Combination of Irinotecan and Melatonin with the Natural Compounds Wogonin and Celastrol for Colon Cancer Treatment
Abstract
1. Introduction
2. Results
2.1. Effect of Tested Compounds and Their Combinations on the Viability of Colon Cancer Cells and Cancer Stem-Like Cells
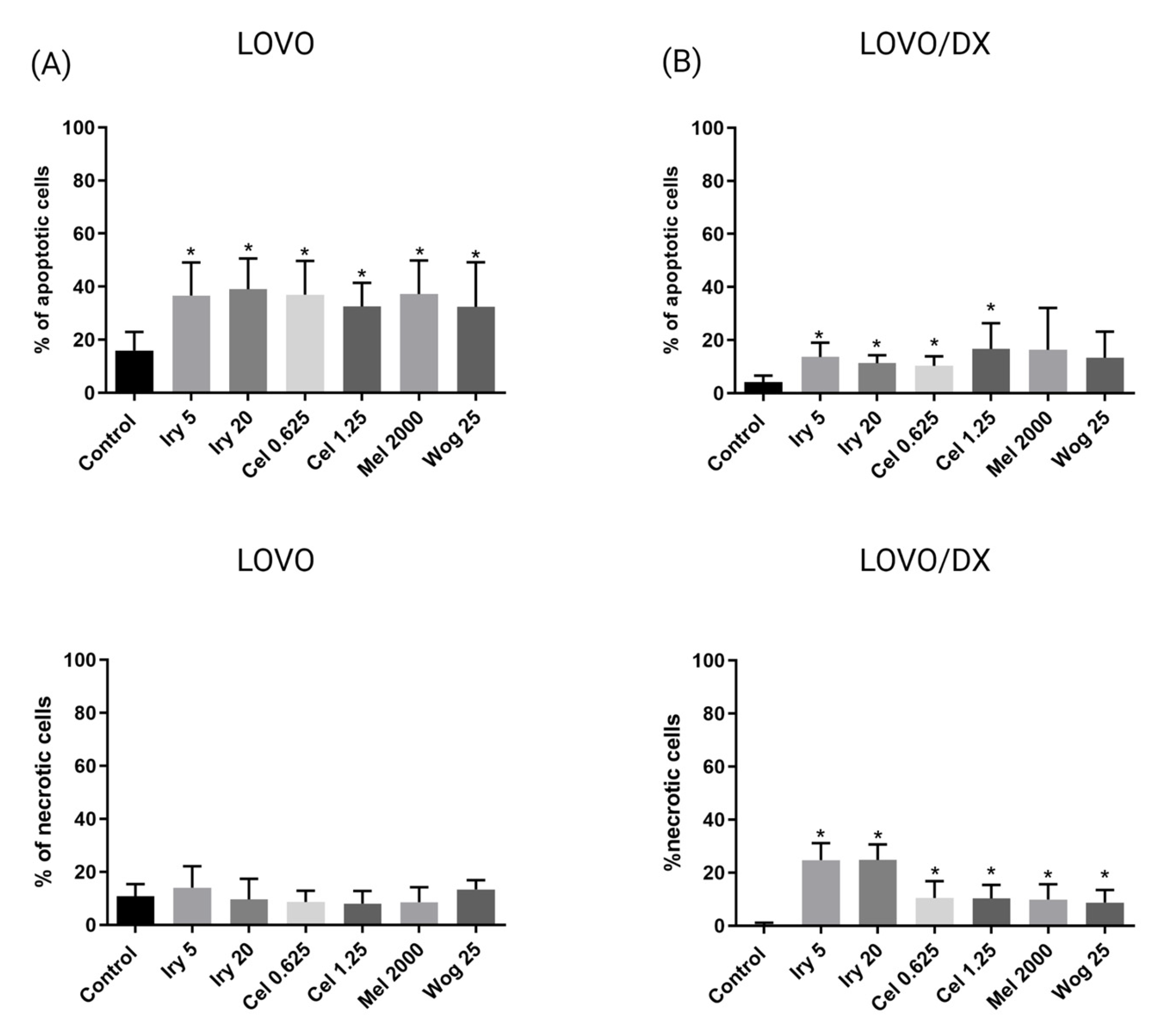
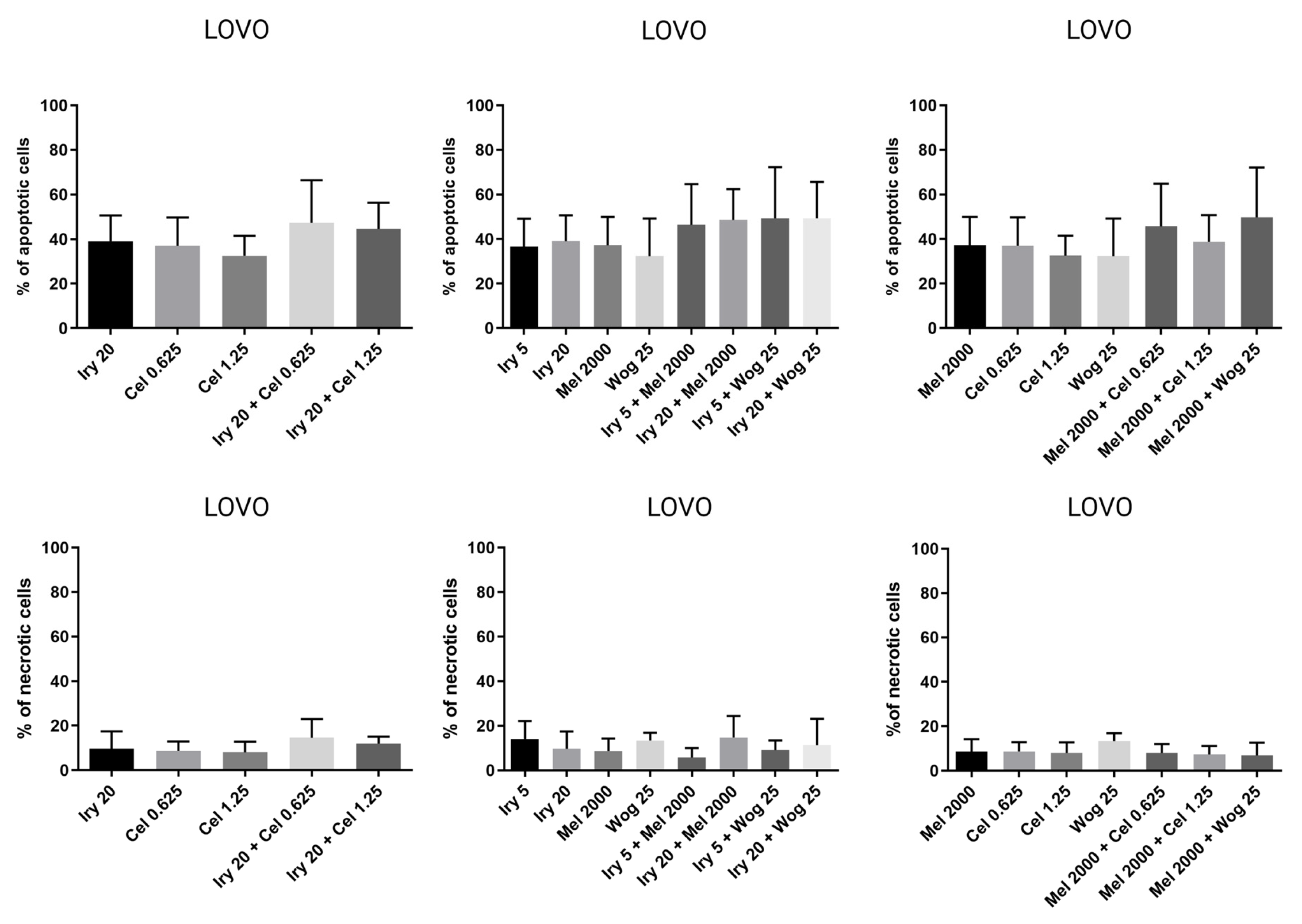
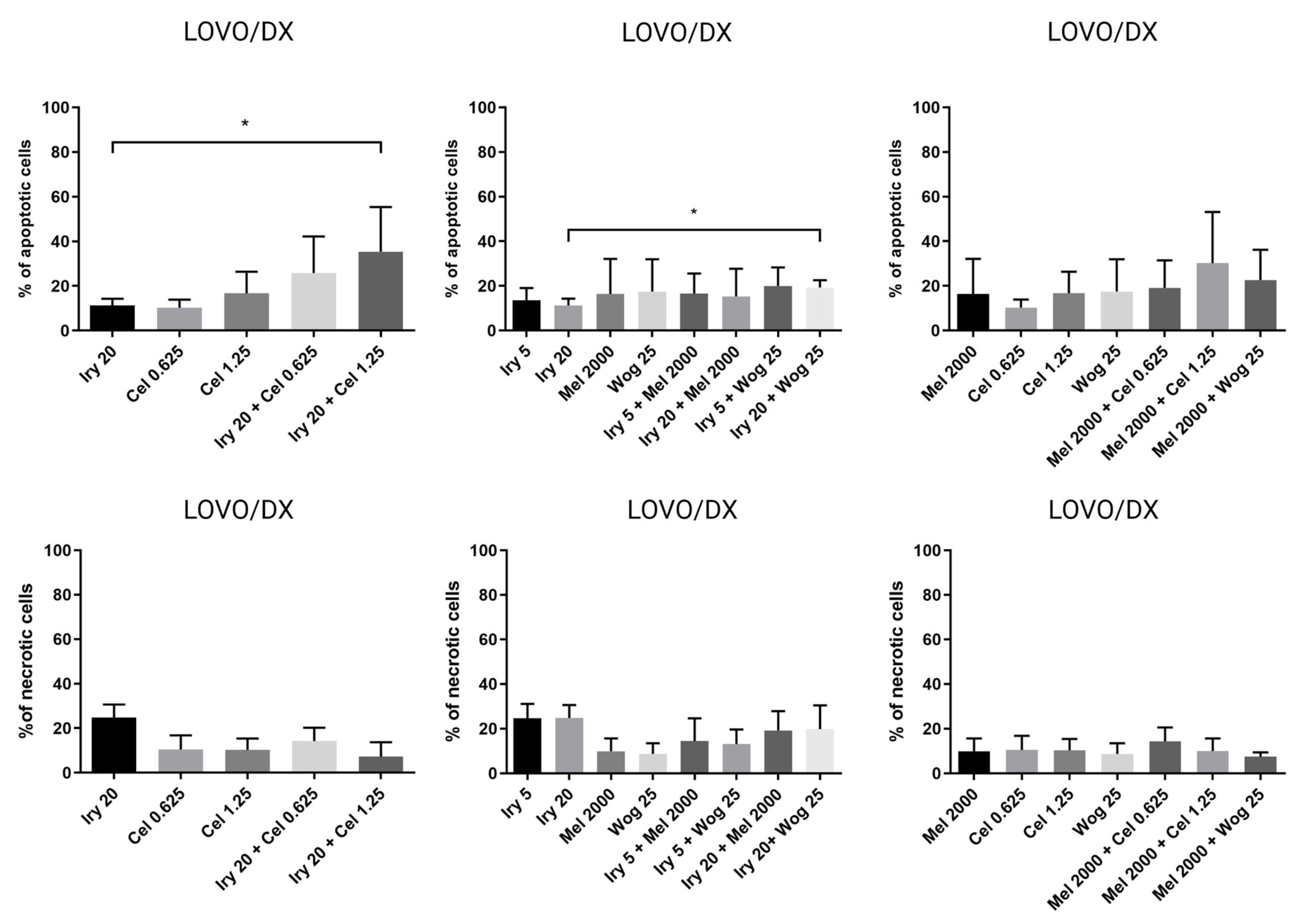
2.2. Pro-Apoptotic and Necrotic Activity of Tested Compounds and Their Combinations in Colon Cancer Cells and Cancer Stem-Like Cells
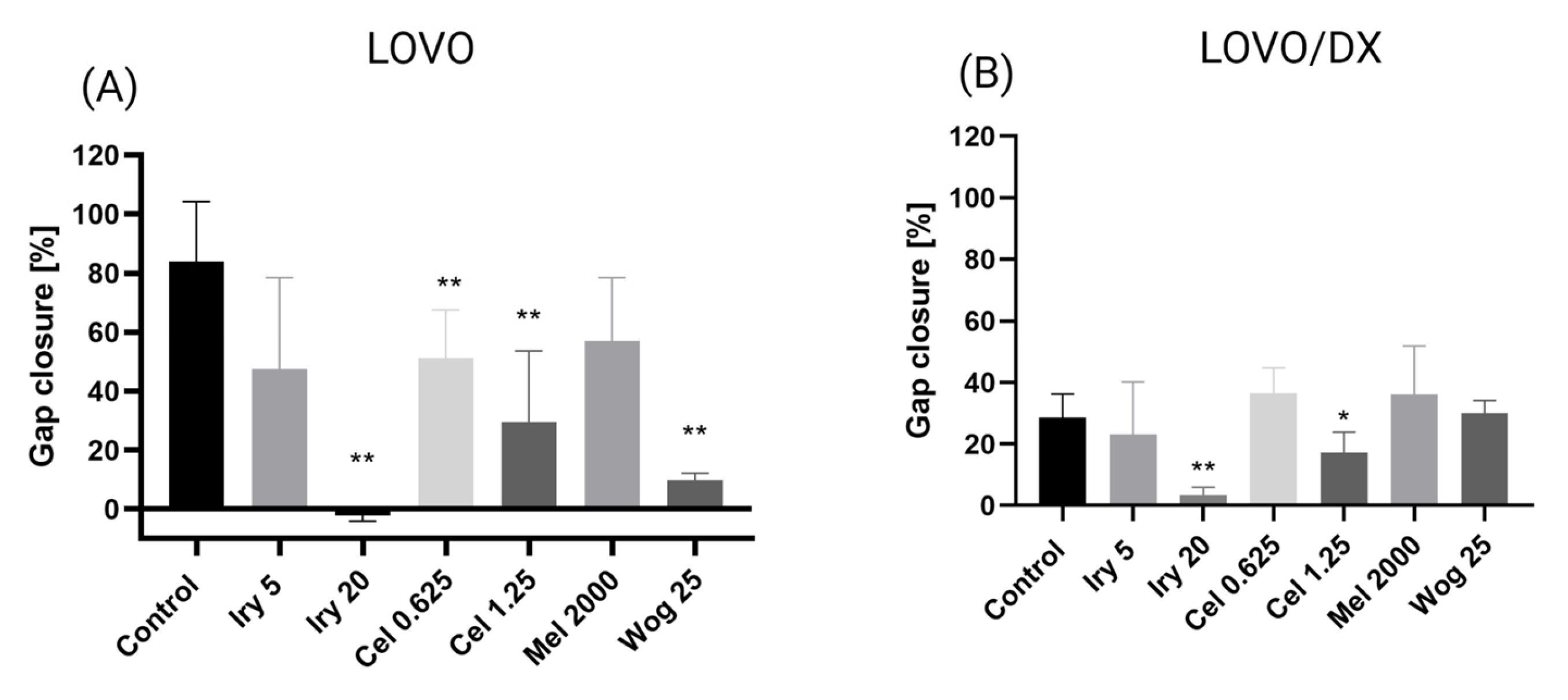
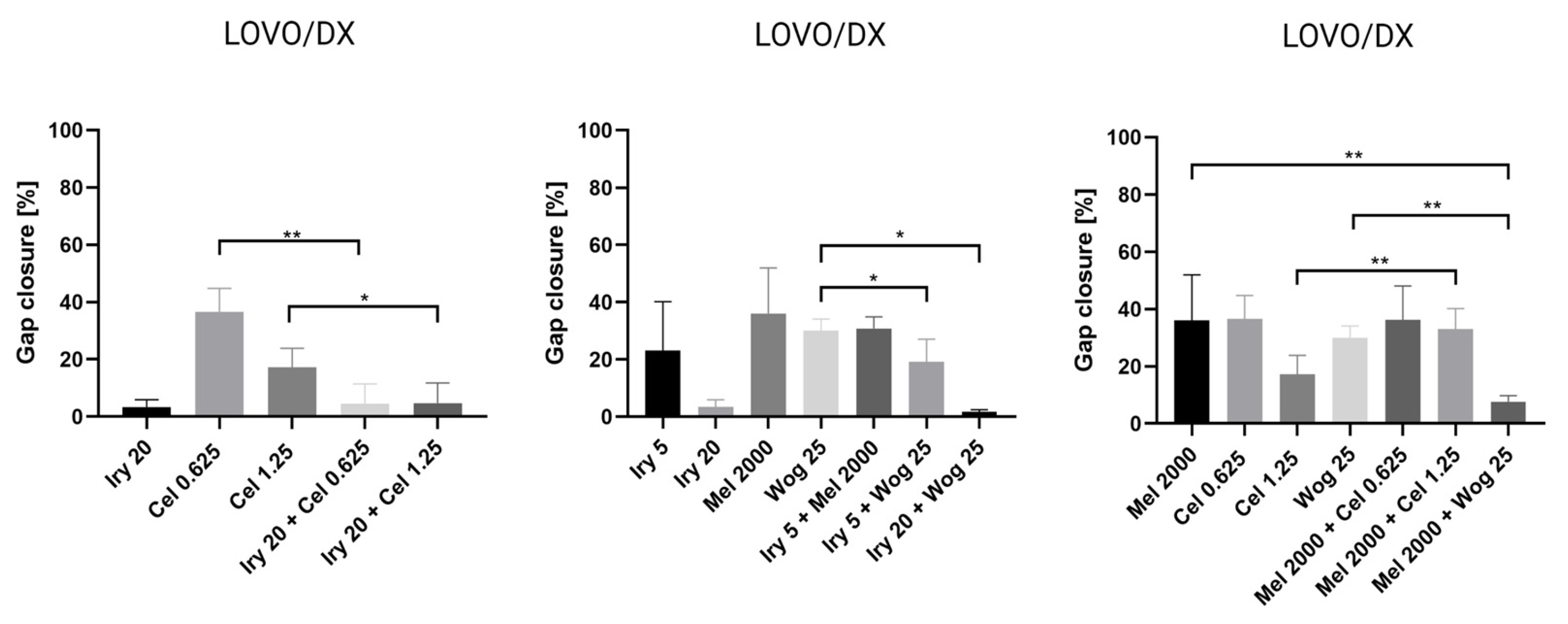
2.3. Inhibition of Cell Migration by Tested Compounds and Their Combinations in Colon Cancer Cells and Cancer Stem-Like Cells
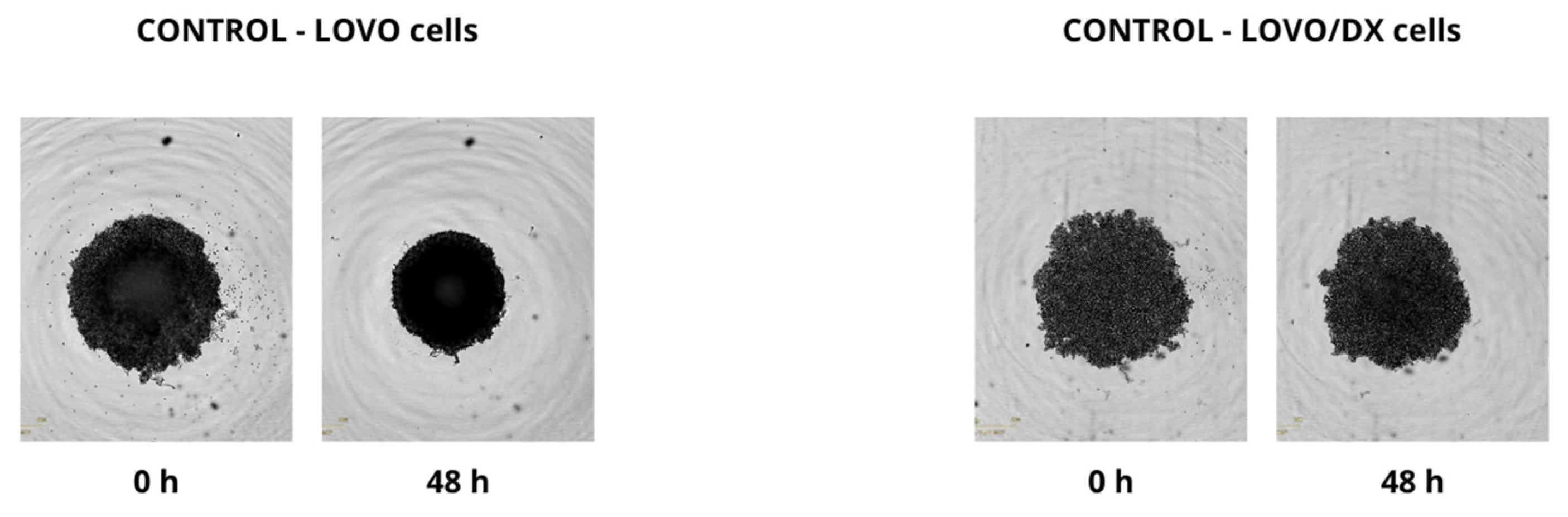
2.4. Effect of Combined Therapy on Spheroid Growth
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture Conditions
4.3. Reagent Preparation
4.4. Cell Viability Assay
4.5. Apoptosis and Necrosis Assay
4.6. Spheroid Preparation and Cell Growth Assessment
4.7. Scratch Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Peterson, C.Y.; Sriram, D.; Mahipal, A. Early stage colon cancer: Current treatment standards, evolving paradigms, and future directions. World J. Gastrointest. Oncol. 2020, 12, 808–832. [Google Scholar] [CrossRef] [PubMed]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, e5416923. [Google Scholar] [CrossRef]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- de Man, F.M.; Goey, A.K.L.; Van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-M.; Koh, W.-P.; Sun, C.-L.; Lee, H.-P.; Yu, M.C. Green tea intake, ACE gene polymorphism and breast cancer risk among Chinese women in Singapore. Carcinogenesis 2005, 26, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Asadi, Z.; Fathi, M.; Rismani, E.; Bigdelou, Z.; Johari, B. Application of decoy oligodeoxynucleotides strategy for inhibition of cell growth and reduction of metastatic properties in nonresistant and erlotinib-resistant SW480 cell line. Cell Biol. Int. 2021, 45, 1001–1014. [Google Scholar] [CrossRef]
- Mousazadeh, N.; Gharbavi, M.; Rashidzadeh, H.; Nosrati, H.; Danafar, H.; Johari, B. Anticancer evaluation of methotrexate and curcumin-coencapsulated niosomes against colorectal cancer cell lines. Nanomedicine 2022, 17, 201–217. [Google Scholar] [CrossRef]
- Efridlender, M.; Ekapulnik, Y.; Ekoltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Farhood, B.; Ahmadi, A.; Potes, Y.; Shabeeb, D.; Musa, A.E. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 2019, 228, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H. Melatonin and Cancer Hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, X.; Zhang, C.; Yu, W.; Guo, W.; Zhang, Z.; Li, Z.; Feng, X.; Hao, J.; Zhang, K.; et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J. Pineal Res. 2017, 62, e12380. [Google Scholar] [CrossRef]
- Wang, J.; Guo, W.; Chen, W.; Yu, W.; Tian, Y.; Fu, L.; Shi, D.; Tong, B.; Xiao, X.; Huang, W.; et al. Melatonin potentiates the antiproliferative and pro-apoptotic effects of ursolic acid in colon cancer cells by modulating multiple signaling pathways. J. Pineal Res. 2013, 54, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Shaheen, S.; Ayatollahi, S.A.; Kobarfard, F.; Imran, M.; Imran, A.; Custódio, L.; López, M.D.; et al. The Therapeutic Potential of Wogonin Observed in Preclinical Studies. Evid.-Based Complement. Altern. Med. 2021, 2021, e9935451. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Di, A.; Zhang, L.; Zhao, G. Effects of wogonin on the growth and metastasis of colon cancer through the Hippo signaling pathway. Bioengineered 2022, 13, 2586–2597. [Google Scholar] [CrossRef]
- Banik, K.; Khatoon, E.; Harsha, C.; Rana, V.; Parama, D.; Thakur, K.K.; Bishayee, A.; Kunnumakkara, A.B. Wogonin and its analogs for the prevention and treatment of cancer: A systematic review. Phytother. Res. 2022, 36, 1854–1883. [Google Scholar] [CrossRef]
- Lim, H.Y.; Ong, P.S.; Wang, L.; Goel, A.; Ding, L.; Wong, A.L.-A.; Ho, P.C.-L.; Sethi, G.; Xiang, X.; Goh, B.C. Celastrol in cancer therapy: Recent developments, challenges and prospects. Cancer Lett. 2021, 521, 252–267. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, S.; Pang, L.; Yang, J.; Li, H.J.; Huo, X.; Qian, S.Y. Celastrol suppresses nitric oxide synthases and the angiogenesis pathway in colorectal cancer. Free Radic. Res. 2019, 53, 324–334. [Google Scholar] [CrossRef]
- Jiang, Z.; Cao, Q.; Dai, G.; Wang, J.; Liu, C.; Lv, L.; Pan, J. Celastrol inhibits colorectal cancer through TGF-β1/Smad signaling. OncoTargets Ther. 2019, 12, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.; Szyjka, A.; Paliszkiewicz, K.; Barg, E. Prooxidative Activity of Celastrol Induces Apoptosis, DNA Damage, and Cell Cycle Arrest in Drug-Resistant Human Colon Cancer Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6793957. [Google Scholar] [CrossRef] [PubMed]
- PubChem, Irinotecan Hydrochloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/74990 (accessed on 27 February 2023).
- PubChem, Melatonin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/896 (accessed on 27 February 2023).
- Celastrol|C29H38O4—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/122724#section=Structures (accessed on 27 February 2023).
- PubChem, Wogonin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281703 (accessed on 27 February 2023).
- Sanz-Garcia, E.; Grasselli, J.; Argilés, G.; Elez, M.E.; Tabernero, J. Current and advancing treatments for metastatic colorectal cancer. Expert Opin. Biol. Ther. 2016, 16, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Garmo, H.; Berglund, M.; Fredriksson, L.A.; Kohnke, H.; Bystrom, P.; Sorbye, H.; Wadelius, M. Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenom. J. 2011, 11, 61–71. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, J.; Yan, T.; Miao, M.; Cao, Y.; Cao, Y.; Zhang, L.; Li, L. Novel strategies to reverse chemoresistance in colorectal cancer. Cancer Med. 2023. [Google Scholar] [CrossRef]
- Zhuang, C.; Guan, X.; Ma, H.; Cong, H.; Zhang, W.; Miao, Z. Small molecule-drug conjugates: A novel strategy for cancer-targeted treatment. Eur. J. Med. Chem. 2019, 163, 883–895. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Ni, J.; Jiang, Z.; Shen, L.; Gao, L.; Yu, M.; Xu, X.; Zou, S.; Hua, D.; Wu, S. β3GnT8 regulates the metastatic potential of colorectal carcinoma cells by altering the glycosylation of CD. Oncol. Rep. 2014, 31, 1795–1801. [Google Scholar] [CrossRef]
- Moreira, H.; Szyjka, A.; Grzesik, J.; Pelc, K.; Żuk, M.; Kulma, A.; Emhemmed, F.; Muller, C.D.; Gąsiorowski, K.; Barg, E. Celastrol and Resveratrol Modulate SIRT Genes Expression and Exert Anticancer Activity in Colon Cancer Cells and Cancer Stem-like Cells. Cancers 2022, 14, 1372. [Google Scholar] [CrossRef]
- Schernhammer, E.S.; Hankinson, S.E. Urinary Melatonin Levels and Postmenopausal Breast Cancer Risk in the Nurses’ Health Study Cohort. Cancer Epidemiol. Biomark. Prev. 2009, 18, 74–79. [Google Scholar] [CrossRef]
- Zhang, N.; Sundquist, J.; Sundquist, K.; Ji, J. Use of Melatonin Is Associated with Lower Risk of Colorectal Cancer in Older Adults. Clin. Transl. Gastroenterol. 2021, 12, e00396. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506. [Google Scholar] [CrossRef] [PubMed]
- Kontek, R.; Jakubczak, M.; Matlawska-Wasowska, K. The antioxidants, vitamin A and E but not vitamin C and melatonin enhance the proapoptotic effects of irinotecan in cancer cells in vitro. Toxicol. Vitr. 2014, 28, 282–291. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Z.; Du, L.; Xu, C.; Wang, Y.; Yang, B.; He, N.; Wang, J.; Ji, K.; Liu, Y.; et al. Melatonin Sensitizes Human Colorectal Cancer Cells to γ-ray Ionizing Radiation In Vitro and In Vivo. Int. J. Mol. Sci. 2018, 19, 3974. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-D.; Hou, X.-Y.; Wang, O.; Wang, D.; Li, D.-T.; Qin, S.-Y.; Lv, B.; Dai, X.-M.; Zhang, Z.-J.; Wan, J.-B.; et al. A four-component combination derived from Huang-Qin Decoction significantly enhances anticancer activity of irinotecan. Chin. J. Nat. Med. 2021, 19, 364–375. [Google Scholar] [CrossRef]
- Moreira, H.; Gębarowski, T.; Szyjka, A.; Flank, M. Effect of baicalein and wogonin on side population quantity in human breast and colon cancers. Bromat. Chem. Toksykol. 2015, 3, 467–472. [Google Scholar]
- Wang, C.-Z.; Calway, T.D.; Wen, X.-D.; Smith, J.; Yu, C.; Wang, Y.; Mehendale, S.R.; Yuan, C.-S. Hydrophobic flavonoids from Scutellaria baicalensis induce colorectal cancer cell apoptosis through a mitochondrial-mediated pathway. Int. J. Oncol. 2013, 42, 1018–1026. [Google Scholar] [CrossRef]
- Rong, L.-W.; Wang, R.-X.; Zheng, X.-L.; Feng, X.-Q.; Zhang, L.; Zhang, L.; Lin, Y.; Li, Z.-P.; Wang, X. Combination of wogonin and sorafenib effectively kills human hepatocellular carcinoma cells through apoptosis potentiation and autophagy inhibition. Oncol. Lett. 2017, 13, 5028–5034. [Google Scholar] [CrossRef]
- Chen, S.; Cooper, M.; Jones, M.; Madhuri, T.K.; Wade, J.; Bachelor, A.; Butler-Manuel, S. Combined activity of oridonin and wogonin in advanced-stage ovarian cancer cells. Cell Biol. Toxicol. 2011, 27, 133–147. [Google Scholar] [CrossRef]
- Kim, E.H.; Jang, H.; Shin, D.; Baek, S.H.; Roh, J.-L. Targeting Nrf2 with wogonin overcomes cisplatin resistance in head and neck cancer. Apoptosis 2016, 21, 1265–1278. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Sun, S.; Liu, G.; Chen, L.; Xia, Y.; Cui, J.; Wang, W.; Jiang, X.; Zhang, L.; et al. Wogonin Induces Apoptosis and Reverses Sunitinib Resistance of Renal Cell Carcinoma Cells via Inhibiting CDK4-RB Pathway. Front. Pharmacol. 2020, 11, 1152. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, R.; Koshiba, C.; Suzuki, C.; Lee, E. Wogonin potentiates the antitumor action of etoposide and ameliorates its adverse effects. Cancer Chemother. Pharmacol. 2011, 67, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, Z.; Wu, S.-S.; Xu, J.; Kong, L.-C.; Wei, P. Celastrol Enhances the Anti-Liver Cancer Activity of Sorafenib. Experiment 2019, 25, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Yao, C.; Liu, C.; Shu, Y. The Synergistic Effects of Celastrol in combination with Tamoxifen on Apoptosis and Autophagy in MCF-7 Cells. J. Immunol. Res. 2021, 2021, e5532269. [Google Scholar] [CrossRef]
- Habra, K.; Pearson, J.R.D.; McArdle, S.E.B. Robust Formation of Optimal Single Spheroids towards Cost-Effective In-Vitro 3-Dimensional Tumor Models. FEBS Open Bio, 2022; in review. [Google Scholar] [CrossRef]
- Incucyte® Single Spheroid Assay, Sartorius Protocol. Available online: https://www.sartorius.com/ (accessed on 20 December 2021).
- Incucyte® Scratch Wound Assay, Sartorius Protocol. Available online: https://www.sartorius.com/ (accessed on 20 December 2021).
- Radstake, W.E.; Gautam, K.; Van Rompay, C.; Vermeesen, R.; Tabury, K.; Verslegers, M.; Baatout, S.; Baselet, B. Comparison of in vitro scratch wound assay experimental procedures. Biochem. Biophys. Rep. 2023, 33, 101423. [Google Scholar] [CrossRef]


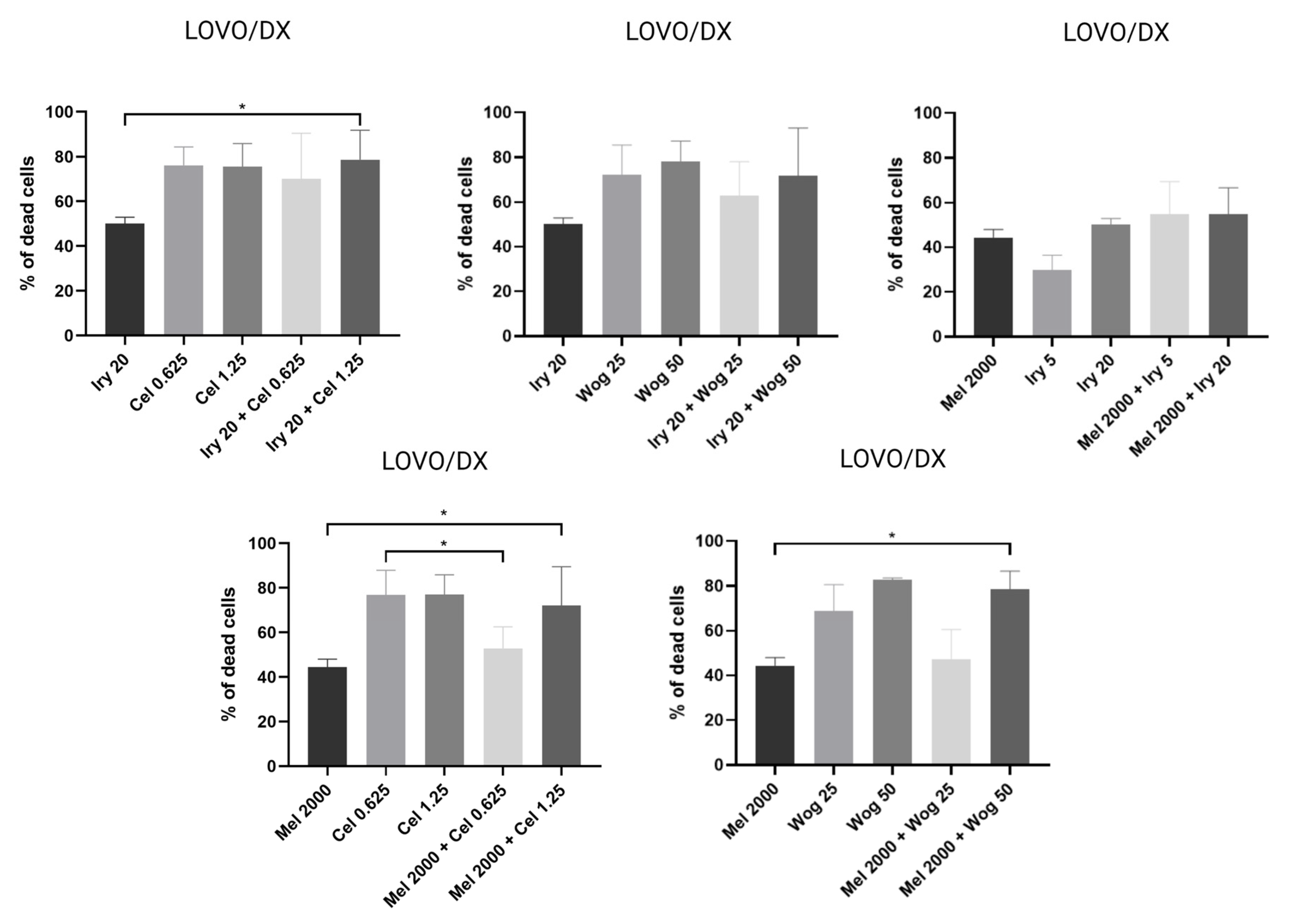





| Irinotecan and Melatonin Alone | Percentage of Dead Cells | |
|---|---|---|
| LOVO Cells | LOVO/DX Cells | |
| IRY 5 µM | 36% | 30% |
| IRY 20 µM | 55% | 50% |
| MEL 2000 µM | 47% | 44% |
| Combination of drugs | ||
| IRY 20 µM + CEL 0.625 µM | 69% | 70% |
| IRY 20 µM + CEL 1.25 µM | 84% | 79% |
| IRY 20 µM + WOG 25 µM | 75% | 63% |
| IRY 20 µM + WOG 50 µM | 75% | 72% |
| MEL 2000 µM + IRY 5 µM | 57% | 55% |
| MEL 2000 µM + IRY 20 µM | 61% | 55% |
| MEL 2000 µM + CEL 0.625 µM | 58% | 53% |
| MEL 2000 µM + CEL 1.25 µM | 83% | 72% |
| MEL 2000 µM + WOG 25 µM | 62% | 47% |
| MEL 2000 µM + WOG 50 µM | 78% | 78% |
| Irinotecan or Melatonin Alone | Percentage of Apoptotic Cells | |
|---|---|---|
| LOVO Cells | LOVO/DX Cells | |
| IRY 5 µM | 37% | 14% |
| IRY 20 µM | 39% | 11% |
| MEL 2000 µM | 37% | 16% |
| Combination of drugs | ||
| IRY 5 µM + WOG 25 µM | 49% | 20% |
| IRY 5 µM + MEL 2000 µM | 46% | 17% |
| IRY 20 µM + WOG 25 µM | 49% | 19% |
| IRY 20 µM + CEL 0.625 µM | 47% | 26% |
| IRY 20 µM + CEL 1.25 µM | 45% | 35% |
| MEL 2000 µM + WOG 25 µM | 50% | 23% |
| MEL 2000 µM + CEL 0.625 µM | 46% | 19% |
| MEL 2000 µM + CEL 1.25 µM | 39% | 30% |
| Irinotecan or Melatonin Alone | Gap Closure | |
|---|---|---|
| LOVO Cells | LOVO/DX Cells | |
| IRY 5 µM | 47.6% | 23.1% |
| IRY 20 µM | −2.2% * | 3.4% |
| MEL 2000 µM | 57.0% | 36.0% |
| Combination of Drugs | ||
| IRY 5 µM + WOG 25 µM | −0.3% * | 19.2% |
| IRY 5 µM + MEL 2000 µM | 48.2% | 30.8% |
| IRY 20 µM + WOG 25 µM | −2.9% * | 1.7% |
| IRY 20 µM + CEL 0.625 µM | 0.6% | 4.5% |
| IRY 20 µM + CEL 1.25 µM | −1.0% * | 4.7% |
| MEL 2000 µM + WOG 25 µM | 25.0% | 7.7% |
| MEL 2000 µM + CEL 0.625 µM | 59.1% | 36.3% |
| MEL 2000 µM + CEL 1.25 µM | 56.5% | 32.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radajewska, A.; Moreira, H.; Bęben, D.; Siwiela, O.; Szyjka, A.; Gębczak, K.; Nowak, P.; Frąszczak, J.; Emhemmed, F.; Muller, C.D.; et al. Combination of Irinotecan and Melatonin with the Natural Compounds Wogonin and Celastrol for Colon Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 9544. https://doi.org/10.3390/ijms24119544
Radajewska A, Moreira H, Bęben D, Siwiela O, Szyjka A, Gębczak K, Nowak P, Frąszczak J, Emhemmed F, Muller CD, et al. Combination of Irinotecan and Melatonin with the Natural Compounds Wogonin and Celastrol for Colon Cancer Treatment. International Journal of Molecular Sciences. 2023; 24(11):9544. https://doi.org/10.3390/ijms24119544
Chicago/Turabian StyleRadajewska, Anna, Helena Moreira, Dorota Bęben, Oliwia Siwiela, Anna Szyjka, Katarzyna Gębczak, Paulina Nowak, Jakub Frąszczak, Fathi Emhemmed, Christian D. Muller, and et al. 2023. "Combination of Irinotecan and Melatonin with the Natural Compounds Wogonin and Celastrol for Colon Cancer Treatment" International Journal of Molecular Sciences 24, no. 11: 9544. https://doi.org/10.3390/ijms24119544
APA StyleRadajewska, A., Moreira, H., Bęben, D., Siwiela, O., Szyjka, A., Gębczak, K., Nowak, P., Frąszczak, J., Emhemmed, F., Muller, C. D., & Barg, E. (2023). Combination of Irinotecan and Melatonin with the Natural Compounds Wogonin and Celastrol for Colon Cancer Treatment. International Journal of Molecular Sciences, 24(11), 9544. https://doi.org/10.3390/ijms24119544








