11549. [Google Scholar] [CrossRef]
- Caldwell, T.M.; Ahn, Y.M.; Bulfer, S.L.; Leary, C.B.; Hood, M.M.; Lu, W.-P.; Vogeti, L.; Vogeti, S.; Kaufman, M.D.; Wise, S.C.; et al. Discovery of vimseltinib (DCC-3014), a highly selective CSF1R switch-control kinase inhibitor, in clinical development for the treatment of Tenosynovial Giant Cell Tumor (TGCT). Bioorganic Med. Chem. Lett. 2022, 74, 128928. [Google Scholar] [CrossRef]
- Smith, B.D.; Kaufman, M.D.; Wise, S.C.; Ahn, Y.M.; Caldwell, T.M.; Leary, C.B.; Lu, W.-P.; Tan, G.; Vogeti, L.; Vogeti, S.; et al. Vimseltinib: A Precision CSF1R Therapy for Tenosynovial Giant Cell Tumors and Diseases Promoted by Macrophages. Mol. Cancer Ther. 2021, 20, 2098–2109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).