Anti-Tumor Immunity and Preoperative Radiovaccination: Emerging New Concepts in the Treatment of Breast Cancer
Abstract
1. Introduction
2. Neoadjuvant Systemic Therapy for BC
3. Unveiling the Role of the Immune Response in pCR
4. Toward ICIs in Combination with NACT
5. Investigative Policies to Reverse Immunological “Coldness”
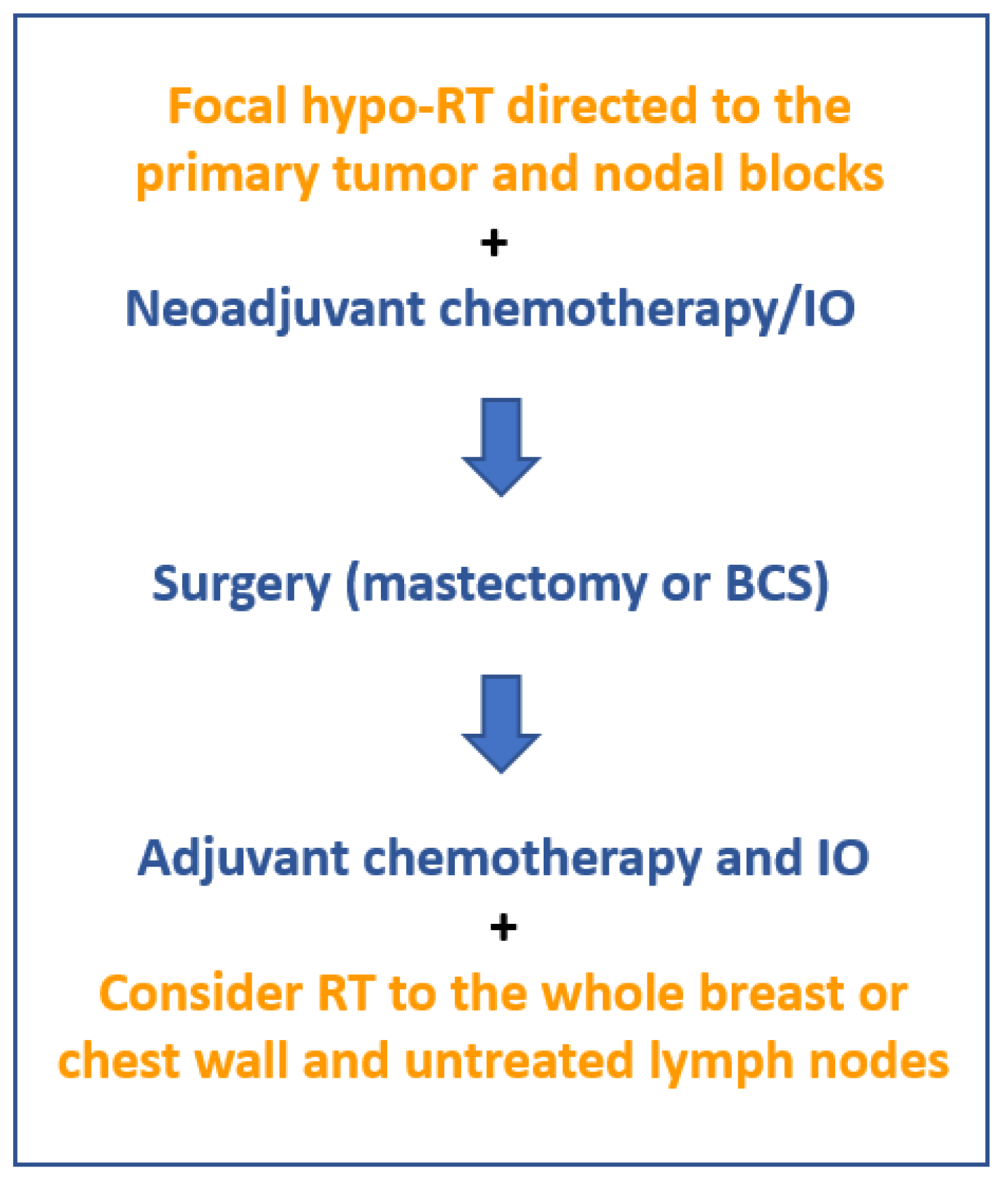
6. Radiovaccination as a Means of Enhancing the Immunological Response
7. Clinical Experience with Preoperative RT
8. Toward Radiovaccination-Oriented Neoadjuvant Immuno-RT (NA-IRT)
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer (Version 4.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 26 November 2022).
- Agostinetto, E.; Gligorov, J.; Piccart, M. Systemic therapy for early-stage breast cancer: Learning from the past to build the future. Nat. Rev. Clin. Oncol. 2022, 19, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B., Jr.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 2001, 2001, 96–102. [Google Scholar] [CrossRef]
- van der Hage, J.A.; van de Velde, C.J.; Julien, J.P.; Tubiana-Hulin, M.; Vandervelden, C.; Duchateau, L. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer trial 10902. J. Clin. Oncol. 2001, 19, 4224–4237. [Google Scholar] [CrossRef]
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Raab, G.; Caputo, A.; Schütte, M.; Hilfrich, J.; Blohmer, J.U.; Gerber, B.; Costa, S.D.; Merkle, E.; Eidtmann, H.; et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: The GEPARDUO study of the German Breast Group. J. Clin. Oncol. 2005, 23, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Baselga, J.; Eiermann, W.; Porta, V.G.; Semiglazov, V.; Lluch, A.; Zambetti, M.; Sabadell, D.; Raab, G.; Cussac, A.L.; et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J. Clin. Oncol. 2009, 27, 2474–2481. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R.; et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13–21. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Sala, I.; Oriecuia, C.; De Pas, T.; Specchia, C.; Graffeo, R.; Pagan, E.; Queirolo, P.; Pennacchioli, E.; et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: Systematic review and meta-analysis. BMJ 2021, 375, e066381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shi, W.; Wali, V.B.; Pongor, L.S.; Li, C.; Lau, R.; Győrffy, B.; Lifton, R.P.; Symmans, W.F.; Pusztai, L.; et al. Predictors of Chemosensitivity in Triple Negative Breast Cancer: An Integrated Genomic Analysis. PLoS Med. 2016, 13, e1002193. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, E.; Haddad, V.; Quesada, S.; Baffert, K.-A.; Lardy-Cléaud, A.; Treilleux, I.; Pissaloux, D.; Attignon, V.; Wang, Q.; Buisson, A.; et al. Alterations in Homologous Recombination-Related Genes and Distinct Platinum Response in Metastatic Triple-Negative Breast Cancers: A Subgroup Analysis of the ProfiLER-01 Trial. J. Pers. Med. 2022, 12, 1595. [Google Scholar] [CrossRef]
- Wang, D.Y.; Jiang, Z.; Ben-David, Y.; Woodgett, J.R.; Zacksenhaus, E. Molecular stratification within triple-negative breast cancer subtypes. Sci. Rep. 2019, 9, 19107. [Google Scholar] [CrossRef]
- Denkert, C.; Von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Mao, Y.; Qu, Q.; Zhang, Y.; Liu, J.; Chen, X.; Shen, K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e115103. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Gkegka, A.G.; Pouliliou, S.; Biziota, E.; Kakolyris, S.; Koukourakis, M. Hypoxia and anaerobic metabolism relate with immunologically cold breast cancer and poor prognosis. Breast Cancer Res. Treat. 2022, 194, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tanikawa, T.; Kryczek, I.; Xia, H.; Li, G.; Wu, K.; Wei, S.; Zhao, L.; Vatan, L.; Wen, B.; et al. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 2018, 28, 87–103.e6. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, P.; Ai, M.; Liu, A.; Budhani, P.; Bartkowiak, T.; Sheng, J.; Ager, C.; Nicholas, C.; Jaiswal, A.R.; Sun, Y.; et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J. Clin. Investig. 2018, 128, 5137–5149. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Chia, S.; Bedard, P.L.; Chu, Q.; Lyle, M.; Tang, L.; Singh, M.; Zhang, Z.; Supuran, C.T.; Renouf, D.J.; et al. A Phase 1 Study of SLC-0111, a Novel Inhibitor of Carbonic Anhydrase IX, in Patients with Advanced Solid Tumors. Am. J. Clin. Oncol. 2020, 43, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Emens, L.A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer 2018, 6, 57. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Kouroupi, M.; Pouliliou, S.; Mitrakas, A.; Hasan, F.; Pappa, A.; Koukourakis, M.I. Ectonucleotidase CD73 and CD39 expression in non-small cell lung cancer relates to hypoxia and immunosuppressive pathways. Life Sci. 2020, 259, 118389. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef]
- Carvajal-Hausdorf, D.E.; Mani, N.; Velcheti, V.; Schalper, K.A.; Rimm, D.L. Objective measurement and clinical significance of IDO1 protein in hormone receptor-positive breast cancer. J. Immunother. Cancer 2017, 5, 81. [Google Scholar] [CrossRef]
- Chen, C.L.; Hsu, S.C.; Ann, D.K.; Yen, Y.; Kung, H.J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541. [Google Scholar] [CrossRef]
- Secondini, C.; Coquoz, O.; Spagnuolo, L.; Spinetti, T.; Peyvandi, S.; Ciarloni, L.; Botta, F.; Bourquin, C.; Rüegg, C. Arginase inhibition suppresses lung metastasis in the 4T1 breast cancer model independently of the immunomodulatory and anti-metastatic effects of VEGFR-2 blockade. Oncoimmunology 2017, 6, e1316437. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.S.; Zong, W.X. Chemotherapeutic approaches for targeting cell death pathways. Oncologist 2006, 11, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, S.; Oltean, T.; Martens, S.; Wiernicki, B.; Goossens, V.; Vanden Berghe, T.; Cappe, B.; Ladik, M.; Riquet, F.B.; Heyndrickx, L.; et al. Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis. 2020, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef] [PubMed]
- Vanmeerbeek, I.; Sprooten, J.; De Ruysscher, D.; Tejpar, S.; Vandenberghe, P.; Fucikova, J.; Spisek, R.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; et al. Trial watch: Chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology 2020, 9, 1703449. [Google Scholar] [CrossRef] [PubMed]
- Vaes, R.D.W.; Hendriks, L.E.L.; Vooijs, M.; De Ruysscher, D. Biomarkers of Radiotherapy-Induced Immunogenic Cell Death. Cells 2021, 10, 930. [Google Scholar] [CrossRef]
- Kim, R.; Kin, T. Current and Future Therapies for Immunogenic Cell Death and Related Molecules to Potentially Cure Primary Breast Cancer. Cancers 2021, 13, 4756. [Google Scholar] [CrossRef]
- Schcolnik-Cabrera, A.; Oldak, B.; Juárez, M.; Cruz-Rivera, M.; Flisser, A.; Mendlovic, F. Calreticulin in phagocytosis and cancer: Opposite roles in immune response outcomes. Apoptosis 2019, 24, 245–255. [Google Scholar] [CrossRef]
- Raghavan, M.; Wijeyesakere, S.J.; Peters, L.R.; Del Cid, N. Calreticulin in the immune system: Ins and outs. Trends Immunol. 2013, 34, 13–21. [Google Scholar] [CrossRef]
- Zanoni, M.; Sarti, A.C.; Zamagni, A.; Cortesi, M.; Pignatta, S.; Arienti, C.; Tebaldi, M.; Sarnelli, A.; Romeo, A.; Bartolini, D.; et al. Irradiation causes senescence, ATP release, and P2X7 receptor isoform switch in glioblastoma. Cell Death Dis. 2022, 13, 80. [Google Scholar] [CrossRef]
- Schnurr, M.; Then, F.; Galambos, P.; Scholz, C.; Siegmund, B.; Endres, S.; Eigler, A. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J. Immunol. 2000, 165, 4704–4709. [Google Scholar] [CrossRef] [PubMed]
- Hubert, P.; Roncarati, P.; Demoulin, S.; Pilard, C.; Ancion, M.; Reynders, C.; Lerho, T.; Bruyere, D.; Lebeau, A.; Radermecker, C.; et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J. Immunother. Cancer 2021, 9, e001966. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuure, R.S.A.; Kleibeuker, E.A.; Buffa, F.M.; Castricum, K.C.M.; Haider, S.; Schulkens, I.A.; Ten Kroode, L.; van den Berg, J.; Jacobs, M.A.J.M.; van Berkel, A.M.; et al. Interferon- and STING-independent induction of type I interferon stimulated genes during fractionated irradiation. J. Exp. Clin. Cancer Res. 2021, 40, 161. [Google Scholar] [CrossRef]
- Koukourakis, I.M.; Tiniakos, D.; Kouloulias, V.; Zygogianni, A. The molecular basis of immuno-radiotherapy. Int. J. Radiat. Biol. 2022, 99, 715–736. [Google Scholar] [CrossRef]
- Quarmby, S.; Kumar, P.; Kumar, S. Radiation-induced normal tissue injury: Role of adhesion molecules in leukocyte-endothelial cell interactions. Int. J. Cancer 1999, 82, 385–395. [Google Scholar] [CrossRef]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Jutzy, J.M.S.; Lemons, J.M.; Luke, J.J.; Chmura, S.J. The Evolution of Radiation Therapy in Metastatic Breast Cancer: From Local Therapy to Systemic Agent. Int. J. Breast Cancer 2018, 2018, 4786819. [Google Scholar] [CrossRef]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11 Pt 1, 728–734. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Krop, I.E.; Trippa, L.; Tan-Wasielewski, Z.; Li, T.; Osmani, W.; Andrews, C.; Dillon, D.; Richardson ET 3rd Pastorello, R.G.; Winer, E.P.; et al. A Phase II Study of Pembrolizumab in Combination with Palliative Radiotherapy for Hormone Receptor-positive Metastatic Breast Cancer. Clin. Breast Cancer 2020, 20, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Page, D.B.; Beal, K.; Linch, S.N.; Spinelli, K.J.; Rodine, M.; Halpenny, D.; Modi, S.; Patil, S.; Young, R.J.; Kaley, T.; et al. Brain radiotherapy, tremelimumab-mediated CTLA-4-directed blockade +/− trastuzumab in patients with breast cancer brain metastases. NPJ Breast Cancer 2022, 8, 50. [Google Scholar] [CrossRef]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef]
- Adair, F.E. Effect of Preoperative Irradiation in Primary Operable Cancer of the Breast. Am. J. Roentgenol. 1936, 35, 359–370. [Google Scholar]
- Wallgren, A.; Arner, O.; Bergström, J.; Blomstedt, B.; Granberg, P.O.; Karnström, L.; Räf, L.; Silfverswärd, C. Preoperative radiotherapy in operable breast cancer: Results in the Stockholm Breast Cancer Trial. Cancer 1978, 42, 1120–1125. [Google Scholar] [CrossRef]
- Semiglazov, V.F.; Topuzov, E.E.; Bavli, J.L.; Moiseyenko, V.M.; Ivanova, O.A.; Seleznev, I.K.; Orlov, A.A.; Barash, N.Y.; Golubeva, O.M.; Chepic, O.F. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann. Oncol. 1994, 5, 591–595. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Kuten, A.; Belkacemi, Y. Primary systemic therapy and whole breast irradiation for locally advanced breast cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2014, 92, 143–152. [Google Scholar] [CrossRef]
- Bourgier, C.; Ghorbel, I.; Heymann, S.; Barhi, M.; Mazouni, C.; Al Ghuzlan, A.A.; Balleyguier, C.; Marsiglia, H.; Delaloge, S. Effect of preoperative rescue concomitant FUN/XUN-based chemo-radiotherapy for neoadjuvant chemotherapy-refractory breast cancer. Radiother. Oncol. 2012, 103, 151–154. [Google Scholar] [CrossRef]
- Fu, W.; Sun, H.; Zhao, Y.; Chen, M.; Yang, L.; Gao, S.; Li, L.; Jin, W. Trends and outcomes of neoadjuvant radiotherapy compared with postoperative radiotherapy for malignant breast cancer. Oncotarget 2018, 9, 24525–24536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.; Chen, H.; Sun, X.; Zhang, Z. Survival comparison between postoperative and preoperative radiotherapy for stage I-III non-inflammatory breast cancer. Sci. Rep. 2022, 12, 14288. [Google Scholar] [CrossRef]
- Jenkins, V.K.; Olson, M.H.; Ellis, H.N.; Cooley, R.N. Effect of therapeutic radiation on peripheral blood lymphocytes in patients with carcinoma of the breast. Acta Radiol. Ther. Phys. Biol. 1975, 14, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.M.; Short, J.H.; Nias, A.H. Recovery of lymphocyte status after radiotherapy. Clin. Radiol. 1980, 31, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.A.; Xi, J.; Luo, J.; Hassan, B.; Thomas, S.; Ma, C.X.; Campian, J.L. Neutrophil-to-lymphocyte ratio as a predictor of survival in patients with triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 174, 443–452. [Google Scholar] [CrossRef]
- Chen, F.; Jin, J.Y.; Hui, T.S.K.; Jing, H.; Zhang, H.; Nong, Y.; Han, Y.; Wang, W.; Ma, L.; Yi, F.; et al. Radiation Induced Lymphopenia Is Associated with the Effective Dose to the Circulating Immune Cells in Breast Cancer. Front. Oncol. 2022, 12, 768956. [Google Scholar] [CrossRef]
- Gutkin, P.M.; Kozak, M.M.; von Eyben, R.; Horst, K.C. Lymphopenia and clinical outcomes in patients with residual nodal disease after neoadjuvant chemotherapy for breast cancer. Cancer Causes Control 2020, 31, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, Q. Comparative analysis of the effect of different radiotherapy regimes on lymphocyte and its subpopulations in breast cancer patients. Clin. Transl. Oncol. 2018, 20, 1219–1225. [Google Scholar] [CrossRef]
- Sun, G.Y.; Wang, S.L.; Song, Y.W.; Jin, J.; Wang, W.H.; Liu, Y.P.; Ren, H.; Fang, H.; Tang, Y.; Zhao, X.R.; et al. Radiation-Induced Lymphopenia Predicts Poorer Prognosis in Patients with Breast Cancer: A Post Hoc Analysis of a Randomized Controlled Trial of Postmastectomy Hypofractionated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 277–285. [Google Scholar] [CrossRef]
- Civil, Y.A.; Jonker, L.W.; Groot Koerkamp, M.P.M.; Duvivier, K.M.; de Vries, R.; Oei, A.L.; Slotman, B.J.; van der Velde, S.; van den Bongard, H.J.G.D. Preoperative Partial Breast Irradiation in Patients with Low-Risk Breast Cancer: A Systematic Review of Literature. Ann. Surg. Oncol. 2023, 30, 3263–3279. [Google Scholar] [CrossRef]
- Demaria, S.; Guha, C.; Schoenfeld, J.; Morris, Z.; Monjazeb, A.; Sikora, A.; Crittenden, M.; Shiao, S.; Khleif, S.; Gupta, S.; et al. Radiation dose and fraction in immunotherapy: One-size regimen does not fit all settings, so how does one choose? J. Immunother. Cancer 2021, 9, e002038. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.W.; Sherer, M.V.; Zamarin, D.; Sharabi, A.B.; Dyer, B.A.; Mell, L.K.; Mayadev, J.S. Immunotherapy and radiation therapy sequencing: State of the data on timing, efficacy, and safety. Cancer 2021, 127, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT03366844 (accessed on 5 January 2023).
- McArthur, H.; Shiao, S.; Karlan, S.; Amersi, F.; Arnold, B.; Basho, R.; Burnison, M.; Chung, A.; Chung, C.; Dang, C.; et al. Pre-operative pembrolizumab (pembro) with radiation therapy (RT) in patients with operable triple-negative breast cancer (TNBC) [abstract]. In Proceedings of the 2019 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 10–14 December 2019; AACR: Philadelphia, PA, USA Cancer Res.2020, 80 (Suppl. 4), Abstract nr P3-09-09. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ct2/show/NCT03804944 (accessed on 5 January 2023).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04443348 (accessed on 5 January 2023).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04454528 (accessed on 5 January 2023).
- Available online: https://clinicaltrials.gov/ct2/show/NCT04807192 (accessed on 5 January 2023).
- Sabree, S.A.; Voigt, A.P.; Blackwell, S.E.; Vishwakarma, A.; Chimenti, M.S.; Salem, A.K.; Weiner, G.J. Direct and indirect immune effects of CMP-001, a virus-like particle containing a TLR9 agonist. J. Immunother. Cancer 2021, 9, e002484. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT03875573 (accessed on 5 January 2023).
- Antonioli, L.; Yegutkin, G.G.; Pacher, P.; Blandizzi, C.; Haskó, G. Anti-CD73 in cancer immunotherapy: Awakening new opportunities. Trends Cancer 2016, 2, 95–109. [Google Scholar] [CrossRef]

| Author/Year/Reference | No Patients | Randomized Groups | Systemic Therapy | Major Findings |
|---|---|---|---|---|
| Fisher et al. and Wolmark et al. NSABP B-18 trial (1997 and 2001) [4,5] | 1523 | Preoperative vs. postoperative chemotherapy | Four cycles of AC |
|
| van der Hage et al. EORTC 10902 trial (2001) [6] | 698 | Preoperative vs. postoperative chemotherapy | FEC chemotherapy |
|
| Rastogi et al. NSABP-B-18 and B-27 trial (2008) [7] | 1560 | Preoperative chemotherapy with or without docetaxel | AC and docetaxel followed by surgery vs. AC followed by surgery and postoperative docetaxel |
|
| von Minckwitz et al. GEPARDUO trial (2005) [8] | 913 | Preoperative chemotherapy with or without docetaxel | Doxorubicin and docetaxel vs. AC followed by docetaxel |
|
| Gianni et al. European Cooperative Trial (2009) [9] | 1355 | Preoperative vs. postoperative chemotherapy | Surgery followed by docetaxel and CMF vs. surgery followed by paclitaxel/doxorubicin and CMF vs. NACT with paclitaxel/doxorubicin and CMF |
|
| Sikov et al. GALGB 40603 (2015) [11] | 443 TNBCs | All patients received NACT. Randomized according to the addition of bevacizumab | NACT with paclitaxel followed by AC with or without paclitaxel and/or Bevacizumab |
|
| von Minckwitz et al. GBG 66 trial (2014) [12] | 296 TNBCs and HER2+ carcinomas | All patients received NACT. Randomized according to the administration of carboplatin | NACT with paclitaxel/liposomal doxorubicin with bevacizumab (TNBCs) or Herceptin/lapatinib (HER2+ cases) with or without carboplatin |
|
| Schmid et al. KEYNOTE-522 trial (2020, 2022) [19,20] | 1174 TNBCs | All patients received neoadjuvant systemic therapy. Randomized according to the administration of pembrolizumab | NACT with AC with or without pembrolizumab |
|
| Nanda et al. I-SPY2 trial (2020) [21] | 250 MammaPrint-high-risk luminal/HER2− BC and TNBC | All patients received neoadjuvant systemic therapy. Randomized according to the administration of pembrolizumab | Taxane- and anthracycline-based NACT with or without pembrolizumab |
|
| Mittendorf et al. IMpassion 031 trial (2020) [22] | 333 TNBCs | All patients received neoadjuvant systemic therapy. Randomized according to the administration of atezolizumab | NACT with Nab-paclitaxel followed by AC with or without atezolizumab |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukourakis, I.M.; Papadimitriou, M.; Desse, D.; Zygogianni, A.; Papadimitriou, C. Anti-Tumor Immunity and Preoperative Radiovaccination: Emerging New Concepts in the Treatment of Breast Cancer. Int. J. Mol. Sci. 2023, 24, 9310. https://doi.org/10.3390/ijms24119310
Koukourakis IM, Papadimitriou M, Desse D, Zygogianni A, Papadimitriou C. Anti-Tumor Immunity and Preoperative Radiovaccination: Emerging New Concepts in the Treatment of Breast Cancer. International Journal of Molecular Sciences. 2023; 24(11):9310. https://doi.org/10.3390/ijms24119310
Chicago/Turabian StyleKoukourakis, Ioannis M., Marios Papadimitriou, Dimitra Desse, Anna Zygogianni, and Christos Papadimitriou. 2023. "Anti-Tumor Immunity and Preoperative Radiovaccination: Emerging New Concepts in the Treatment of Breast Cancer" International Journal of Molecular Sciences 24, no. 11: 9310. https://doi.org/10.3390/ijms24119310
APA StyleKoukourakis, I. M., Papadimitriou, M., Desse, D., Zygogianni, A., & Papadimitriou, C. (2023). Anti-Tumor Immunity and Preoperative Radiovaccination: Emerging New Concepts in the Treatment of Breast Cancer. International Journal of Molecular Sciences, 24(11), 9310. https://doi.org/10.3390/ijms24119310






