Spermatocytic Tumor: A Review
Abstract
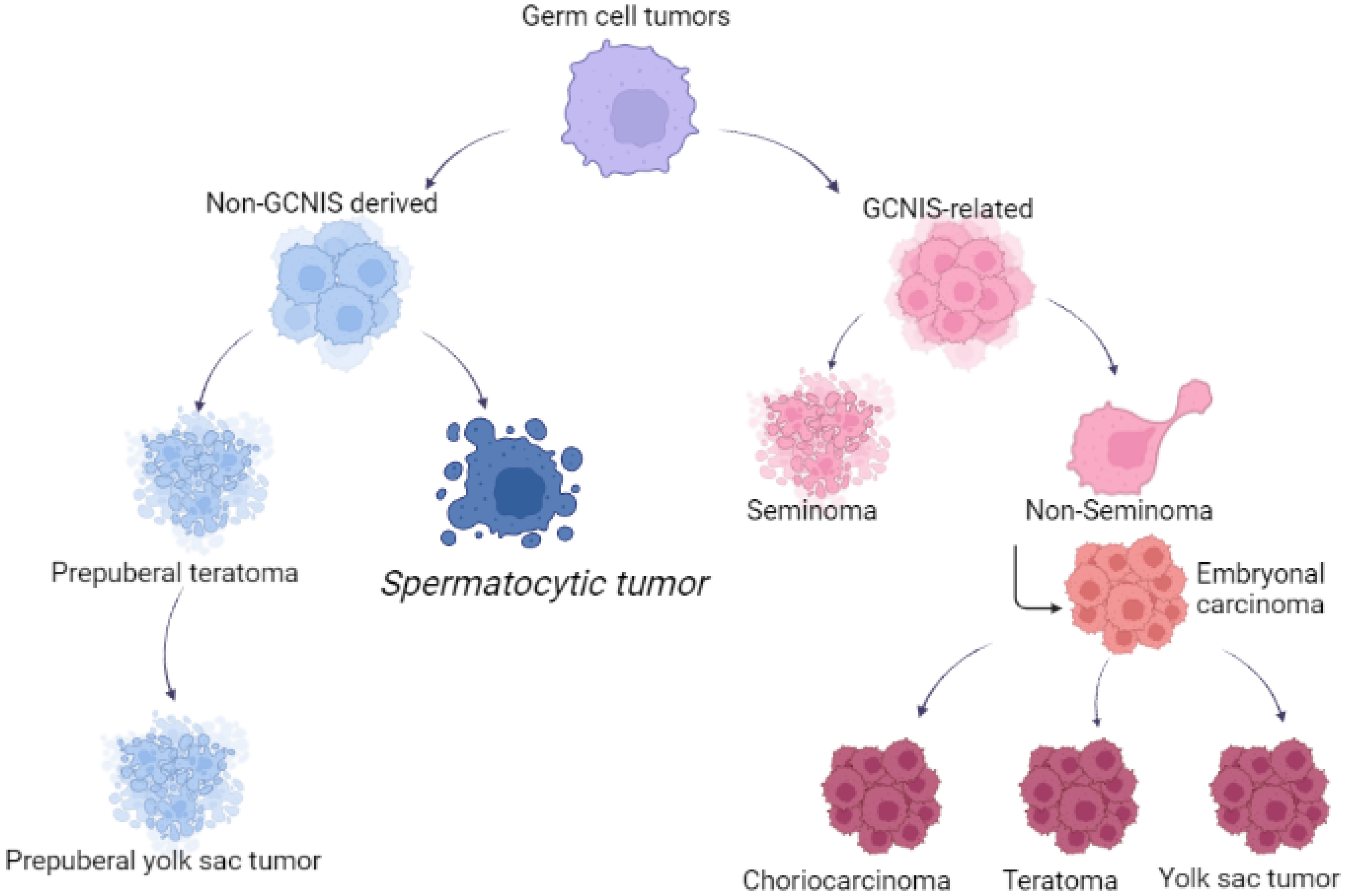
1. Introduction
2. Epidemiology
3. Pathophysiology
4. Histopathology
5. Clinical Features
6. Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moch, H.; Amin, M.B.; Berney, D.M.; Comperat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of the tumors of the urinary system and male genital organs part A: Renal, penile and testicular tumors. Eur. Urol. 2022, 82, 458. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Frazier, A.L.; Shaikh, F. Germ cell tumors in adolescent and young adults. J. Oncol. Pract. 2019, 15, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Kullis, T.; Laversanne, M.; Gurney, J.; Sarfati, D.; McGlynn, K.; Bray, F. Global patterns in testicular cancer incidence and mortality in 2020. Int. J. Cancer 2022, 151, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary and male genital organs, part A: Renal, penile and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Talerman, A. Spermatocytic seminoma: Clinicopathological study of 22 cases. Cancer 1980, 45, 2169–2176. [Google Scholar] [CrossRef]
- Burke, A.; Mostofi, F.K. Spermatocytic seminoma, a clinicopathologic study of 79 cases. J. Urol. Pathol. 1993, 1, 21–32. [Google Scholar]
- Rabade, K.; Panjwani, P.K.; Menon, S.; Prakash, G.; Pal, M.; Bakshi, G.; Desai, S. Spermatocytic tumor of testis: A case series of 26 cases elucidating unusual patterns with diagnostic and treatment dilemmas. J. Cancer Res. Ther. 2022, 18, S449–S454. [Google Scholar]
- Wetherell, D.; Lawrentschuk, N.; Gyomber, D. Spermatocytic seminoma with sarcoma: An indication for adjuvant chemotherapy in localized disease. Korean J. Urol. 2013, 54, 884–887. [Google Scholar] [CrossRef]
- Carrière, P.; Baade, P.; Fritschi, L. Population based incidence and age distribution of spermatocytic seminoma. J. Urol. 2007, 178, 125–128. [Google Scholar] [CrossRef]
- Secondino, S.; Rosti, G.; Tralongo, A.C.; Nolè, F.; Alaimo, D.; Carminati, O.; Naspro, R.L.J.; Pedrazzoli, P. Testicular tumors in the “elderly” population. Front. Oncol. 2022, 12, 972151. [Google Scholar] [CrossRef]
- Hu, R.; Ulbright, T.M.; Young, R.H. Spermatocytic seminoma: A report of 85 cases emphasizing its morphologic spectrum including some aspects not widely known. Am. J. Surg. Pathol. 2019, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.M.; Patel, H.D.; Koehne, E.L.; Doshi, C.; Belshoff, A.; Seffren, C.M.; Baker, M.; Gorbonos, A.; Gupta, G. Contemporary trends in presentation and management of spermatocytic seminoma. Urology 2020, 146, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ghazarian, A.A.; Rusner, C.; Trabert, B.; Braunlin, M.; McGlynn, K.A.; Stang, A. Testicular cancer among US men aged 50 years and older. Cancer Epidemiol. 2018, 55, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Raiss, G.G.; Andaloussi, M.M.B.; Raissouni, S.S.; Mrabti, H.H.; Errihani, H.H. Spermatocytic seminoma at the National Institute of Oncology in Morocco. BMC Res. Notes 2011, 4, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.K. The puzzling incidence of testicular cancer in New Zealand: What can we learn? Andrology 2019, 7, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Masson, P. A study of seminomas. Rev. Can. Biol. 1946, 5, 361–387. [Google Scholar]
- Rosenberg, C.; Mostert, M.C.; Schut, T.B.; van de Pol, M.; van Echten, J.; de Jong, B.; Raap, A.K.; Tanke, H.; Oosterhuis, J.W.; Looijenga, L.H. Chromosomal constitution of human spermatocytic seminomas: Comparative genomic hybridization supported by conventional and interphase cytogenetics. Genes Chromosomes Cancer 1998, 23, 286–291. [Google Scholar] [CrossRef]
- Freitag, C.E.; Sukov, W.R.; Bryce, A.H.; Berg, J.V.; Vanderbilt, C.M.; Shen, W.; Smadbeck, J.B.; Greipp, P.T.; Ketterling, R.P.; Jenkins, R.B.; et al. Assessment of isochromosome 12p and 12q abnormalities in germ cell tumors using fluorescence in situ hybridization, single-nucleotide polymorphism array, and next-generation sequencing-mate-pair sequencing. Hum. Pathol. 2021, 112, 20–24. [Google Scholar] [CrossRef]
- Looijenga, L.H.; Hersmus, R.; Gillis, A.J.M.; Pfundt, R.; Stoop, H.J.; van Gurp, R.J.H.L.M.; Veltman, J.; Baverloo, H.B.; van Drunen, E.; van Kessel, A.G.; et al. Genomic and expression profiling of human spermatocytic seminomas: Primary spermatocyte as tumorigenic precursor and DMRT1 as candidate chromosome 9 gene. Cancer Res. 2006, 66, 290–302. [Google Scholar] [CrossRef]
- Dundr, P.; Pesl, M.; Povýsil, C.; Prokopová, P.; Pavlík, I.; Soukup, V.; Dvoracek, J. Anaplastic variant of spermatocytic seminoma. Pathol. Res. Pract. 2007, 203, 621–624. [Google Scholar] [CrossRef]
- Chennoufi, M.; Boukhannous, I.; Mokhtari, M.; El Moudane, A.; Barki, A. Spermatocytic seminoma of testis associated with undifferentiated sarcoma revealed in metastatic disease: A review and case report analysis. Urol. Case Rep. 2021, 38, 101732. [Google Scholar] [CrossRef] [PubMed]
- Ulbright, T.M.; Young, R.H. Seminoma with tubular, microcystic, and related patterns: A study of 28 cases of unusual morphologic variants that often cause confusion with yolk sac tumor. Am. J. Surg. Pathol. 2005, 29, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.S.; Ulbright, T.M.; Young, R.H.; Idrees, M.T. Testicular embryonal carcinoma: A morphologic study of 180 cases highlighting unusual and unemphasized aspects. Am. J. Surg. Pathol. 2014, 38, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.A.; Harris, N.L.; Young, R.H.; Coen, J.; Zietman, A.; Scully, R.E. Malignant lymphoma of the testis, epididymis, and spermatic cord. A clinicopathologic study of 69 cases with immunophenotypic analysis. Am. J. Surg. Pathol. 1994, 18, 376–390. [Google Scholar] [CrossRef]
- Cheah, C.Y.; Wirth, A.; Seymours, J.F. Primary testicular lymphoma. Blood 2014, 123, 486–492. [Google Scholar] [CrossRef]
- Al-Obaidy, K.; Idrees, M. Testicular tumors: A contemporary update on morphologic, immunohistochemical and molecular features. Adv. Anat. Pathol. 2021, 28, 258–275. [Google Scholar] [CrossRef]
- Giannoulatou, E.; Maher, G.J.; Ding, Z.; Gillis, A.d.J.; Dorssers, L.C.J.; Hoischen, A.; Rajpert-De Meyts, E.; WGS500 Consortium; McVean, G.; Wilkie, A.O.M.; et al. Whole-genome sequencing of spermatocytic tumors provides insights into the mutational processes operating in the male germline. PLoS ONE 2017, 12, e0178169. [Google Scholar] [CrossRef]
- Narins, H.; Chevli, K.; Gilbert, R.; Duff, M.; Toenniessen, A.; Hu, Y. Bilateral spermatocytic seminoma: A case report. Res. Rep. Urol. 2014, 6, 63–65. [Google Scholar]
- Bergner, D.M.; Duck, G.B.; Rao, M. Bilateral sequential spermatocytic seminoma. J. Urol. 1980, 124, 565. [Google Scholar] [CrossRef]
- Aggarwal, N.; Parwani, A.V. Spermatocytic seminoma. Arch. Pathol. Lab. Med. 2009, 133, 1985–1988. [Google Scholar] [CrossRef]
- Grogg, J.B.; Schneider, K.; Bode, P.K.; Wettstein, M.S.; Kranzbuhler, B.; Eberli, D.; Sulser, T.; Beyer, J.; Hermanns, T.; Fankhauser, C.D. A systematic review of treatment outcomes in localized and metastatic spermatocytic tumors of the testis. J. Cancer Res. Clin. Oncol. 2019, 145, 3037–3045. [Google Scholar] [CrossRef]
- Eble, J.N. Spermatocytic seminoma. Hum. Pathol. 1994, 25, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.J.; Gildersleve, J.; Jameson, C.F.; Horwich, A. Spermatocytic seminoma in a maldescended testis. Br. J. Urol. 1993, 72, 657–659. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; Higgins, C.D.; Pike, M.C. Risk of testicular cancer in cohort of boys with cryptorchidism. BMJ 1997, 314, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, P.; Rosti, G.; Soresini, E.; Ciani, S.; Secondino, S. Serum tumour markers in germ cell tumours: From diagnosis to cure. Crit. Rev. Oncol. Hematol. 2021, 159, 103224. [Google Scholar] [CrossRef] [PubMed]
- Gru, A.A.; Williams, E.S.; Cao, D. Mixed gonadal germ cell tumor composed of a spermatocytic tumor-like component and germinoma arising in gonadoblastoma in a phenotypic woman with a 46, XX peripheral karyotype: Report of the first case. Am. J. Surg. Pathol. 2017, 41, 1290–1297. [Google Scholar] [CrossRef]
- Patrikidou, A.; Cazzaniga, W.; Berney, D.; Boormans, J.; de Angst, I.; Di Nardo, D.; Fankhauser, C.; Fischer, S.; Gravina, C.; Gremmels, H.; et al. Europenan association of urology guidelines on testicular cancer: 2023 update. Eur. Urol. 2023. S0302-2838(23)02732-X. [Google Scholar] [CrossRef]
- Chung, P.; Daugaard, G.; Tyldesley, S.; Atenafu, E.G.; Panzarella, T.; Kollmannsberger, C.; Warde, P. Evaluation of a prognostic model for risk of relapse in stage I seminoma surveillance. Cancer Med. 2015, 4, 155–160. [Google Scholar] [CrossRef]
- Patel, H.D.; Srivastava, A.; Alam, R.; Joice, G.A.; Schwen, Z.R.; Semerjian, A.; Allaf, M.E.; Pierorazio, P.M. Radiotherapy for stage I and II testicular seminomas: Secondary malignancies and survival. Urol. Oncol. 2017, 35, 606.e1–606.e7. [Google Scholar] [CrossRef]
- Pendlebury, S.; Horwich, A.; Dearnaley, D.P.; Nicholls, J.; Fisher, C. Spermatocytic seminoma: A clinicopathological review of ten patients. Clin. Oncol. 1996, 8, 316–318. [Google Scholar] [CrossRef]
- Jeong, Y.; Cheon, J.; Kim, T.O. Conventional cisplatin-based combination chemotherapy is effective in the treatment of metastatic spermatocytic seminoma with extensive rhabdomyosarcomatous transformation. Cancer Res. Treat. 2015, 47, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Grogg, J.B.; Rothermundt, C.; Clarke, N.W. Treatment and follow up of rare testis tumors. J. Cancer Res. Clin. Oncol. 2022, 148, 667–671. [Google Scholar] [CrossRef] [PubMed]
| Spermatocytic Tumor | Seminoma | |
|---|---|---|
| CD117/ckit | + | + |
| D2-40/podoplanin | − | + |
| OCT3/4 | − | + |
| PLAP | − | + |
| FGFR3 | + | − |
| HRAS | + | − |
| DMRT1 | + | − |
| Spermatocytic Tumor | Seminoma | |
|---|---|---|
| Median Age (years) | 54 | 35 |
| Frequency of all GCT (%) | <1% | 60% |
| Presence of GCNIS | No | Yes |
| Extragonadal site (% of all GCT) | No | 2–5% |
| Expression of isochromosome 12p | Absent | Present |
| Sarcomatous component | 5–8% | No |
| Bilaterality (synchronous and metachronous) | 8–10% | 5% |
| Stromal inflammatory reaction | Absent | Present |
| Association to cryptorchidism | No | Yes |
| Overall Survival at 5-y for Clinical Stage I (%) | 95% | 99% |
| Overall Survival at 5-y for advanced disease (%) | Poor | 72–86% * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secondino, S.; Viglio, A.; Neri, G.; Galli, G.; Faverio, C.; Mascaro, F.; Naspro, R.; Rosti, G.; Pedrazzoli, P. Spermatocytic Tumor: A Review. Int. J. Mol. Sci. 2023, 24, 9529. https://doi.org/10.3390/ijms24119529
Secondino S, Viglio A, Neri G, Galli G, Faverio C, Mascaro F, Naspro R, Rosti G, Pedrazzoli P. Spermatocytic Tumor: A Review. International Journal of Molecular Sciences. 2023; 24(11):9529. https://doi.org/10.3390/ijms24119529
Chicago/Turabian StyleSecondino, Simona, Alessandra Viglio, Giuseppe Neri, Giulia Galli, Carlotta Faverio, Federica Mascaro, Richard Naspro, Giovanni Rosti, and Paolo Pedrazzoli. 2023. "Spermatocytic Tumor: A Review" International Journal of Molecular Sciences 24, no. 11: 9529. https://doi.org/10.3390/ijms24119529
APA StyleSecondino, S., Viglio, A., Neri, G., Galli, G., Faverio, C., Mascaro, F., Naspro, R., Rosti, G., & Pedrazzoli, P. (2023). Spermatocytic Tumor: A Review. International Journal of Molecular Sciences, 24(11), 9529. https://doi.org/10.3390/ijms24119529








