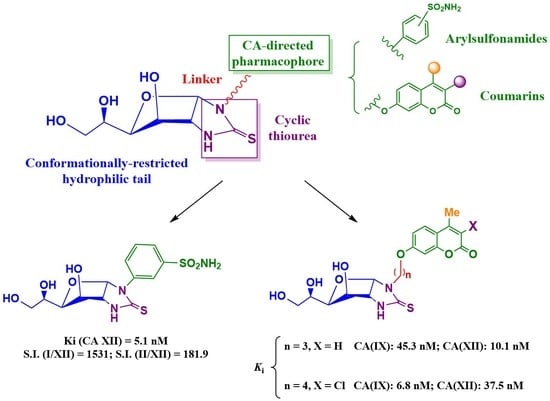
Conformationally Restricted Glycoconjugates Derived from Arylsulfonamides and Coumarins: New Families of Tumour-Associated Carbonic Anhydrase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
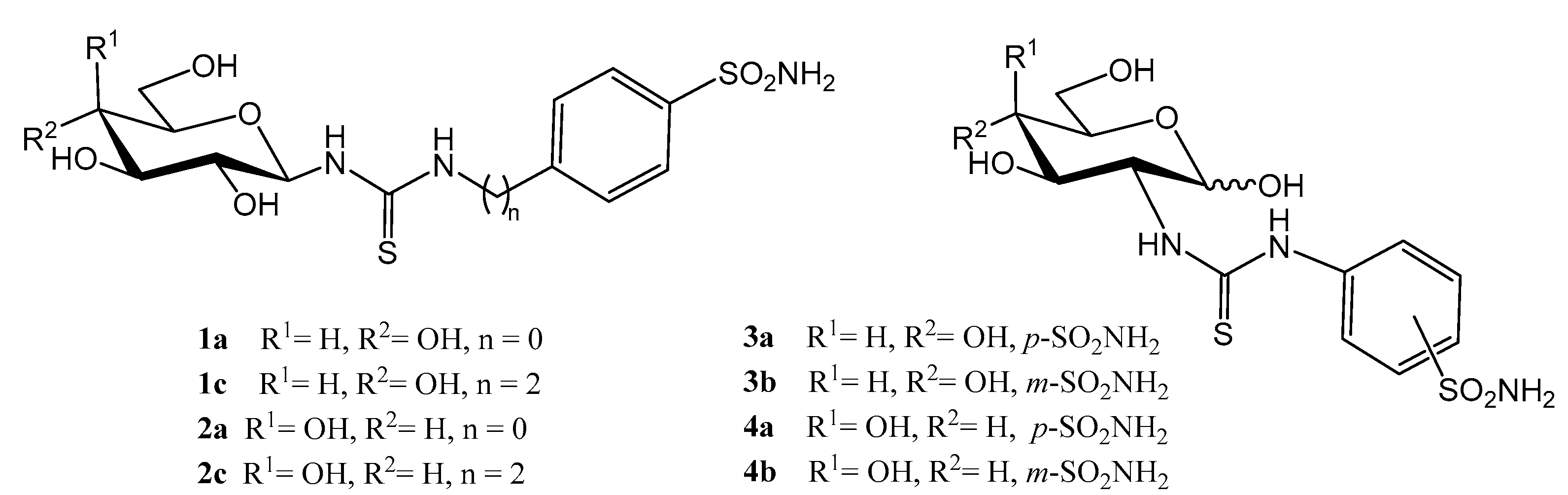
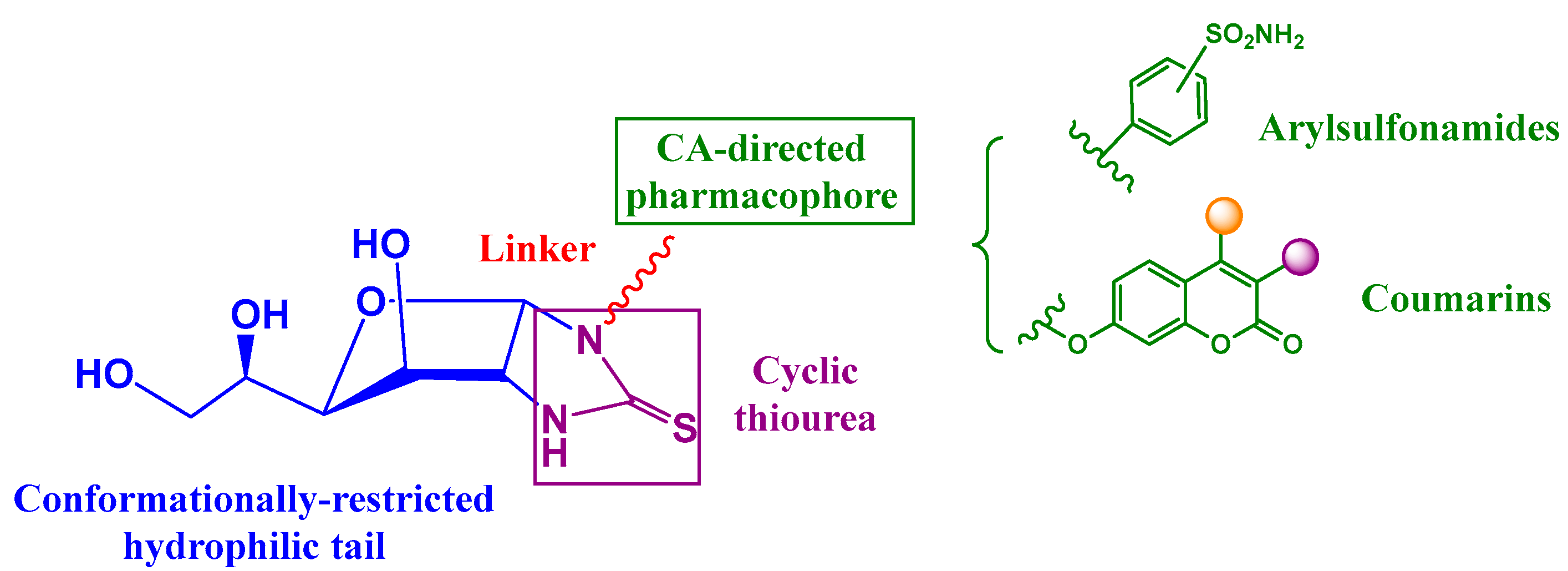
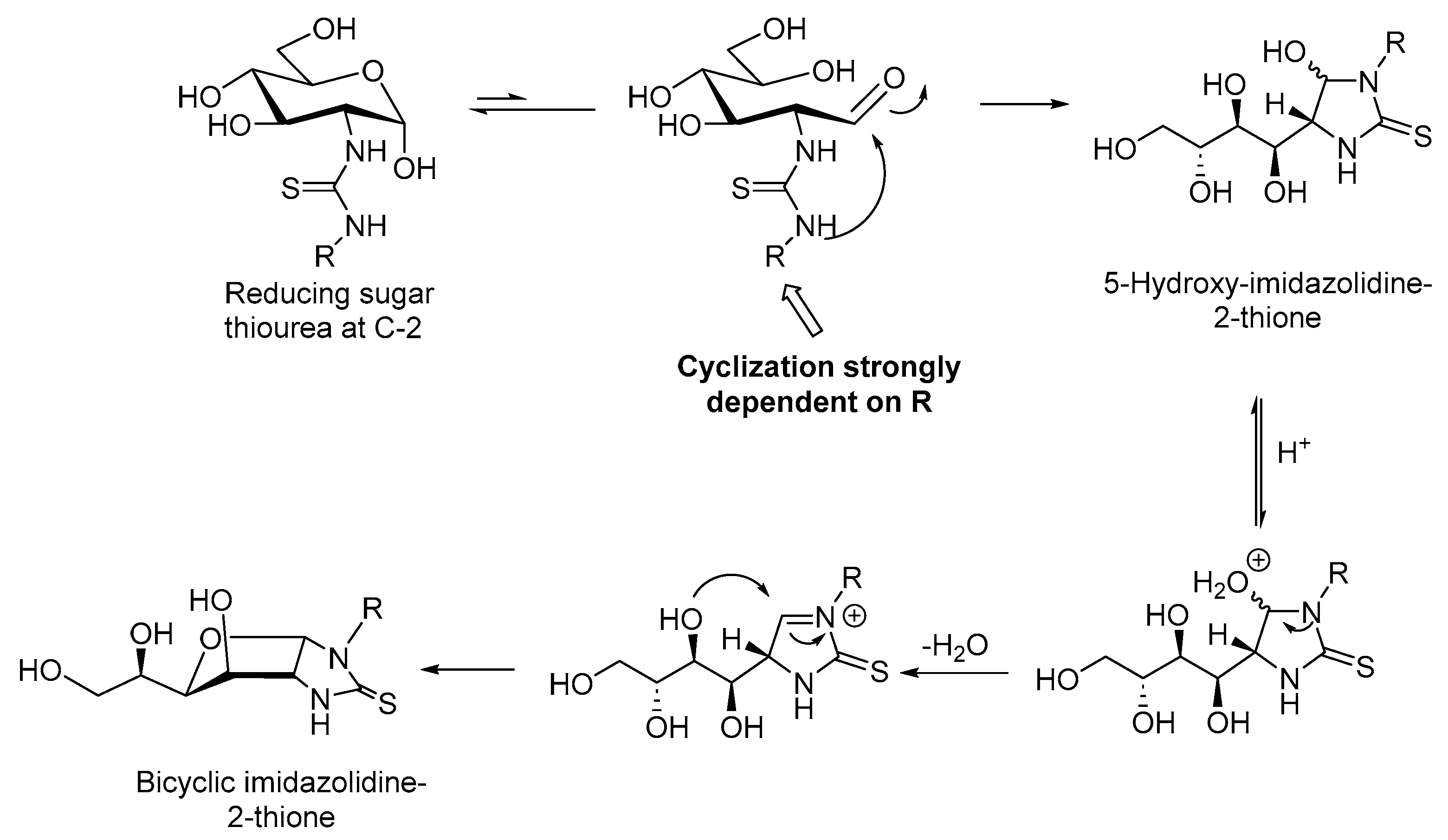
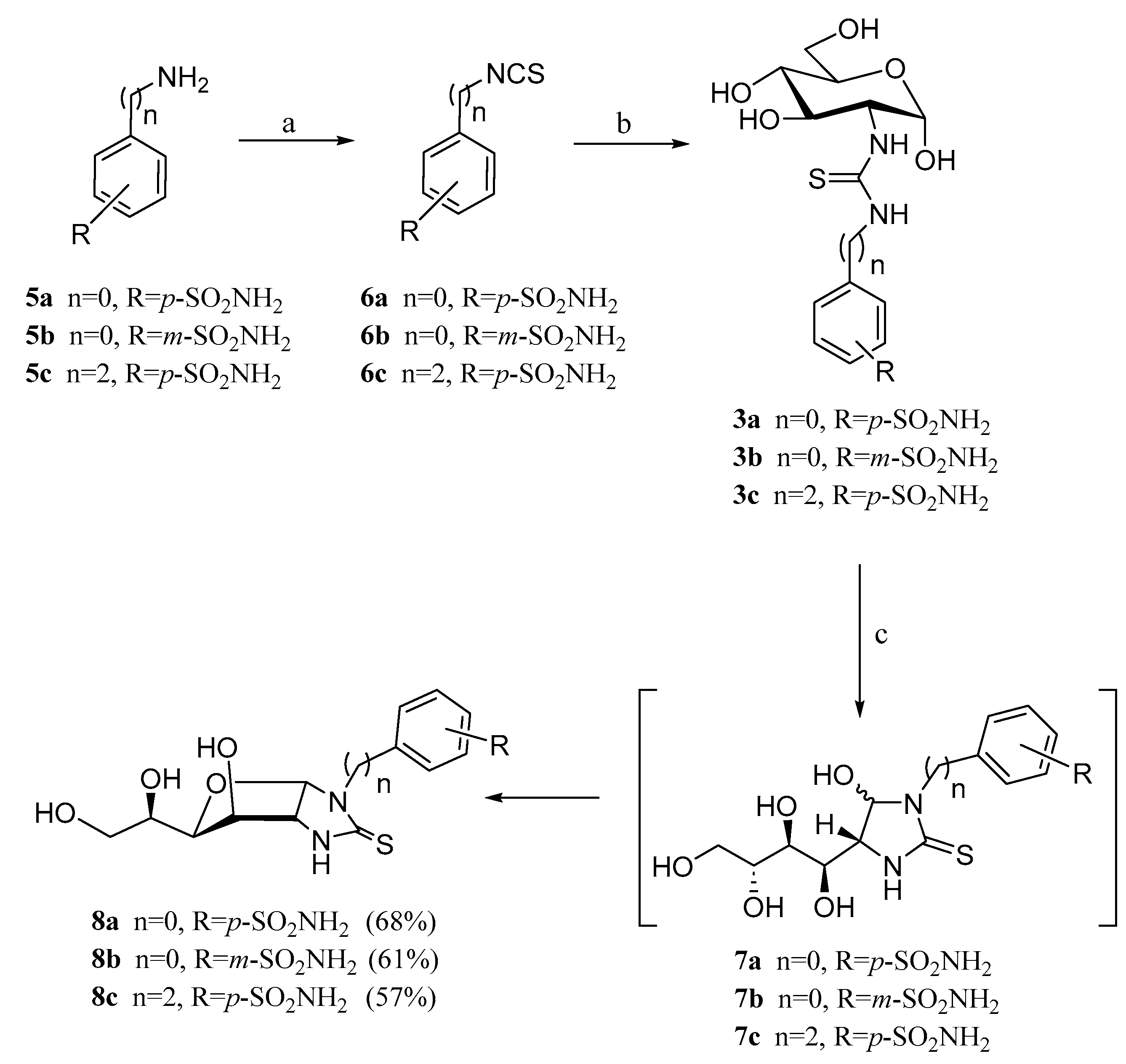
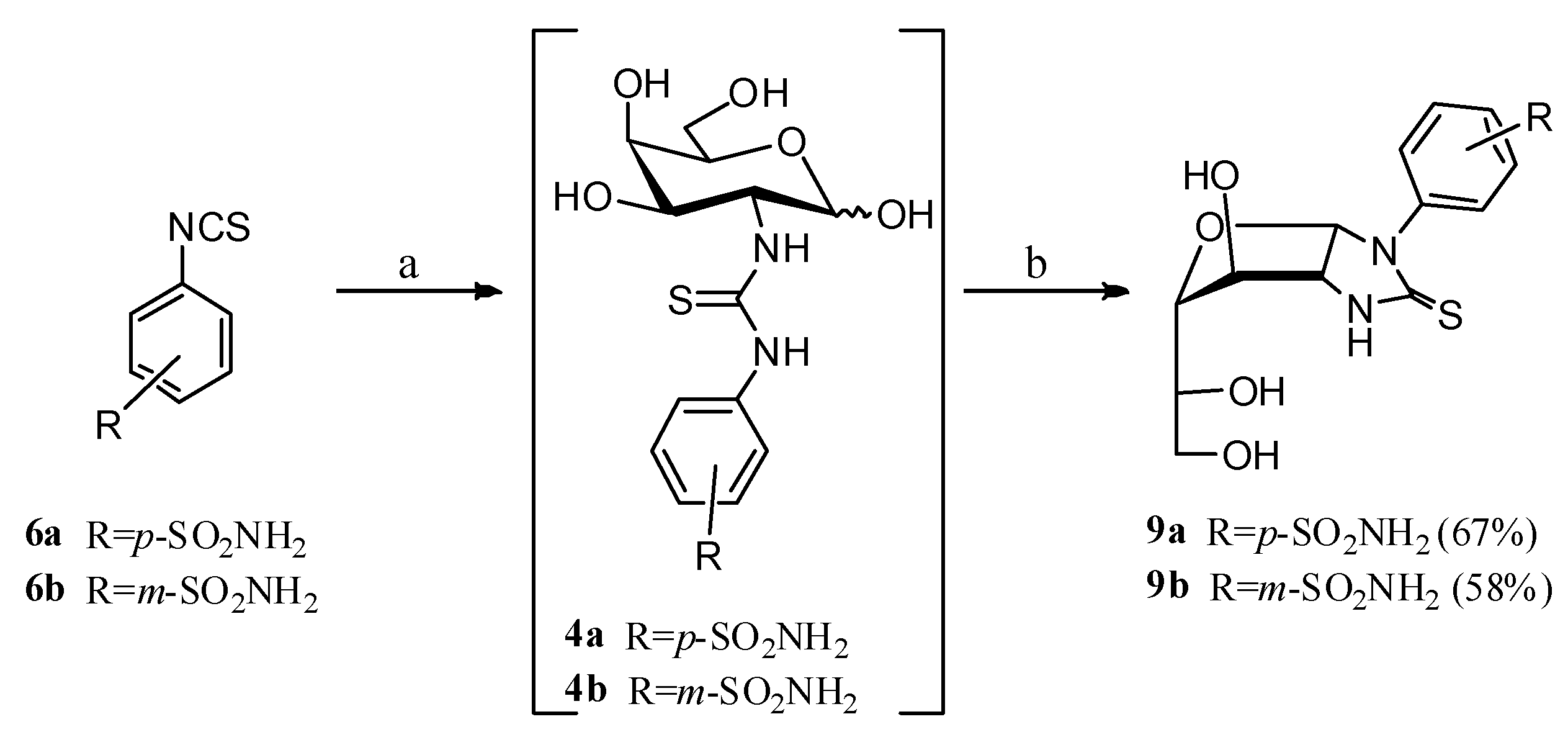
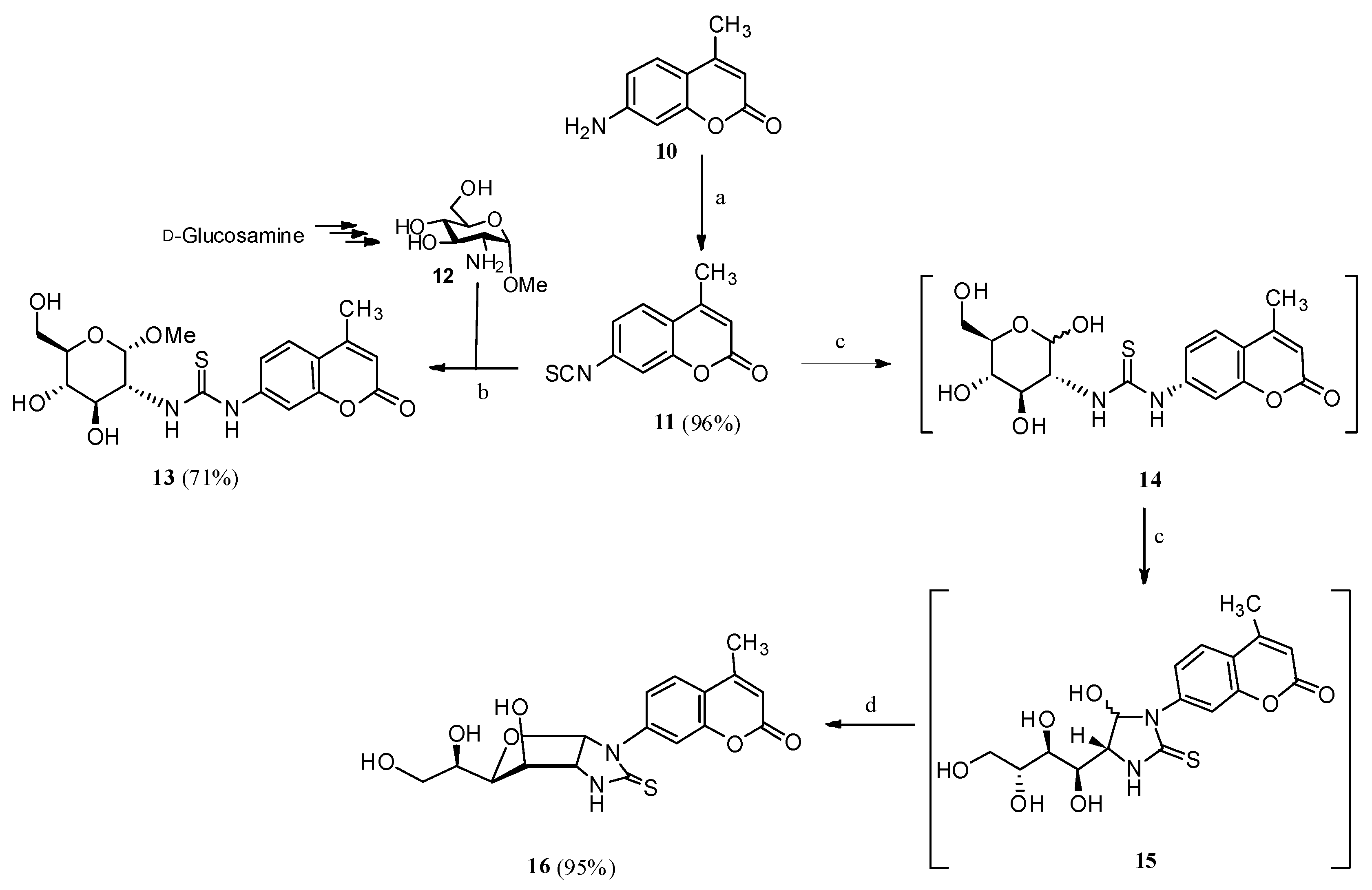
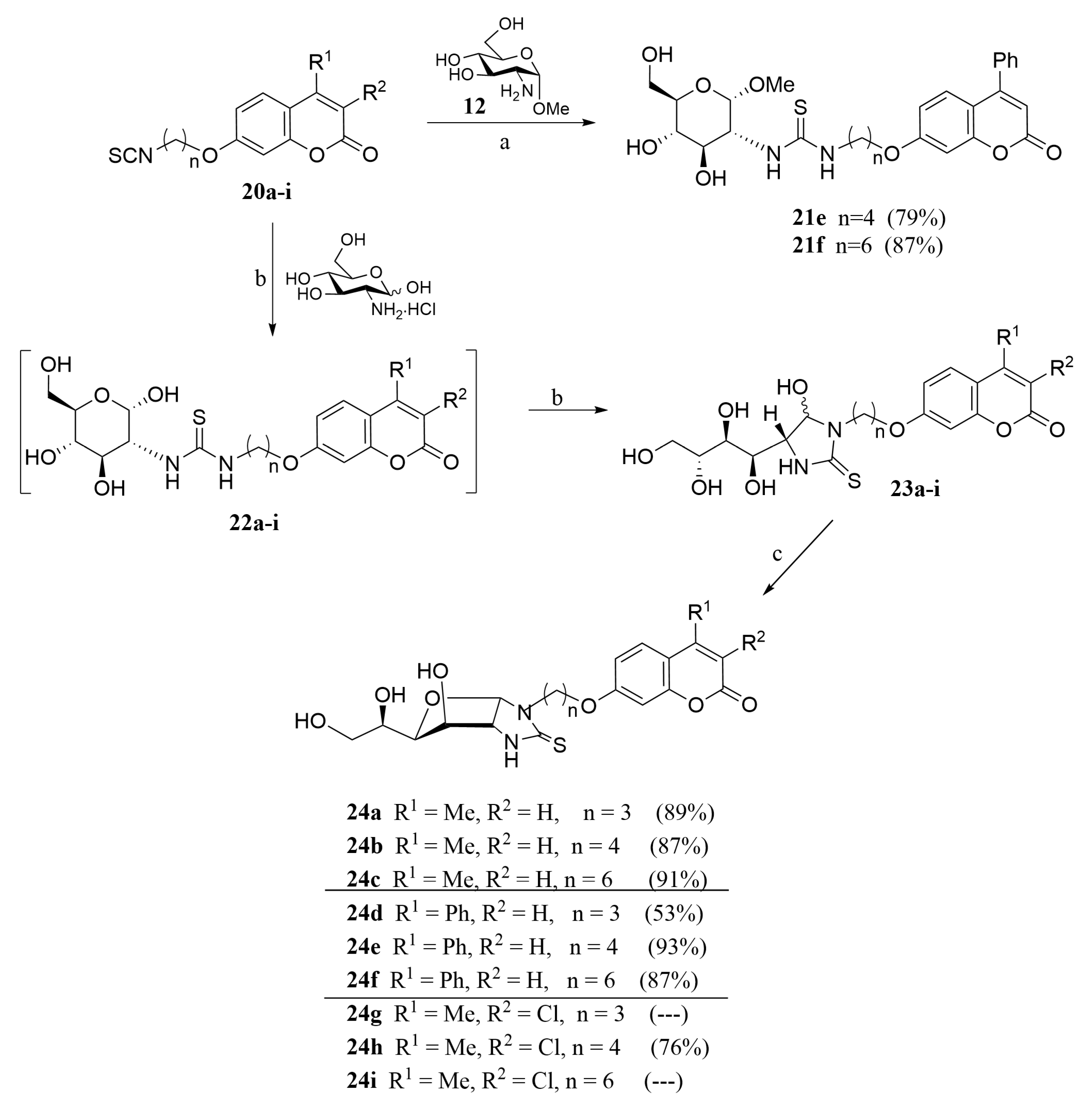
2.1. Drug Design and Chemistry
2.2. Biological Assessments
In Vitro Carbonic Anhydrase Inhibition
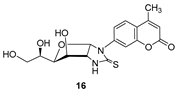
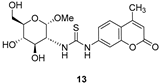
- For compounds lacking linkers, almost no difference in activities can be found for non-cyclic (13) and bicyclic (16) thioureas.
- The insertion of a linker between the carbohydrate and the coumarin residues of byclicic structures proved to be benefitial for the inhibition of both membrane-bound enzymes (16 vs. 24a–c).
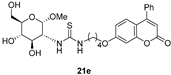
- The presence of a Ph residue on C-3 both in linear thioureas (21e,f) and imidazolidine-2-thiones (24d,e) provoked an impariment of the inhibitory profile against CA IX and XII, reaching the submicromolar range. This is probably due to steric clash within the active site.
- Considering the effect of the substituents (n = 4), the observed order of activity is:
- CA IX: R1 = Me, R2 = Cl (24h) > R1 = Me, R2 = H (24b) > R1 = Ph, R2 = H (24e). Indeed, compound 24h provided the strongest CA IX inhibitor of the series (Ki = 6.8 nM), roughly 3.8-fold stronger than AAZ.
- CA XII: R1 = Me, R2 = H (24b) > R1 = Me, R2 = Cl (24h) > R1 = Ph, R2 = H (24e).
- The best template for the inhibition of CA XII was proved to be a short linkage (n = 3), and the monosubtitution of coumarin on C-3 with small substituents (Me, 24a), with Ki = 10.1 nM
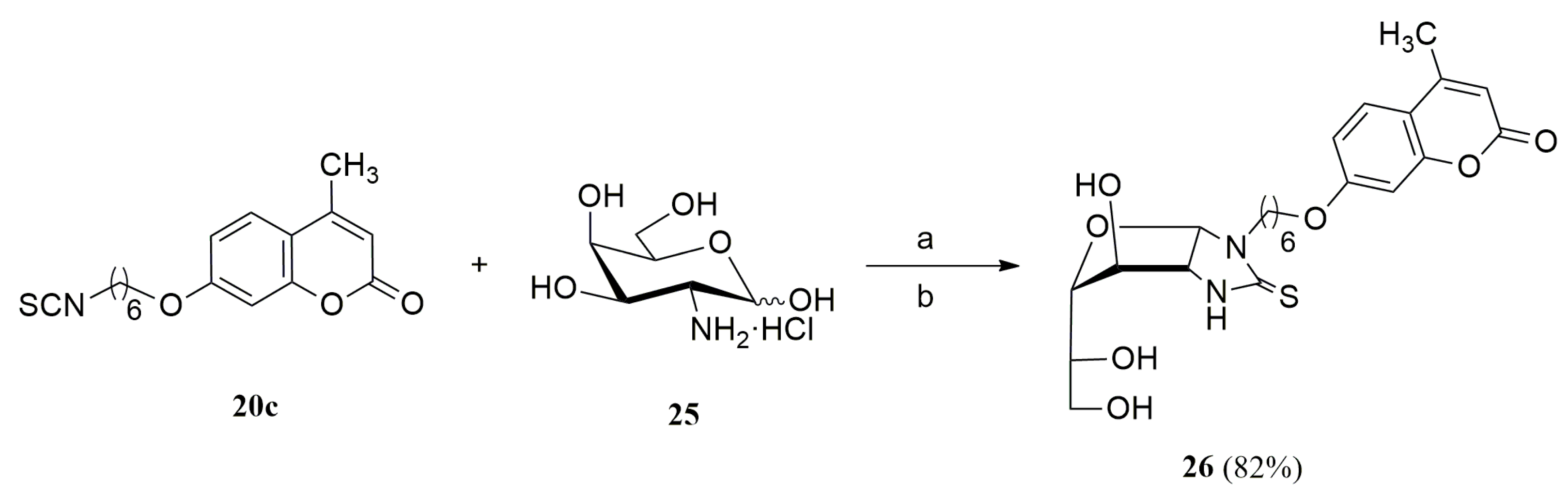
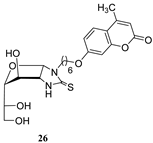
- Little differences in activity were found by changing the carbohydrate configuration (24c vs. 26).
2.3. Antiproliferative Activities
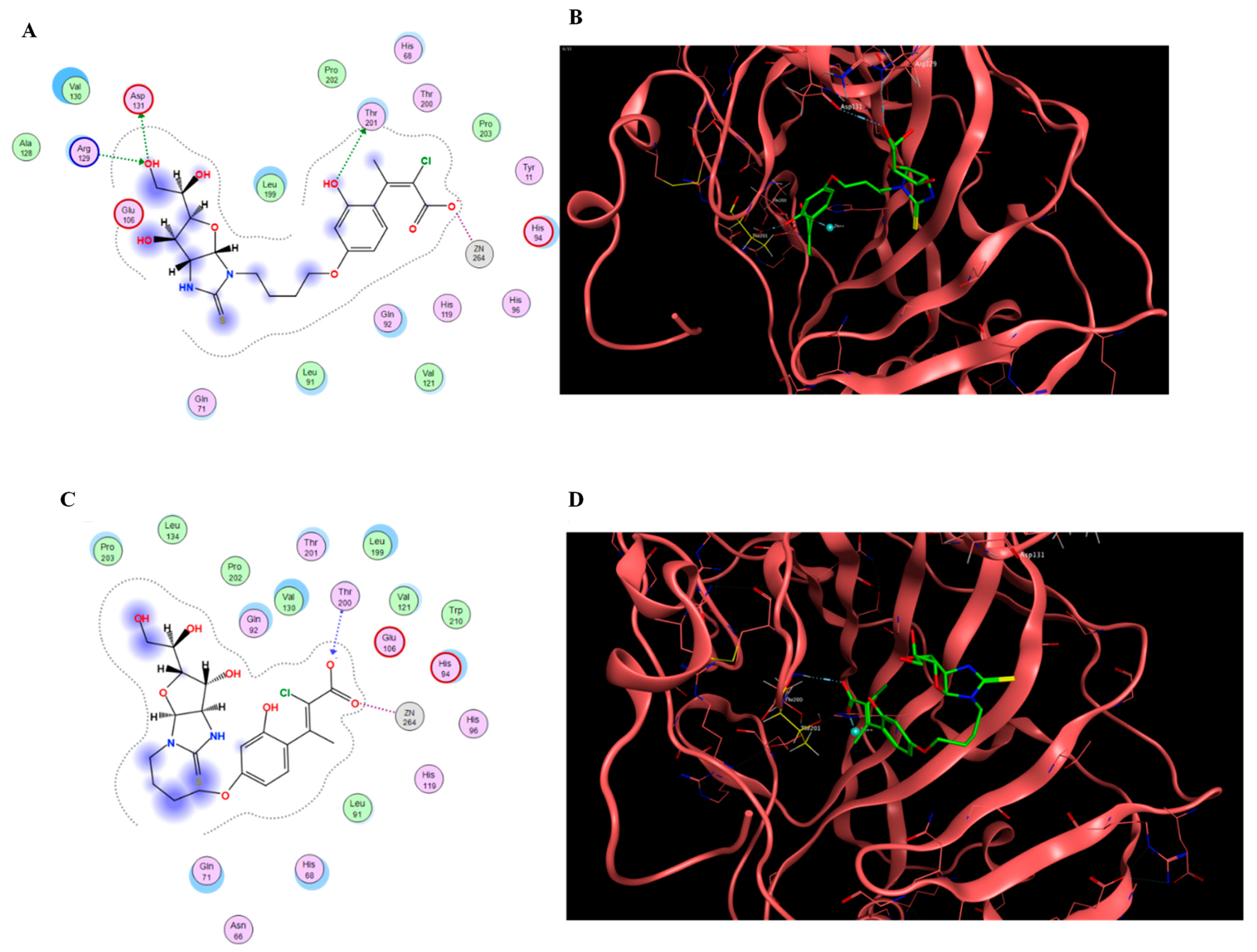
2.4. Docking Simulations
3. Materials and Methods
3.1. Chemistry
3.1.1. General Methods
3.1.2. General Procedure for the Preparation of Imidazolidine-2-Thiones 8a–c, 9a,b
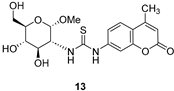
3.1.3. N-(Methyl 2-deoxy-α-d-Glucopyranosid-2-yl)-N′-(4-methyl-2′-oxo-2′H-chromen-7′-yl)thiourea (13)
3.1.4. 1-(4′-Methyl-2′-oxo-2′H-chromen-7′-yl)-(1″,2″-dideoxy-α-d-glucofurano)[2,1-d]imidazolidine-2-thione (16)
3.1.5. General Procedure for the Preparation of 7-Hidroxycoumarins via Pechmann Condensation
3.1.6. General Procedure for the O-Alkylation of 7-Hydroxycoumarins with α,ω-Dibromoalkanes (17a–i)
3.1.7. General Procedure for the Preparation of Azides 18a–i
3.1.8. General Procedure for the Preparation of Amines 19a–i
3.1.9. General Procedure for the Preparation of Isothiocyanates 20a–i
3.1.10. General Procedure for the Preparation of Coumarin-Derived Glycol-Thioureas 21e,f
3.1.11. General Procedure for the Preparation of Coumarin-Derived Imidazolidine-2-Thiones 24a–i, 26
3.2. CA Inhibition Assays
3.3. Antiproliferative Assays
3.4. Docking Simulations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.K.; Lee, C.; Lim, S.W.; Adhikari, A.; Andring, J.T.; McKenna, R.; Ghim, C.-M.; Kim, C.U. Elucidating the role of metal ions in carbonic anhydrase catalysis. Nat. Commun. 2020, 11, 4557. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.D.; Pinard, M.; McKenna, R.; Silverman, D. Catalytic mechanism of α-class carbonic anhydrases: CO2 hydration and proton transfer. Subcell. Biochem. 2014, 75, 31–52. [Google Scholar] [PubMed]
- Geers, C.; Gros, G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol. Rev. 2000, 80, 681–715. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hong, J.H. The fundamental role of bicarbonate transporters and associated carbonic anhydrase enzymes in maintaining ion and pH homeostasis in non-secretory organs. Int. J. Mol. Sci. 2020, 21, 339. [Google Scholar] [CrossRef]
- Supuran, C.T. Novel carbonic anhydrase inhibitors. Future Med. Chem. 2021, 13, 1935–1937. [Google Scholar] [CrossRef]
- Angeli, A.; Carta, F.; Supuran, C.T. Carbonic anhydrases: Versatile and useful biocatalysts in Chemistry and Biochemistry. Catalyst 2020, 10, 1008. [Google Scholar] [CrossRef]
- Domsic, J.F.; McKenna, R. Sequestration of carbon dioxide by the hydrophobic pocket of the carbonic anhydrases. Biochim. Biophys. Acta. 2010, 1804, 326. [Google Scholar] [CrossRef]
- Angeli, A.; Supuran, C.T. Click chemistry approaches for developing carbonic anhydrase inhibitors and their applications. J. Enzyme Inhib. Med. Chem. 2023, 38, 2166503. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Supuran, C.T.; Capasso, C. An overview on the recently discovered iota-carbonic anhydrases. J. Enzyme Inhib. Med. Chem. 2021, 36, 1988–1995. [Google Scholar] [CrossRef]
- Esbaugh, A.J.; Tufts, B.L. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir. Physiol. Neurobiol. 2006, 154, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Ghorai, S.; Pulya, S.; Ghosh, K.; Panda, P.; Ghosh, B.; Gayen, S. Structure-activity relationship of human carbonic anhydrase-II inhibitors: Detailed insight for future development as anti-glaucoma agents. Bioorg. Chem. 2020, 95, 103557. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Cerri, C.; Nencetti, S.; Orlandini, E. Carbonic anhydrase inhibitors and epilepsy: State of the art and future perspectives. Molecules 2021, 26, 6380. [Google Scholar] [CrossRef]
- Supuran, C.T. Anti-obesity carbonic anhydrase inhibitors: Challenges and opportunities. J. Enzyme Inhib. Med. Chem. 2022, 37, 2478–2488. [Google Scholar] [CrossRef]
- Artasensi, A.; Angeli, A.; Lammi, C.; Bollati, C.; Gervasoni, S.; Baron, G.; Matucci, R.; Supuran, C.T.; Vistoli, G.; Fumagalli, L. Discovery of a potent and highly selective dipeptidyl peptidase IV and carbonic anhydrase inhibitor as “antidiabesity” agents based on repurposing and morphing of WB-4101. J. Med. Chem. 2022, 65, 13946–13966. [Google Scholar] [CrossRef]
- Bonardi, A.; Micheli, L.; Di Cesare Mannelli, L.; Ghelardini, C.; Gratteri, P.; Nocentini, A.; Supuran, C.T. Development of hydrogen sulfide-releasing carbonic anhydrases IX-and XII-selective inhibitors with enhanced antihyperalgesic action in a rat model of arthritis. J. Med. Chem. 2022, 65, 13143–13157. [Google Scholar] [CrossRef]
- Akgul, O.; Lucarini, E.; Mannelli, L.; Di, C.; Ghelardini, C.; D’Ambrosio, K.; Buonanno, M.; Monti, S.M.; De Simone, G.; Angeli, A.; et al. Sultam based carbonic anhydrase VII inhibitors for the management of neuropathic pain. Eur. J. Med. Chem. 2022, 227, 113956. [Google Scholar] [CrossRef]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med. Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Mojzych, M.; Marciniak, B.; Drozda, R.; Kontek, R. Targeting carbonic anhydrase IX and XII isoforms with small molecule inhibitors and monoclonal antibodies. J. Enzyme Inhib. Med. Chem. 2022, 37, 1278–1298. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Mujumdar, P.; Annovazzi, L.; Kopecka, J.; Mellai, M.; Schiffer, D.; Poulsen, S.-A.; Riganti, C. Carbonic anhydrase XII inhibitors overcome P-glycoprotein-mediated resistance to temozolomide in glioblastoma. Mol. Cancer Ther. 2018, 17, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Kumar, A.; Siwach, K.; Supuran, C.T.; Sharma, P.K. A decade of tail-approach based design of selective as well as potent tumor associated carbonic anhydrase inhibitors. Bioorg. Chem. 2022, 126, 105920. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, M.B.; Poulsen, S.-A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-Zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, D.; Nuti, E.; Rossello, A. An overview of carbohydrate-based carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 1906–1922. [Google Scholar] [CrossRef]
- Moeker, J.; Teruya, K.; Rossit, S.; Wilkinson, B.L.; López, M.; Bornaghi, L.F.; Innocenti, A.; Supuran, C.T.; Poulsen, S.-A. Design and synthesis of thiourea compounds that inhibit transmembrane anchored carbonic anhydrases. Bioorg. Med. Chem. 2012, 20, 2392–2404. [Google Scholar] [CrossRef]
- Smaine, F.Z.; Winum, Y.J.; Montero, J.L.; Regainia, Z.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Selective inhibition of the extracellular, tumor-associated isoforms IX and XII over isozymes I and II with glycosyl-thioureido-sulfonamides. Bioorg. Med. Chem. Lett. 2007, 17, 5096–5100. [Google Scholar] [CrossRef]
- Fang, Z.; Song, Y.; Zhan, P.; Zhang, Q.; Liu, X. Conformational restriction: An effective tactic in ‘follow-on’-based drug discovery. Future Med. Chem. 2014, 6, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.G.; Zafra, E.; López, Ó.; Robina, I.; Fuentes, J. Stereoselective synthesis of imidazolidine, imidazoline and imidazole C-and N-pseudonucleosides. Tetrahedron Asymm. 1999, 10, 3011–3023. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.G.; López, Ó. Heterocycles from carbohydrate isothiocyanates. In Topics in Heterocyclic Chemistry; El Ashry, E.S.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 7, pp. 67–100. [Google Scholar]
- Fernández-Bolaños Guzmán, F.; García Rodríguez, S.; Fernández-Bolaños, J.; Díanez, M.J.; López-Castro, A. Reaction of 2-amino-2-deoxy-d-glucose with aryl and acyl isothiocyanates, and aryl isocyanates: Structure of the intermediate products. Carbohydr. Res. 1991, 210, 125–143. [Google Scholar] [CrossRef]
- Maza, S.; López, Ó.; Martos, S.; Maya, I.; Fernández-Bolaños, J.G. Synthesis of the first selenium-containing acyclic nucleosides and anomeric spironucleosides from carbohydrate precursors. Eur. J. Org. Chem. 2009, 2009, 5239–5246. [Google Scholar] [CrossRef]
- Baldwin, J.E. Rules for ring closure. J. Chem. Soc. Chem. Commun. 1976, 18, 734–741. [Google Scholar] [CrossRef]
- Karalı, N.; Akdemir, A.; Göktas, F.; Elma, P.E.; Angeli, A.; Kızılırmak, M.; Supuran, C.T. Novel sulfonamide-containing 2-indolinones that selectively inhibit tumor-associated alpha carbonic anhydrases. Bioorg. Med. Chem. 2017, 25, 3714–3718. [Google Scholar] [CrossRef]
- Bozdag, M.; Alafeefy, A.M.; Altamimi, A.M.; Carta, F.; Supuran, C.T.; Vullo, D. Synthesis of new 3-(2-mercapto-4-oxo-4H-quinazolin-3-yl)-benzenesulfonamides with strong inhibition properties against the tumor associated carbonic anhydrases IX and XII. Bioorg. Med. Chem. 2017, 25, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, M.; Alafeefy, A.M.; Carta, F.; Ceruso, M.; Al-Tamimi, A.-M.S.; Al-Kahtani, A.A.; Alasmary, F.A.S.; Supuran, C.T. Synthesis 4-[2-(2-mercapto-4-oxo-4H-quinazolin-3-yl)-ethyl]-benzenesulfonamides with subnanomolar carbonic anhydrase II and XII inhibitory properties. Bioorg. Med. Chem. 2016, 24, 4100–4107. [Google Scholar] [CrossRef]
- Voutsadaki, S.; Tsikalas, G.K.; Klontzas, E.; Froudakis, G.E.; Katerinopoulos, H.E. A “turn-on” coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media. Chem. Commun. 2010, 46, 3292–3294. [Google Scholar] [CrossRef]
- Inouye, Y.; Onodera, K.; Kitaoka, S.; Hinaro, S. Some fatty acid derivatives of d-glucosamine. J. Am. Chem. Soc. 1956, 70, 4722–4724. [Google Scholar] [CrossRef]
- Gibbs, C.F.; Hough, L.; Richardson, A.C. A new synthesis of a 2,3-epimino-α-d-allopyranoside. Carbohydr. Res. 1965, 1, 290–296. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent advances in the synthesis of coumarin derivatives from different starting materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef]
- Bock, K.; Pedersen, C. Carbon-13 Nuclear Magnetic Resonance spectroscopy of monossacharides. Adv. Carbohydr. Chem. Biochem. 1983, 41, 27–66. [Google Scholar]
- Scozzafava, A.; Supuran, C.T. Glaucoma and the applications of carbonic anhydrase inhibitors. Sub-Cell. Biochem. 2014, 75, 349–359. [Google Scholar]
- Liu, C.; Wei, Y.; Wang, J.; Pi, L.; Huang, J.; Wang, P. Carbonic anhydrases III and IV autoantibodies in rheumatoid arthritis, systemic lupus erythematosus, diabetes, hypertensive renal disease, and heart failure. Clin. Dev. Immunol. 2012, 2012, 354594. [Google Scholar] [CrossRef]
- Krasavin, M.; Kalinin, S.; Sharonova, T.; Supuran, C.T. Inhibitory activity against carbonic anhydrase IX and XII as a candidate selection criterion in the development of new anticancer agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 1555–1561. [Google Scholar] [CrossRef]
- El-Damasy, A.K.; Kim, H.J.; Nocentini, A.; Seo, S.H.; Eldehna, W.M.; Bang, E.-K.; Supuran, C.T.; Keum, G. Discovery of new 6-ureido/amidocoumarins as highly potent and selective inhibitors for the tumour-relevant carbonic anhydrases IX and XII. J. Enzyme Inhib. Med. Chem. 2023, 38, 2154603. [Google Scholar] [CrossRef]
- Arrighi, G.; Puerta, A.; Petrini, A.; Hicke, F.J.; Nocentini, A.; Fernandes, M.X.; Padrón, J.M.; Supuran, C.T.; Fernández-Bolaños, J.G.; López, Ó. Squaramide-tethered sulfonamides and coumarins: Synthesis, inhibition of tumor-associated CAs IX and XII and docking simulations. Int. J. Mol. Sci. 2022, 23, 7685. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Aguilar, A.; Merino-Montiel, P.; Montiel-Smith, S.; Meza-Reyes, S.; Vega-Báez, J.L.; Puerta, A.; Fernandes, M.X.; Padrón, J.M.; Petreni, A.; Nocentini, A.; et al. 2-Aminobenzoxazole-appended coumarins as potent and selective inhibitors of tumour-associated carbonic anhydrases. J. Enzyme Inhib. Med. Chem. 2022, 37, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Thacker, P.S.; Goud, N.S.; Argulwar, O.S.; Soman, J.; Angeli, A.; Alvala, M.; Arifuddin, M.; Supuran, C.T. Synthesis and biological evaluation of some coumarin hybrids as selective carbonic anhydrase IX and XII inhibitors. Bioorg. Chem. 2020, 104, 104272. [Google Scholar]
- Puerta, A.; Galán, A.R.; Abdilla, R.; Demanuele, K.; Fernandes, M.X.; Bosica, G.; Padrón, J.M. Naphthol-derived Betti bases as potential SLC6A14 blockers. J. Mol. Clin. Med. 2019, 2, 35–40. [Google Scholar]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzyme Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef]
- Petreni, A.; Osman, S.M.; Alasmary, F.A.; Almutairi, T.M.; Nocentini, A.; Supuran, C.T. Binding site comparison for coumarin inhibitors and amine/amino acid activators of human carbonic anhydrases. Eur. J. Med. Chem. 2021, 226, 113875. [Google Scholar] [CrossRef]
- Buran, K.; Bua, S.; Poli, G.; Önen Bayram, F.E.; Tuccinardi, T.; Supuran, C.T. Novel 8-substituted coumarins that selectively inhibit human carbonic anhydrase IX and XII. Int. J. Mol. Sci. 2019, 20, 1208. [Google Scholar] [CrossRef] [PubMed]
- Hicke, F.J.; Puerta, A.; Dinić, J.; Pešić, M.; Padrón, J.M.; López, Ó.; Fernández-Bolaños, J.G. Straightforward access to novel mitochondriotropics derived from 2-arylethanol as potent and selective antiproliferative agents. Eur. J. Med. Chem. 2022, 228, 113980. [Google Scholar] [CrossRef] [PubMed]



 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Ki (nM) | S.I. b | ||||||||
| hCA I | hCA II | hCA IV | hCA IX | hCA XII | I/IX | I/XII | II/IX | II/XII | ||
| d-Gluco | 8a (n = 0, R = p-SO2NH2) | 765.5 | 60.0 | 347.6 | 175.0 | 502.5 | 4.4 | 1.5 | 0.3 | 0.1 |
| 8b (n = 0, R = m-SO2NH2) | 4578 | 5053 | 45.1 | 252.6 | 216.0 | 18.1 | 21.2 | 20.0 | 23.4 | |
| 8c (n = 2, R = p-SO2NH2) | 84.7 | 8.9 d | 1050 | 61.4 | 51.4 | 1.4 | 1.6 | 0.1 | 0.1 | |
| d-Galacto | 9a (n = 0, R = p-SO2NH2) | 90.3 | 116.0 | 4572 | 5657 | 2.9 | 0.02 | 31.1 | 0.02 | 40.0 |
| 9b (n = 0, R = m-SO2NH2) | 7807 | 927.6 | 27,250 | 9627 | 5.1 | 0.8 | 1531 | 0.1 | 181.9 | |
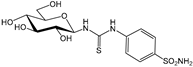 1a [26] | 7680 | 7.0 | --- c | 282 | 8.2 | 27.2 | 937 | 0.02 | 0.9 | |
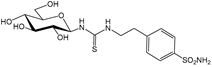 1c [26] | 9.0 | 108 | --- | 8.7 | 9.7 | 1.0 | 0.9 | 12.4 | 11.1 | |
 2a [26] | 6840 | 222 | --- | 7.0 | 20.1 | 977.1 | 340 | 31.7 | 11.0 | |
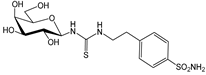 2c [26] | 5790 | 9.3 | --- | 2.8 | 10.2 | 2067.9 | 568 | 3.3 | 0.9 | |
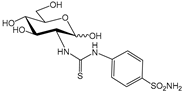 3a [27] | 2700 | 9700 | --- | 77 | 7.9 | 35.1 | 342 | 126 | 1228 | |
 3b [27] | 100 | 8600 | --- | 9.0 | 207 | 11.1 | 0.5 | 956 | 41.5 | |
 4a [27] | 3600 | 7700 | --- | 74 | 104 | 48.6 | 34.6 | 104 | 74.0 | |
 4b [27] | 4300 | 940 | --- | 42 | 14 | 102.4 | 307.1 | 22.4 | 67.1 | |
| AAZ | 250 | 12.1 | 74.0 | 25.8 | 5.7 | 9.7 | 43.9 | 0.5 | 2.1 | |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Ki (nM) | S.I. b | ||||||
| hCA I | hCA II | hCA IX | hCA XII | I/IX | I/XII | II/IX | II/XII | |
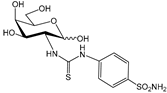
 | >100,000 | >100,000 | 73.9 | 56.1 | >1353 | >1783 | >1353 | >1783 |
 | >100,000 | >100,000 | 89.1 | 91.0 | >1122 | >1099 | >1122 | >1099 |
 | >100,000 | >100,000 | 105.8 | 227.8 | >945 | >439 | >945 | >439 |
 | >100,000 | 17,300 | 116.5 | 163.9 | >858 | >610 | 148 | 106 |
| 24a R1 = Me, R2 = H, n = 3 | >100,000 | >100,000 | 45.3 | 10.1 c | >2208 | >9901 | >2208 | >9901 |
| 24b R1 = Me, R2 = H, n = 4 | >100,000 | >100,000 | 70.7 | 25.7 | >1414 | >3891 | >1414 | >3891 |
| 24c R1 = Me, R2 = H, n = 6 | >100,000 | >100,000 | 22.4 | 72.1 | >4464 | >1387 | >4464 | >1387 |
| 24d R1 = Ph, R2 = H, n = 3 | >100,000 | >100,000 | 91.4 | 260.3 | >1094 | >384 | >1094 | >384 |
| 24e R1 = Ph, R2 = H, n = 4 | >100,000 | >100,000 | 177.3 | 140.4 | >564 | >712 | >564 | >712 |
| 24h R1 = Me, R2 = Cl, n = 4 | >100,000 | >100,000 | 6.8 | 37.5 | >14,706 | >2667 | >14,706 | >2667 |
 | >100,000 | >100,000 | 28.6 | 61.4 | >3947 | >1629 | >3947 | >1629 |
| Compound | Drug-Sensitive Cell Lines | Multidrug-Resistant Cell Lines | ||||
|---|---|---|---|---|---|---|
| A549 (Lung, Non-Small) | HBL-100 (Breast) | HeLa (Cervix) | SW1573 (Lung, Non-Small) | T-47D (Breast) | WiDr (Colon) | |
 | 79 ± 36 | 86 ± 25 | 83 ± 30 | 79 ± 37 | >100 | >100 |
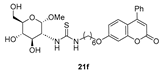 | 34 ± 4.0 | 23 ± 3.2 | 25 ± 8.0 | 9.7 a | 30 ± 7.4 | 36 ± 16 |
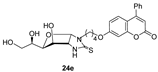 | 64 ± 31 | 23 ± 0.8 | 31 ± 0.2 | 5.7 ± 1.8 | 70 ± 23 | 47 ± 11 |
| Enzyme/Compound | 24h (Closed) | 24h (Open E) | 24h (Open Z) |
|---|---|---|---|
| hCA IX | −8.4781 | −10.1987 | −9.6243 |
| hCA XII | −7.1633 | −9.3104 | −9.8885 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Montiel, M.; Romero-Hernández, L.L.; Giovannuzzi, S.; Begines, P.; Puerta, A.; Ahuja-Casarín, A.I.; Fernandes, M.X.; Merino-Montiel, P.; Montiel-Smith, S.; Nocentini, A.; et al. Conformationally Restricted Glycoconjugates Derived from Arylsulfonamides and Coumarins: New Families of Tumour-Associated Carbonic Anhydrase Inhibitors. Int. J. Mol. Sci. 2023, 24, 9401. https://doi.org/10.3390/ijms24119401
Martínez-Montiel M, Romero-Hernández LL, Giovannuzzi S, Begines P, Puerta A, Ahuja-Casarín AI, Fernandes MX, Merino-Montiel P, Montiel-Smith S, Nocentini A, et al. Conformationally Restricted Glycoconjugates Derived from Arylsulfonamides and Coumarins: New Families of Tumour-Associated Carbonic Anhydrase Inhibitors. International Journal of Molecular Sciences. 2023; 24(11):9401. https://doi.org/10.3390/ijms24119401
Chicago/Turabian StyleMartínez-Montiel, Mónica, Laura L. Romero-Hernández, Simone Giovannuzzi, Paloma Begines, Adrián Puerta, Ana I. Ahuja-Casarín, Miguel X. Fernandes, Penélope Merino-Montiel, Sara Montiel-Smith, Alessio Nocentini, and et al. 2023. "Conformationally Restricted Glycoconjugates Derived from Arylsulfonamides and Coumarins: New Families of Tumour-Associated Carbonic Anhydrase Inhibitors" International Journal of Molecular Sciences 24, no. 11: 9401. https://doi.org/10.3390/ijms24119401
APA StyleMartínez-Montiel, M., Romero-Hernández, L. L., Giovannuzzi, S., Begines, P., Puerta, A., Ahuja-Casarín, A. I., Fernandes, M. X., Merino-Montiel, P., Montiel-Smith, S., Nocentini, A., Padrón, J. M., Supuran, C. T., Fernández-Bolaños, J. G., & López, Ó. (2023). Conformationally Restricted Glycoconjugates Derived from Arylsulfonamides and Coumarins: New Families of Tumour-Associated Carbonic Anhydrase Inhibitors. International Journal of Molecular Sciences, 24(11), 9401. https://doi.org/10.3390/ijms24119401