Targeting IGF2BP3 in Cancer
Abstract
1. Introduction
2. Physiological Roles of IGF2BP3
3. IGF2BP3 in Different Human Cancers
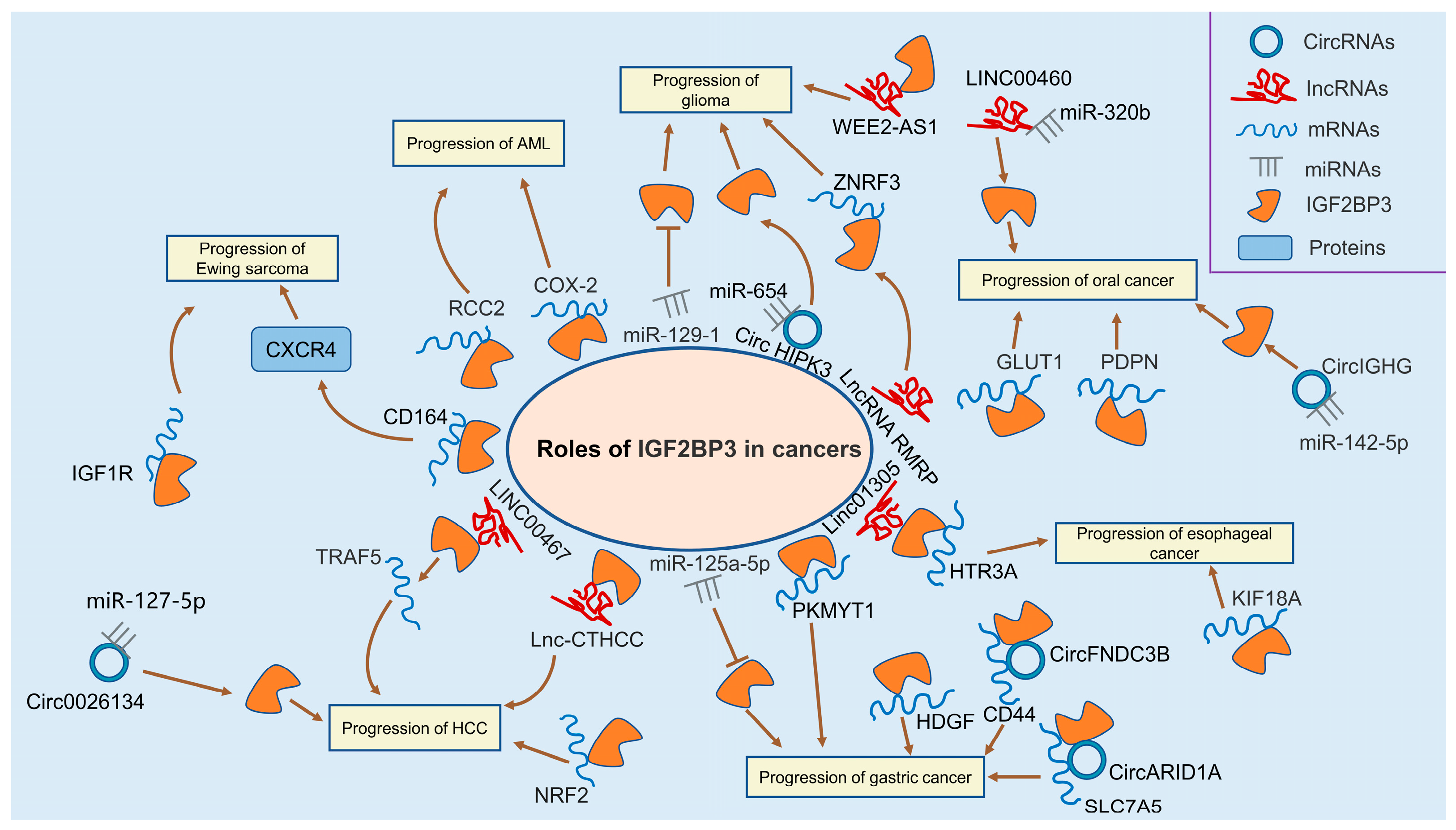
3.1. Leukemia
3.2. Glioma
3.3. Meningioma
3.4. Ewing Sarcoma
3.5. Hepatocellular Carcinoma
3.6. Gastric Cancer
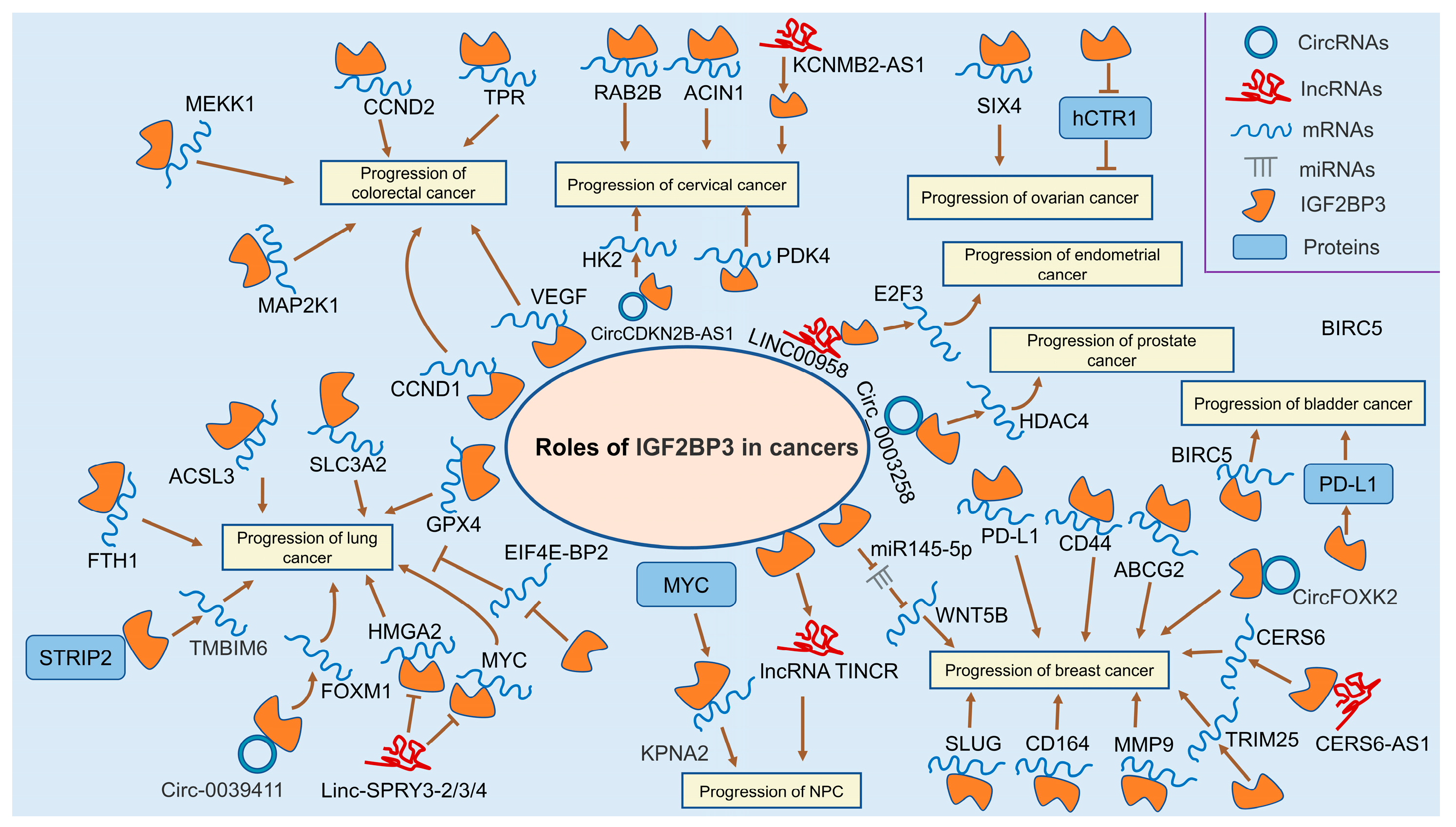
3.7. Colorectal Cancer
3.8. Oral Cancer
3.9. Esophageal Cancer
3.10. Lung Cancer
3.11. Nasopharyngeal Cancer
3.12. Breast Cancer
3.13. Cervical Cancer
3.14. Ovarian Cancer
3.15. Endometrial Cancer
3.16. Prostate Cancer
3.17. Kidney Cancer
3.18. Bladder Cancer
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Mir, C.; Garcia-Mayea, Y.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. RNA-binding proteins: Underestimated contributors in tumorigenesis. Semin. Cancer Biol. 2022, 86, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Z.; Lei, Z.; Zhang, H.T. RNA-binding proteins and cancer metastasis. Semin. Cancer Biol. 2022, 86, 748–768. [Google Scholar] [CrossRef] [PubMed]
- Geula, S.; Moshitch-Moshkovitz, S.; Dominissini, D.; Mansour, A.A.; Kol, N.; Salmon-Divon, M.; Hershkovitz, V.; Peer, E.; Mor, N.; Manor, Y.S.; et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 2015, 347, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Li, Z.; Weng, H.; Su, R.; Weng, X.; Zuo, Z.; Li, C.; Huang, H.; Nachtergaele, S.; Dong, L.; Hu, C.; et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N(6)-Methyladenosine RNA Demethylase. Cancer Cell 2017, 31, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, B.; Kim, V.N. Emerging roles of RNA modification: M(6)A and U-tail. Cell 2014, 158, 980–987. [Google Scholar] [CrossRef]
- Warda, A.S.; Kretschmer, J.; Hackert, P.; Lenz, C.; Urlaub, H.; Höbartner, C.; Sloan, K.E.; Bohnsack, M.T. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017, 18, 2004–2014. [Google Scholar] [CrossRef]
- Liang, Y.; Zhan, G.; Chang, K.J.; Yang, Y.P.; Wang, L.; Lin, J.; Hsu, C.H. The roles of m6A RNA modifiers in human cancer. J. Chin. Med. Assoc. JCMA 2020, 83, 221–226. [Google Scholar] [CrossRef]
- Lence, T.; Paolantoni, C.; Worpenberg, L.; Roignant, J.Y. Mechanistic insights into m(6)A RNA enzymes. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 222–229. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.L.; Song, S.H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dai, Q.; Zheng, G.; He, C.; Parisien, M.; Pan, T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015, 518, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- Müeller-Pillasch, F.; Lacher, U.; Wallrapp, C.; Micha, A.; Zimmerhackl, F.; Hameister, H.; Varga, G.; Friess, H.; Büchler, M.; Beger, H.G.; et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997, 14, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.L.; Wächter, K.; Mühleck, B.; Pazaitis, N.; Köhn, M.; Lederer, M.; Hüttelmaier, S. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of cancer progression? Cell. Mol. Life Sci. 2013, 70, 2657–2675. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Cao, D.; Du, B.B.; Chen, C.W.; Liu, D. The role of Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) as m(6)A readers in cancer. Int. J. Biol. Sci. 2022, 18, 2744–2758. [Google Scholar] [CrossRef]
- Hansen, T.V.; Hammer, N.A.; Nielsen, J.; Madsen, M.; Dalbaeck, C.; Wewer, U.M.; Christiansen, J.; Nielsen, F.C. Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol. Cell. Biol. 2004, 24, 4448–4464. [Google Scholar] [CrossRef]
- Nielsen, F.C.; Nielsen, J.; Kristensen, M.A.; Koch, G.; Christiansen, J. Cytoplasmic trafficking of IGF-II mRNA-binding protein by conserved KH domains. J. Cell Sci. 2002, 115, 2087–2097. [Google Scholar] [CrossRef]
- Mueller-Pillasch, F.; Pohl, B.; Wilda, M.; Lacher, U.; Beil, M.; Wallrapp, C.; Hameister, H.; Knöchel, W.; Adler, G.; Gress, T.M. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech. Dev. 1999, 88, 95–99. [Google Scholar] [CrossRef]
- Mori, H.; Sakakibara, S.; Imai, T.; Nakamura, Y.; Iijima, T.; Suzuki, A.; Yuasa, Y.; Takeda, M.; Okano, H. Expression of mouse igf2 mRNA-binding protein 3 and its implications for the developing central nervous system. J. Neurosci. Res. 2001, 64, 132–143. [Google Scholar] [CrossRef]
- Yaniv, K.; Fainsod, A.; Kalcheim, C.; Yisraeli, J.K. The RNA-binding protein Vg1 RBP is required for cell migration during early neural development. Development 2003, 130, 5649–5661. [Google Scholar] [CrossRef]
- Ren, F.; Lin, Q.; Gong, G.; Du, X.; Dan, H.; Qin, W.; Miao, R.; Xiong, Y.; Xiao, R.; Li, X.; et al. Igf2bp3 maintains maternal RNA stability and ensures early embryo development in zebrafish. Commun. Biol. 2020, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Vong, Y.H.; Sivashanmugam, L.; Leech, R.; Zaucker, A.; Jones, A.; Sampath, K. The RNA-binding protein Igf2bp3 is critical for embryonic and germline development in zebrafish. PLoS Genet. 2021, 17, e1009667. [Google Scholar] [CrossRef] [PubMed]
- Elagib, K.E.; Lu, C.H.; Mosoyan, G.; Khalil, S.; Zasadzińska, E.; Foltz, D.R.; Balogh, P.; Gru, A.A.; Fuchs, D.A.; Rimsza, L.M.; et al. Neonatal expression of RNA-binding protein IGF2BP3 regulates the human fetal-adult megakaryocyte transition. J. Clin. Investig. 2017, 127, 2365–2377. [Google Scholar] [CrossRef] [PubMed]
- Tangprasittipap, A.; Kaewprommal, P.; Sripichai, O.; Sathirapongsasuti, N.; Satirapod, C.; Shaw, P.J.; Piriyapongsa, J.; Hongeng, S. Comparison of gene expression profiles between human erythroid cells derived from fetal liver and adult peripheral blood. PeerJ 2018, 6, e5527. [Google Scholar] [CrossRef] [PubMed]
- Hammer, N.A.; Hansen, T.; Byskov, A.G.; Rajpert-De Meyts, E.; Grøndahl, M.L.; Bredkjaer, H.E.; Wewer, U.M.; Christiansen, J.; Nielsen, F.C. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction 2005, 130, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Pelcovits, A.; Niroula, R. Acute Myeloid Leukemia: A Review. Rhode Isl. Med. J. 2020, 103, 38–40. [Google Scholar]
- Zhang, N.; Shen, Y.; Li, H.; Chen, Y.; Zhang, P.; Lou, S.; Deng, J. The m6A reader IGF2BP3 promotes acute myeloid leukemia progression by enhancing RCC2 stability. Exp. Mol. Med. 2022, 54, 194–205. [Google Scholar] [CrossRef]
- Ko, C.Y.; Wang, W.L.; Li, C.F.; Jeng, Y.M.; Chu, Y.Y.; Wang, H.Y.; Tseng, J.T.; Wang, J.M. IL-18-induced interaction between IMP3 and HuR contributes to COX-2 mRNA stabilization in acute myeloid leukemia. J. Leukoc. Biol. 2016, 99, 131–141. [Google Scholar] [CrossRef]
- Liu, H. Emerging agents and regimens for AML. J. Hematol. Oncol. 2021, 14, 49. [Google Scholar] [CrossRef]
- Gou, J.; Li, H.; Bi, J.; Pang, X.; Li, X.; Wang, Y. Transfer of IGF2BP3 Through Ara-C-Induced Apoptotic Bodies Promotes Survival of Recipient Cells. Front. Oncol. 2022, 12, 801226. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Philipp, J.; Bassi, J.S.; Nibber, N.; Draper, J.M.; Lin, T.L.; Palanichamy, J.K.; Jaiswal, A.K.; Silva, O.; Paing, M.; et al. The RNA-binding protein IGF2BP3 is critical for MLL-AF4-mediated leukemogenesis. Leukemia 2022, 36, 68–79. [Google Scholar] [CrossRef]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xu, J.; Cui, Z.; Wu, S.; Xie, T.; Zhang, X. Multi-omics analysis of N6-methyladenosine reader IGF2BP3 as a promising biomarker in pan-cancer. Front. Immunol. 2023, 14, 1071675. [Google Scholar] [CrossRef] [PubMed]
- Kouhkan, F.; Mobarra, N.; Soufi-Zomorrod, M.; Keramati, F.; Hosseini Rad, S.M.; Fathi-Roudsari, M.; Tavakoli, R.; Hajarizadeh, A.; Ziaei, S.; Lahmi, R.; et al. MicroRNA-129-1 acts as tumour suppressor and induces cell cycle arrest of GBM cancer cells through targeting IGF2BP3 and MAPK1. J. Med. Genet. 2016, 53, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Huang, Y.; Zhu, P.; Zou, Y.; Shao, T.; Wang, O. CircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem. Biophys. Res. Commun. 2018, 503, 1570–1574. [Google Scholar] [CrossRef]
- Nomura, M.; Saito, K.; Aihara, K.; Nagae, G.; Yamamoto, S.; Tatsuno, K.; Ueda, H.; Fukuda, S.; Umeda, T.; Tanaka, S.; et al. DNA demethylation is associated with malignant progression of lower-grade gliomas. Sci. Rep. 2019, 9, 1903. [Google Scholar] [CrossRef]
- Chua, J.; Nafziger, E.; Leung, D. Evidence-Based Practice: Temozolomide Beyond Glioblastoma. Curr. Oncol. Rep. 2019, 21, 30. [Google Scholar] [CrossRef]
- Liu, T.; Hu, J.; Han, B.; Tan, S.; Jia, W.; Xin, Y. A positive feedback loop of lncRNA-RMRP/ZNRF3 axis and Wnt/β-catenin signaling regulates the progression and temozolomide resistance in glioma. Cell Death Dis. 2021, 12, 952. [Google Scholar] [CrossRef]
- Koschmann, C.; Zamler, D.; MacKay, A.; Robinson, D.; Wu, Y.M.; Doherty, R.; Marini, B.; Tran, D.; Garton, H.; Muraszko, K.; et al. Characterizing and targeting PDGFRA alterations in pediatric high-grade glioma. Oncotarget 2016, 7, 65696–65706. [Google Scholar] [CrossRef]
- Greish, K.; Jasim, A.; Parayath, N.; Abdelghany, S.; Alkhateeb, A.; Taurin, S.; Nehoff, H. Micellar formulations of Crizotinib and Dasatinib in the management of glioblastoma multiforme. J. Drug Target. 2018, 26, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, R.; Qiu, W.; Pan, Z.; Zhao, S.; Qi, Y.; Qiu, J.; Zhang, S.; Guo, Q.; Fan, Y.; et al. The N(6)-methyladenosine-mediated lncRNA WEE2-AS1 promotes glioblastoma progression by stabilizing RPN2. Theranostics 2022, 12, 6363–6379. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Daubon, T.; Hemadou, A.; Romero Garmendia, I.; Saleh, M. Glioblastoma Immune Landscape and the Potential of New Immunotherapies. Front. Immunol. 2020, 11, 585616. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, R.; Li, B.; Qi, Y.; Qiu, W.; Guo, Q.; Zhang, S.; Zhao, S.; Xu, H.; Li, M.; et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol. Cancer 2022, 21, 16. [Google Scholar] [CrossRef]
- Johnson, R.M.; Phillips, H.S.; Bais, C.; Brennan, C.W.; Cloughesy, T.F.; Daemen, A.; Herrlinger, U.; Jenkins, R.B.; Lai, A.; Mancao, C.; et al. Development of a gene expression-based prognostic signature for IDH wild-type glioblastoma. Neuro Oncol. 2020, 22, 1742–1756. [Google Scholar] [CrossRef]
- Marosi, C.; Hassler, M.; Roessler, K.; Reni, M.; Sant, M.; Mazza, E.; Vecht, C. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef]
- Hao, S.; Smith, T.W.; Chu, P.G.; Liu, Q.; Ok, C.Y.; Woda, B.A.; Lu, D.; Lin, P.; Wang, S.A.; Dresser, K.; et al. The oncofetal protein IMP3: A novel molecular marker to predict aggressive meningioma. Arch. Pathol. Lab. Med. 2011, 135, 1032–1036. [Google Scholar] [CrossRef]
- Morales, E.; Olson, M.; Iglesias, F.; Dahiya, S.; Luetkens, T.; Atanackovic, D. Role of immunotherapy in Ewing sarcoma. J. Immunother. Cancer 2020, 8, e000653. [Google Scholar] [CrossRef]
- Mancarella, C.; Pasello, M.; Ventura, S.; Grilli, A.; Calzolari, L.; Toracchio, L.; Lollini, P.L.; Donati, D.M.; Picci, P.; Ferrari, S.; et al. Insulin-Like Growth Factor 2 mRNA-Binding Protein 3 is a Novel Post-Transcriptional Regulator of Ewing Sarcoma Malignancy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3704–3716. [Google Scholar] [CrossRef]
- Mancarella, C.; Caldoni, G.; Ribolsi, I.; Parra, A.; Manara, M.C.; Mercurio, A.M.; Morrione, A.; Scotlandi, K. Insulin-Like Growth Factor 2 mRNA-Binding Protein 3 Modulates Aggressiveness of Ewing Sarcoma by Regulating the CD164-CXCR4 Axis. Front. Oncol. 2020, 10, 994. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, C.; Pasello, M.; Manara, M.C.; Toracchio, L.; Sciandra, E.F.; Picci, P.; Scotlandi, K. Insulin-Like Growth Factor 2 mRNA-Binding Protein 3 Influences Sensitivity to Anti-IGF System Agents Through the Translational Regulation of IGF1R. Front. Endocrinol. 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Jeng, Y.M.; Chang, C.C.; Hu, F.C.; Chou, H.Y.; Kao, H.L.; Wang, T.H.; Hsu, H.C. RNA-binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology 2008, 48, 1118–1127. [Google Scholar] [CrossRef]
- Jiang, W.; Cheng, X.; Wang, T.; Song, X.; Zheng, Y.; Wang, L. LINC00467 promotes cell proliferation and metastasis by binding with IGF2BP3 to enhance the mRNA stability of TRAF5 in hepatocellular carcinoma. J. Gene Med. 2020, 22, e3134. [Google Scholar] [CrossRef]
- Xia, A.; Yuan, W.; Wang, Q.; Xu, J.; Gu, Y.; Zhang, L.; Chen, C.; Wang, Z.; Wu, D.; He, Q.; et al. The cancer-testis lncRNA lnc-CTHCC promotes hepatocellular carcinogenesis by binding hnRNP K and activating YAP1 transcription. Nat. Cancer 2022, 3, 203–218. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, L.; Yang, G.; Zhou, B.; Wang, J.; Qu, X.; Yan, Z.; Qian, S.; Liu, R. Hsa_circ_0026134 expression promoted TRIM25- and IGF2BP3-mediated hepatocellular carcinoma cell proliferation and invasion via sponging miR-127-5p. Biosci. Rep. 2020, 40, BSR20191418. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Robinton, D.A.; Seligson, M.T.; Wu, L.; Li, L.; Rakheja, D.; Comerford, S.A.; Ramezani, S.; Sun, X.; Parikh, M.S.; et al. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell 2014, 26, 248–261. [Google Scholar] [CrossRef]
- Shaalan, Y.M.; Handoussa, H.; Youness, R.A.; Assal, R.A.; El-Khatib, A.H.; Linscheid, M.W.; El Tayebi, H.M.; Abdelaziz, A.I. Destabilizing the interplay between miR-1275 and IGF2BPs by Tamarix articulata and quercetin in hepatocellular carcinoma. Nat. Prod. Res. 2018, 32, 2217–2220. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Ge, C.; Chen, L.; Fang, T.; Li, H.; Tian, H.; Liu, J.; Chen, T.; Jiang, G.; et al. An isocorydine derivative (d-ICD) inhibits drug resistance by downregulating IGF2BP3 expression in hepatocellular carcinoma. Oncotarget 2015, 6, 25149–25160. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yang, H.; Shao, Y.; Sun, W.; Jiang, Y.; Li, J. IGF2BP3-NRF2 axis regulates ferroptosis in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2022, 627, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, K.; Zhang, W.; Yang, K.W.; Mu, D.A.; Jiang, G.J.; Shi, R.S.; Ke, D. The m6A/m5C/m1A Regulated Gene Signature Predicts the Prognosis and Correlates With the Immune Status of Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 918140. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, B.; Lv, J.; Gao, J.; Qin, L. Systematic analyses to explore immune gene sets-based signature in hepatocellular carcinoma, in which IGF2BP3 contributes to tumor progression. Clin. Immunol. 2022, 241, 109073. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, J.; Liu, S.; Li, W. Identification of Crucial Genes Associated With Immune Cell Infiltration in Hepatocellular Carcinoma by Weighted Gene Co-expression Network Analysis. Front. Genet. 2020, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, F.; Jiao, D.; Li, Q.; Ma, H. The Aberrant Expression of MicroRNA-125a-5p/IGF2BP3 Axis in Advanced Gastric Cancer and Its Clinical Relevance. Technol. Cancer Res. Treat. 2020, 19, 1533033820917332. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gong, C.; Li, Z.; Liu, J.; Chen, Y.; Huang, Y.; Luo, Q.; Wang, S.; Hou, Y.; Yang, S.; et al. Demethylase ALKBH5 suppresses invasion of gastric cancer via PKMYT1 m6A modification. Mol. Cancer 2022, 21, 34. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, C.; Ding, Q.; Zhao, Y.; Wang, Z.; Chen, J.; Jiang, Z.; Zhang, Y.; Xu, G.; Zhang, J.; et al. METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut 2020, 69, 1193–1205. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, F.; Huang, B.; Pan, X.; Li, W.; Yu, T.; Wang, X.; Ran, L.; Qian, K.; Li, H.; et al. CircARID1A binds to IGF2BP3 in gastric cancer and promotes cancer proliferation by forming a circARID1A-IGF2BP3-SLC7A5 RNA-protein ternary complex. J. Exp. Clin. Cancer Res. 2022, 41, 251. [Google Scholar] [CrossRef]
- Yu, T.; Ran, L.; Zhao, H.; Yin, P.; Li, W.; Lin, J.; Mao, H.; Cai, D.; Ma, Q.; Pan, X.; et al. Circular RNA circ-TNPO3 suppresses metastasis of GC by acting as a protein decoy for IGF2BP3 to regulate the expression of MYC and SNAIL. Mol. Ther. Nucleic Acids 2021, 26, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Qin, H.; Li, Y.; Zhang, Y.; Zhuang, X.; Liu, L.; Lu, K.; Li, L.; Deng, X.; Liu, F.; et al. FNDC3B circular RNA promotes the migration and invasion of gastric cancer cells via the regulation of E-cadherin and CD44 expression. J. Cell. Physiol. 2019, 234, 19895–19910. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, T.; Siu, H.L.; Wong, C.C.; Dong, Y.; Wu, F.; Zhang, B.; Wu, W.K.; Cheng, A.S.; Yu, J.; et al. IGF2BP3 functions as a potential oncogene and is a crucial target of miR-34a in gastric carcinogenesis. Mol. Cancer 2017, 16, 77. [Google Scholar] [CrossRef]
- Ishii, S.; Yamashita, K.; Harada, H.; Ushiku, H.; Tanaka, T.; Nishizawa, N.; Yokoi, K.; Washio, M.; Ema, A.; Mieno, H.; et al. The H19-PEG10/IGF2BP3 axis promotes gastric cancer progression in patients with high lymph node ratios. Oncotarget 2017, 8, 74567–74581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hong, L.L.; Zheng, J.S.; Ling, Z.N.; Zhang, Z.L.; Qi, Y.N.; Zhang, X.Y.; Zhu, T.Y.; Wang, J.L.; Han, J.; et al. Comprehensive transcriptomic profiling and mutational landscape of primary gastric linitis plastica. Gastric Cancer 2022, 26, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, E.A.; Carneiro, F.P.; Magalhães, A.V.; Carneiro Mde, V.; Takano, G.H.; Vianna, L.M.; Seidler, H.B.; Castro, T.M.; Muniz-Junqueira, M.I.; Amorim, R.F.; et al. IMP3 expression in gastric cancer: Association with clinicopathological features and HER2 status. J. Cancer Res. Clin. Oncol. 2014, 140, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Wu, D.; Min, Z.; Tan, J.; Yu, B. RNA N6-methyladenosine reader IGF2BP3 regulates cell cycle and angiogenesis in colon cancer. J. Exp. Clin. Cancer Res. 2020, 39, 203. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, S.; Tan, C.; Gu, Y.; He, X.; Du, X.; Li, D.; Wei, P. RNA-binding protein IMP3 is a novel regulator of MEK1/ERK signaling pathway in the progression of colorectal Cancer through the stabilization of MEKK1 mRNA. J. Exp. Clin. Cancer Res. 2021, 40, 200. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Lin, Y.; Yang, Y.; Zhang, Z.; Wang, Q.; Zhang, H.; Jiang, K.; Ye, Y.; Wang, S.; et al. hsa_circ_0000231 Promotes colorectal cancer cell growth through upregulation of CCND2 by IGF2BP3/miR-375 dual pathway. Cancer Cell Int. 2022, 22, 27. [Google Scholar] [CrossRef]
- Li, K.; Huang, F.; Li, Y.; Li, D.; Lin, H.; Ni, R.; Zhang, Q.; Zhao, M.; Huang, S.; Zou, L.; et al. Stabilization of oncogenic transcripts by the IGF2BP3/ELAVL1 complex promotes tumorigenicity in colorectal cancer. Am. J. Cancer Res. 2020, 10, 2480–2494. [Google Scholar] [PubMed]
- Desi, N.; Tong, Q.Y.; Teh, V.; Chan, J.J.; Zhang, B.; Tabatabaeian, H.; Tan, H.Q.; Kapeli, K.; Jin, W.; Lim, C.Y.; et al. Global analysis of RNA-binding proteins identifies a positive feedback loop between LARP1 and MYC that promotes tumorigenesis. Cell. Mol. Life Sci. 2022, 79, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Yu, M.; Xu, M.; Xiao, Y.; Ma, W.; Huang, L.; Li, X.; Ye, X. Berberine inhibits proliferation and induces G0/G1 phase arrest in colorectal cancer cells by downregulating IGF2BP3. Life Sci. 2020, 260, 118413. [Google Scholar] [CrossRef]
- Fu, R.; Yang, P.; Sajid, A.; Li, Z. Avenanthramide A Induces Cellular Senescence via miR-129-3p/Pirh2/p53 Signaling Pathway To Suppress Colon Cancer Growth. J. Agric. Food Chem. 2019, 67, 4808–4816. [Google Scholar] [CrossRef]
- Liang, Q.; Du, X.; Mao, L.; Wang, G. Molecular characterization of colorectal cancer: A five-gene prognostic signature based on RNA-binding proteins. Saudi J. Gastroenterol. 2021, 27, 223–233. [Google Scholar] [CrossRef]
- Busuioc, C.; Ciocan-Cartita, C.A.; Braicu, C.; Zanoaga, O.; Raduly, L.; Trif, M.; Muresan, M.S.; Ionescu, C.; Stefan, C.; Crivii, C.; et al. Epithelial-Mesenchymal Transition Gene Signature Related to Prognostic in Colon Adenocarcinoma. J. Pers. Med. 2021, 11, 476. [Google Scholar] [CrossRef]
- Lochhead, P.; Imamura, Y.; Morikawa, T.; Kuchiba, A.; Yamauchi, M.; Liao, X.; Qian, Z.R.; Nishihara, R.; Wu, K.; Meyerhardt, J.A.; et al. Insulin-like growth factor 2 messenger RNA binding protein 3 (IGF2BP3) is a marker of unfavourable prognosis in colorectal cancer. Eur. J. Cancer 2012, 48, 3405–3413. [Google Scholar] [CrossRef]
- Bevanda Glibo, D.; Bevanda, D.; Vukojević, K.; Tomić, S. IMP3 protein is an independent prognostic factor of clinical stage II rectal cancer. Sci. Rep. 2021, 11, 10844. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.; Zou, A.; Mai, Z.; Huang, Z.; Sun, L.; Zhao, J. circIGHG-Induced Epithelial-to-Mesenchymal Transition Promotes Oral Squamous Cell Carcinoma Progression via miR-142-5p/IGF2BP3 Signaling. Cancer Res. 2021, 81, 344–355. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, J.; Liu, L.; Ma, X.; Gui, Y.; Liu, H.; Zhao, W. m(6)A-modified circFOXK2 targets GLUT1 to accelerate oral squamous cell carcinoma aerobic glycolysis. Cancer Gene Ther. 2023, 30, 163–171. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Xianglan, Z.; Park, K.K.; Chung, W.Y. Functional invadopodia formation through stabilization of the PDPN transcript by IMP-3 and cancer-stromal crosstalk for PDPN expression. Carcinogenesis 2012, 33, 2135–2146. [Google Scholar] [CrossRef]
- Tarsitano, A.; Asioli, S.; Morandi, L.; Monti, V.; Righi, A.; Morselli Labate, A.M.; Nardi, E.; Foschini, M.P.; Marchetti, C. Laminin-5 and insulin-like growth factor-II mRNA binding protein-3 (IMP3) expression in preoperative biopsy specimens from oral cancer patients: Their role in neural spread risk and survival stratification. J. Cranio Maxillo Facial Surg. 2016, 44, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Clauditz, T.S.; Wang, C.J.; Gontarewicz, A.; Blessmann, M.; Tennstedt, P.; Borgmann, K.; Tribius, S.; Sauter, G.; Dalchow, C.; Knecht, R.; et al. Expression of insulin-like growth factor II mRNA-binding protein 3 in squamous cell carcinomas of the head and neck. J. Oral Pathol. Med. 2013, 42, 125–132. [Google Scholar] [CrossRef]
- Wu, K.; Wang, X.; Yu, H.; Yu, Z.; Wang, D.; Xu, X. LINC00460 facilitated tongue squamous cell carcinoma progression via the miR-320b/IGF2BP3 axis. Oral Dis. 2022, 28, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Huang, G.W.; Chen, Q.Q.; Ma, C.C.; Xie, L.H.; Gu, J. linc01305 promotes metastasis and proliferation of esophageal squamous cell carcinoma through interacting with IGF2BP2 and IGF2BP3 to stabilize HTR3A mRNA. Int. J. Biochem. Cell Biol. 2021, 136, 106015. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.X.; Cao, X.; Du, M.Y.; Ma, C.X.; Zhu, H.M.; Peng, Y.; Hu, X.Y.; He, X.; Yin, L. KIF18A knockdown reduces proliferation, migration, invasion and enhances radiosensitivity of esophageal cancer. Biochem. Biophys. Res. Commun. 2021, 557, 192–198. [Google Scholar] [CrossRef]
- Kono, K.; Iinuma, H.; Akutsu, Y.; Tanaka, H.; Hayashi, N.; Uchikado, Y.; Noguchi, T.; Fujii, H.; Okinaka, K.; Fukushima, R.; et al. Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J. Transl. Med. 2012, 10, 141. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, Y.; Xie, Y.; Zhang, L.; Gao, M.; Li, S.; Wang, F. m6A Regulators Is Differently Expressed and Correlated With Immune Response of Esophageal Cancer. Front. Cell Dev. Biol. 2021, 9, 650023. [Google Scholar] [CrossRef]
- Guo, W.; Tan, F.; Huai, Q.; Wang, Z.; Shao, F.; Zhang, G.; Yang, Z.; Li, R.; Xue, Q.; Gao, S.; et al. Comprehensive Analysis of PD-L1 Expression, Immune Infiltrates, and m6A RNA Methylation Regulators in Esophageal Squamous Cell Carcinoma. Front. Immunol. 2021, 12, 669750. [Google Scholar] [CrossRef]
- Wakita, A.; Motoyama, S.; Sato, Y.; Nagaki, Y.; Fujita, H.; Terata, K.; Imai, K.; Maeda, E.; Minamiya, Y. IGF2BP3 Expression Correlates With Poor Prognosis in Esophageal Squamous Cell Carcinoma. J. Surg. Res. 2021, 259, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cui, J.; Wang, H.; Ma, L.; Zhang, X.; Guo, W.; Xue, X.; Wang, Y.; Qiu, S.; Tian, X.; et al. IGF2BP3 is an essential N(6)-methyladenosine biotarget for suppressing ferroptosis in lung adenocarcinoma cells. Mater. Today. Bio 2022, 17, 100503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Q.; He, Y.; Shi, Q.; Yin, C.; Xie, Y.; Yu, H.; Bao, Y.; Wang, X.; Tang, C.; et al. STRIP2 motivates non-small cell lung cancer progression by modulating the TMBIM6 stability through IGF2BP3 dependent. J. Exp. Clin. Cancer Res. 2023, 42, 19. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Huang, H.; Feng, H.; Wei, Z. Circ-MMP2 (circ-0039411) induced by FOXM1 promotes the proliferation and migration of lung adenocarcinoma cells in vitro and in vivo. Cell Death Dis. 2020, 11, 426. [Google Scholar] [CrossRef]
- Brownmiller, T.; Juric, J.A.; Ivey, A.D.; Harvey, B.M.; Westemeier, E.S.; Winters, M.T.; Stevens, A.M.; Stanley, A.N.; Hayes, K.E.; Sprowls, S.A.; et al. Y Chromosome LncRNA Are Involved in Radiation Response of Male Non-Small Cell Lung Cancer Cells. Cancer Res. 2020, 80, 4046–4057. [Google Scholar] [CrossRef]
- Mizutani, R.; Imamachi, N.; Suzuki, Y.; Yoshida, H.; Tochigi, N.; Oonishi, T.; Suzuki, Y.; Akimitsu, N. Oncofetal protein IGF2BP3 facilitates the activity of proto-oncogene protein eIF4E through the destabilization of EIF4E-BP2 mRNA. Oncogene 2016, 35, 3495–3502. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, Y.; Wang, C.; Wang, Z.; Li, Y.; Jiang, Z.; Zhao, W.; Pan, Z. Isoliquiritigenin inhibits non-small cell lung cancer progression via m(6)A/IGF2BP3-dependent TWIST1 mRNA stabilization. Phytomedicine 2022, 104, 154299. [Google Scholar] [CrossRef]
- Tomita, Y.; Harao, M.; Senju, S.; Imai, K.; Hirata, S.; Irie, A.; Inoue, M.; Hayashida, Y.; Yoshimoto, K.; Shiraishi, K.; et al. Peptides derived from human insulin-like growth factor-II mRNA binding protein 3 can induce human leukocyte antigen-A2-restricted cytotoxic T lymphocytes reactive to cancer cells. Cancer Sci. 2011, 102, 71–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Yang, Z.; Zhang, G.; Wu, P.; Luo, Y.; Zeng, Q.; Wang, L.; Xue, Q.; Zhang, Y.; et al. m(6)A regulators as predictive biomarkers for chemotherapy benefit and potential therapeutic targets for overcoming chemotherapy resistance in small-cell lung cancer. J. Hematol. Oncol. 2021, 14, 190. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Luo, Y.; Wu, P.; Zhang, G.; Zeng, Q.; Wang, L.; Yang, Z.; Xue, L.; Zheng, B.; et al. m(6)A regulator expression profile predicts the prognosis, benefit of adjuvant chemotherapy, and response to anti-PD-1 immunotherapy in patients with small-cell lung cancer. BMC Med. 2021, 19, 284. [Google Scholar] [CrossRef]
- Wang, Z.; Mu, L.; Feng, H.; Yao, J.; Wang, Q.; Yang, W.; Zhou, H.; Li, Q.; Xu, L. Expression patterns of platinum resistance-related genes in lung adenocarcinoma and related clinical value models. Front. Genet. 2022, 13, 993322. [Google Scholar] [CrossRef]
- Chang, E.T.; Ye, W.; Zeng, Y.X.; Adami, H.O. The Evolving Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Peng, Y.; Li, Y.; Sun, W.; Zhu, H.; Wu, J.; Zong, D.; Wu, L.; He, X. MYC-activated RNA N6-methyladenosine reader IGF2BP3 promotes cell proliferation and metastasis in nasopharyngeal carcinoma. Cell Death Discov. 2022, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, Z.; Peng, H.; Guo, L.; Wang, P. IGF2BP3 promotes cell metastasis and is associated with poor patient survival in nasopharyngeal carcinoma. J. Cell. Mol. Med. 2022, 26, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Hu, G.Q.; Zhang, N.; Zhu, X.D.; Yang, K.Y.; Jin, F.; Shi, M.; Chen, Y.P.; Hu, W.H.; et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N. Engl. J. Med. 2019, 381, 1124–1135. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Li, Z.X.; Guan, J.L.; Liu, X.; Li, J.Y.; Chen, Y.; Lin, L.; Kou, J.; Lv, J.W.; Zhang, L.L.; et al. Long Noncoding RNA TINCR-Mediated Regulation of Acetyl-CoA Metabolism Promotes Nasopharyngeal Carcinoma Progression and Chemoresistance. Cancer Res. 2020, 80, 5174–5188. [Google Scholar] [CrossRef]
- Samanta, S.; Sun, H.; Goel, H.L.; Pursell, B.; Chang, C.; Khan, A.; Greiner, D.L.; Cao, S.; Lim, E.; Shultz, L.D.; et al. IMP3 promotes stem-like properties in triple-negative breast cancer by regulating SLUG. Oncogene 2016, 35, 1111–1121. [Google Scholar] [CrossRef]
- Samanta, S.; Sharma, V.M.; Khan, A.; Mercurio, A.M. Regulation of IMP3 by EGFR signaling and repression by ERβ: Implications for triple-negative breast cancer. Oncogene 2012, 31, 4689–4697. [Google Scholar] [CrossRef]
- Samanta, S.; Guru, S.; Elaimy, A.L.; Amante, J.J.; Ou, J.; Yu, J.; Zhu, L.J.; Mercurio, A.M. IMP3 Stabilization of WNT5B mRNA Facilitates TAZ Activation in Breast Cancer. Cell Rep. 2018, 23, 2559–2567. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, D.; Han, C.; Zhao, Z.; Wang, X.; Jiang, T.; Li, Q.; Liu, S.; Chen, L.; Chen, Y.; et al. Blockade of miR-3614 maturation by IGF2BP3 increases TRIM25 expression and promotes breast cancer cell proliferation. EBioMedicine 2019, 41, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Huang, J.; Pan, W.; Li, X.; Zhou, T. Long noncoding RNA CERS6-AS1 functions as a malignancy promoter in breast cancer by binding to IGF2BP3 to enhance the stability of CERS6 mRNA. Cancer Med. 2020, 9, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Jiang, J.; Yang, Y.; Wang, W.; Jia, Z. CircRNA circFOXK2 facilitates oncogenesis in breast cancer via IGF2BP3/miR-370 axis. Aging 2021, 13, 18978–18992. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Ha Thi, H.T.; Hong, S. IMP2 and IMP3 cooperate to promote the metastasis of triple-negative breast cancer through destabilization of progesterone receptor. Cancer Lett. 2018, 415, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Afzali, F.; Salimi, M. Unearthing Regulatory Axes of Breast Cancer circRNAs Networks to Find Novel Targets and Fathom Pivotal Mechanisms. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.M.; Wong, E.M.; Joo, J.E.; Dugué, P.A.; Jung, C.H.; O’Callaghan, N.; Dowty, J.; Giles, G.G.; Hopper, J.L.; Southey, M.C. Genome-wide DNA methylation assessment of ‘BRCA1-like’ early-onset breast cancer: Data from the Australian Breast Cancer Family Registry. Exp. Mol. Pathol. 2018, 105, 404–410. [Google Scholar] [CrossRef]
- Nicoletto, R.E.; Ofner, C.M., 3rd. Cytotoxic mechanisms of doxorubicin at clinically relevant concentrations in breast cancer cells. Cancer Chemother. Pharmacol. 2022, 89, 285–311. [Google Scholar] [CrossRef]
- Valinezhad Sani, F.; Palizban, A.; Mosaffa, F.; Jamialahmadi, K. Glucosamine attenuates drug resistance in Mitoxantrone-resistance breast cancer cells. J. Pharm. Pharmacol. 2021, 73, 922–927. [Google Scholar] [CrossRef]
- Samanta, S.; Pursell, B.; Mercurio, A.M. IMP3 protein promotes chemoresistance in breast cancer cells by regulating breast cancer resistance protein (ABCG2) expression. J. Biol. Chem. 2013, 288, 12569–12573. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Wu, Y.; Sun, X.; Su, Q.; You, C.; Xin, H. CD44(+) fibroblasts increases breast cancer cell survival and drug resistance via IGF2BP3-CD44-IGF2 signalling. J. Cell. Mol. Med. 2017, 21, 1979–1988. [Google Scholar] [CrossRef]
- de Lint, K.; Poell, J.B.; Soueidan, H.; Jastrzebski, K.; Vidal Rodriguez, J.; Lieftink, C.; Wessels, L.F.; Beijersbergen, R.L. Sensitizing Triple-Negative Breast Cancer to PI3K Inhibition by Cotargeting IGF1R. Mol. Cancer Ther. 2016, 15, 1545–1556. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Ao, X.; Chen, Q.; Yu, Y.; Ao, L.; Xing, W.; Guo, W.; Wu, X.; Pu, C.; Hu, X.; et al. METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N(6)-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol. Cancer 2022, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Sjekloča, N.; Tomić, S.; Mrklić, I.; Vukmirović, F.; Vučković, L.; Lovasić, I.B.; Maras-Šimunić, M. Prognostic value of IMP3 immunohistochemical expression in triple negative breast cancer. Medicine 2020, 99, e19091. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, R.; Sangen, M.; Namimatsu, S.; Yanagihara, K.; Yamashita, K.; Sakatani, T.; Takei, H.; Naito, Z. Prognostic value of IMP3 expression as a determinant of chemosensitivity in triple-negative breast cancer. Pathol. Res. Pract. 2017, 213, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Huang, Y.; Jin, Z.; Wang, C.; Wang, H.; Xu, J. METTL3 regulates the malignancy of cervical cancer via post-transcriptional regulation of RAB2B. Eur. J. Pharmacol. 2020, 879, 173134. [Google Scholar] [CrossRef]
- Zhu, J.; Han, S. Downregulation of LncRNA DARS-AS1 Inhibits the Tumorigenesis of Cervical Cancer via Inhibition of IGF2BP3. OncoTargets Ther. 2021, 14, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, Y.; Chen, P.; Yang, W.; Du, J.; Zhang, D. Methyltransferase-like 3 induces the development of cervical cancer by enhancing insulin-like growth factor 2 mRNA-binding proteins 3-mediated apoptotic chromatin condensation inducer 1 mRNA stability. Bioengineered 2022, 13, 7034–7048. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Wu, D.; Zhang, D.; Sun, M. Long Noncoding RNA KCNMB2-AS1 Stabilized by N(6)-Methyladenosine Modification Promotes Cervical Cancer Growth Through Acting as a Competing Endogenous RNA. Cell Transplant. 2020, 29, 963689720964382. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Yang, S.; Cen, Y.; Zhu, T.; Wang, L.; Xia, L.; Liu, Y.; Zou, J.; Xu, J.; et al. CircCDKN2B-AS1 interacts with IMP3 to stabilize hexokinase 2 mRNA and facilitate cervical squamous cell carcinoma aerobic glycolysis progression. J. Exp. Clin. Cancer Res. 2020, 39, 281. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Li, J.; Chen, Z.; Chen, F.; Tu, J.; Lin, S.; Wang, H. N(6)-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat. Commun. 2020, 11, 2578. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Han, J.; Hu, X. IGF2BP3-stabilized SIX4 promotes the proliferation, migration, invasion and tube formation of ovarian cancer cells. Mol. Med. Rep. 2022, 26, 232. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Backes, F.J.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Chitiyo, V.C.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 972–980. [Google Scholar] [CrossRef]
- Hsu, K.F.; Shen, M.R.; Huang, Y.F.; Cheng, Y.M.; Lin, S.H.; Chow, N.H.; Cheng, S.W.; Chou, C.Y.; Ho, C.L. Overexpression of the RNA-binding proteins Lin28B and IGF2BP3 (IMP3) is associated with chemoresistance and poor disease outcome in ovarian cancer. Br. J. Cancer 2015, 113, 414–424. [Google Scholar] [CrossRef]
- Wiedemeyer, K.; Wang, L.; Kang, E.Y.; Liu, S.; Ou, Y.; Kelemen, L.E.; Feil, L.; Anglesio, M.S.; Glaze, S.; Ghatage, P.; et al. Prognostic and Theranostic Biomarkers in Ovarian Clear Cell Carcinoma. Int. J. Gynecol. Pathol. 2022, 41, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.C.M.; van der Putten, L.J.M.; van Egerschot, A.; Van de Vijver, K.K.; Santacana, M.; Bronsert, P.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. Addition of IMP3 to L1CAM for discrimination between low- and high-grade endometrial carcinomas: A European Network for Individualised Treatment of Endometrial Cancer collaboration study. Hum. Pathol. 2019, 89, 90–98. [Google Scholar] [CrossRef]
- Wang, C.; Kong, F.; Ma, J.; Miao, J.; Su, P.; Yang, H.; Li, Q.; Ma, X. IGF2BP3 enhances the mRNA stability of E2F3 by interacting with LINC00958 to promote endometrial carcinoma progression. Cell Death Discov. 2022, 8, 279. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Yu, Y.Z.; Lv, D.J.; Wang, C.; Song, X.L.; Xie, T.; Wang, T.; Li, Z.M.; Guo, J.D.; Fu, D.J.; Li, K.J.; et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol. Cancer 2022, 21, 12. [Google Scholar] [CrossRef]
- Szarvas, T.; Tschirdewahn, S.; Niedworok, C.; Kramer, G.; Sevcenco, S.; Reis, H.; Shariat, S.F.; Rübben, H.; vom Dorp, F. Prognostic value of tissue and circulating levels of IMP3 in prostate cancer. Int. J. Cancer 2014, 135, 1596–1604. [Google Scholar] [CrossRef]
- Gu, Y.; Niu, S.; Wang, Y.; Duan, L.; Pan, Y.; Tong, Z.; Zhang, X.; Yang, Z.; Peng, B.; Wang, X.; et al. DMDRMR-Mediated Regulation of m(6)A-Modified CDK4 by m(6)A Reader IGF2BP3 Drives ccRCC Progression. Cancer Res. 2021, 81, 923–934. [Google Scholar] [CrossRef]
- Xie, X.; Lin, J.; Fan, X.; Zhong, Y.; Chen, Y.; Liu, K.; Ren, Y.; Chen, X.; Lai, D.; Li, X.; et al. LncRNA CDKN2B-AS1 stabilized by IGF2BP3 drives the malignancy of renal clear cell carcinoma through epigenetically activating NUF2 transcription. Cell Death Dis. 2021, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Tschirdewahn, S.; Panic, A.; Püllen, L.; Harke, N.N.; Hadaschik, B.; Riesz, P.; Horváth, A.; Szalontai, J.; Nyirády, P.; Baba, H.A.; et al. Circulating and tissue IMP3 levels are correlated with poor survival in renal cell carcinoma. Int. J. Cancer 2019, 145, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Sitnikova, L.; Mendese, G.; Liu, Q.; Woda, B.A.; Lu, D.; Dresser, K.; Mohanty, S.; Rock, K.L.; Jiang, Z. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin. Cancer Res. 2008, 14, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, Y.; Zhang, C.; Zha, H.; Zhou, X.; Fu, B.; Guo, J.; Wang, G. IGF2BP3 facilitates cell proliferation and tumorigenesis via modulation of JAK/STAT signalling pathway in human bladder cancer. J. Cell. Mol. Med. 2020, 24, 13949–13960. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, J.; Huang, Z.; Han, Z.; Xin, J.; Yuan, L.; Du, M.; Chu, H.; Wang, M.; Zhang, Z. Fine particulate matter induces METTL3-mediated m(6)A modification of BIRC5 mRNA in bladder cancer. J. Hazard. Mater. 2022, 437, 129310. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, Y.; Liu, X.; Wang, W.; Jiang, X.; Xia, Y.; Zhou, G.; Chen, S.; Shi, B. Comprehensive analysis of N(6)-methyladenosine regulators with the tumor immune landscape and correlation between the insulin-like growth factor 2 mRNA-binding protein 3 and programmed death ligand 1 in bladder cancer. Cancer Cell Int. 2022, 22, 72. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Yankova, E.; Blackaby, W.; Albertella, M.; Rak, J.; De Braekeleer, E.; Tsagkogeorga, G.; Pilka, E.S.; Aspris, D.; Leggate, D.; Hendrick, A.G.; et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 2021, 593, 597–601. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, B.; Liu, W.; Zhang, M.; Shen, Z.; Han, Z.; Jiang, Q.; Yang, Q.; Song, C.; Wang, R.; et al. Identification of A Novel Small-Molecule Binding Site of the Fat Mass and Obesity Associated Protein (FTO). J. Med. Chem. 2015, 58, 7341–7348. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, J.; Chen, W.; Xu, Y.; Shen, Y.; Xu, X. Targeting IGF2BP3 in Cancer. Int. J. Mol. Sci. 2023, 24, 9423. https://doi.org/10.3390/ijms24119423
Liu X, Chen J, Chen W, Xu Y, Shen Y, Xu X. Targeting IGF2BP3 in Cancer. International Journal of Molecular Sciences. 2023; 24(11):9423. https://doi.org/10.3390/ijms24119423
Chicago/Turabian StyleLiu, Xin, Jiayu Chen, Wenliang Chen, Yangtao Xu, Yang Shen, and Ximing Xu. 2023. "Targeting IGF2BP3 in Cancer" International Journal of Molecular Sciences 24, no. 11: 9423. https://doi.org/10.3390/ijms24119423
APA StyleLiu, X., Chen, J., Chen, W., Xu, Y., Shen, Y., & Xu, X. (2023). Targeting IGF2BP3 in Cancer. International Journal of Molecular Sciences, 24(11), 9423. https://doi.org/10.3390/ijms24119423





