Oncogenic Viruses and the Epigenome: How Viruses Hijack Epigenetic Mechanisms to Drive Cancer
Abstract
1. Introduction
| Oncogenic Virus | Genome | Family | Associated Cancer Types | Global Infection Prevalence | Global Attributable Fraction | Refs. |
|---|---|---|---|---|---|---|
| EBV | dsDNA ~170 kb | Herpesviridae |
|
|
| [3,9] |
| HPVs | dsDNA ~8 kb | Papillomaviridae |
|
|
| [3,10,11] |
| HBV | dsDNA ~3.2 kb | Hepadnaviridae |
|
|
| [12,13] |
| HCV | ssRNA ~9.6 kb | Flaviviridae |
|
|
| [13,14] |
| HTLV-1 | ssRNA ~9 kb | Retroviridae |
|
|
| [3,15] |
| KSHV | dsDNA ~165 kb | Herpesviridae |
|
|
| [2,3] |
| MCPyV | dsDNA ~5.4 kb | Polyomaviridae |
|
|
| [16] |
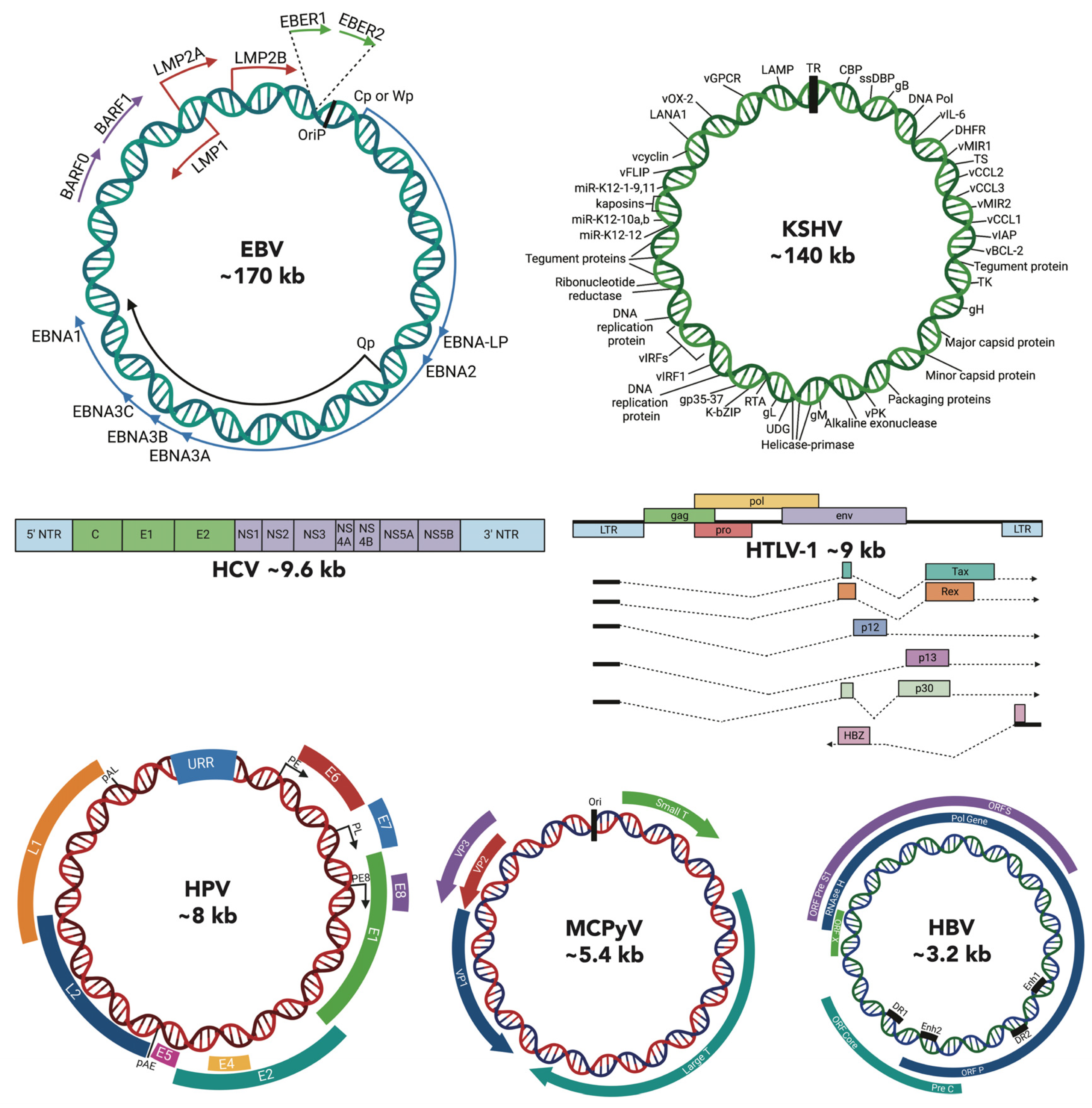
2. Cancer Epigenetics
2.1. DNA Methylation
2.2. Histone Post-Translational Modifications
2.3. Non-Coding RNAs
3. Epigenetics and Viral Life Cycles: HPV in Cervical Cancer as a Case Study
3.1. The HPV Life Cycle—Productive Infection vs. Neoplastic Progression
3.2. Epigenetic Modulation of HPV and Host Gene Expression in Cervical Cancer
4. Impacts of Virally Mediated Epigenetic Changes on Cancer Pathology
4.1. Epithelial-to-Mesenchymal Transition

4.2. Escape from Apoptosis
4.3. Altered Cellular Metabolism
4.4. Angiogenesis
4.5. Inflammation
4.6. Generation of Genomic Instability
5. Epigenetic Biomarkers and Therapeutic Targets of Virally Driven Cancers
5.1. Diagnostic and Prognostic Biomarkers
5.2. Epigenetic Therapeutic Targets
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krump, N.A.; You, J. Molecular Mechanisms of Viral Oncogenesis in Humans. Nat. Rev. Microbiol. 2018, 16, 684–698. [Google Scholar] [CrossRef]
- Lunn, R.M.; Jahnke, G.D.; Rabkin, C.S. Tumour Virus Epidemiology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160266. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global Burden of Cancer Attributable to Infections in 2018: A Worldwide Incidence Analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-Associated Head and Neck Cancer: A Virus-Related Cancer Epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Rous, P. A Sarcoma of the Fowl Transmissible by an Agent Separable from the Tumor Cells. J. Exp. Med. 1911, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.A.; Achong, B.G.; Barr, Y.M. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet 1964, 1, 702–703. [Google Scholar] [CrossRef]
- Chang, Y.; Moore, P.S.; Weiss, R.A. Human Oncogenic Viruses: Nature and Discovery. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160264. [Google Scholar] [CrossRef]
- Flanagan, J.M. Host Epigenetic Modifications by Oncogenic Viruses. Br. J. Cancer 2007, 96, 183–188. [Google Scholar] [CrossRef]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the Global Burden of Epstein–Barr Virus-Related Cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Shewale, J.B.; Gillison, M.L. Dynamic Factors Affecting HPV-Attributable Fraction for Head and Neck Cancers. Curr. Opin. Virol. 2019, 39, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Razavi-Shearer, D.; Gamkrelidze, I.; Nguyen, M.H.; Chen, D.-S.; Van Damme, P.; Abbas, Z.; Abdulla, M.; Abou Rached, A.; Adda, D.; Aho, I.; et al. Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory HCV Collaborators. Global Prevalence and Genotype Distribution of Hepatitis C Virus Infection in 2015: A Modelling Study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Pandeya, N.; Whiteman, D.C. International Increases in Merkel Cell Carcinoma Incidence Rates between 1997 and 2016. J. Investig. Dermatol. 2021, 141, 2596–2601.e1. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in Cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Zhao, S.; Allis, C.D.; Wang, G.G. The Language of Chromatin Modification in Human Cancers. Nat. Rev. Cancer 2021, 21, 413–430. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.X.; Ashworth, A. Marked for Death: Targeting Epigenetic Changes in Cancer. Nat. Rev. Drug Discov. 2017, 16, 241–263. [Google Scholar] [CrossRef]
- Young, L.S.; Rickinson, A.B. Epstein-Barr Virus: 40 Years on. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Mesri, E.A.; Cesarman, E.; Boshoff, C. Kaposi’s Sarcoma and Its Associated Herpesvirus. Nat. Rev. Cancer 2010, 10, 707–719. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Javed, T.; Rehman, S.; Nawaz, Z.; Riazuddin, S. An Overview of HCV Molecular Biology, Replication and Immune Responses. Virol. J. 2011, 8, 161. [Google Scholar] [CrossRef]
- Matsuoka, M.; Jeang, K.-T. Human T-Cell Leukemia Virus Type 1 (HTLV-1) and Leukemic Transformation: Viral Infectivity, Tax, HBZ and Therapy. Oncogene 2011, 30, 1379–1389. [Google Scholar] [CrossRef]
- Mac, M.; Moody, C.A. Epigenetic Regulation of the Human Papillomavirus Life Cycle. Pathogens 2020, 9, 483. [Google Scholar] [CrossRef]
- Krump, N.A.; You, J. From Merkel Cell Polyomavirus Infection to Merkel Cell Carcinoma Oncogenesis. Front. Microbiol. 2021, 12, 739695. [Google Scholar] [CrossRef]
- Al-Sadeq, D.W.; Taleb, S.A.; Zaied, R.E.; Fahad, S.M.; Smatti, M.K.; Rizeq, B.R.; Al Thani, A.A.; Yassine, H.M.; Nasrallah, G.K. Hepatitis B Virus Molecular Epidemiology, Host-Virus Interaction, Coinfection, and Laboratory Diagnosis in the MENA Region: An Update. Pathogens 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Calderon, A.; Lieberman, P.M. Control of Viral Latency by Episome Maintenance Proteins. Trends Microbiol. 2020, 28, 150–162. [Google Scholar] [CrossRef]
- You, J.; Croyle, J.L.; Nishimura, A.; Ozato, K.; Howley, P.M. Interaction of the Bovine Papillomavirus E2 Protein with Brd4 Tethers the Viral DNA to Host Mitotic Chromosomes. Cell 2004, 117, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-S.; Lu, F.; Lieberman, P.M. Epigenetic Regulation of EBV and KSHV Latency. Curr. Opin. Virol. 2013, 3, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.S.; Chang, Y. Why Do Viruses Cause Cancer? Highlights of the First Century of Human Tumour Virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA Methylation and Cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef]
- Noushmehr, H.; Weisenberger, D.J.; Diefes, K.; Phillips, H.S.; Pujara, K.; Berman, B.P.; Pan, F.; Pelloski, C.E.; Sulman, E.P.; Bhat, K.P.; et al. Identification of a CpG Island Methylator Phenotype That Defines a Distinct Subgroup of Glioma. Cancer Cell 2010, 17, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Turcan, S.; Rohle, D.; Goenka, A.; Walsh, L.A.; Fang, F.; Yilmaz, E.; Campos, C.; Fabius, A.W.M.; Lu, C.; Ward, P.S.; et al. IDH1 Mutation Is Sufficient to Establish the Glioma Hypermethylator Phenotype. Nature 2012, 483, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Ahuja, N.; Ohe-Toyota, M.; Herman, J.G.; Baylin, S.B.; Issa, J.P. CpG Island Methylator Phenotype in Colorectal Cancer. Proc. Natl. Acad. Sci. USA 1999, 96, 8681–8686. [Google Scholar] [CrossRef]
- Toyota, M.; Ahuja, N.; Suzuki, H.; Itoh, F.; Ohe-Toyota, M.; Imai, K.; Baylin, S.B.; Issa, J.P. Aberrant Methylation in Gastric Cancer Associated with the CpG Island Methylator Phenotype. Cancer Res. 1999, 59, 5438–5442. [Google Scholar]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Bhende, P.M.; Seaman, W.T.; Delecluse, H.-J.; Kenney, S.C. The EBV Lytic Switch Protein, Z, Preferentially Binds to and Activates the Methylated Viral Genome. Nat. Genet. 2004, 36, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Flower, K.; Thomas, D.; Heather, J.; Ramasubramanyan, S.; Jones, S.; Sinclair, A.J. Epigenetic Control of Viral Life-Cycle by a DNA-Methylation Dependent Transcription Factor. PLoS ONE 2011, 6, e25922. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Wei, W.; Anderton, J.; Vockerodt, M.; Rowe, M.; Murray, P.G.; Woodman, C.B. Epigenetic and Transcriptional Changes Which Follow Epstein-Barr Virus Infection of Germinal Center B Cells and Their Relevance to the Pathogenesis of Hodgkin’s Lymphoma. J. Virol. 2011, 85, 9568–9577. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lian, Z.; Han, S.; Waye, M.M.Y.; Wang, H.; Wu, M.-C.; Wu, K.; Ding, J.; Arbuthnot, P.; Kew, M.; et al. Downregulation of E-Cadherin by Hepatitis B Virus X Antigen in Hepatocellular Carcinoma. Oncogene 2006, 25, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Marsit, C.J.; McClean, M.D.; Furniss, C.S.; Kelsey, K.T. Epigenetic Inactivation of the SFRP Genes Is Associated with Drinking, Smoking and HPV in Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 2006, 119, 1761–1766. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Li, H.-P.; Lu, Y.-J.; Hsueh, C.; Liang, Y.; Chen, C.-L.; Tsao, S.W.; Tse, K.-P.; Yu, J.-S.; Chang, Y.-S. Activation of DNA Methyltransferase 1 by EBV LMP1 Involves c-Jun NH(2)-Terminal Kinase Signaling. Cancer Res. 2006, 66, 11668–11676. [Google Scholar] [CrossRef]
- ENCODE Project Consortium; Birney, E.; Stamatoyannopoulos, J.A.; Dutta, A.; Guigó, R.; Gingeras, T.R.; Margulies, E.H.; Weng, Z.; Snyder, M.; Dermitzakis, E.T.; et al. Identification and Analysis of Functional Elements in 1% of the Human Genome by the ENCODE Pilot Project. Nature 2007, 447, 799–816. [Google Scholar] [CrossRef]
- Tempera, I.; Lieberman, P.M. Epigenetic Regulation of EBV Persistence and Oncogenesis. Semin. Cancer Biol. 2014, 26, 22–29. [Google Scholar] [CrossRef]
- Goodall, G.J.; Wickramasinghe, V.O. RNA in Cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- van Zonneveld, A.J.; Kölling, M.; Bijkerk, R.; Lorenzen, J.M. Circular RNAs in Kidney Disease and Cancer. Nat. Rev. Nephrol. 2021, 17, 814–826. [Google Scholar] [CrossRef]
- Marquitz, A.R.; Raab-Traub, N. The Role of miRNAs and EBV BARTs in NPC. Semin. Cancer Biol. 2012, 22, 166–172. [Google Scholar] [CrossRef] [PubMed]
- David, R. miRNAs Help KSHV Lay Low. Nat. Rev. Microbiol. 2010, 8, 158–159. [Google Scholar] [CrossRef]
- Lajer, C.B.; Garnæs, E.; Friis-Hansen, L.; Norrild, B.; Therkildsen, M.H.; Glud, M.; Rossing, M.; Lajer, H.; Svane, D.; Skotte, L.; et al. The Role of miRNAs in Human Papilloma Virus (HPV)-Associated Cancers: Bridging between HPV-Related Head and Neck Cancer and Cervical Cancer. Br. J. Cancer 2012, 106, 1526–1534. [Google Scholar] [CrossRef]
- Yao, S.; Jia, X.; Wang, F.; Sheng, L.; Song, P.; Cao, Y.; Shi, H.; Fan, W.; Ding, X.; Gao, S.-J.; et al. CircRNA ARFGEF1 Functions as a ceRNA to Promote Oncogenic KSHV-Encoded Viral Interferon Regulatory Factor Induction of Cell Invasion and Angiogenesis by Upregulating Glutaredoxin 3. PLoS Pathog. 2021, 17, e1009294. [Google Scholar] [CrossRef]
- Flores, E.R.; Lambert, P.F. Evidence for a Switch in the Mode of Human Papillomavirus Type 16 DNA Replication during the Viral Life Cycle. J. Virol. 1997, 71, 7167–7179. [Google Scholar] [CrossRef]
- D’Costa, Z.J.; Jolly, C.; Androphy, E.J.; Mercer, A.; Matthews, C.M.; Hibma, M.H. Transcriptional Repression of E-Cadherin by Human Papillomavirus Type 16 E6. PLoS ONE 2012, 7, e48954. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.; Hoppe-Seyler, K.; Schuller, B.; Lohrey, C.; Maroldt, J.; Dürst, M.; Hoppe-Seyler, F. Activation of the Enhancer of Zeste Homologue 2 Gene by the Human Papillomavirus E7 Oncoprotein. Cancer Res. 2008, 68, 9964–9972. [Google Scholar] [CrossRef] [PubMed]
- Romanczuk, H.; Howley, P.M. Disruption of Either the E1 or the E2 Regulatory Gene of Human Papillomavirus Type 16 Increases Viral Immortalization Capacity. Proc. Natl. Acad. Sci. USA 1992, 89, 3159–3163. [Google Scholar] [CrossRef]
- Dong, G.; Broker, T.R.; Chow, L.T. Human Papillomavirus Type 11 E2 Proteins Repress the Homologous E6 Promoter by Interfering with the Binding of Host Transcription Factors to Adjacent Elements. J. Virol. 1994, 68, 1115–1127. [Google Scholar] [CrossRef]
- Kim, K.; Garner-Hamrick, P.A.; Fisher, C.; Lee, D.; Lambert, P.F. Methylation Patterns of Papillomavirus DNA, Its Influence on E2 Function, and Implications in Viral Infection. J. Virol. 2003, 77, 12450–12459. [Google Scholar] [CrossRef]
- Vinokurova, S.; von Knebel Doeberitz, M. Differential Methylation of the HPV 16 Upstream Regulatory Region during Epithelial Differentiation and Neoplastic Transformation. PLoS ONE 2011, 6, e24451. [Google Scholar] [CrossRef]
- Kalantari, M.; Calleja-Macias, I.E.; Tewari, D.; Hagmar, B.; Lie, K.; Barrera-Saldana, H.A.; Wiley, D.J.; Bernard, H.-U. Conserved Methylation Patterns of Human Papillomavirus Type 16 DNA in Asymptomatic Infection and Cervical Neoplasia. J. Virol. 2004, 78, 12762–12772. [Google Scholar] [CrossRef]
- Groves, I.J.; Knight, E.L.A.; Ang, Q.Y.; Scarpini, C.G.; Coleman, N. HPV16 Oncogene Expression Levels during Early Cervical Carcinogenesis Are Determined by the Balance of Epigenetic Chromatin Modifications at the Integrated Virus Genome. Oncogene 2016, 35, 4773–4786. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Huang, S.M.; Baglia, L.A.; McCance, D.J. The E6 Protein of Human Papillomavirus Type 16 Binds to and Inhibits Co-Activation by CBP and p300. EMBO J. 1999, 18, 5061–5072. [Google Scholar] [CrossRef]
- Zimmermann, H.; Degenkolbe, R.; Bernard, H.U.; O’Connor, M.J. The Human Papillomavirus Type 16 E6 Oncoprotein Can down-Regulate p53 Activity by Targeting the Transcriptional Coactivator CBP/p300. J. Virol. 1999, 73, 6209–6219. [Google Scholar] [CrossRef]
- Bernat, A.; Avvakumov, N.; Mymryk, J.S.; Banks, L. Interaction between the HPV E7 Oncoprotein and the Transcriptional Coactivator p300. Oncogene 2003, 22, 7871–7881. [Google Scholar] [CrossRef]
- Bauknecht, T.; Angel, P.; Royer, H.D.; zur Hausen, H. Identification of a Negative Regulatory Domain in the Human Papillomavirus Type 18 Promoter: Interaction with the Transcriptional Repressor YY1. EMBO J. 1992, 11, 4607–4617. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J.; Tan, S.H.; Tan, C.H.; Bernard, H.U. YY1 Represses Human Papillomavirus Type 16 Transcription by Quenching AP-1 Activity. J. Virol. 1996, 70, 6529–6539. [Google Scholar] [CrossRef] [PubMed]
- Pentland, I.; Campos-León, K.; Cotic, M.; Davies, K.-J.; David Wood, C.; Groves, I.J.; Burley, M.; Coleman, N.; Stockton, J.D.; Noyvert, B.; et al. Disruption of CTCF-YY1–dependent Looping of the Human Papillomavirus Genome Activates Differentiation-Induced Viral Oncogene Transcription. PLoS Biol. 2018, 16, e2005752. [Google Scholar] [CrossRef]
- Luo, X.; Hong, L.; Cheng, C.; Li, N.; Zhao, X.; Shi, F.; Liu, J.; Fan, J.; Zhou, J.; Bode, A.M.; et al. DNMT1 Mediates Metabolic Reprogramming Induced by Epstein–Barr Virus Latent Membrane Protein 1 and Reversed by Grifolin in Nasopharyngeal Carcinoma. Cell Death Dis. 2018, 9, 619. [Google Scholar] [CrossRef]
- Anderton, E.; Yee, J.; Smith, P.; Crook, T.; White, R.E.; Allday, M.J. Two Epstein–Barr Virus (EBV) Oncoproteins Cooperate to Repress Expression of the Proapoptotic Tumour-Suppressor Bim: Clues to the Pathogenesis of Burkitt’s Lymphoma. Oncogene 2007, 27, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Paschos, K.; Smith, P.; Anderton, E.; Middeldorp, J.M.; White, R.E.; Allday, M.J. Epstein-Barr Virus Latency in B Cells Leads to Epigenetic Repression and CpG Methylation of the Tumour Suppressor Gene Bim. PLoS Pathog. 2009, 5, e1000492. [Google Scholar] [CrossRef]
- Paschos, K.; Parker, G.A.; Watanatanasup, E.; White, R.E.; Allday, M.J. BIM Promoter Directly Targeted by EBNA3C in Polycomb-Mediated Repression by EBV. Nucleic Acids Res. 2012, 40, 7233–7246. [Google Scholar] [CrossRef]
- Zhang, R.; Su, J.; Xue, S.-L.; Yang, H.; Ju, L.-L.; Ji, Y.; Wu, K.-H.; Zhang, Y.-W.; Zhang, Y.-X.; Hu, J.-F.; et al. HPV E6/p53 Mediated down-Regulation of miR-34a Inhibits Warburg Effect through Targeting LDHA in Cervical Cancer. Am. J. Cancer Res. 2016, 6, 312–320. [Google Scholar]
- Liu, Y.; Guo, J.-Z.; Liu, Y.; Wang, K.; Ding, W.; Wang, H.; Liu, X.; Zhou, S.; Lu, X.-C.; Yang, H.-B.; et al. Nuclear Lactate Dehydrogenase A Senses ROS to Produce α-Hydroxybutyrate for HPV-Induced Cervical Tumor Growth. Nat. Commun. 2018, 9, 4429. [Google Scholar] [CrossRef] [PubMed]
- Laurson, J.; Khan, S.; Chung, R.; Cross, K.; Raj, K. Epigenetic Repression of E-Cadherin by Human Papillomavirus 16 E7 Protein. Carcinogenesis 2010, 31, 918–926. [Google Scholar] [CrossRef]
- Burgers, W.A.; Blanchon, L.; Pradhan, S.; de Launoit, Y.; Kouzarides, T.; Fuks, F. Viral Oncoproteins Target the DNA Methyltransferases. Oncogene 2007, 26, 1650–1655. [Google Scholar] [CrossRef]
- Jung, J.K.; Arora, P.; Pagano, J.S.; Jang, K.L. Expression of DNA Methyltransferase 1 Is Activated by Hepatitis B Virus X Protein via a Regulatory Circuit Involving the p16INK4a-Cyclin D1-CDK 4/6-pRb-E2F1 Pathway. Cancer Res. 2007, 67, 5771–5778. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-O.; Kwun, H.J.; Jung, J.K.; Choi, K.H.; Min, D.S.; Jang, K.L. Hepatitis B Virus X Protein Represses E-Cadherin Expression via Activation of DNA Methyltransferase 1. Oncogene 2005, 24, 6617–6625. [Google Scholar] [CrossRef]
- Liang, H.-W.; Wang, N.; Wang, Y.; Wang, F.; Fu, Z.; Yan, X.; Zhu, H.; Diao, W.; Ding, Y.; Chen, X.; et al. Hepatitis B Virus-Human Chimeric Transcript HBx-LINE1 Promotes Hepatic Injury via Sequestering Cellular microRNA-122. J. Hepatol. 2016, 64, 278–291. [Google Scholar] [CrossRef]
- Arora, P.; Kim, E.-O.; Jung, J.K.; Jang, K.L. Hepatitis C Virus Core Protein Downregulates E-Cadherin Expression via Activation of DNA Methyltransferase 1 and 3b. Cancer Lett. 2008, 261, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, D.; Nakagawa, S.; Hori, M.; Kurokawa, N.; Soejima, A.; Nakano, K.; Yamochi, T.; Nakashima, M.; Kobayashi, S.; Tanaka, Y.; et al. Polycomb-Dependent Epigenetic Landscape in Adult T-Cell Leukemia. Blood 2016, 127, 1790–1802. [Google Scholar] [CrossRef]
- Vernin, C.; Thenoz, M.; Pinatel, C.; Gessain, A.; Gout, O.; Delfau-Larue, M.-H.; Nazaret, N.; Legras-Lachuer, C.; Wattel, E.; Mortreux, F. HTLV-1 bZIP Factor HBZ Promotes Cell Proliferation and Genetic Instability by Activating OncomiRs. Cancer Res. 2014, 74, 6082–6093. [Google Scholar] [CrossRef]
- Tanaka-Nakanishi, A.; Yasunaga, J.-I.; Takai, K.; Matsuoka, M. HTLV-1 bZIP Factor Suppresses Apoptosis by Attenuating the Function of FoxO3a and Altering Its Localization. Cancer Res. 2014, 74, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, D.L.; Cannon, M.; Liu, Y.-F.; Renne, R.; Chadburn, A.; Boshoff, C.; Cesarman, E. KSHV LANA Inhibits TGF-β Signaling through Epigenetic Silencing of the TGF-β Type II Receptor. Blood J. Am. Soc. Hematol. 2008, 111, 4731–4740. [Google Scholar] [CrossRef]
- Choi, H.S.; Jain, V.; Krueger, B.; Marshall, V.; Kim, C.H.; Shisler, J.L.; Whitby, D.; Renne, R. Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling. PLoS Pathog. 2015, 11, e1005255. [Google Scholar] [CrossRef]
- Cheng, J.; Park, D.E.; Berrios, C.; White, E.A.; Arora, R.; Yoon, R.; Branigan, T.; Xiao, T.; Westerling, T.; Federation, A.; et al. Merkel Cell Polyomavirus Recruits MYCL to the EP400 Complex to Promote Oncogenesis. PLoS Pathog. 2017, 13, e1006668. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in Cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef]
- Au Yeung, C.L.; Tsang, W.P.; Tsang, T.Y.; Co, N.N.; Yau, P.L.; Kwok, T.T. HPV-16 E6 Upregulation of DNMT1 through Repression of Tumor Suppressor p53. Oncol. Rep. 2010, 24, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lim, J.S.; Lim, S.-Y.; Tiwari, I.; Jang, K.L. Hepatitis C Virus Core Protein Stimulates Cell Growth by down-Regulating p16 Expression via DNA Methylation. Cancer Lett. 2011, 310, 61–68. [Google Scholar] [CrossRef]
- Suzuki, H.; Watkins, D.N.; Jair, K.-W.; Schuebel, K.E.; Markowitz, S.D.; Chen, W.D.; Pretlow, T.P.; Yang, B.; Akiyama, Y.; Van Engeland, M.; et al. Epigenetic Inactivation of SFRP Genes Allows Constitutive WNT Signaling in Colorectal Cancer. Nat. Genet. 2004, 36, 417–422. [Google Scholar] [CrossRef]
- Suzuki, H.; Toyota, M.; Carraway, H.; Gabrielson, E.; Ohmura, T.; Fujikane, T.; Nishikawa, N.; Sogabe, Y.; Nojima, M.; Sonoda, T.; et al. Frequent Epigenetic Inactivation of Wnt Antagonist Genes in Breast Cancer. Br. J. Cancer 2008, 98, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.-T.; Lai, H.-C.; Sytwu, H.-K.; Yan, M.-D.; Shih, Y.-L.; Chang, C.-C.; Yu, M.-H.; Liu, H.-S.; Chu, D.-W.; Lin, Y.-W. SFRP1 and SFRP2 Suppress the Transformation and Invasion Abilities of Cervical Cancer Cells through Wnt Signal Pathway. Gynecol. Oncol. 2009, 112, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhou, F.; Nie, D.; Chen, Q.; Cai, X.; Shan, X.; Zhou, Z.; Chen, K.; Huang, A.; Li, S.; et al. Hepatitis C Virus Core Protein Epigenetically Silences SFRP1 and Enhances HCC Aggressiveness by Inducing Epithelial–mesenchymal Transition. Oncogene 2013, 33, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Chen, L.; Shan, X.; Shan, X.; Tang, J.; Zhou, F.; Chen, Q.; Quan, H.; Nie, D.; Zhang, W.; et al. Epigenetic Silencing of SFRP1 and SFRP5 by Hepatitis B Virus X Protein Enhances Hepatoma Cell Tumorigenicity through Wnt Signaling Pathway. Int. J. Cancer 2014, 135, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Sasaki, S.; Suzuki, H.; Toyota, M.; Maruyama, R.; Nojima, M.; Yamamoto, H.; Omata, M.; Tokino, T.; Imai, K.; et al. Frequent Epigenetic Inactivation of SFRP Genes in Hepatocellular Carcinoma. J. Gastroenterol. 2008, 43, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A. Identification and Characterization of a New Member of the TNF Family That Induces Apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.Y.-W.; Siu, K.-L.; Kok, K.-H.; Lung, R.W.-M.; Tsang, C.M.; To, K.-F.; Kwong, D.L.-W.; Tsao, S.W.; Jin, D.-Y. An Epstein-Barr Virus–encoded microRNA Targets PUMA to Promote Host Cell Survival. J. Exp. Med. 2008, 205, 2551–2560. [Google Scholar] [CrossRef]
- Marquitz, A.R.; Mathur, A.; Nam, C.S.; Raab-Traub, N. The Epstein–Barr Virus BART microRNAs Target the pro-Apoptotic Protein Bim. Virology 2011, 412, 392–400. [Google Scholar] [CrossRef]
- Fitzsimmons, L.; Boyce, A.J.; Wei, W.; Chang, C.; Croom-Carter, D.; Tierney, R.J.; Herold, M.J.; Bell, A.I.; Strasser, A.; Kelly, G.L.; et al. Coordinated Repression of BIM and PUMA by Epstein–Barr Virus Latent Genes Maintains the Survival of Burkitt Lymphoma Cells. Cell Death Differ. 2017, 25, 241–254. [Google Scholar] [CrossRef]
- Snellenberg, S.; Cillessen, S.A.G.M.; Van Criekinge, W.; Bosch, L.; Meijer, C.J.L.M.; Snijders, P.J.F.; Steenbergen, R.D.M. Methylation-Mediated Repression of PRDM14 Contributes to Apoptosis Evasion in HPV-Positive Cancers. Carcinogenesis 2014, 35, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.A.; Dai, Z.; Locasale, J.W. The Impact of Cellular Metabolism on Chromatin Dynamics and Epigenetics. Nat. Cell Biol. 2017, 19, 1298–1306. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-Associated IDH1 Mutations Produce 2-Hydroxyglutarate. Nature 2010, 465, 966. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 Mutations Result in a Hypermethylation Phenotype, Disrupt TET2 Function, and Impair Hematopoietic Differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic Reprogramming and Cancer Progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Hulse, M.; Caruso, L.B.; Madzo, J.; Tan, Y.; Johnson, S.; Tempera, I. Poly(ADP-Ribose) Polymerase 1 Is Necessary for Coactivating Hypoxia-Inducible Factor-1-Dependent Gene Expression by Epstein-Barr Virus Latent Membrane Protein 1. PLoS Pathog. 2018, 14, e1007394. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Lang, F.; Pei, Y.; Jha, H.C.; Robertson, E.S. Metabolic Reprogramming of Kaposi’s Sarcoma Associated Herpes Virus Infected B-Cells in Hypoxia. PLoS Pathog. 2018, 14, e1007062. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Samols, M.A.; Skalsky, R.L.; Maldonado, A.M.; Riva, A.; Lopez, M.C.; Baker, H.V.; Renne, R. Identification of Cellular Genes Targeted by KSHV-Encoded microRNAs. PLoS Pathog. 2007, 3, e65. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Zhu, Y.; Jones, T.; Bai, Z.; Huang, Y.; Gao, S.-J. A Kaposi’s Sarcoma-Associated Herpesvirus microRNA and Its Variants Target the Transforming Growth Factor β Pathway to Promote Cell Survival. J. Virol. 2012, 86, 11698–11711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, R.; Lin, X.; Liang, D.; Deng, Q.; Lan, K. Kaposi’s Sarcoma-Associated Herpesvirus-Encoded microRNA miR-K12-11 Attenuates Transforming Growth Factor Beta Signaling through Suppression of SMAD5. J. Virol. 2012, 86, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; Benelli, R.; Borsotti, P.; Rusnati, M.; Presta, M.; Giavazzi, R.; Ruco, L.; Albini, A. Thrombospondin-1 Inhibits Kaposi’s Sarcoma (KS) Cell and HIV-1 Tat-Induced Angiogenesis and Is Poorly Expressed in KS Lesions. J. Pathol. 1999, 188, 76–81. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Lo, A.K.F.; To, K.F.; Lo, K.W.; Lung, R.W.M.; Hui, J.W.Y.; Liao, G.; Hayward, S.D. Modulation of LMP1 Protein Expression by EBV-Encoded microRNAs. Proc. Natl. Acad. Sci. USA 2007, 104, 16164–16169. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Zeng, Z.; Li, Q.; Gong, Z.; Liao, Q.; Li, X.; Chen, P.; Xiang, B.; Zhang, W.; et al. Epstein-Barr Virus Encoded miR-BART11 Promotes Inflammation-Induced Carcinogenesis by Targeting FOXP1. Oncotarget 2016, 7, 36783–36799. [Google Scholar] [CrossRef] [PubMed]
- Bumrungthai, S.; Ekalaksananan, T.; Evans, M.F.; Chopjitt, P.; Tangsiriwatthana, T.; Patarapadungkit, N.; Kleebkaow, P.; Luanratanakorn, S.; Kongyingyoes, B.; Worawichawong, S.; et al. Up-Regulation of miR-21 Is Associated with Cervicitis and Human Papillomavirus Infection in Cervical Tissues. PLoS ONE 2015, 10, e0127109. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic Instability--an Evolving Hallmark of Cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-H.; Liu, X.; Yan, H.-X.; Li, W.-Y.; Zeng, X.; Yang, Y.; Zhao, J.; Liu, S.-P.; Zhuang, X.-H.; Lin, C.; et al. Genomic and Oncogenic Preference of HBV Integration in Hepatocellular Carcinoma. Nat. Commun. 2016, 7, 12992. [Google Scholar] [CrossRef]
- Naipauer, J.; Salyakina, D.; Journo, G.; Rosario, S.; Williams, S.; Abba, M.; Shamay, M.; Mesri, E.A. High-Throughput Sequencing Analysis of a “hit and Run” Cell and Animal Model of KSHV Tumorigenesis. PLoS Pathog. 2020, 16, e1008589. [Google Scholar] [CrossRef]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-Wide Analysis of HPV Integration in Human Cancers Reveals Recurrent, Focal Genomic Instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef]
- Álvarez, E.G.; Demeulemeester, J.; Otero, P.; Jolly, C.; García-Souto, D.; Pequeño-Valtierra, A.; Zamora, J.; Tojo, M.; Temes, J.; Baez-Ortega, A.; et al. Aberrant Integration of Hepatitis B Virus DNA Promotes Major Restructuring of Human Hepatocellular Carcinoma Genome Architecture. Nat. Commun. 2021, 12, 6910. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.; van Buuren, N.; Gamelin, L.; Soulette, C.; May, L.; Han, D.; Yu, M.; Choy, R.; Cheng, G.; Bhardwaj, N.; et al. Targeted Long-Read Sequencing Reveals Comprehensive Architecture, Burden, and Transcriptional Signatures from Hepatitis B Virus-Associated Integrations and Translocations in Hepatocellular Carcinoma Cell Lines. J. Virol. 2021, 95, e0029921. [Google Scholar] [CrossRef] [PubMed]
- Lleras, R.A.; Smith, R.V.; Adrien, L.R.; Schlecht, N.F.; Burk, R.D.; Harris, T.M.; Childs, G.; Prystowsky, M.B.; Belbin, T.J. Unique DNA Methylation Loci Distinguish Anatomic Site and HPV Status in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2013, 19, 5444–5455. [Google Scholar] [CrossRef]
- Journo, G.; Tushinsky, C.; Shterngas, A.; Avital, N.; Eran, Y.; Karpuj, M.V.; Frenkel-Morgenstern, M.; Shamay, M. Modulation of Cellular CpG DNA Methylation by Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2018, 92, e00008-18. [Google Scholar] [CrossRef] [PubMed]
- Birdwell, C.E.; Queen, K.J.; Kilgore, P.C.S.R.; Rollyson, P.; Trutschl, M.; Cvek, U.; Scott, R.S. Genome-Wide DNA Methylation as an Epigenetic Consequence of Epstein-Barr Virus Infection of Immortalized Keratinocytes. J. Virol. 2014, 88, 11442–11458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, Q.; Cheung, K.-F.; Kang, W.; Lung, R.W.M.; Tong, J.H.M.; To, K.F.; Sung, J.J.Y.; Yu, J. Genome-Wide Identification of Epstein-Barr Virus-Driven Promoter Methylation Profiles of Human Genes in Gastric Cancer Cells. Cancer 2013, 119, 304–312. [Google Scholar] [CrossRef]
- Sartor, M.A.; Dolinoy, D.C.; Jones, T.R.; Colacino, J.A.; Prince, M.E.P.; Carey, T.E.; Rozek, L.S. Genome-Wide Methylation and Expression Differences in HPV(+) and HPV(-) Squamous Cell Carcinoma Cell Lines Are Consistent with Divergent Mechanisms of Carcinogenesis. Epigenetics 2011, 6, 777–787. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Kostareli, E.; Holzinger, D.; Bogatyrova, O.; Hielscher, T.; Wichmann, G.; Keck, M.; Lahrmann, B.; Grabe, N.; Flechtenmacher, C.; Schmidt, C.R.; et al. HPV-Related Methylation Signature Predicts Survival in Oropharyngeal Squamous Cell Carcinomas. J. Clin. Investig. 2013, 123, 2488–2501. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, D.; Sklias, A.; Lima, S.C.; Beghelli-de la Forest Divonne, S.; Cahais, V.; Fernandez-Jimenez, N.; Cros, M.-P.; Ecsedi, S.; Cuenin, C.; Bouaoun, L.; et al. Unique DNA Methylation Signature in HPV-Positive Head and Neck Squamous Cell Carcinomas. Genome Med. 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Verhaegen, M.E.; Vo, J.N.; Tien, J.C.; Pratt, D.; Su, F.; Dhanasekaran, S.M.; Cao, X.; Mangelberger, D.; VanGoor, J.; et al. Viral Status Predicts the Patterns of Genome Methylation and Decitabine Response in Merkel Cell Carcinoma. J. Investig. Dermatol. 2021, 142, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.H.; Lee, S.; Cho, N.-Y.; Gandamihardja, T.; Long, T.I.; Weisenberger, D.J.; Campan, M.; Laird, P.W. DNA Methylation Profiles of Gastric Carcinoma Characterized by Quantitative DNA Methylation Analysis. Lab. Investig. 2008, 88, 161–170. [Google Scholar] [CrossRef]
- Chang, M.-S.; Uozaki, H.; Chong, J.-M.; Ushiku, T.; Sakuma, K.; Ishikawa, S.; Hino, R.; Barua, R.R.; Iwasaki, Y.; Arai, K.; et al. CpG Island Methylation Status in Gastric Carcinoma with and without Infection of Epstein-Barr Virus. Clin. Cancer Res. 2006, 12, 2995–3002. [Google Scholar] [CrossRef]
- Badal, V.; Chuang, L.S.H.; Tan, E.H.-H.; Badal, S.; Villa, L.L.; Wheeler, C.M.; Li, B.F.L.; Bernard, H.-U. CpG Methylation of Human Papillomavirus Type 16 DNA in Cervical Cancer Cell Lines and in Clinical Specimens: Genomic Hypomethylation Correlates with Carcinogenic Progression. J. Virol. 2003, 77, 6227–6234. [Google Scholar] [CrossRef]
- Kaur, P.; Paliwal, A.; Durantel, D.; Hainaut, P.; Scoazec, J.-Y.; Zoulim, F.; Chemin, I.; Herceg, Z. DNA Methylation of Hepatitis B Virus (HBV) Genome Associated with the Development of Hepatocellular Carcinoma and Occult HBV Infection. J. Infect. Dis. 2010, 202, 700–704. [Google Scholar] [CrossRef]
- Clarke, M.A.; Wentzensen, N.; Mirabello, L.; Ghosh, A.; Wacholder, S.; Harari, A.; Lorincz, A.; Schiffman, M.; Burk, R.D. Human Papillomavirus DNA Methylation as a Potential Biomarker for Cervical Cancer. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2125–2137. [Google Scholar] [CrossRef]
- Lam, W.K.J.; Jiang, P.; Chan, K.C.A.; Peng, W.; Shang, H.; Heung, M.M.S.; Cheng, S.H.; Zhang, H.; Tse, O.Y.O.; Raghupathy, R.; et al. Methylation Analysis of Plasma DNA Informs Etiologies of Epstein-Barr Virus-Associated Diseases. Nat. Commun. 2019, 10, 3256. [Google Scholar] [CrossRef]
- Shamay, M.; Kanakry, J.A.; Low, J.S.W.; Horowitz, N.A.; Journo, G.; Ahuja, A.; Eran, Y.; Barzilai, E.; Dann, E.J.; Stone, J.; et al. CpG Methylation in Cell-Free Epstein-Barr Virus DNA in Patients with EBV-Hodgkin Lymphoma. Blood Adv. 2020, 4, 1624–1627. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Wu, H.-C.; Shen, J.; Ahsan, H.; Tsai, W.Y.; Yang, H.-I.; Wang, L.-Y.; Chen, S.-Y.; Chen, C.-J.; Santella, R.M. Predicting Hepatocellular Carcinoma by Detection of Aberrant Promoter Methylation in Serum DNA. Clin. Cancer Res. 2007, 13, 2378–2384. [Google Scholar] [CrossRef]
- Rostami, A.; Bratman, S.V.; Han, K. Liquid Biopsy Goes Viral: Next-Generation Sequencing to Enhance HPV Detection. Clin. Cancer Res. 2021, 27, 5158–5160. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical Utility of Circulating Non-Coding RNAs—An Update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Ferrajoli, A.; Ivan, C.; Ciccone, M.; Shimizu, M.; Kita, Y.; Ohtsuka, M.; D’Abundo, L.; Qiang, J.; Lerner, S.; Nouraee, N.; et al. Epstein–Barr Virus MicroRNAs Are Expressed in Patients with Chronic Lymphocytic Leukemia and Correlate with Overall Survival. EBioMedicine 2015, 2, 572–582. [Google Scholar] [CrossRef]
- Zhang, G.; Zong, J.; Lin, S.; Verhoeven, R.J.A.; Tong, S.; Chen, Y.; Ji, M.; Cheng, W.; Tsao, S.-W.; Lung, M.; et al. Circulating Epstein-Barr Virus microRNAs miR-BART7 and miR-BART13 as Biomarkers for Nasopharyngeal Carcinoma Diagnosis and Treatment. Int. J. Cancer 2015, 136, E301–E312. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.S.; Moore, D.W.; Broker, T.R.; Chow, L.T. Vorinostat, a Pan-HDAC Inhibitor, Abrogates Productive HPV-18 DNA Amplification. Proc. Natl. Acad. Sci. USA 2018, 115, E11138–E11147. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Perrine, S.P.; Williams, R.M.; Faller, D.V. Histone Deacetylase Inhibitors Are Potent Inducers of Gene Expression in Latent EBV and Sensitize Lymphoma Cells to Nucleoside Antiviral Agents. Blood 2012, 119, 1008–1017. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Toffanin, S.; Cabellos, L.; Alsinet, C.; Hoshida, Y.; Villanueva, A.; Minguez, B.; Tsai, H.-W.; Ward, S.C.; Thung, S.; et al. Combination Therapy for Hepatocellular Carcinoma: Additive Preclinical Efficacy of the HDAC Inhibitor Panobinostat with Sorafenib. J. Hepatol. 2012, 56, 1343–1350. [Google Scholar] [CrossRef]
- Nishioka, C.; Ikezoe, T.; Yang, J.; Komatsu, N.; Bandobashi, K.; Taniguchi, A.; Kuwayama, Y.; Togitani, K.; Koeffler, H.P.; Taguchi, H. Histone Deacetylase Inhibitors Induce Growth Arrest and Apoptosis of HTLV-1-Infected T-Cells via Blockade of Signaling by Nuclear Factor kappaB. Leuk. Res. 2008, 32, 287–296. [Google Scholar] [CrossRef]
- Shin, H.J.; DeCotiis, J.; Giron, M.; Palmeri, D.; Lukac, D.M. Histone Deacetylase Classes I and II Regulate Kaposi’s Sarcoma-Associated Herpesvirus Reactivation. J. Virol. 2014, 88, 1281–1292. [Google Scholar] [CrossRef]
- Bhatt, S.; Ashlock, B.M.; Toomey, N.L.; Diaz, L.A.; Mesri, E.A.; Lossos, I.S.; Ramos, J.C. Efficacious proteasome/HDAC Inhibitor Combination Therapy for Primary Effusion Lymphoma. J. Clin. Investig. 2013, 123, 2616–2628. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Taura, K.; Kodama, Y.; Schnabl, B.; Brenner, D.A. Hepatitis C Virus-Induced Oxidative Stress Suppresses Hepcidin Expression through Increased Histone Deacetylase Activity. Hepatology 2008, 48, 1420–1429. [Google Scholar] [CrossRef]
- Lindsay, C.D.; Kostiuk, M.A.; Harris, J.; O’Connell, D.A.; Seikaly, H.; Biron, V.L. Efficacy of EZH2 Inhibitory Drugs in Human Papillomavirus-Positive and Human Papillomavirus-Negative Oropharyngeal Squamous Cell Carcinomas. Clin. Epigenet. 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- La Thangue, N.B.; Kerr, D.J. Predictive Biomarkers: A Paradigm Shift towards Personalized Cancer Medicine. Nat. Rev. Clin. Oncol. 2011, 8, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The Changing Therapeutic Landscape of Head and Neck Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef] [PubMed]
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-Cancer Analysis of Whole Genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef]

| Oncogenic Virus | Oncoprotein | Mechanism | Impact on Host Epigenome | Impact on Cancer Pathology | Refs. |
|---|---|---|---|---|---|
| EBV |
|
|
|
| [43,67] |
|
|
|
| [68,69,70] | |
| HPVs (high-risk) |
|
|
|
| [71] |
|
|
|
| [72] | |
|
|
| [54] | ||
|
|
|
| [53,73,74] | |
| HBV |
|
|
|
| [75,76] |
|
|
| [77] | ||
| HCV |
|
|
|
| [78] |
| HTLV-1 |
|
|
|
| [79] |
|
|
| [80] | ||
|
|
|
| [81] | |
| KSHV |
|
|
|
| [82] |
|
|
|
| [83] | |
|
|
|
| [51] | |
| MCPyV |
|
|
|
| [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLennan, S.A.; Marra, M.A. Oncogenic Viruses and the Epigenome: How Viruses Hijack Epigenetic Mechanisms to Drive Cancer. Int. J. Mol. Sci. 2023, 24, 9543. https://doi.org/10.3390/ijms24119543
MacLennan SA, Marra MA. Oncogenic Viruses and the Epigenome: How Viruses Hijack Epigenetic Mechanisms to Drive Cancer. International Journal of Molecular Sciences. 2023; 24(11):9543. https://doi.org/10.3390/ijms24119543
Chicago/Turabian StyleMacLennan, Signe A., and Marco A. Marra. 2023. "Oncogenic Viruses and the Epigenome: How Viruses Hijack Epigenetic Mechanisms to Drive Cancer" International Journal of Molecular Sciences 24, no. 11: 9543. https://doi.org/10.3390/ijms24119543
APA StyleMacLennan, S. A., & Marra, M. A. (2023). Oncogenic Viruses and the Epigenome: How Viruses Hijack Epigenetic Mechanisms to Drive Cancer. International Journal of Molecular Sciences, 24(11), 9543. https://doi.org/10.3390/ijms24119543





