Reverse-Polarization High-Performance Layer Electrochromatography—A New Approach to Anion Separation
Abstract
1. Introduction
- investigation of reverse-polarization HPLEC separation mode with our prototype equipment, using a set of solutes similar to those used before in normal-polarization HPLEC [2];
- comparison of the results with OPLC in similar separation conditions;
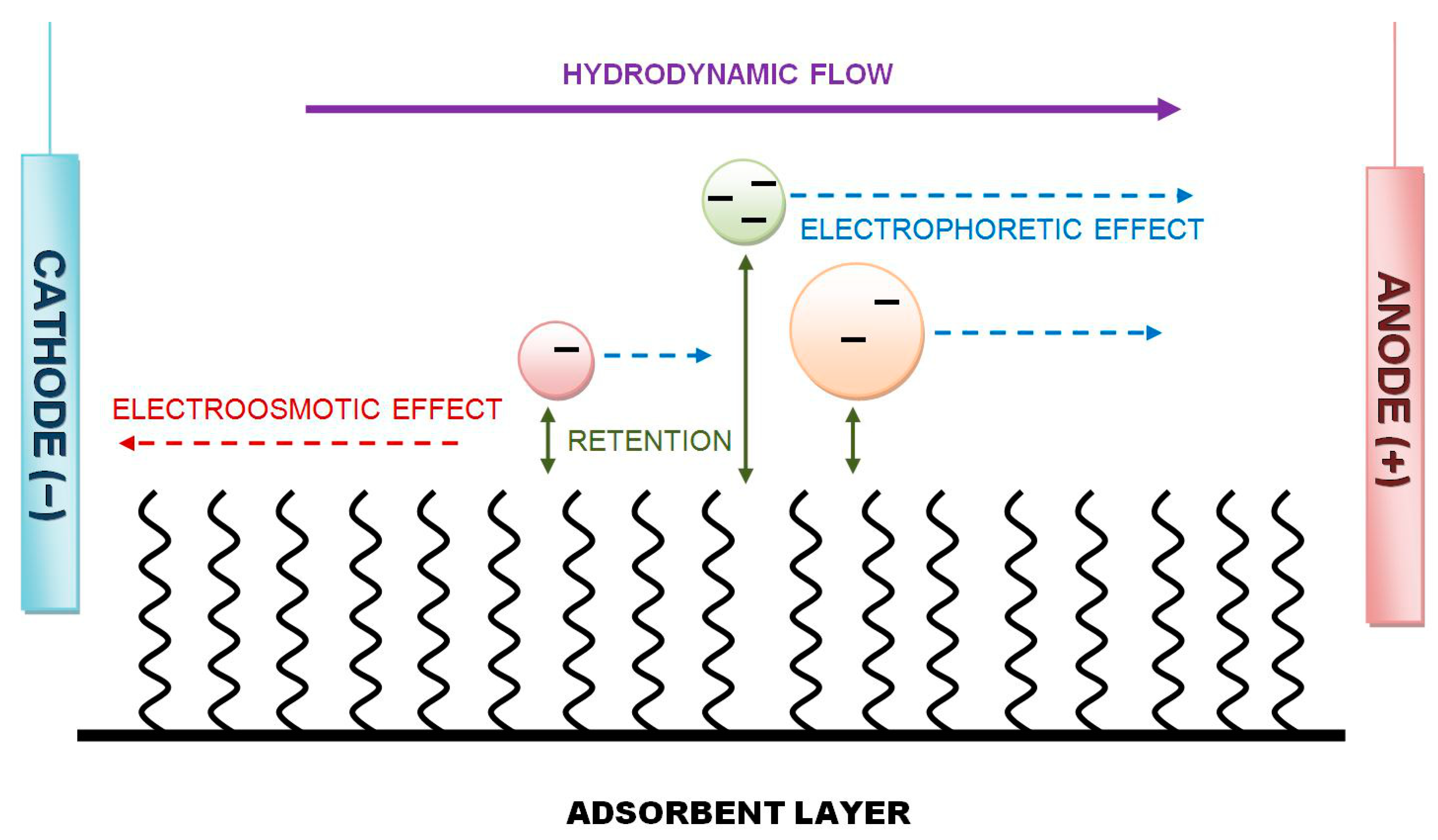
- investigation of the influence of the change in the electric field direction, especially the influence of the redirection of the electroosmotic effect against the hydrodynamic flow of the mobile phase on the total flow profile and direction, backpressure and velocity limit of the mobile phase flow, velocity and direction of solute migration, and overall performance of separation;
- evaluation of any possible side effects of the approach;
- discussion of the advantages and disadvantages of the reversed-polarization HPLEC mode and its practical potential.
2. Results and Discussion
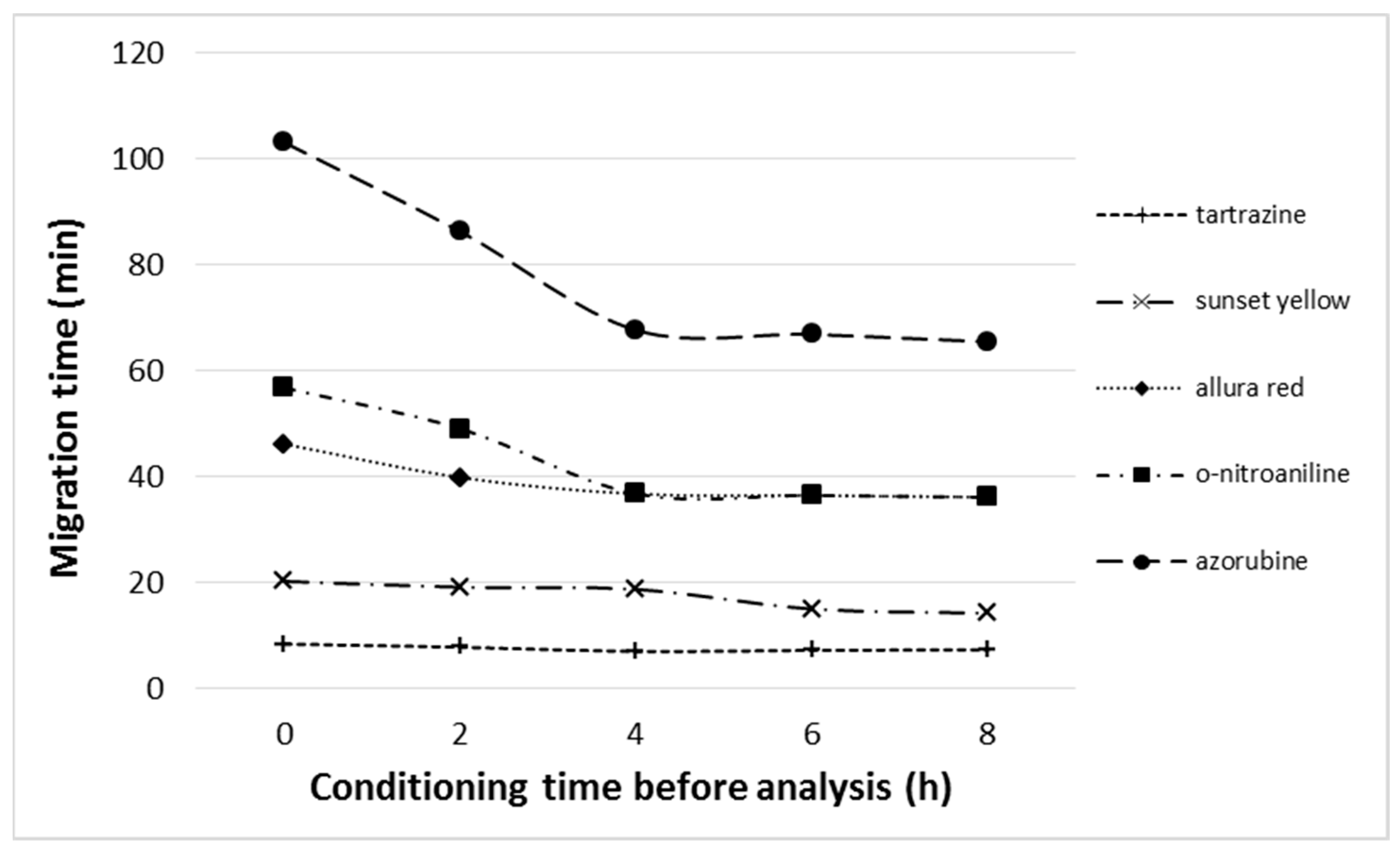
2.1. Advantages of Our Equipment and Reverse-Polarity HPLEC
2.2. Drawbacks and Limitations of Reverse-Polarization HPLEC
3. Materials and Methods
3.1. Equipment and Chemicals
- The prototype HPLEC equipment was designed and constructed in the Department of Physical Chemistry, Medical University of Lublin, and was presented and described in our previous paper [1]. For our current experiments, reverse-polarization of the separation system was set so that the cathode was placed near the inlet side of the separation chamber and the anode was placed in the outlet electrode compartment near the sample outlet tubing. All other elements of the equipment were set in a standard way, as described before [1].
- The external pressure supply unit was purchased from P.W. Rafkop (Lubartów, Poland). A high-voltage power supply EV262 was bought from Consort (Turnhout, Belgium). Two quaternary HPLC pumps Azura P6.1L, an automatic six-channel selection valve Azura V2.1S, an autosampler Azura AS6.1L, and six UV detectors Azura UVD 2.1S (with analytical flow cells—path length 3 mm, capacity 2 µL) were bought from Knauer (Berlin, Germany). A circular thermostat AD07R-20 was bought from PolyScience (Niles, MI, USA).
- Certified analytical standard dyes were purchased from the Institute of Dyes and Organic Products (Zgierz, Poland). Ammonium acetate (analytical grade) and methanol (for HPLC—super gradient) were purchased from POCH (Gliwice, Poland). Water used in all experiments was purified using an HLP demineralizer from Hydrolab (Gdańsk, Poland). Glass-backed HPTLC RP-18 W plates were purchased from Merck (Darmstadt, Germany).
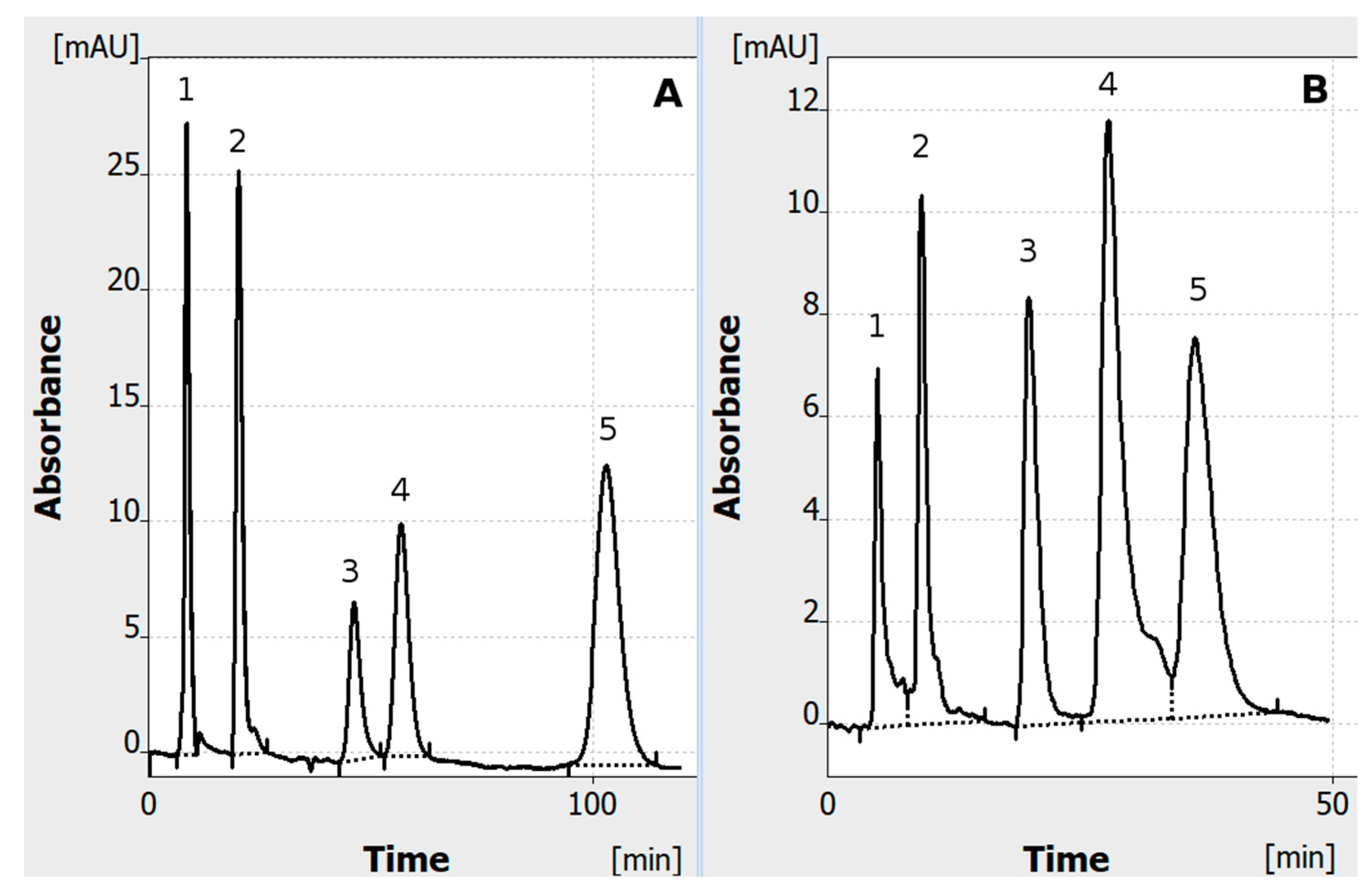
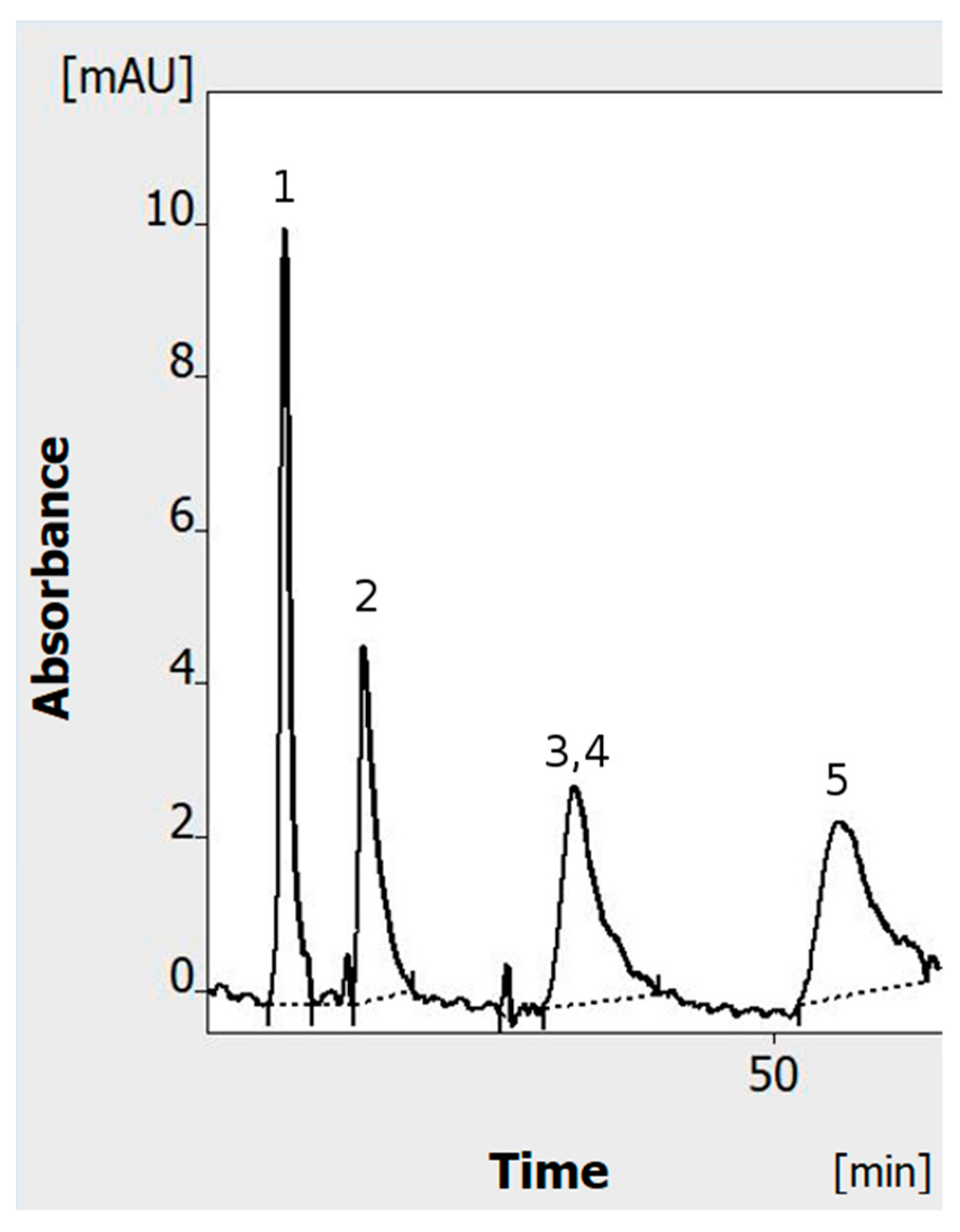
- A standard set of solutes was used (mainly food dyes; azo dyes), similar to the one used before in normal-polarization HPLEC [2] (indigotine was replaced by tartrazine, as properties/migration time of both are similar). The dye mixture was prepared by mixing and diluting of 0.5% dye stocks solutions (w/v). All stocks and the final mixture were prepared in a water/methanol solution (1/1 v/v). Finally, a dye mixture with the following composition was used: 1—tartrazine 0.0125% (w/v); 2—sunset yellow 0.025% (w/v); 3—allura red 0.025% (w/v); 4—azorubine; 0.1% (w/v); 5—o-nitroaniline—0.0125% (w/v). Except o-nitroaniline, all the solutes were azo dyes with strong acidic sulfo groups.
3.2. Experimental Conditions
- The experiments were performed in the single-channel mode at the temperature of 25 °C, applying 100 bar pressure to the cushion pressurizing the adsorbent layer. The mobile phase was a mixture of water/methanol (7/3 v/v) with the addition of 40 mM ammonium acetate (final concentration). The HPLEC experiments were performed with 4 kV polarization voltage with the cathode on the inlet side of the separation system.
- Before separation, the adsorbent layer was washed (conditioned) with the mobile phase until a completely flat baseline was obtained (for at least 1 h). The composition and flow of the mobile phase were the same as those used in the experiments. Before HPLEC separation, the voltage gradient was also applied from 0 V to the final value used for separation for 1 h. After the conditioning, chromatographic plates were used for multiple analyses. All statistics were made on the basis of five independent analyses (using the single or multiple chromatographic plates, respectively).
- In the experiments, a 1 µL sample was applied using the autosampler. The sample was injected into the stream of the mobile phase and pumped by the sample pump. The sample in the stream of the mobile phase was applied to the chromatographic plate. The mobile phase flow provided by the sample pump (after sample application) was sustained during the whole experiment.
- For both the OPLC and HPLEC experiments, the venting valve of the inlet side electrode compartment was opened, facilitating a constant wash of the electrode with the mobile phase. The flow through the venting valve was restricted to about 2% of the total mobile phase flow. Backpressure measured at the mobile phase inlet was 83 bar for OPLC and 90 bar for HPLEC separations.
- Separated solutes were detected at the 256 nm wavelength with a sampling rate of 2 Hz and time constant of 1 s. The migration time (tM) was established automatically by the software. The identification of the solutes was based on the comparison between tM of mixture components and single standards separated in the same conditions.
- Most of the HPLEC equipment modules were controlled by a computer with ClarityChrom software 7.4.1.88. Only the high-voltage power supply, the pressure supply unit, and the circular thermostat were programed independently.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gwarda, R.Ł.; Dzido, T.H. High Performance (High Pressure) Layer Electrochromatography Separation Technique: Equipment and Preliminary Results. Anal. Chem. 2022, 94, 9091–9096. [Google Scholar] [CrossRef] [PubMed]
- Gwarda, R.Ł.; Dzido, T.H. Comparison of Overpressured-Layer Chromatography and High-Performance/High-Pressure Layer Electrochromatography Using the New Prototype Equipment in Various Operational Modes. Molecules 2022, 27, 4032. [Google Scholar] [CrossRef] [PubMed]
- Mincsovics, E.; Tyihák, E. Analytical and Preparative Overpressured Layer Chromatography (OPLC). In Recent Advances in Thin-Layer Chromatography; Dallas, F.A.A., Read, H., Ruane, R.J., Wilson, I.D., Eds.; Springer: Boston, MA, USA, 1988; pp. 57–65. ISBN 978-1-4899-2223-6. [Google Scholar]
- Tyihák, E.; Mincsovics, E. Overpressured Layer Chromatography: From the Ultra-micro Chamber to On-line Use. LC-GC Int. 1991, 4, 24–33. [Google Scholar]
- Mincsovics, E.; Tyihak, E. Overpressured Layer Chromatography (OPLC)—A Flexible Tool of Analysis and Isolation. Nat. Prod. Commun. 2011, 6, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Kalasz, H. Forced-Flow Planar Chromatography in the Rear View Mirror. J. Chromatogr. Sci. 2015, 53, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Tyihák, E.; Mincsovics, E.; Móricz, Á.M. Overpressured Layer Chromatography: From the Pressurized Ultramicro Chamber to BioArena System. J. Chromatogr. A 2012, 1232, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nurok, D.; Koers, J.M.; Novotny, A.L.; Carmichael, M.A.; Kosiba, J.J.; Santini, R.E.; Hawkins, G.L.; Replogle, R.W. Apparatus and Initial Results for Pressurized Planar Electrochromatography. Anal. Chem. 2004, 76, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Dzido, T.H.; Plocharz, P.W.; Slazak, P. Apparatus for Pressurized Planar Electrochromatography in a Completely Closed System. Anal. Chem. 2006, 78, 4713–4721. [Google Scholar] [CrossRef] [PubMed]
- Dzido, T.H.; Płocharz, P.; Chomicki, A.; Hałka-Grysinska, A.; Polak, B. Pressurized Planar Electrochromatography. J. Chromatogr. A 2011, 1218, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Dzido, T.H.; Mróz, J.; Jóźwiak, G.W. Adaptation of a Horizontal DS Chamber to Planar Electrochromatography in a Closed System. J. Planar. Chromatogr. 2004, 17, 404–410. [Google Scholar] [CrossRef]
- Kevin, D. Altria Analysis of Inorganic Anions by Capillary Electrophoresis. LCGC Eur. 2011, 24, 32–36. [Google Scholar]
- Gwarda, R.Ł.; Szwerc, W.; Aletańska-Kozak, M.; Klimek-Turek, A.; Torbicz, A.; Chomicki, A.; Kocjan, R.; Matosiuk, D.; Dzido, T.H. The Influence of Metallic Impurities on the Free Silanol Activity of Commercial Thin-Layer Chromatography Adsorbents Demonstrated by Retention Changes of Basic/Amphoteric Compounds Such as Peptides. J. Planar. Chromatogr. 2017, 30, 378–385. [Google Scholar] [CrossRef]
- Ru, Q.-H.; Luo, G.-A.; Fu, Y.-R. Effect of Various Modes of Pressurization on the Separation in Capillary Electrochromatography. J. Chromatogr. A 2001, 924, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.B.; Riaz, M. Determination of Cations and Anions in Environmental Samples by HPLC: Review. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 1045–1064. [Google Scholar] [CrossRef]
- Paull, B.; Nesterenko, P.N. Ion Chromatography. In Liquid Chromatography; Elsevier: Amsterdam, The Netherlands, 2013; pp. 157–191. ISBN 978-0-12-415807-8. [Google Scholar]
- Kaniansky, D.; Masár, M.; Marák, J.; Bodor, R. Capillary Electrophoresis of Inorganic Anions. J. Chromatogr. A 1999, 834, 133–178. [Google Scholar] [CrossRef] [PubMed]
- Altria, K.D. Overview of Capillary Electrophoresis and Capillary Electrochromatography. J. Chromatogr. A 1999, 856, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Breadmore, M.C.; Hilder, E.F.; Macka, M.; Haddad, P.R. Determination of Inorganic Anions by Capillary Electrochromatography. TrAC Trends Anal. Chem. 2001, 20, 355–364. [Google Scholar] [CrossRef]

| OPLC after Conditioning with Voltage, Single Plate | HPLEC Single Plate | HPLEC between Plates/Days | |
|---|---|---|---|
| tartrazine | 1.37% | 1.00% | 1.35% |
| sunset yellow | 2.02% | 2.46% | 3.08% |
| allura red | 1.01% | 1.20% | 3.03% |
| o-nitroaniline | 1.01% | 1.07% | 2.15% |
| azorubine | 3.45% | 2.08% | 2.46% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwarda, R.Ł.; Dzido, T.H. Reverse-Polarization High-Performance Layer Electrochromatography—A New Approach to Anion Separation. Int. J. Mol. Sci. 2023, 24, 9389. https://doi.org/10.3390/ijms24119389
Gwarda RŁ, Dzido TH. Reverse-Polarization High-Performance Layer Electrochromatography—A New Approach to Anion Separation. International Journal of Molecular Sciences. 2023; 24(11):9389. https://doi.org/10.3390/ijms24119389
Chicago/Turabian StyleGwarda, Radosław Łukasz, and Tadeusz Henryk Dzido. 2023. "Reverse-Polarization High-Performance Layer Electrochromatography—A New Approach to Anion Separation" International Journal of Molecular Sciences 24, no. 11: 9389. https://doi.org/10.3390/ijms24119389
APA StyleGwarda, R. Ł., & Dzido, T. H. (2023). Reverse-Polarization High-Performance Layer Electrochromatography—A New Approach to Anion Separation. International Journal of Molecular Sciences, 24(11), 9389. https://doi.org/10.3390/ijms24119389






