Anti-Candida albicans Effects and Mechanisms of Theasaponin E1 and Assamsaponin A
Abstract
1. Introduction
2. Results
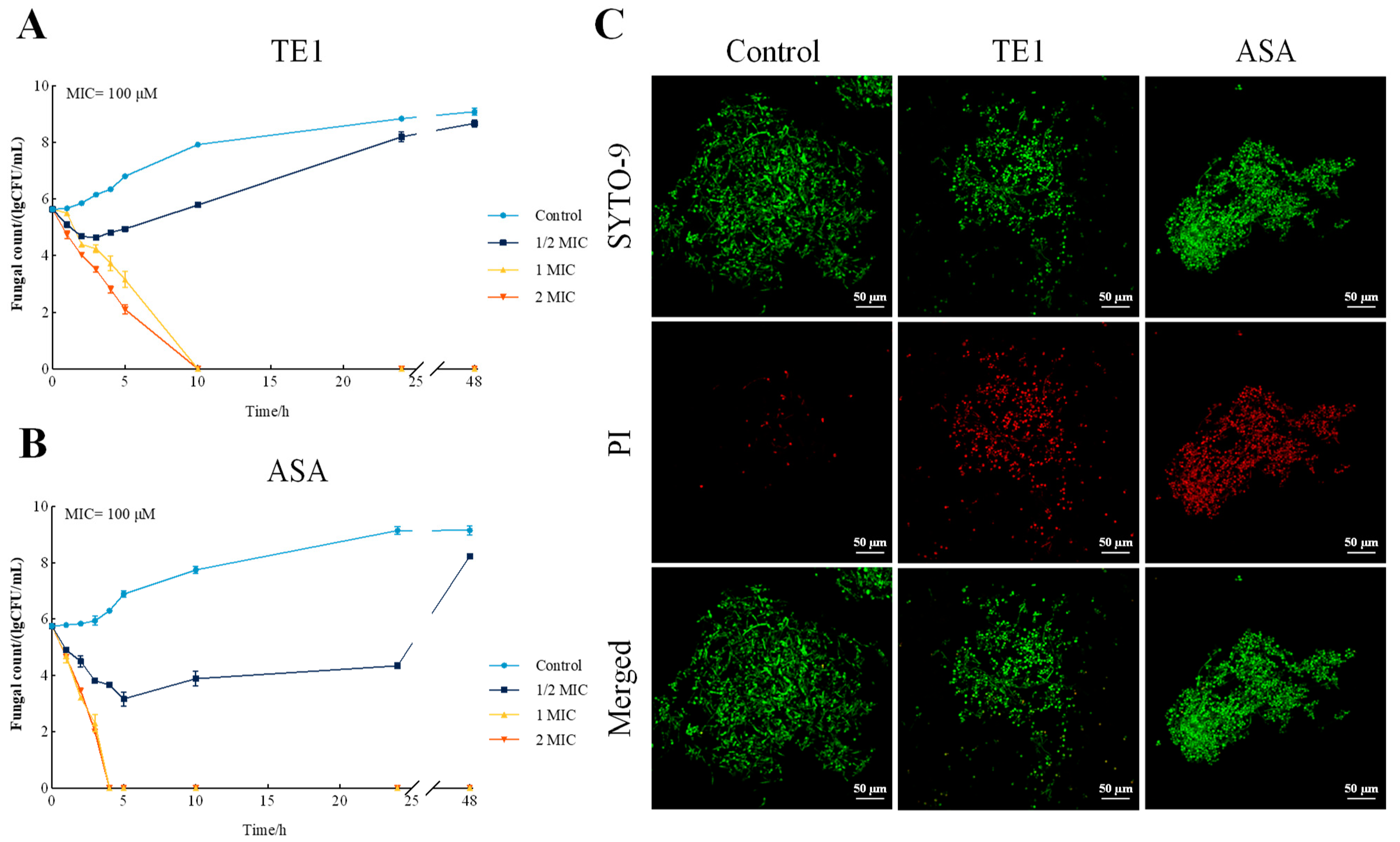
2.1. Antifungal Activities of TE1 and ASA
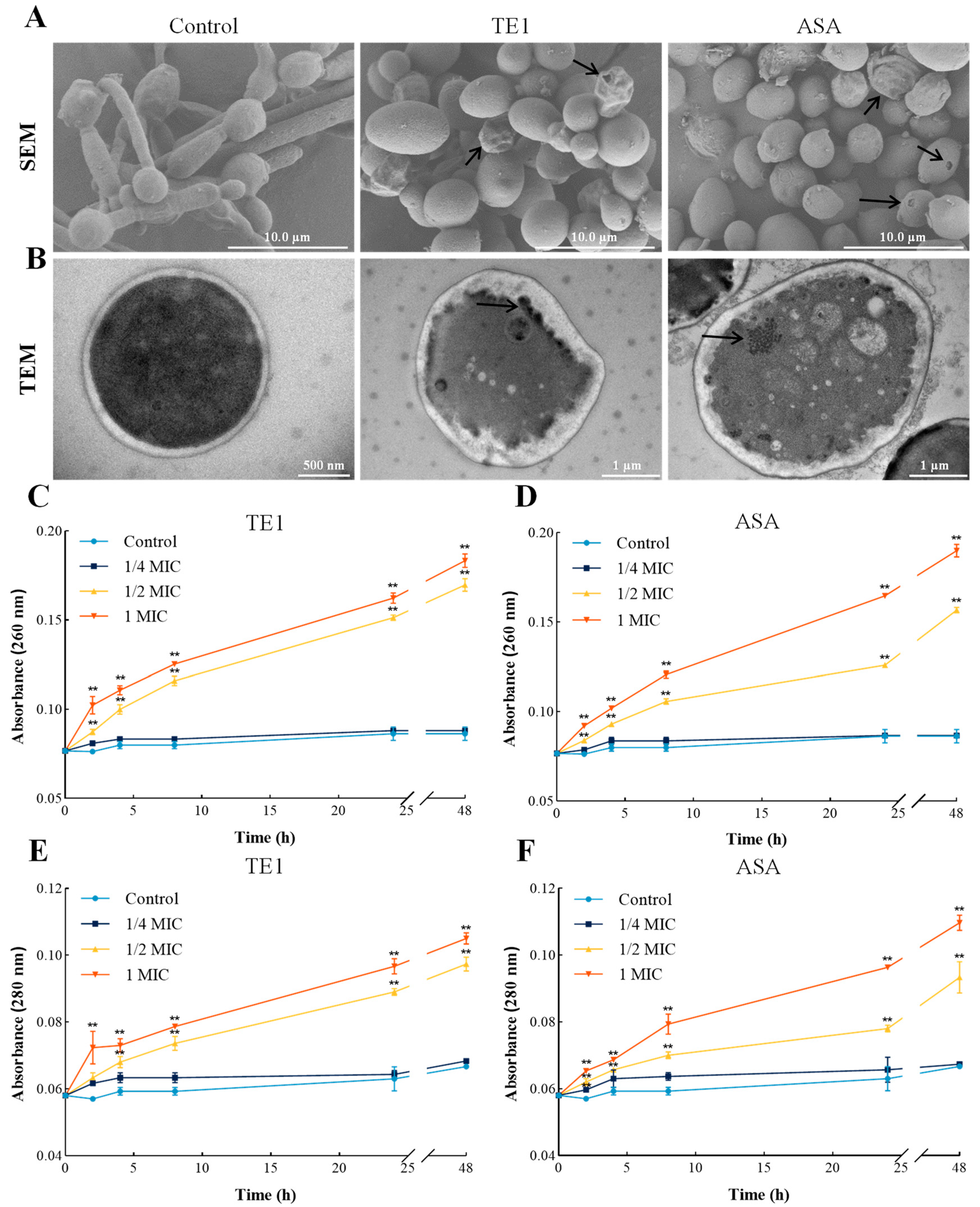
2.2. Effects of TE1 and ASA on the Cell Wall and Membrane of C. albicans
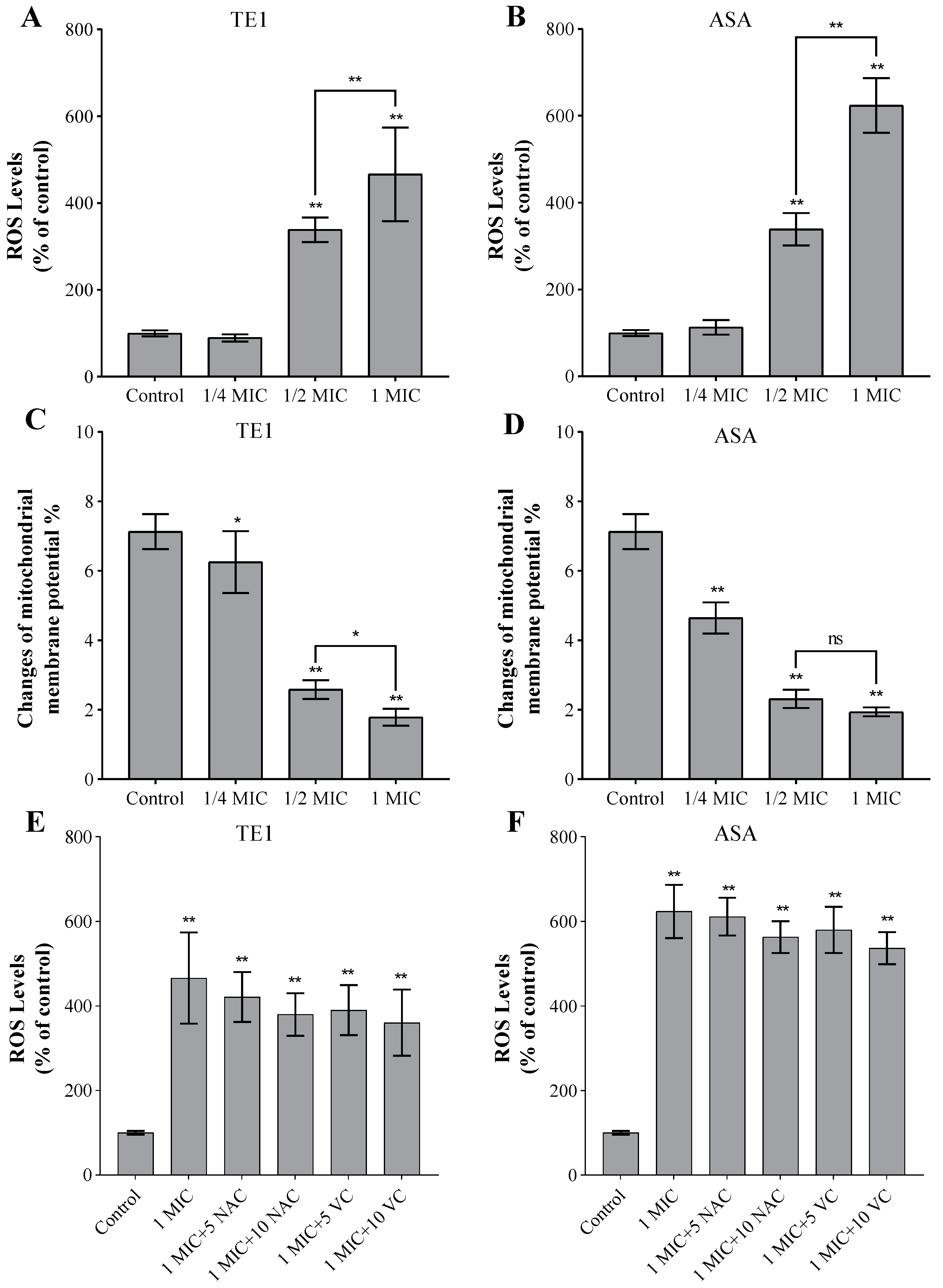
2.3. Effect of TE1 and ASA on Intracellular Reactive Oxygen Species (ROS) and Mitochondria
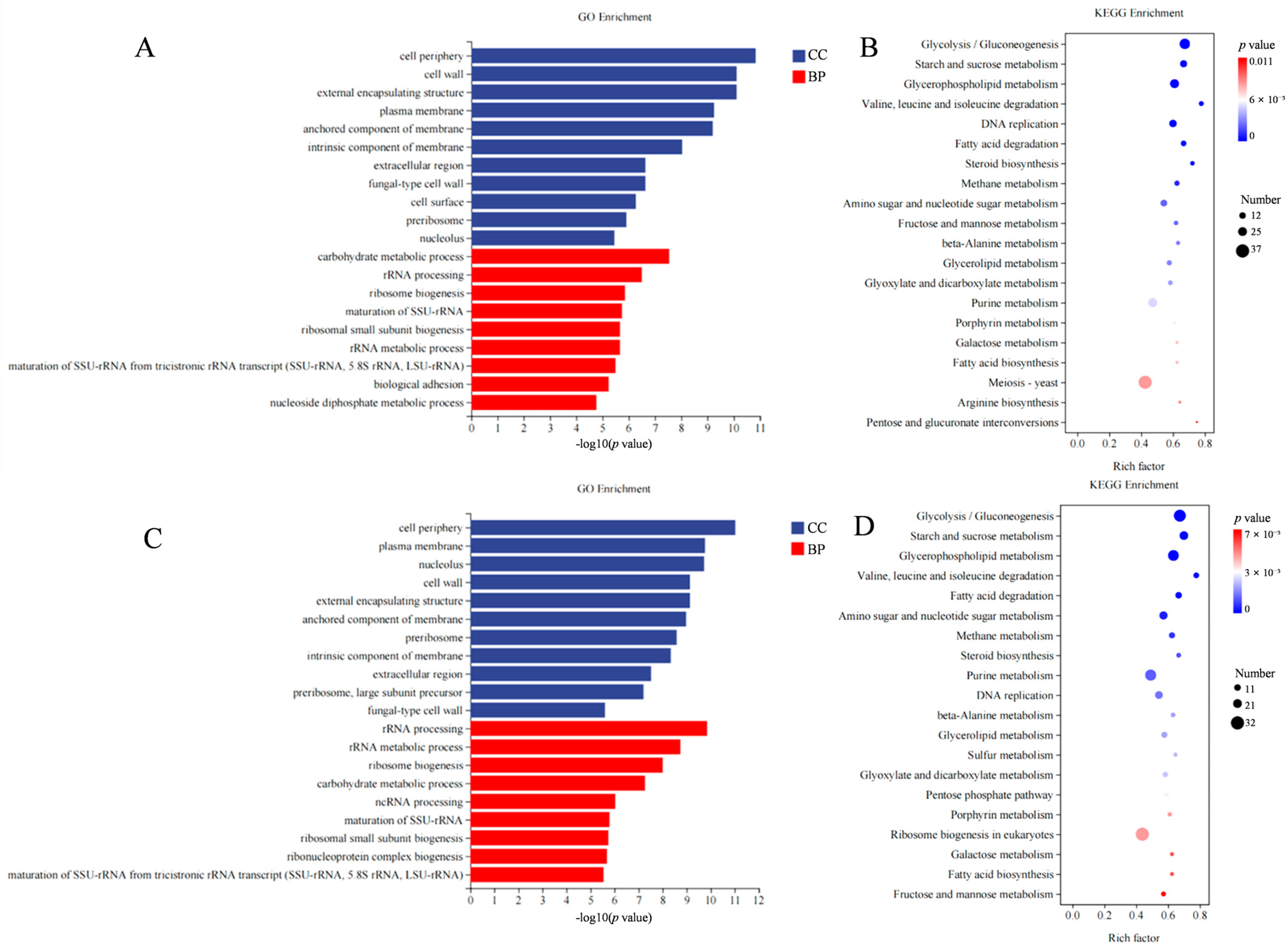
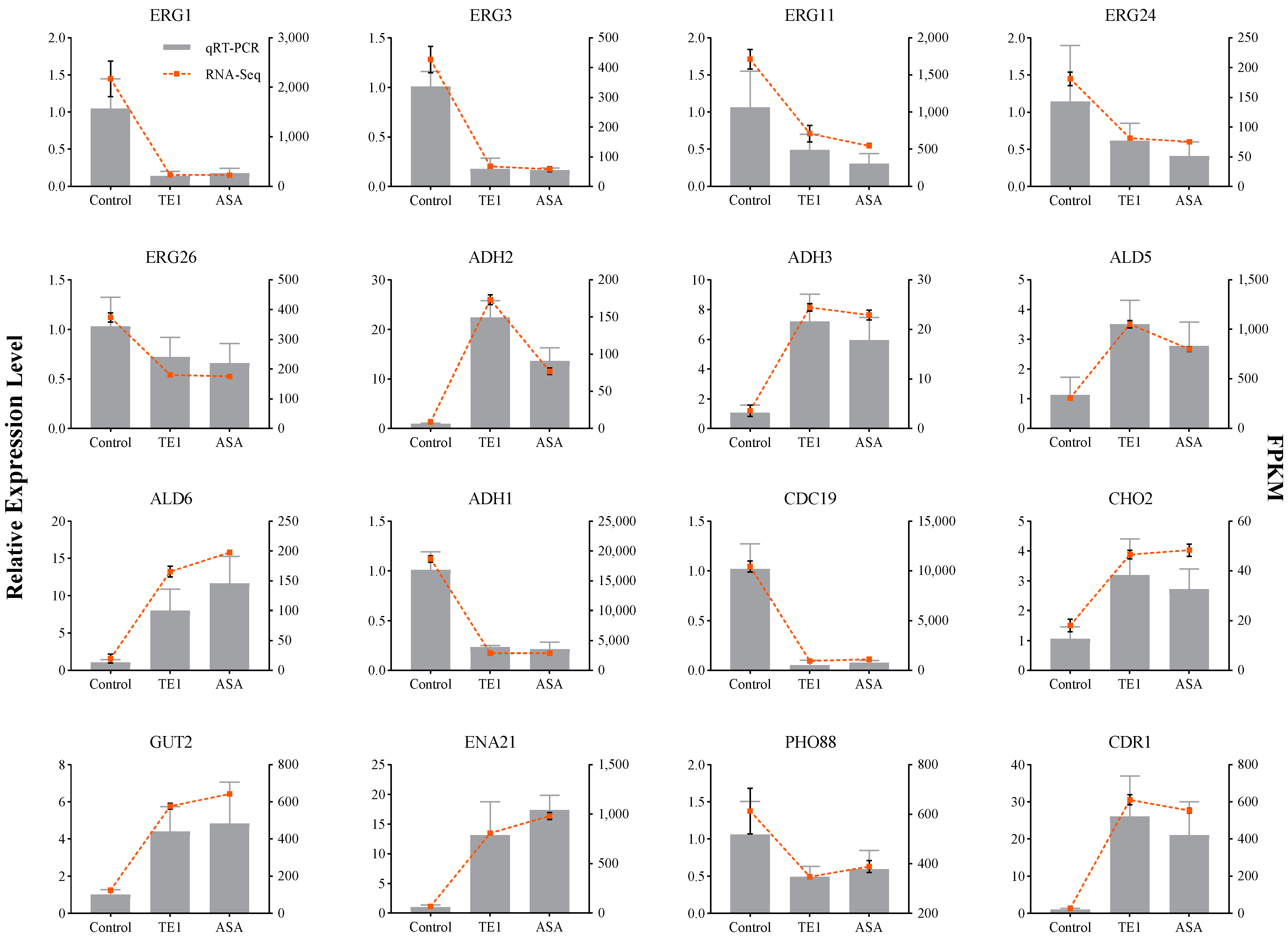
2.4. Transcriptomic Analysis of C. albicans with TE1 and ASA Treatment
3. Discussion
4. Materials and Methods
4.1. Strains and Chemicals
4.2. Preparation of TE1 and ASA
4.3. Antifungal Susceptibility Tests
4.4. Time–Kill Curves
4.5. Live/Dead Fungicidal Fluorescent Imaging
4.6. Morphology Observation of C. albicans ATCC 10231
4.7. Cellular Leakage Effect
4.8. Sorbitol Protection Assay
4.9. Ergosterol Binding Assay
4.10. Reactive Oxygen Species and Membrane Potential Assays
4.11. The Effect of the Antioxidant on the Fungicidal Activity of Saponins
4.12. Transcriptome Analysis
4.13. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) Validation
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erb Downward, J.R.; Falkowski, N.R.; Mason, K.L.; Muraglia, R.; Huffnagle, G.B. Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans. Sci. Rep. 2013, 3, 2191. [Google Scholar] [CrossRef] [PubMed]
- da Silva Dantas, A.; Lee, K.K.; Raziunaite, I.; Schaefer, K.; Wagener, J.; Yadav, B.; Gow, N.A. Cell biology of Candida albicans-host interactions. Curr. Opin. Microbiol. 2016, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Gaffen, S.L.; Swidergall, M. Innate immunity to mucosal Candida Infections. J. Fungi. 2017, 3, 60. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S.; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Liu, H.P. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef]
- Pfaller, M.A. Nosocomial candidiasis: Emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 1996, 22, S89–S94. [Google Scholar] [CrossRef]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- Hu, W.L.; Liu, J.X.; Ye, J.; Wu, Y.M.; Guo, Y.Q. Effect of tea saponin on rumen fermentation in vitro. Anim. Feed Sci. Technol. 2005, 120, 333–339. [Google Scholar] [CrossRef]
- Yang, W.S.; Ko, J.; Kim, E.; Kim, J.H.; Park, J.G.; Sung, N.Y.; Kim, H.G.; Yang, S.; Rho, H.S.; Hong, Y.D.; et al. 21-O-angeloyltheasapogenol E3, a novel triterpenoid saponin from the seeds of tea plants, inhibits macrophage-mediated inflammatory responses in a NF-κB-dependent manner. Mediat. Inflamm. 2014, 2014, 658351. [Google Scholar] [CrossRef]
- Yang, H.; Cai, R.; Kong, Z.Y.; Chen, Y.; Cheng, C.; Qi, S.H.; Gu, B. Teasaponin ameliorates murine colitis by regulating gut microbiota and suppressing the immune system response. Front. Med. 2020, 7, 584369. [Google Scholar] [CrossRef]
- Yang, H.; Shao, X.; Yu, G.H. Effect of tea saponin on blood pressure in spontaneously hypertensive rats. Chin. J. Clin. Healthc. 2007, 116, 388–395. (In Chinese) [Google Scholar]
- Zhao, W.H.; Li, N.; Zhang, X.R.; Wang, W.L.; Li, J.Y.; Si, Y.Y. Cancer chemopreventive theasaponin derivatives from the total tea seed saponin of Camellia sinensis. J. Funct. Foods 2015, 12, 192–198. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kanegae, T.; Nagoya, T.; Shimamura, M.; Kato, T.; Watanabe, S.; Kawaguchi, M. Effects of seed saponins of Thea sinensis L. (Ryokucha saponin) on alcohol absorption and metabolism. Alcohol Alcohol. 1993, 28, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.P.; Jiang, X.D.; Chen, J.J.; Zhang, S.S. Control of postharvest grey mould decay of nectarine by tea polyphenol combined with tea saponin. Lett. Appl. Microbiol. 2013, 57, 502–509. [Google Scholar] [CrossRef]
- Zhang, X.F.; Yang, S.L.; Han, Y.Y.; Zhao, L.; Lu, G.L.; Xia, T.; Gao, L.P. Qualitative and quantitative analysis of triterpene saponins from tea seed pomace (Camellia oleifera Abel) and their activities against bacteria and fungi. Molecules 2014, 19, 7568–7580. [Google Scholar] [CrossRef]
- Sagesaka, Y.M.; Uemura, T.; Suzuki, Y.; Sugiura, T.; Yoshida, M.; Yamaguchi, K.; Kyuki, K. Antimicrobial and anti-inflammatory actions of tea-leaf saponin. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1996, 116, 238–243. [Google Scholar] [CrossRef]
- Li, S.H.; Guo, K.M.; Yin, N.N.; Shan, M.Z.; Zhu, Y.; Li, Y. Teasaponin induces reactive oxygen species-mediated mitochondrial dysfunction in Candida albicans. J. Chin. Pharm. Sci. 2021, 30, 895–903. [Google Scholar] [CrossRef]
- Guo, N.; Tong, T.T.; Ren, N.; Tu, Y.Y.; Li, B. Saponins from seeds of Genus Camellia: Phytochemistry and bioactivity. Phytochemistry 2018, 149, 42–55. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Qin, F.; Wang, Q.; Zhang, C.L.; Fang, C.Y.; Zhang, L.P.; Chen, H.L.; Zhang, M.; Cheng, F. Efficacy of antifungal drugs in the treatment of vulvovaginal candidiasis: A Bayesian network meta-analysis. Infect. Drug Resist. 2018, 11, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.Y.; Li, S.S.; Lin, Z.T.; Liu, M.X.; Wang, D.D.; Wang, H.; Chen, S.Z. Development of propidium iodide as a fluorescence probe for the on-line screening of non-specific DNA-intercalators in Fufang Banbianlian Injection. J. Chromatogr. A. 2016, 1463, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Turecka, K.; Chylewska, A.; Kawiak, A.; Waleron, K.F. Antifungal activity and mechanism of action of the Co(III) coordination complexes with diamine chelate ligands against reference and clinical strains of Candida spp. Front. Microbiol. 2018, 9, 1594. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, A.A.; de Oliveira, H.M.B.F.; de Sousa, J.P.; Meireles, D.; de Azevedo Maia, G.L.; Filho, J.M.B.; Lima, E.O. In vitro anti-Candida activity and mechanism of action of the flavonoid isolated from Praxelis clematidea against Candida albicans species. J. App. Pharm. Sci. 2016, 6, 66–69. [Google Scholar] [CrossRef]
- Simons, V.; Morrissey, J.P.; Latijnhouwers, M.; Csukai, M.; Cleaver, A.; Yarrow, C.; Osbourn, A. Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2006, 50, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Keukens, E.A.; De, V.T.; Fabrie, C.H.; Demel, R.A.; Jongen, W.M.; De, K.B. Dual specificity of sterol-mediated glycoalkaloid induced membrane disruption. Biochimica et Biophysica Acta (BBA) Biomembr. 1992, 1110, 127–136. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Garvey, E.P.; Hoekstra, W.J.; Schotzinger, R.J.; Sobel, J.D.; Lilly, E.A.; Fidel, P.L., Jr. Efficacy of the clinical agent VT-1161 against fluconazole-sensitive and -resistant Candida albicans in a murine model of vaginal candidiasis. Antimicrob. Agents Chemother. 2015, 59, 5567–5573. [Google Scholar] [CrossRef]
- Peláez, F.; Cabello, A.; Platas, G.; Díez, M.T.; del Val, A.G.; Basilio, A.; Martán, I.; Vicente, F.; Bills, G.E.; Giacobbe, R.A.; et al. The discovery of enfumafungin, a novel antifungal compound produced by an endophytic Hormonema species biological activity and taxonomy of the producing organisms. Syst. Appl. Microbiol. 2000, 23, 333–343. [Google Scholar] [CrossRef]
- Jiménez-Ortigosa, C.; Paderu, P.; Motyl, M.R.; Perlin, D.S. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida Species and Aspergillus species isolates. Antimicrob. Agents Chemother. 2014, 58, 1248–1251. [Google Scholar] [CrossRef]
- Shibata, T.; Takahashi, T.; Yamada, E.; Kimura, A.; Nishikawa, H.; Hayakawa, H.; Nomura, N.; Mitsuyama, J. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob. Agents Chemother. 2012, 56, 5892–5897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.D.; Cao, Y.B.; Xu, Z.; Sun, H.H.; An, M.M.; Yan, L.; Chen, H.S.; Gao, P.H.; Wang, Y.; Jia, X.M.; et al. In vitro and in vivo antifungal activities of the eight steroid saponins from Tribulus terrestris L. with potent activity against fluconazole-resistant fungal. Biol. Pharm. Bull. 2005, 28, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Okoli, I.; Tegos, G.P.; Holson, E.B.; Wagner, F.F.; Hamblin, M.R.; Mylonakis, E. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem. Biol. 2010, 5, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Li, L.; Su, L.Q.; Li, H.F.; Zhao, Y.; Wu, Y.D.; Liu, R.N.; Zou, F.; Ni, G. Synthesis and in vitro synergistic antifungal activity of analogues of Panax stipulcanatus saponin against fluconazole-resistant Candida albicans. Carbohydr. Res. 2022, 517, 108575. [Google Scholar] [CrossRef]
- Dawgul, M.A.; Grzywacz, D.; Liberek, B.; Kamysz, W.; Myszka, H. Activity of diosgenyl 2-amino-2-deoxy-β-D-glucopyranoside, its hydrochloride, and N,N-dialkyl derivatives against non-albicans Candida Isolates. Med. Chem. 2018, 14, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011, 49, 171–177. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Favel, A.; Steinmetz, M.D.; Regli, P.; Vidal-Ollivier, E.; Balansard, G. In vitro antifungal activity of triterpenoid saponins. Planta Med. 1994, 60, 50–53. [Google Scholar] [CrossRef]
- Zhang, J.D.; Xu, Z.; Cao, Y.B.; Chen, H.S.; Yan, L.; An, M.M.; Gao, P.H.; Wang, Y.; Jia, X.M.; Jiang, Y.Y. Antifungal activities and action mechanisms of compounds from Tribulus terrestris L. J. Ethnopharmacol. 2006, 103, 76–84. [Google Scholar] [CrossRef]
- Li, Y.; Shan, M.Z.; Zhu, Y.; Yao, H.K.; Li, H.C.; Gu, B.; Zhu, Z.B. Kalopanaxsaponin A induces reactive oxygen species mediated mitochondrial dysfunction and cell membrane destruction in Candida albicans. PLoS ONE 2020, 15, e0243066. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.J.; Jiang, Z.H.; Huang, Z.A.; Chen, T.; Liao, Y.X. Resource and development of tea meal (tea bran or tea seed). Guangzhou Chem. Ind. 2021, 49, 26–27. (In Chinese) [Google Scholar]
- Lin, Z.; Tan, X.H.; Zhang, Y.; Li, F.P.; Luo, P.; Liu, H.Z. Molecular targets and related biologic activities of fucoidan: A Review. Mar. Drugs 2020, 18, 376. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, N.P.; Bruhn, A.; Cole, A.; Nielsen, M.O. Targeted and untargeted metabolic profiling to discover bioactive compounds in seaweeds and hemp using gas and liquid chromatography-mass spectrometry. Metabolites 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Liu, L.J.; OuYang, X.K.; Qu, Y.L.; Chen, Y.; Ding, G.F. Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Cui, N.S.; Wang, P.; Song, S.L.; Liang, H.; Ji, A.G. Sulfated polysaccharide isolated from the sea cucumber Stichopus japonicus against PC12 hypoxia/reoxygenation injury by inhibition of the MAPK signaling pathway. Cell. Mol. Neurobiol. 2015, 35, 1081–1092. [Google Scholar] [CrossRef]
- Ye, Z.P.; Wang, W.; Yuan, Q.X.; Ye, H.; Sun, Y.; Zhang, H.C.; Zeng, X.X. Box–Behnken design for extraction optimization, characterization and in vitro antioxidant activity of Cicer arietinum L. hull polysaccharides. Carbohydr. Polym. 2016, 147, 354–364. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, T.; Murakami, S.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 2000, 381, 67–74. [Google Scholar] [CrossRef]
- Sokolov, S.S.; Volynsky, P.E.; Zangieva, O.T.; Severin, F.F.; Glagoleva, E.S.; Knorre, D.A. Cytostatic effects of structurally different ginsenosides on yeast cells with altered sterol biosynthesis and transport. Biochimica et Biophysica Acta (BBA) Biomembr. 2022, 1864, 183993. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, K.; Yamamoto, Y.; Kato, Y.; Nagatomo, A.; Matsuda, H. Floratheasaponins A-C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the tea plant (Camellia sinensis). J. Nat. Prod. 2005, 68, 1360–1365. [Google Scholar] [CrossRef]
- Cui, C.J.; Zong, J.F.; Sun, Y.; Zhang, L.; Ho, C.T. Triterpenoid saponins from the genus Camellia: Structures, biological activities, and molecular simulation for structure-activity relationship. Food Funct. 2018, 9, 3069–3091. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Horne, R.W. Action of saponin on biological cell membranes. Nature 1962, 196, 952–953. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Ultrastructure of membrane lesions in immune lysis, osmotic lysis and drug-induced lysis. Fed. Proc. 1974, 33, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cai, M.J.; Zhou, L.L.; Wang, H.D. The structure and function of cell membranes studied by atomic force microscopy. Semin. Cell Dev. Biol. 2018, 73, 31–44. [Google Scholar] [CrossRef]
- Ma, M.M.; Wen, X.F.; Xie, Y.T.; Guo, Z.; Zhao, R.B.; Yu, P.; Gong, D.M.; Deng, S.G.; Zeng, Z.L. Antifungal activity and mechanism of monocaprin against food spoilage fungi. Food Control. 2018, 84, 561–568. [Google Scholar] [CrossRef]
- Yu, Z.L.; Wu, X.H.; He, J.H. Study on the antifungal activity and mechanism of tea saponin from Camellia oleifera cake. Eur. Food. Res. Technol. 2022, 248, 783–795. [Google Scholar] [CrossRef]
- Behbehani, J.M.; Irshad, M.; Shreaz, S.; Karched, M. Anticandidal activity of capsaicin and its effect on ergosterol biosynthesis and membrane integrity of Candida albicans. Int. J. Mol. Sci. 2023, 24, 1046. [Google Scholar] [CrossRef]
- Daum, G.; Lees, N.D.; Bard, M.; Dickson, R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 1998, 14, 1471–1510. [Google Scholar] [CrossRef]
- Prasad, R.; Shah, A.H.; Rawal, M.K. Antifungals: Mechanism of action and drug resistance. Adv. Exp. Med. Biol. 2016, 892, 327–349. [Google Scholar] [CrossRef]
- Espinoza, C.; González, M.C.R.; Mendoza, G.; Creus, A.H.; Trigos, Á.; Fernández, J.J. Exploring photosensitization as an efficient antifungal method. Sci. Rep. 2018, 8, 14489. [Google Scholar] [CrossRef]
- Veen, M.; Lang, C. Interactions of the ergosterol biosynthetic pathway with other lipid pathways. Biochem. Soc. Trans. 2005, 33, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Dong, D.F.; Yu, B.Q.; Cai, G.; Wang, X.F.; Ji, Y.H.; Peng, Y.B. Mechanisms of azole resistance in 52 clinical isolates of Candida tropicalis in China. J. Antimicrob. Chemother. 2013, 68, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, F.; Griffin, D.C.; Loraine, J.; Rittig, M.; Delves-Broughton, J.; Bonev, B.B. Recognition of membrane sterols by polyene antifungals amphotericin B and natamycin, a 13C MAS NMR Study. Front. Cell Dev. Biol. 2016, 4, 57. [Google Scholar] [CrossRef]
- Lin, F.; Wang, R. Hemolytic mechanism of dioscin proposed by molecular dynamics simulations. J. Mol. Model. 2009, 16, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Dantas Ada, S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules. 2015, 5, 142–165. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. Methods Mol. Biol. 2012, 810, 183–205. [Google Scholar] [CrossRef]
- Wu, X.J. Separation, Identification and Quantification of Triterpene Saponins in Seeds of Camellia sinensis. Master’s Thesis, Zhejiang University, Hangzhou, China, 2018. (In Chinese). [Google Scholar]
- Alastruey-Izquierdo, A.; Melhem, M.S.; Bonfietti, L.X.; Rodriguez-Tudela, J.L. Susceptibility test for fungi: Clinical and laboratorial correlations in medical mycology. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 57–64. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]

| Compounds | MIC | MFC |
|---|---|---|
| AMB | 0.5 μg/mL | 0.5 μg/mL |
| CPF | 0.25 μg/mL | 0.5 μg/mL |
| FLC | >2000 μg/mL | >2000 μg/mL |
| Saponin mixture (purity > 96%) | 125 μg/mL | 250 μg/mL |
| TE1 | 100 μM | 100 μM |
| ASA | 100 μM | 100 μM |
| MIC TE1 | MIC ASA | MIC CPF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 2 | Day 7 | Day 2 | Day 7 | Day 2 | Day 7 | ||||||
| (-) S | (+) S | (-) S | (+) S | (-) S | (+) S | (-) S | (+) S | (-) S | (+) S | (-) S | (+) S |
| 100 μM | 100 μM | 100 μM | 100 μM | 100 μM | 100 μM | 100 μM | 100 μM | 0.25 μg/mL | 8 μg/mL | 0.5 μg/mL | 8 μg/mL |
| Compounds | Ergosterol Concentration (μg/mL) | |||||
|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | 200 | 250 | |
| TE1 | 100 μM | 100 μM | 100 μM | 100 μM | 100 μM | 200 μM |
| ASA | 100 μM | 100 μM | 100 μM | 100 μM | 100 μM | 200 μM |
| AMB | 0.5 μg/mL | 2 μg/mL | 4 μg/mL | 8 μg/mL | 16 μg/mL | 32 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Gao, Y.; Yuan, M.; Zheng, Z.; Yin, J. Anti-Candida albicans Effects and Mechanisms of Theasaponin E1 and Assamsaponin A. Int. J. Mol. Sci. 2023, 24, 9350. https://doi.org/10.3390/ijms24119350
Chen Y, Gao Y, Yuan M, Zheng Z, Yin J. Anti-Candida albicans Effects and Mechanisms of Theasaponin E1 and Assamsaponin A. International Journal of Molecular Sciences. 2023; 24(11):9350. https://doi.org/10.3390/ijms24119350
Chicago/Turabian StyleChen, Yuhong, Ying Gao, Mingan Yuan, Zhaisheng Zheng, and Junfeng Yin. 2023. "Anti-Candida albicans Effects and Mechanisms of Theasaponin E1 and Assamsaponin A" International Journal of Molecular Sciences 24, no. 11: 9350. https://doi.org/10.3390/ijms24119350
APA StyleChen, Y., Gao, Y., Yuan, M., Zheng, Z., & Yin, J. (2023). Anti-Candida albicans Effects and Mechanisms of Theasaponin E1 and Assamsaponin A. International Journal of Molecular Sciences, 24(11), 9350. https://doi.org/10.3390/ijms24119350









