Haemophilia and Fragility Fractures: From Pathogenesis to Multidisciplinary Approach
Abstract
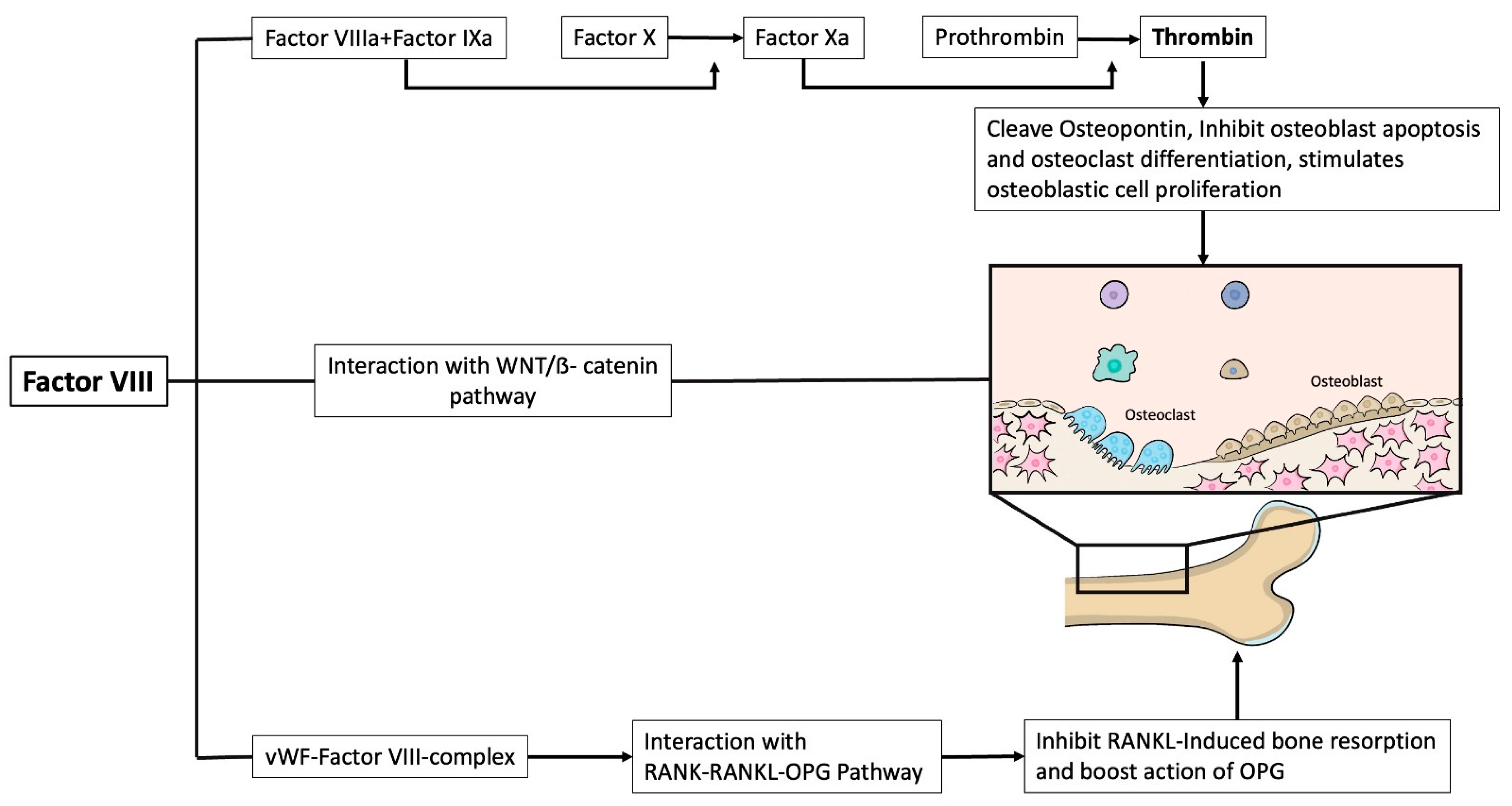
1. Introduction
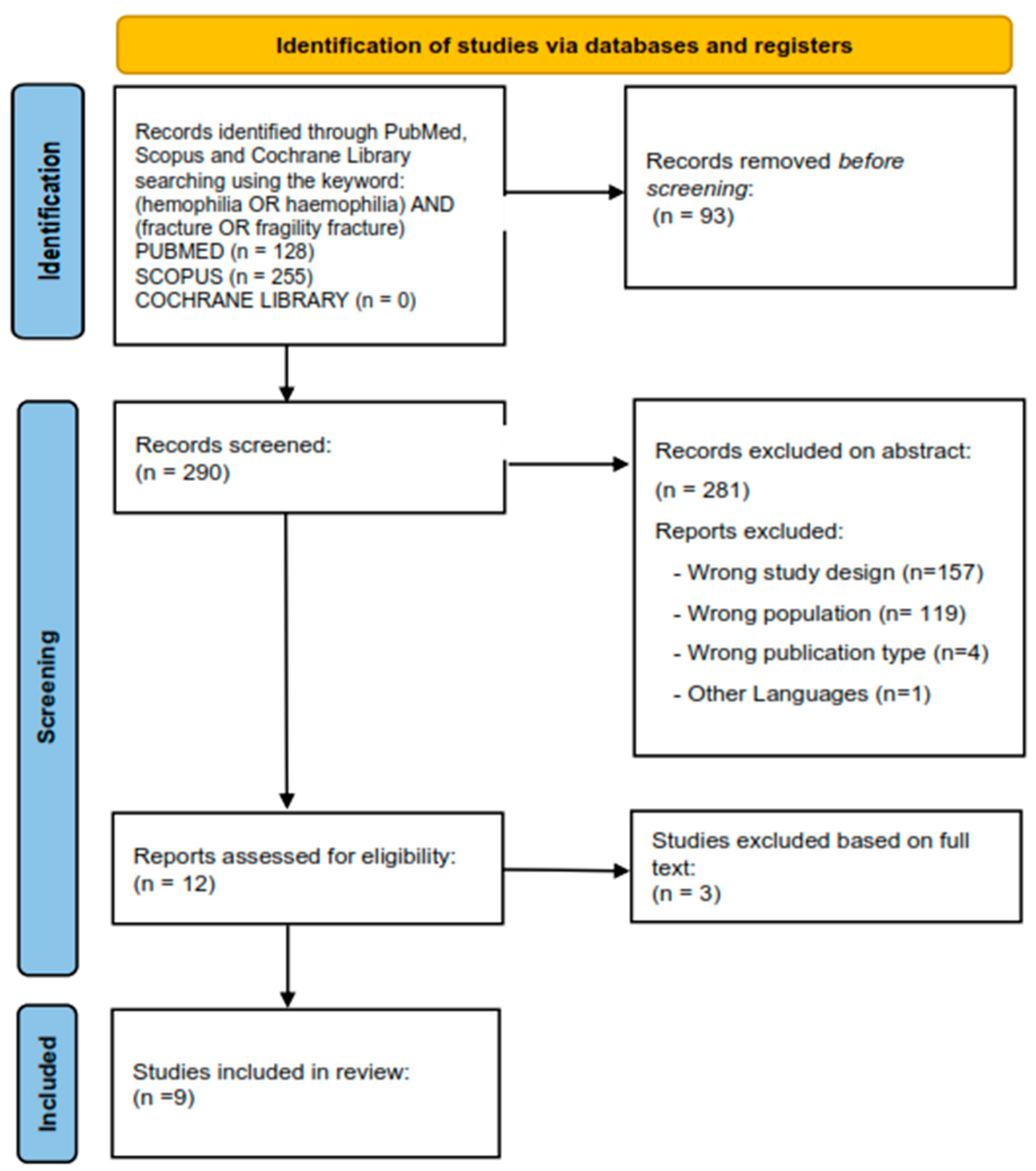
2. Search Strategy
3. Evidence of Fractures in Haemophilia
| Publication | Study Design | Patient Features | Fracture Incidence | Results | Correlations between Haemophilia and Fractures |
|---|---|---|---|---|---|
| Pai et al., 2022 [35] | Retrospective study (compared the incidence of all-site fractures, repeated fractures and osteoporotic fractures occurring in all PWH) | 9152 (832 vs. 8320) Age: <20 years 4576 (50.00%) 20–39 years 3135 (34.25%) 40–64 years 1210 (13.22%) ≥65 years 231 (2.52%) Sex: NA | The incidence of fractures in PWH is inconclusive, and no research has yet explored repeated fractures among PWH. | Screening, prevention and treatment of osteoporosis and further osteoporotic fractures among PWH remain essential in order to improve quality of life and achieve healthy aging in this particular population. | PWH had a higher risk of osteoporotic fracture, but the haemophilia only had a neutral effect in all sites of fracture and repeated fractures. |
| Tuan et al., 2019 [32] | Nationwide population-based cohort study based on the data in the Taiwan National Health Insurance Research Database (PWH vs. control patients without haemophilia) | 375 (75 vs. 300) Age: 35.7 (11.2–48.3) vs. 35.7(11.2–48.3) Sex: M:45 F: 30 vs. M:120 F:180 | The cumulative incidence was significantly higher for PWH diagnosed more than 5 years | Strong association between haemophilia and the development of osteoporotic fractures after haemophilia diagnosis. The relative risk of osteoporotic fracture after haemophilia in PWH increased with age in those aged <65 years compared with non-PWH. Clinicians should pay particular attention to osteoporotic fractures after haemophilia in PWH as they age. | PWH have significantly lower bone mineral density. Lower bone mineral density may lead to fragile in-bone structure and even osteoporotic fractures. |
| Angelini et al., 2016 [54] | Review (focused on common complications affecting the older haemophilia population, including joint disease, cardiovascular disease, malignancy, renal insufficiency and liver disease) | Comparison of age distribution of PWH between 2011 and 2015 (Age <45 vs. Age 45–64 vs. Age >65) Sex: NA | Markedly increased risk of fracture in PWH, which corresponding with both disease severity and increasing age | The elderly PWH must cope with chronic joint arthropathy, which provokes falls and fractures, and complications related to HIV and HCV infections, which greatly impact the incidence of cancer and liver disease. | Aside from functional impairment, PWH are also at higher risk of fractures secondary to decreased bone mineral density. |
| Strauss et al., 2016 [57] | Retrospective study (PWH with surgical fracture fixation compared to a matched non-haemophilic control group) | 46 fractures after low-energy trauma in 44 PWH vs. 46 non-haemophilic patients Age: 42.4 ± 20.5 years (range, 7–85 years) vs. 43.8 ± 20.7 years (range, 14–84 years) Sex: M: 38 F: 6 vs. M:23 F: 23 | Higher incidence of fractures in PWH compared to a population without haemophilia | There was no significant difference regarding the duration of the preoperative hospital stay between PWH and controls. | End-stage haemophilic arthropathy, muscle atrophy and joint contracture on the one hand increase the risk of falling and on the other hand lead to osteoporosis, making the bone more susceptible to fracture after trivial trauma. |
| Anagnostis et al., 2015 [28] | Review (on the current understanding of the association between haemophilia A or B and low bone mass, as well as the optimal approach and management of bone disease in these patients) | Age: NA Sex: NA | Data for increased fracture risk in PWH are currently not robust because of the rarity of the disease and the patients’ relatively young age; it can be speculated that it should be higher than in the general population | Regular exercise, prophylactic factor replacement therapy for severe haemophilia, fall prevention strategies and optimising calcium and vitamin D intake are recommended. | Except for low bone mineral density, PWH seem to be at increased fracture risk. Individualized multidisciplinary approach and careful assessment and management of risk factors associated with increased fracture risk are recommended. |
| Angelini et al., 2015 [55] | Review (focus on common complications affecting the older PWH, including cardiovascular disease, malignancy, liver disease, renal insufficiency, and joint disease) | Age: mean age 54.5 years (range 31 to 72) vs. mean age 46.7 years (range 23 to 76) Sex: M: 11 F: 18 | Higher incidence of fractures in PWH | Elderly PWHs should be treated similarly to their peers without haemophilia, with the addition of factor replacement therapy as appropriate. Primary prevention of risk factors should be emphasized, and close coordination between specialties is essential | In the older PWH, chronic joint arthropathy provokes falls and thus an increased fracture risk |
| Caviglia et al., 2015 [56] | Retrospective study (on 28 years’ experience treating PWH who suffered fractures and evaluating the impact of access to treatment) | 151 fractures in 141 PWH (25 vs. 35 vs. 33 vs. 31 vs. 27) Age: NA Sex: NA | Higher incidence of LL fractures in the first period analysed (1986–1990); over time, the ratio LL/UL changed as UL fractures became more frequent. This change is due access to treatment and specifically to the prophylaxis. | Fractures in PWH became more common in the UL than in the LL, lowering the age at which they occur and being less frequent. The advent of new and accessible treatments decreased the development of orthopedic complications and favours improvement in the quality of life of PWH. | Daily activities for PWH are reduced due to the impact of multiple bleeding episodes in the musculoskeletal system, leading to arthropathy and contractures. In addition, muscle wasting, osteoporosis, joint stiffness and malalignment can increase the risk of fractures. |
| Gay et al., 2015 [52] | Retrospective study (on increased fracture rates in PWH: a 10-year single institution retrospective analysis) | 382 patients (316 with haemophilia A vs. 66 with haemophilia B) Age: 20 (±18) vs. 26 (±21) Sex: NA | PWH had an increased fracture rate compared with the control population. PWH with mild to moderate haemophilia had a significantly reduced risk of fracture compared with those with severe disease. | Increased fracture risk with increasing severity of haemophilia, coupled with the evidence for bleeding-independent mechanisms of decreased skeletal health, suggest factor replacement may directly impact bone health and fracture risk in PWH. | PWH have a higher risk of reduced bone mineral density than the general population. It is currently unclear how this predilection for reduced bone mineral density translates into fracture rates. |
| Lee et al., 2007 [53] | Case report (comprehensive report on the management of a cohort of patients with fracture of neck of femur in haemophilia) | 11 Age: mean age was 30 years (range: 16–55) Sex: NA | In PWH, most of the femoral neck fractures (9 out of 11) are seen almost two decades earlier than in general population (where they occur in patients over 50 years of age) | In PWH, femoral neck fractures can be treated as in the general population, with modest dose of factor replacement. Postoperatively, prolonged use of plaster immobilization should be avoided and early mobilization of the ipsilateral knee joint should be initiated. | Although physical activity is often reduced in PWH, poor musculature, osteoporosis and haemophilic bone changes may predispose to an increased risk of fractures. |
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mancuso, M.E.; Mahlangu, J.N.; Pipe, S.W. The changing treatment landscape in haemophilia: From standard half-life clotting factor concentrates to gene editing. Lancet 2021, 397, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.D.; Connors, J.M. Factor XI Deficiency. Hematol. Oncol. Clin. N. Am. 2021, 35, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Castaman, G.; Matino, D. Hemophilia A and B: Molecular and clinical similarities and differences. Haematologica 2019, 104, 1702–1709. [Google Scholar] [CrossRef]
- Kovacs, C.S. Hemophilia, low bone mass, and osteopenia/osteoporosis. Transfus. Apher. Sci. 2008, 38, 33–40. [Google Scholar] [CrossRef]
- Berntorp, E.; Fischer, K.; Hart, D.P.; Mancuso, M.E.; Stephensen, D.; Shapiro, A.D.; Blanchette, V. Haemophilia. Nat. Rev. Dis. Primers 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Ashritha, A.; Delhi Kumar, C.G.; Sahoo, J.; Nalini, P. Evaluation of Bone Mineral Density in Children With Hemophilia: An Observational Case-Control Study. J. Pediatr. Hematol. Oncol. 2019, 41, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.P.; Jijina, F.; Ghosh, K.; Madkaikar, M.; Shrikhande, M.; Nema, M. Osteoporosis in young haemophiliacs from western India. Am. J. Hematol. 2007, 82, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Recht, M.; Liel, M.S.; Turner, R.T.; Klein, R.F.; Taylor, J.A. The bone disease associated with factor VIII deficiency in mice is secondary to increased bone resorption. Haemophilia 2013, 19, 908–912. [Google Scholar] [CrossRef]
- Taves, S.; Sun, J.; Livingston, E.W.; Chen, X.; Amiaud, J.; Brion, R.; Hannah, W.B.; Bateman, T.A.; Heymann, D.; Monahan, P.E. Hemophilia A and B mice, but not VWF−/−mice, display bone defects in congenital development and remodeling after injury. Sci. Rep. 2019, 9, 14428. [Google Scholar] [CrossRef]
- Senger, D.R.; Perruzzi, C.A.; Papadopoulos-Sergiou, A.; Van de Water, L. Adhesive properties of osteopontin: Regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol. Biol. Cell 1994, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Pagel, C.N.; de Niese, M.R.; Abraham, L.A.; Chinni, C.; Song, S.J.; Pike, R.N.; Mackie, E.J. Inhibition of osteoblast apoptosis by thrombin. Bone 2003, 33, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gao, P.; Zhang, Q.; Jiang, Y.; Wang, O.; Xia, W.; Li, M. Pathogenesis and treatment of osteoporosis in patients with hemophilia. Arch. Osteoporos. 2023, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Montilla, M.; Atienza-Navarro, I.; Garcia-Cozar, F.J.; Castro, C.; Rodriguez-Martorell, F.J.; Ruiz, F.A. Polyphosphate Activates von Willebrand Factor Interaction with Glycoprotein Ib in the Absence of Factor VIII In Vitro. Int. J. Mol. Sci. 2022, 23, 14118. [Google Scholar] [CrossRef] [PubMed]
- Baud’huin, M.; Duplomb, L.; Teletchea, S.; Charrier, C.; Maillasson, M.; Fouassier, M.; Heymann, D. Factor VIII-von Willebrand factor complex inhibits osteoclastogenesis and controls cell survival. J. Biol. Chem. 2009, 284, 31704–31713. [Google Scholar] [CrossRef] [PubMed]
- Semenov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef]
- El-Mikkawy, D.M.E.; Elbadawy, M.A.; Abd El-Ghany, S.M.; Samaha, D. Serum Sclerostin Level and Bone Mineral Density in Pediatric Hemophilic Arthropathy. Indian J. Pediatr. 2019, 86, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Vakalopoulou, S.; Christoulas, D.; Paschou, S.A.; Papatheodorou, A.; Garipidou, V.; Kokkoris, P.; Terpos, E. The role of sclerostin/dickkopf-1 and receptor activator of nuclear factor kB ligand/osteoprotegerin signalling pathways in the development of osteoporosis in patients with haemophilia A and B: A cross-sectional study. Haemophilia 2018, 24, 316–322. [Google Scholar] [CrossRef]
- Giordano, P.; Brunetti, G.; Lassandro, G.; Notarangelo, L.D.; Luciani, M.; Mura, R.M.; Lazzareschi, I.; Santagostino, E.; Piacente, L.; Ventura, A.; et al. High serum sclerostin levels in children with haemophilia A. Br. J. Haematol. 2016, 172, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Nogami, K.; Shima, M. Current and future therapies for haemophilia-Beyond factor replacement therapies. Br. J. Haematol. 2023, 200, 23–34. [Google Scholar] [CrossRef]
- Hermans, C.; de Moerloose, P.; Dolan, G. Clinical management of older persons with haemophilia. Crit. Rev. Oncol. Hematol. 2014, 89, 197–206. [Google Scholar] [CrossRef]
- Knobe, K.; Berntorp, E. Haemophilia and joint disease: Pathophysiology, evaluation, and management. J. Comorb. 2011, 1, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef]
- van Vulpen, L.F.D.; Holstein, K.; Martinoli, C. Joint disease in haemophilia: Pathophysiology, pain and imaging. Haemophilia 2018, 24 (Suppl. S6), 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lafeber, F.P.; Miossec, P.; Valentino, L.A. Physiopathology of haemophilic arthropathy. Haemophilia 2008, 14 (Suppl. S4), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gualtierotti, R.; Solimeno, L.P.; Peyvandi, F. Hemophilic arthropathy: Current knowledge and future perspectives. J. Thromb. Haemost. 2021, 19, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, C.; Ying, J.; Wang, P.; Xu, T.; Chen, D.; Jin, H.; Tong, P. Inflammatory focal bone destruction in femoral heads with end-stage haemophilic arthropathy: A study on clinic samples with micro-CT and histological analyses. Haemophilia 2015, 21, e472–e478. [Google Scholar] [CrossRef] [PubMed]
- Zwagemaker, A.F.; Kloosterman, F.R.; Hemke, R.; Gouw, S.C.; Coppens, M.; Romano, L.G.R.; Kruip, M.; Cnossen, M.H.; Leebeek, F.W.G.; Hutten, B.A.; et al. Joint status of patients with nonsevere hemophilia A. J. Thromb. Haemost. 2022, 20, 1126–1137. [Google Scholar] [CrossRef]
- Anagnostis, P.; Karras, S.; Paschou, S.A.; Goulis, D.G. Haemophilia A and B as a cause for secondary osteoporosis and increased fracture risk. Blood Coagul. Fibrinolysis 2015, 26, 599–603. [Google Scholar] [CrossRef]
- Paschou, S.A.; Anagnostis, P.; Karras, S.; Annweiler, C.; Vakalopoulou, S.; Garipidou, V.; Goulis, D.G. Bone mineral density in men and children with haemophilia A and B: A systematic review and meta-analysis. Osteoporos. Int. 2014, 25, 2399–2407. [Google Scholar] [CrossRef]
- Posma, J.J.; Posthuma, J.J.; Spronk, H.M. Coagulation and non-coagulation effects of thrombin. J. Thromb. Haemost. 2016, 14, 1908–1916. [Google Scholar] [CrossRef]
- Katsarou, O.; Terpos, E.; Chatzismalis, P.; Provelengios, S.; Adraktas, T.; Hadjidakis, D.; Kouramba, A.; Karafoulidou, A. Increased bone resorption is implicated in the pathogenesis of bone loss in hemophiliacs: Correlations with hemophilic arthropathy and HIV infection. Ann. Hematol. 2010, 89, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tuan, S.H.; Hu, L.Y.; Sun, S.F.; Huang, W.Y.; Chen, G.B.; Li, M.H.; Liou, I.H. Risk of osteoporotic fractures as a consequence of haemophilia: A nationwide population-based cohort study. Haemophilia 2019, 25, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, A.; Xourafa, A.; Rapisarda, R.; Zanoli, L.; Signorelli, S.S.; Castellino, P. Hematological Diseases and Osteoporosis. Int. J. Mol. Sci. 2020, 21, 3538. [Google Scholar] [CrossRef]
- Ardoino, I.; Franchi, C.; Nobili, A.; Mannucci, P.M.; Corli, O.; Investigators, R. Pain and Frailty in Hospitalized Older Adults. Pain Ther. 2020, 9, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Pai, Y.Y.; Wang, J.D.; Ho, H.E.; Chou, Y.J.; Ho, W.C.; Chan, W.C.; Chu, W.M.; Tsan, Y.T. Risk of Fractures, Repeated Fractures and Osteoporotic Fractures among Patients with Hemophilia in Taiwan: A 14-Year Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2022, 20, 525. [Google Scholar] [CrossRef]
- Mosekilde, L.; Torring, O.; Rejnmark, L. Emerging anabolic treatments in osteoporosis. Curr. Drug Saf. 2011, 6, 62–74. [Google Scholar] [CrossRef]
- D’Amelio, P.; Tamone, C.; Sassi, F.; D’Amico, L.; Roato, I.; Patane, S.; Ravazzoli, M.; Veneziano, L.; Ferracini, R.; Pescarmona, G.P.; et al. Teriparatide increases the maturation of circulating osteoblast precursors. Osteoporos. Int. 2012, 23, 1245–1253. [Google Scholar] [CrossRef]
- Smith, M.R.; Egerdie, B.; Hernandez Toriz, N.; Feldman, R.; Tammela, T.L.; Saad, F.; Heracek, J.; Szwedowski, M.; Ke, C.; Kupic, A.; et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2009, 361, 745–755. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. Osteoporosis in hemophilia: What is its importance in clinical practice? Expert Rev. Hematol. 2022, 15, 697–710. [Google Scholar] [CrossRef]
- Gamal Andrawes, N.; Hashem Fayek, M.; Salah El-Din, N.; Atef Mostafa, R. Effect of low-dose factor VIII prophylaxis therapy on bone mineral density and 25(OH) vitamin D level in children with severe haemophilia A. Haemophilia 2020, 26, 325–332. [Google Scholar] [CrossRef]
- Strauss, A.C.; Muellejans, P.; Koob, S.; Goldmann, G.; Pennekamp, P.H.; Wallny, T.A.; Oldenburg, J.; Strauss, A.C. Osteoporosis Remains Constant in Patients with Hemophilia-Long-Term Course in Consideration of Comorbidities. Hamostaseologie, 2023; ahead of print. [Google Scholar] [CrossRef]
- Petkovic, M.J.; Tran, H.A.; Ebeling, P.R.; Zengin, A. Osteoporosis management and falls prevention in patients with haemophilia: Review of haemophilia guidelines. Haemophilia 2022, 28, 388–396. [Google Scholar] [CrossRef]
- Masi, L.; Ferrari, S.; Javaid, M.K.; Papapoulos, S.; Pierroz, D.D.; Brandi, M.L.; IOF Skeletal Rare Diseases Working Group. Bone fragility in patients affected by congenital diseases non skeletal in origin. Orphanet. J. Rare Dis. 2021, 16, 11. [Google Scholar] [CrossRef]
- Ulivieri, F.M.; Rebagliati, G.A.A.; Piodi, L.P.; Solimeno, L.P.; Pasta, G.; Boccalandro, E.; Fasulo, M.R.; Mancuso, M.E.; Santagostino, E. Usefulness of bone microarchitectural and geometric DXA-derived parameters in haemophilic patients. Haemophilia 2018, 24, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, E.; Alito, A.; Zanella, C.; Busi, L.; Mangone, G.; Scarselli, M.; Pasquetti, P. Lower limbs heterometry correction in patients with osteoporosis and increased risk of falls. Clin. Cases Miner Bone Metab. 2017, 14, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Kempton, C.L.; Antoniucci, D.M.; Rodriguez-Merchan, E.C. Bone health in persons with haemophilia. Haemophilia 2015, 21, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Franco, P. Osteoporosis in haemophilic patient, rehabilitative aspects. Clin. Cases Miner. Bone Metab. 2012, 9, 96–99. [Google Scholar]
- Berntorp, E.; Hermans, C.; Solms, A.; Poulsen, L.; Mancuso, M.E. Optimising prophylaxis in haemophilia A: The ups and downs of treatment. Blood Rev. 2021, 50, 100852. [Google Scholar] [CrossRef]
- Alito, A.; Basile, G.C.; Bruschetta, D.; Berescu, G.L.; Cavallaro, F.; Postorino, A.D.; Scarcella, E.; Ragonese, M.; Cannavo, S.; Tisano, A. Association between pain, arthropathy and health-related quality of life in patients suffering from acromegaly. A cross-sectional study. Folia Med. 2023, 65, 37–45. [Google Scholar] [CrossRef]
- Atilla, B.; Guney-Deniz, H. Musculoskeletal treatment in haemophilia. EFORT Open Rev. 2019, 4, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.D.; Lee, S.C.; Liel, M.S.; Sochacki, P.; Recht, M.; Taylor, J.A. Increased fracture rates in people with haemophilia: A 10-year single institution retrospective analysis. Br. J. Haematol. 2015, 170, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.N.; Srivastava, A.; Nithyananth, M.; Kumar, P.; Cherian, V.M.; Viswabandya, A.; Mathews, V.; George, B.; Venkatesh, K.; Nair, S.C.; et al. Fracture neck of femur in haemophilia A—Experience from a cohort of 11 patients from a tertiary centre in India. Haemophilia 2007, 13, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.; Konkle, B.A.; Sood, S.L. Aging among persons with hemophilia: Contemporary concerns. Semin. Hematol. 2016, 53, 35–39. [Google Scholar] [CrossRef]
- Angelini, D.; Sood, S.L. Managing older patients with hemophilia. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 41–47. [Google Scholar] [CrossRef]
- Caviglia, H.; Landro, M.E.; Galatro, G.; Candela, M.; Neme, D. Epidemiology of fractures in patients with haemophilia. Injury 2015, 46, 1885–1890. [Google Scholar] [CrossRef]
- Strauss, A.C.; Pennekamp, P.H.; Placzek, R.; Schmolders, J.; Friedrich, M.J.; Oldenburg, J.; Burger, C.; Muller, M.C. Perioperative management and outcome of fracture treatment in patients with haemophilia without inhibitors. Haemophilia 2016, 22, e30–e35. [Google Scholar] [CrossRef]
- Anagnostis, P.; Karras, S.N.; Vakalopoulou, S.; Terpos, E. Haemophilia and low bone mass. Ok, but what about fracture risk? Haemophilia 2016, 22, 11–14. [Google Scholar] [CrossRef]
- Gebetsberger, J.; Schirmer, M.; Wurzer, W.J.; Streif, W. Low Bone Mineral Density in Hemophiliacs. Front. Med. 2022, 9, 794456. [Google Scholar] [CrossRef]
- Abdelrazik, N.; Reda, M.; El-Ziny, M.; Rabea, H. Evaluation of bone mineral density in children with hemophilia: Mansoura University children hospital (MUCH) experience, Mansoura, Egypt. Hematology 2007, 12, 431–437. [Google Scholar] [CrossRef]
- Anagnostis, P.; Vakalopoulou, S.; Slavakis, A.; Charizopoulou, M.; Kazantzidou, E.; Chrysopoulou, T.; Vyzantiadis, T.A.; Moka, E.; Agapidou, A.; Garipidou, V. Reduced bone mineral density in patients with haemophilia A and B in Northern Greece. Thromb. Haemost. 2012, 107, 545–551. [Google Scholar] [CrossRef]
- Lee, A.; Boyd, S.K.; Kline, G.; Poon, M.C. Premature changes in trabecular and cortical microarchitecture result in decreased bone strength in hemophilia. Blood 2015, 125, 2160–2163. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C. Complications of Muscle Hematomas in Hemophilia. Cardiovasc. Hematol. Disord. Drug Targets 2020, 20, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.J.; McLaughlin, P.; Simmonds, J.V.; Prouse, P.J.; Prelevic, G.; Gill, S.; Chowdary, P. A case-control study assessing bone mineral density in severe haemophilia A in the UK. Haemophilia 2015, 21, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Gaudio, A.; Agostino, R.M.; Morabito, N.; Bellone, F.; Lasco, A. Trabecular bone score and quantitative ultrasound measurements in the assessment of bone health in breast cancer survivors assuming aromatase inhibitors. J. Endocrinol. Investig. 2019, 42, 1337–1343. [Google Scholar] [CrossRef]
- Boccalandro, E.; Mancuso, M.E.; Riva, S.; Pisaniello, D.M.; Ronchetti, F.; Santagostino, E.; Peyvandi, F.; Solimeno, L.P.; Mannucci, P.M.; Pasta, G. Ageing successfully with haemophilia: A multidisciplinary programme. Haemophilia 2018, 24, 57–62. [Google Scholar] [CrossRef]
- Ruffilli, A.; Traina, F.; Pilla, F.; Fenga, D.; Faldini, C. Marchetti Vicenzi elastic retrograde nail in the treatment of humeral shaft fractures: Review of the current literature. Musculoskelet. Surg. 2015, 99, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, T.B.; Zhang, P.; Dang, Y.; Chen, J.H.; Xue, F.; Zhang, P.X.; Yang, M.; Xu, H.L.; Fu, Z.G.; et al. Characteristics and perioperative management of hemophilia patients with fractures. Beijing Da Xue Xue Bao Yi Xue Ban 2015, 47, 281–284. [Google Scholar]
- Stephensen, D.; Bladen, M.; McLaughlin, P. Recent advances in musculoskeletal physiotherapy for haemophilia. Ther. Adv. Hematol. 2018, 9, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Lobet, S.; Lambert, C.; Hermans, C. Stop only advising physical activity in adults with haemophilia... prescribe it now! The role of exercise therapy and nutrition in chronic musculoskeletal diseases. Haemophilia 2016, 22, e554–e556. [Google Scholar] [CrossRef]
- Boccalandro, E.A.; Begnozzi, V.; Garofalo, S.; Pasca, S.; Peyvandi, F. The evolution of physiotherapy in the multidisciplinary management of persons with haemophilia (PWH): A scoping review. Haemophilia 2023, 29, 11–20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alito, A.; Bellone, F.; Portaro, S.; Leonardi, G.; Cannavò, V.; Coppini, F.; Leonetti, D.; Catalano, A.; Squadrito, G.; Fenga, D. Haemophilia and Fragility Fractures: From Pathogenesis to Multidisciplinary Approach. Int. J. Mol. Sci. 2023, 24, 9395. https://doi.org/10.3390/ijms24119395
Alito A, Bellone F, Portaro S, Leonardi G, Cannavò V, Coppini F, Leonetti D, Catalano A, Squadrito G, Fenga D. Haemophilia and Fragility Fractures: From Pathogenesis to Multidisciplinary Approach. International Journal of Molecular Sciences. 2023; 24(11):9395. https://doi.org/10.3390/ijms24119395
Chicago/Turabian StyleAlito, Angelo, Federica Bellone, Simona Portaro, Giulia Leonardi, Vittorio Cannavò, Francesca Coppini, Danilo Leonetti, Antonino Catalano, Giovanni Squadrito, and Domenico Fenga. 2023. "Haemophilia and Fragility Fractures: From Pathogenesis to Multidisciplinary Approach" International Journal of Molecular Sciences 24, no. 11: 9395. https://doi.org/10.3390/ijms24119395
APA StyleAlito, A., Bellone, F., Portaro, S., Leonardi, G., Cannavò, V., Coppini, F., Leonetti, D., Catalano, A., Squadrito, G., & Fenga, D. (2023). Haemophilia and Fragility Fractures: From Pathogenesis to Multidisciplinary Approach. International Journal of Molecular Sciences, 24(11), 9395. https://doi.org/10.3390/ijms24119395









