miRNA-Guided Regulation of Mesenchymal Stem Cells Derived from the Umbilical Cord: Paving the Way for Stem-Cell Based Regeneration and Therapy
Abstract
1. Introduction
2. miRNA-Guided Differentiation Potential of UCMSCs
2.1. Osteogenic/Osteoblastic Differentiation
2.2. Hepatic Differentiation
2.3. Neural Differentiation
2.4. Chondrogenic Differentiation
2.5. Epithelial Differentiation
2.6. Differentiation toward Other Lineages
3. Applications of UCMSCs in Regenerative and Therapeutic Medicine and the Role of miRNAs
3.1. Ischemic/Reperfusion (I/R) Injuries
3.2. Acute Organ Injuries
| miRNA | Tissue Origin | Vehicle Type | Target (Gene/Pathway) | Function | Clinical Application | Reference |
|---|---|---|---|---|---|---|
| miR-17-3p | UCMSCs | Exosomes | STAT1 | Inflammation/Apoptosis Suppression, Oxidative Injury Reduction | Diabetic Retinopathy | [132] |
| miR-17-5p | UCMSCs | Exosomes | SIRT7 | ROS Reduction, Proliferation Promotion | Premature Ovarian Insufficiency | [133] |
| miR-18b | UCMSCs | EVs | MAP3K1/NF-κB/p65 | Apoptosis/Inflammation Inhibition | Diabetic Retinopathy | [134] |
| UCMSCs | EVs | Notch2/TIM3/mTORC1 | Proliferation/Migration Promotion, Blood Pressure Reduction | Pre-Eclampsia | [135] | |
| miR-21 | UCMSCs | EVs | TGF-β2 | Myoblast Differentiation Inhibition | Lung Fibrosis | [136] |
| UCMSCs | Exosomes | LATS1 | Estrogen Secretion Promotion | Premature Ovarian Insufficiency | [137] | |
| UCMSCs | Exosomes | p38 MAPK | Apoptosis/ER Stress Suppression | Diabetes | [138] | |
| miR-23 | UCMSCs | EVs | TGF-βR2 | Myoblast Differentiation Inhibition | Lung Fibrosis | [136] |
| miR-24-3p | UCMSCs | Exosomes | Keap-1 | Lipid Accumulation/ROS Generation/Inflammation Inhibition | Non-Alcoholic Fatty Liver Disease | [139] |
| miR-26a-5p | UCMSCs | Exosomes | METTL14/NLRP3 | Cell Survival Promotion, Pyroptosis Inhibition | Intervertebral Disc Degeneration | [140] |
| miR-27b | UCMSCs | Exosomes | HOXC6 | EMT Suppression | Subretinal Fibrosis | [141] |
| miR-29a | UCMSCs | EVs | HBP1/Wnt/β-catenin | Proliferation Promotion, Apoptosis Inhibition, Ovarian Function Restoration | Premature Ovarian Insufficiency | [142] |
| Placenta-derived MSCs | Exosomes | - | Differentiation Promotion, Utrophin Increase, Fibrosis/Inflammation Inhibition | Duchenne Muscular Dystrophy | [143] | |
| miR-30c-5p | UCMSCs | EVs | PLCG1/PKC/NF-κB | Inflammation Suppression | Diabetic Retinopathy | [144] |
| miR-100 | UCMSCs | EVs | HS3ST2 | Proliferation/Invasion/Migration/EMT Promotion | Endometriosis | [145] |
| miR-100-5p | UCMSCs | - | NOX4/NLRP3, GSDMD | Inflammation/Oxidative Stress/Apoptosis Inhibition | Premature Ovarian Insufficiency | [146] |
| UCMSCs | EVs | - | M2 Polarization Promotion, Treg Generation | SS Dry Eye | [147] | |
| UCMSCs | EVs | NOX4 | ROS/Oxidative Stress/Apoptosis Inhibition | Heart Failure | [148] | |
| UCMSCs | Exosomes | FZD5/Wnt/β-catenin | Migration Inhibition, Apoptosis Promotion, Inflammation Suppression | Atherosclerosis | [149] | |
| miR-101 | UCMSCs | EVs | BRD4/NF-κB/CXCL11 | Proliferation/Migration Promotion | Pre-Eclampsia | [150] |
| miR-125b | UCMSCs | EVs | IL-6R, IFV genes | Viral Activities/Infection Inhibition | Respiratory Virus-associated Diseases | [151] |
| miR-126 | UCMSCs | Exosomes | HMGB1 | Inflammation Suppression | Retinal Inflammation | [152] |
| miR-126-3p | UCMSCs | Exosomes | PIK3R2, PI3K/AKT/mTOR | Proliferation/Angiogenesis Promotion, Apoptosis Suppression | Premature Ovarian Insufficiency | [153] |
| miR-133 | UCMSCs | Exosomes | - | Proliferation/Survival Promotion, Bregs Production | Immune Thrombocytopenia | [154] |
| miR-133b | UCMSCs | Exosomes | SGK1 | Proliferation/Cell Cycle Progression/Migration/Invasion Promotion, Apoptosis Inhibition | Pre-Eclampsia | [155] |
| miR-140-5p | UCMSCs | Exosomes | FSTL3 | Cell Growth/Angiogenesis Promotion, Inflammation Suppression | Pre-Eclampsia | [156] |
| miR-146a | UCBMSCs | - | - | Inflammation Suppression | Inflammatory Diseases | [157] |
| UCMSCs | Exosomes | SUMO1/β-catenin | Colitis Deterioration/CAC Progression Inhibition | Colitis | [158] | |
| UCMSCs | Exosomes | TRAF6, IRAK1, NF-κB | Fibroblast Activation/Inflammation/Fibrosis Inhibition | Urethral Stricture Diseases | [159] | |
| WJMSCs | Exosomes | - | M2 Macrophage Polarization Promotion, Inflammation Inhibition | Inflammatory Disorders/Sepsis | [160] | |
| miR-146a-5p | UCMSCs | Exosomes | TRAF6 | Neuroinflammation/Pyroptosis Suppression, Autophagy Promotion | Inflammatory Pain | [161] |
| UCMSCs | Exosomes | NOTCH1 | Bleeding/Inflammation/M1 Polarization Suppression, M2 Polarization Promotion | SLE-associated DAH | [162] | |
| UCMSCs | - | TRAF6/STAT1 | M2 Polarization Promotion, Renal Function Improvement, Inflammation Suppression | Diabetic Nephropathy | [163] | |
| UCMSCs | EVs | (TGF-β1/Smad2/3) | Allergic Inflammation/Fibrosis/Airway Remodeling Suppression | Asthma | [164] | |
| miR-147 | WJMSCs | EVs | - | Inflammation Suppression, Macrophage Activation | Abdominal Aortic Aneurysm | [165] |
| miR-148a-5p | UCMSCs | - | Notch2 | Proliferation Promotion, Apoptosis/Fibrosis Inhibition | Liver Fibrosis | [166] |
| miR-153-3p | UCMSCs | - | Snai1 | EMT Suppression | Peritoneal Fibrosis | [167] |
| UCMSCs | - | PELI1 | Proliferation/Migration Inhibition, Tfh/Treg Imbalance Promotion | SLE | [168] | |
| miR-181a | UCMSCs | - | - | T Lymphocyte Regulation | SLE | [169] |
| miR-195 | UCMSCs | Exosomes | TFPI2 | Hypoxic Damage Reduction | Pre-Eclampsia | [170] |
| miR-199 | UCMSCs | - | KGF | (Fibrosis Promotion) | Cirrhosis | [171] |
| miR-199a-5p | UCMSCs | - | Sirt1/p53 | CD4+ T-cell Senescence Promotion | SLE | [172] |
| miR-203a-3p.2 | UCMSCs | Exosomes | casp11/4 | Macrophage Pyroptosis/Inflammation Inhibition | IBD | [173] |
| miR-204 | WJMSCs, BMMSCs | Exosomes | STAT3 | Proliferation Inhibition | Pulmonary Hypertension | [174] |
| anti-miR-206 | UCMSCs | - | BDNF, (Egr-1, PSD-95) | Neuroprotection, Neuronal Function Promotion | Age-related Cognitive Decline | [175] |
| anti-miR-210 | UCMSCs | EVs | - | Immunosuppressive Properties | Psoriasis | [176] |
| miR-223 | UCMSCs, (BMMSCs) | Exosomes | ICAM-1 | T cell Adhesion/Migration/Infiltration Inhibition, Inflammatory Factors Suppression | Acute Graft-versus-Host Disease | [177] |
| anti-miR-301a-3p | UCMSCs | - | IGF-1, PI3K/Akt/FOXO3a | Burn-induced Apoptosis/Organ Vascular Permeability Inhibition | Vascular Endothelial Barrier Dysfunction | [178] |
| miR-302d-3p | UCMSCs | Exosomes | FLT4, VEGFR3/AKT | Migration/Tube Formation/Lymphangiogenesis Inhibition | IBD | [179] |
| miR-326 | UCMSCs | Exosomes | NEDD8, NF-κB | Neddylation/Inflammation Inhibition | IBD | [180] |
| miR-335-5p | UCMSCs | Exosomes | ADAM19 | Inflammation/EMT Inhibition | Renal Fibrosis | [181] |
| miR-342-3p | UCMSCs | Exosomes | EDNRA | Thrombus Formation Inhibition, Angiogenesis Promotion | Deep Vein Thrombosis | [182] |
| miR-378 | UCMSCs | EVs | PSMD14/TGF-β1/Smad2/3 | Mesangial Hyperplasia/Fibrosis/Proliferation Suppression | Mesangial Proliferative Glomerulonephritis (MsPGN) | [183] |
| miR-378a-5p | UCMSCs | Exosomes | NLRP3 | Macrophage Pyroptosis/Inflammation Inhibition, Cell Survival Promotion | IBD/Colitis | [184] |
| miR-455-3p | UCMSCs | - | PAK2 | Profibrogenic Markers Suppression | Liver Fibrosis | [185] |
| miR-499 | WJMSCs | - | TGFβR 1/3 | Creatine Kinase Decrease, Muscle Regeneration, Apoptosis/Fibrosis Inhibition | Duchenne Muscular Dystrophy | [186] |
| miR-627-5p | UCMSCs | Exosomes | FTO | Cell Survival Promotion, Apoptosis Inhibition, Glucose/Lipid Metabolism Improvement | Non-Alcoholic Fatty Liver Disease | [187] |
| miR-1246 | UCMSCs | Exosomes | PRSS23/Snail/α-SMA | Angiogenesis Promotion, Apoptosis/Hypoxic Damage Reduction | Chronic Heart Failure | [188] |
| miR-1348a-3p | UCMSCs | Exosomes | Serpine1 | Vascular Smooth Muscle Cell Phenotypic Switching/Migration Inhibition | Neointimal Hyperplasia | [189] |
3.3. Regenerative Medicine (Wound Healing/Bone Regeneration)
3.4. Anti-Cancer Treatment
| miRNA | Tissue Origin | Vehicle Type | Target (Gene/Pathway) | Function | Clinical Application | Reference |
|---|---|---|---|---|---|---|
| miR-10a-5p | UCMSCs | Exosomes | PTEN | Cell Growth/Cell Survival Promotion | Glioma | [205] |
| miR-15a-5p | UCMSCs | Exosomes | CHEK1 | Proliferation/Invasion/Migration/EMT Inhibition, Apoptosis Promotion | Cholangiocarcinoma | [206] |
| UCMSCs | Exosomes | SEPT2 | Proliferation/Migration/Invasion Inhibition, Apoptosis Promotion | Wilms Tumor | [220] | |
| miR-21-5p | UCMSCs | Exosomes | ZNF367 | Migration/Invasion Inhibition | Breast Cancer | [194] |
| miR-30c-5p | UCMSCs | EVs | PELI1/PI3K/AKT | Proliferation/Migration Inhibition | Papillary Thyroid Carcinoma | [216] |
| miR-100-5p | UCMSCs | Exosomes | - | Proliferation/Migration Promotion | Pancreatic Ductal Adenocarcinoma | [222] |
| miR-124 | WJMSCs | - | CDK6 | Proliferation/Migration Suppression, Chemosensitivity Promotion | Glioblastoma Multiform | [204] |
| miR-125b | WJMSCs | EVs | HIF1α | Proliferation/EMT/Angiogenesis Inhibition | Triple Negative Breast Cancer | [223] |
| miR-127-3p | UCMSCs | EVs | ITGA6/TGF-β1/Smad | Proliferation/Invasion/Migration/EMT Inhibition, Apoptosis Promotion | Cholangiocarcinoma | [207] |
| miR-139-5p | UCMSCs | Exosomes | PRC1 | Proliferation/Migration/Invasion Suppression | Bladder Cancer | [215] |
| miR-145-5p | UCMSCs | Exosomes | TGF-β/Smad3 | Proliferation/Invasion Inhibition, Apoptosis/Cell Cycle Arrest Promotion | Pancreatic Ductal Adenocarcinoma | [219] |
| miR-145a-5p | UCMSCs | Exosomes | USP6/GLS1 | Apoptosis/Chemosensitivity Promotion | Chronic Myeloid Leukemia | [221] |
| miR-146a | UCMSCs | Exosomes | LAMC2/PI3K/Akt | Cell Growth Suppression, Chemosensitivity Promotion | Ovarian Cancer | [202] |
| miR-148b-3p | UCMSCs | Exosomes | TRIM59 | Proliferation/Invasion/Migration Inhibition, Apoptosis Promotion | Breast Cancer | [195] |
| miR-181a | UCMSCs | Exosomes | KDM5C | Cell Growth Suppression | Nasopharyngeal Carcinoma | [210] |
| miR-200c | UCMSCs | - | Wnt/β-catenin | Tumor Growth/Metastasis Inhibition | Ovarian Cancer | [201] |
| anti-miR-224-5p | UCMSCs | Exosomes | HOXA5 | Proliferation Inhibition, Apoptosis Promotion | Breast Cancer | [198] |
| miR-302a | UCMSCs | EVs | cyclin D1/AKT | Proliferation/Migration Inhibition | Endometrial Cancer | [200] |
| miR-320a | UCMSCs | Exosomes | SOX4/Wnt/β-catenin | Proliferation/Metastasis Inhibition | Lung Cancer | [208] |
| miR-375 | UCMSCs | Exosomes | ENAH | Proliferation/Invasion/Migration/Tumorsphere Formation Inhibition, Apoptosis Promotion | Esophageal Squamous Cell Carcinoma | [212] |
| anti-miR-375 | UCMSCs | Exosomes | PTPN4/STAT3 | Proliferation/Migration/Invasion/Chemoresistance Suppression, Apoptosis Promotion | Prostate Cancer | [217] |
| miR-410 | UCMSCs | EVs | PTEN | Proliferation Promotion, Apoptosis Suppression | Lung Adenocarcinoma | [209] |
| miR-431-5p | UCMSCs | Exosomes | PRDX1 | Cell Growth Suppression, Prognostic Marker | Colorectal Cancer | [213] |
| miR-451a | UCMSCs | Exosomes | ADAM10 | Paclitaxel Resistance/Cell Cycle Transition/Proliferation/Migration/Invasion Inhibition, Apoptosis Promotion | Hepatocellular Carcinoma | [218] |
| miR-503-3p | UCBMSCs | Exosomes | MEST | Cell Growth Suppression | Endometrial Cancer | [199] |
| miR-655-3p | UCMSCs | EVs | HIF-1α/LMO4/HDAC2 | Cell Growth/Metastasis Suppression | Esophageal Cancer | [211] |
| miR-3182 | UCMSCs | Exosomes | mTOR, S6KB1 | Proliferation/Migration Inhibition, Apoptosis Promotion | Triple Negative Breast Cancer | [197] |
| miR-6785-5p | UCMSCs | Exosomes | INHBA | Angiogenesis/Metastasis Suppression | Gastric Cancer | [214] |
| miR-11401 | UCMSCs | EVs | SCOTIN/p53 | Doxorubicin-induced Apoptosis Inhibition | Chemotherapy | [224] |
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campagnoli, C.; Roberts, I.A.; Kumar, S.; Bennett, P.R.; Bellantuono, I.; Fisk, N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001, 98, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, G.Y.; Delorme, B.; Lopez, A.; Hérault, O.; Bonnet, P.; Charbord, P.; Eder, V.; Domenech, J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 2006, 24, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Gökçinar-Yagci, B.; Özyüncü, Ö.; Çelebi-Saltik, B. Isolation, characterisation and comparative analysis of human umbilical cord vein perivascular cells and cord blood mesenchymal stem cells. Cell Tissue Bank. 2016, 17, 345–352. [Google Scholar] [CrossRef]
- In ‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Christodoulou, I.; Kolisis, F.N.; Papaevangeliou, D.; Zoumpourlis, V. Comparative Evaluation of Human Mesenchymal Stem Cells of Fetal (Wharton’s Jelly) and Adult (Adipose Tissue) Origin during Prolonged In Vitro Expansion: Considerations for Cytotherapy. Stem Cells Int. 2013, 2013, 246134. [Google Scholar] [CrossRef]
- Amit, M.; Carpenter, M.K.; Inokuma, M.S.; Chiu, C.P.; Harris, C.P.; Waknitz, M.A.; Itskovitz-Eldor, J.; Thomson, J.A. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000, 227, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Anderson, C.; Medicetty, S.; Seshareddy, K.B.; Weiss, R.J.; VanderWerff, I.; Troyer, D.; McIntosh, K.R. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells 2008, 26, 2865–2874. [Google Scholar] [CrossRef]
- Mansilla, E.; Marin, G.H.; Sturla, F.; Drago, H.E.; Gil, M.A.; Salas, E.; Gardiner, M.C.; Piccinelli, G.; Bossi, S.; Salas, E.; et al. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant. Proc. 2005, 37, 292–294. [Google Scholar] [CrossRef]
- Girousse, A.; Mathieu, M.; Sastourné-Arrey, Q.; Monferran, S.; Casteilla, L.; Sengenès, C. Endogenous Mobilization of Mesenchymal Stromal Cells: A Pathway for Interorgan Communication? Front. Cell Dev. Biol. 2020, 8, 598520. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The role of miRNAs in regulating gene expression networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S. Posttranscriptional upregulation by microRNAs. RNA 2012, 3, 311–330. [Google Scholar] [CrossRef]
- Vasudevan, S.; Steitz, J.A. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell 2007, 128, 1105–1118. [Google Scholar] [CrossRef]
- Orom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Bottini, S.; Hamouda-Tekaya, N.; Mategot, R.; Zaragosi, L.E.; Audebert, S.; Pisano, S.; Grandjean, V.; Mauduit, C.; Benahmed, M.; Barbry, P.; et al. Post-transcriptional gene silencing mediated by microRNAs is controlled by nucleoplasmic Sfpq. Nat. Commun. 2017, 8, 1189. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.S.; Malakar, A.K.; Chakraborty, S. Interplay between miRNAs and human diseases. J. Cell. Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Collino, F.; Bruno, S.; Deregibus, M.C.; Tetta, C.; Camussi, G. MicroRNAs and mesenchymal stem cells. Vitam. Horm. 2011, 87, 291–320. [Google Scholar] [CrossRef]
- Yang, C.; Luo, M.; Chen, Y.; You, M.; Chen, Q. MicroRNAs as Important Regulators Mediate the Multiple Differentiation of Mesenchymal Stromal Cells. Front. Cell Dev. Biol. 2021, 9, 619842. [Google Scholar] [CrossRef]
- Oveili, E.; Vafaei, S.; Bazavar, H.; Eslami, Y.; Mamaghanizadeh, E.; Yasamineh, S.; Gholizadeh, O. The potential use of mesenchymal stem cells-derived exosomes as microRNAs delivery systems in different diseases. Cell Commun. Signal. CCS 2023, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Alipour, M. Overexpression Effects of miR-424 and BMP2 on the Osteogenesis of Wharton’s Jelly-Derived Stem Cells. BioMed Res. Int. 2021, 2021, 7031492. [Google Scholar] [CrossRef]
- Hwang, S.; Park, S.K.; Lee, H.Y.; Kim, S.W.; Lee, J.S.; Choi, E.K.; You, D.; Kim, C.S.; Suh, N. miR-140-5p suppresses BMP2-mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett. 2014, 588, 2957–2963. [Google Scholar] [CrossRef]
- Huang, M.; Qing, Y.; Shi, Q.; Cao, Y.; Song, K. miR-342-3p elevates osteogenic differentiation of umbilical cord mesenchymal stem cells via inhibiting Sufu in vitro. Biochem. Biophys. Res. Commun. 2017, 491, 571–577. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Yang, H.; Cao, Y.; Yu, D.; Zhao, Y.; Cao, Y. MicroRNA-196a-5p overexpression in Wharton’s jelly umbilical cord stem cells promotes their osteogenic differentiation and new bone formation in bone defects in the rat calvarium. Cell Tissue Res. 2022, 390, 245–260. [Google Scholar] [CrossRef]
- Zhao, G.; Luo, W.-D.; Yuan, Y.; Lin, F.; Guo, L.-M.; Ma, J.-J.; Chen, H.-B.; Tang, H.; Shu, J. LINC02381, a sponge of miR-21, weakens osteogenic differentiation of hUC-MSCs through KLF12-mediated Wnt4 transcriptional repression. J. Bone Miner. Metab. 2022, 40, 66–80. [Google Scholar] [CrossRef]
- Meng, Y.B.; Li, X.; Li, Z.Y.; Zhao, J.; Yuan, X.B.; Ren, Y.; Cui, Z.D.; Liu, Y.D.; Yang, X.J. microRNA-21 promotes osteogenic differentiation of mesenchymal stem cells by the PI3K/β-catenin pathway. J. Orthop. Res. 2015, 33, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Yahao, G.; Xinjia, W. The Role and Mechanism of Exosomes from Umbilical Cord Mesenchymal Stem Cells in Inducing Osteogenesis and Preventing Osteoporosis. Cell Transplant. 2021, 30, 9636897211057465. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, W.; Zhong, Y.; Zhang, Y.; Gao, D.; He, T.; Liu, Y.; Zou, Z.; Mo, Y.; Peng, S. Linc02349 promotes osteogenesis of human umbilical cord-derived stem cells by acting as a competing endogenous RNA for miR-25-3p and miR-33b-5p. Cell Prolif. 2020, 53, e12814. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.L.; Meng, Y.L.; Ge, J.H. Upregulation of miR-132 attenuates osteoblast differentiation of UC-MSCs. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1580–1587. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Fan, J.; Li, T.; Fan, L.; Wang, S.; Weng, X.; Han, Q.; Zhao, R.C. miR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death Differ. 2015, 22, 1935–1945. [Google Scholar] [CrossRef]
- Raut, A.; Khanna, A. Enhanced expression of hepatocyte-specific microRNAs in valproic acid mediated hepatic trans-differentiation of human umbilical cord derived mesenchymal stem cells. Exp. Cell Res. 2016, 343, 237–247. [Google Scholar] [CrossRef]
- Khosravi, M.; Azarpira, N.; Shamdani, S.; Hojjat-Assari, S.; Naserian, S.; Karimi, M.H. Differentiation of umbilical cord derived mesenchymal stem cells to hepatocyte cells by transfection of miR-106a, miR-574-3p, and miR-451. Gene 2018, 667, 1–9. [Google Scholar] [CrossRef]
- Wang, H.; Ban, W.; Wang, T.; Li, Z.; Dang, X. miR-20b/106a modulate Ngn2 gene expression during neural differentiation of human umbilical cord mesenchymal stem cells. Neuroreport 2017, 28, 1225–1231. [Google Scholar] [CrossRef]
- Choi, S.W.; Shin, J.H.; Kim, J.J.; Shin, T.H.; Seo, Y.; Kim, H.S.; Kang, K.S. Direct cell fate conversion of human somatic stem cells into cone and rod photoreceptor-like cells by inhibition of microRNA-203. Oncotarget 2016, 7, 42139–42149. [Google Scholar] [CrossRef]
- Cao, B.; Dai, X. Platelet lysate induces chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells by regulating the lncRNA H19/miR-29b-3p/SOX9 axis. FEBS Open Bio 2020, 10, 2656–2665. [Google Scholar] [CrossRef]
- Jing, H.; Zhang, X.; Luo, K.; Luo, Q.; Yin, M.; Wang, W.; Zhu, Z.; Zheng, J.; He, X. miR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials 2020, 231, 119682. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, J.J.; Seo, M.S.; Park, S.B.; Shin, T.H.; Shin, J.H.; Seo, Y.; Kim, H.S.; Kang, K.S. Inhibition by miR-410 facilitates direct retinal pigment epithelium differentiation of umbilical cord blood-derived mesenchymal stem cells. J. Vet. Sci. 2017, 18, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, X.; Yang, L.; Mou, Y.; Li, Y.; Dang, R.; Li, C. Hypoxia promotes the skewed differentiation of umbilical cord mesenchymal stem cells toward type II alveolar epithelial cells by regulating microRNA-145. Gene 2017, 630, 68–75. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, W.; Dong, J.; Wang, J.; Wang, B. miR-200b-3p Induces the Formation of Insulin-Producing Cells from Umbilical Cord Mesenchymal Stem Cells by Targeting ZEB2. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 33–46. [Google Scholar] [CrossRef]
- Bai, C.; Gao, Y.; Li, X.; Wang, K.; Xiong, H.; Shan, Z.; Zhang, P.; Wang, W.; Guan, W.; Ma, Y. MicroRNAs can effectively induce formation of insulin-producing cells from mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 3457–3468. [Google Scholar] [CrossRef]
- Hu, K.; Xu, C.; Ni, H.; Xu, Z.; Wang, Y.; Xu, S.; Ji, K.; Xiong, J.; Liu, H. Mir-218 contributes to the transformation of 5-Aza/GF induced umbilical cord mesenchymal stem cells into hematopoietic cells through the MITF pathway. Mol. Biol. Rep. 2014, 41, 4803–4816. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Chen, M.; Ren, S.; Cheng, P.; Zhang, H. miR-301b~miR-130b—PPARγ axis underlies the adipogenic capacity of mesenchymal stem cells with different tissue origins. Sci. Rep. 2017, 7, 1160. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Issa Bhaloo, S.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 2018, 293, 8089–8102. [Google Scholar] [CrossRef]
- Qing, Y.; Huang, M.; Cao, Y.; Du, T.; Song, K. Effects of miRNA-342-3p in modulating Hedgehog signaling pathway of human umbilical cord mesenchymal stem cells by down-regulating Sufu. Oral Dis. 2019, 25, 1147–1157. [Google Scholar] [CrossRef]
- Hong, I.S.; Lee, H.Y.; Choi, S.W.; Kim, H.S.; Yu, K.R.; Seo, Y.; Jung, J.W.; Kang, K.S. The effects of hedgehog on RNA binding protein Msi1 during the osteogenic differentiation of human cord blood-derived mesenchymal stem cells. Bone 2013, 56, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, H.; Liu, H.; Zhang, C.; Cao, Y.; Fan, Z.; Shi, R. miR-196b-5p inhibits proliferation of Wharton’s jelly umbilical cord stem cells. FEBS Open Bio 2021, 11, 278–288. [Google Scholar] [CrossRef]
- Asgharzadeh, A.; Alizadeh, S.; Keramati, M.R.; Soleimani, M.; Atashi, A.; Edalati, M.; Kashani Khatib, Z.; Rafiee, M.; Barzegar, M.; Razavi, H. Upregulation of miR-210 promotes differentiation of mesenchymal stem cells (MSCs) into osteoblasts. Bosn. J. Basic Med. Sci. 2018, 18, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.; Khanna, A. High-throughput sequencing to identify microRNA signatures during hepatic differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells. Hepatol.Res. Off. J. Jpn. Soc. Hepatol. 2017, 47, 910–927. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhou, X.; Li, J.; Wang, L.; Wang, J.; Li, Q.; Chu, J.; Zheng, L.; Wu, Q.; Han, Z.; et al. Dynamic microRNA profiles of hepatic differentiated human umbilical cord lining-derived mesenchymal stem cells. PLoS ONE 2012, 7, e44737. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Shi, Y.; Zhou, X.; Wang, X.; Wang, J.; Lan, Y.; Wang, M.; Zheng, L.; Li, H.; Wu, Q.; et al. A set of microRNAs mediate direct conversion of human umbilical cord lining-derived mesenchymal stem cells into hepatocytes. Cell Death Dis. 2013, 4, e918. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, L.; Zhou, X.; Yang, Q.; Wang, L.; Guo, G.; Hou, Y.; Cai, W.; Han, Z.; Shi, Y.; et al. Induction of hepatocyte-like cells from human umbilical cord-derived mesenchymal stem cells by defined microRNAs. J. Cell. Mol. Med. 2017, 21, 881–893. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, R.; Zhang, S.; Shu, Q.; Zhang, D.; Xu, G. Altered expression of microRNAs in the neuronal differentiation of human Wharton’s Jelly mesenchymal stem cells. Neurosci. Lett. 2015, 600, 69–74. [Google Scholar] [CrossRef]
- Chang, S.-J.; Weng, S.-L.; Hsieh, J.-Y.; Wang, T.-Y.; Chang, M.D.-T.; Wang, H.-W. MicroRNA-34a modulates genes involved in cellular motility and oxidative phosphorylation in neural precursors derived from human umbilical cord mesenchymal stem cells. BMC Med. Genom. 2011, 4, 65. [Google Scholar] [CrossRef]
- Sanooghi, D.; Lotfi, A.; Bagher, Z.; Barati, S.; Karimi, A.; Faghihi, F.; Lotfi, E.; Joghataei, M.T. Large-scale analysis of MicroRNA expression in motor neuron-like cells derived from human umbilical cord blood mesenchymal stem cells. Sci. Rep. 2022, 12, 5894. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J.J.; Seo, M.S.; Park, S.B.; Kang, T.W.; Lee, J.Y.; Lee, B.C.; Kang, I.; Shin, T.H.; Kim, H.S.; et al. miR-410 Inhibition Induces RPE Differentiation of Amniotic Epithelial Stem Cells via Overexpression of OTX2 and RPE65. Stem Cell Rev. Rep. 2015, 11, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Goulielmaki, M.; Devetzi, M.; Panagiotidis, M.; Koliakos, G.; Zoumpourlis, V. Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Res. Ther. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Goulielmaki, M. Suitability of Human Mesenchymal Stem Cells Derived from Fetal Umbilical Cord (Wharton’s Jelly) as an Alternative In Vitro Model for Acute Drug Toxicity Screening. Cells 2022, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, Y.; Ma, K.; Li, Q.; Li, B.; Hu, W.; Fu, X.; Zhang, C. Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Facilitate Diabetic Wound Healing Through MiR-17-5p-mediated Enhancement of Angiogenesis. Stem Cell Rev. Rep. 2022, 18, 1025–1040. [Google Scholar] [CrossRef]
- Huang, L.; Yang, L.; Ding, Y.; Jiang, X.; Xia, Z.; You, Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle 2020, 19, 339–353. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Y.; Chen, L.; Li, D.; Feng, H.; Lu, Z.; Fan, T.; Chen, Z.; Livingston, M.J.; Geng, Q. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J. Cell. Physiol. 2020, 235, 3698–3710. [Google Scholar] [CrossRef]
- Du, T.; Zhou, J.; Chen, W.X.; Zhang, X.L.; Ji, T.Y.; Liu, J.; Rong, L.; Wang, L.D.; Zhou, R.J.; Ding, D.G. Microvesicles derived from human umbilical cord mesenchymal stem cells ameliorate renal ischemia-reperfusion injury via delivery of miR-21. Cell Cycle 2020, 19, 1285–1297. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Zhang, L.; Wu, T.; Wu, T.; Zhang, W.; Decker, A.M.; He, J.; Liu, J.; Wu, Y.; et al. Human Stem Cells Overexpressing miR-21 Promote Angiogenesis in Critical Limb Ischemia by Targeting CHIP to Enhance HIF-1α Activity. Stem Cells 2016, 34, 924–934. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, D. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote the Proliferation of Schwann Cells by Regulating the PI3K/AKT Signaling Pathway via Transferring miR-21. Stem Cells Int. 2021, 2021, 1496101. [Google Scholar] [CrossRef]
- Liu, X.; Li, X. Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Deliver miR-21 to Promote Corneal Epithelial Wound Healing through PTEN/PI3K/Akt Pathway. Stem Cells Int. 2022, 2022, 1252557. [Google Scholar] [CrossRef]
- Kuang, M.J.; Huang, Y.; Zhao, X.G.; Zhang, R.; Ma, J.X.; Wang, D.C.; Ma, X.L. Exosomes derived from Wharton’s jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. Int. J. Biol. Sci. 2019, 15, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, Y.; Hao, Z.; Zhou, P.; Wang, P.; Fang, S.; Li, L.; Xu, S.; Xia, Y. Correction to “Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis”. ACS Appl. Mater. Interfaces 2022, 14, 14834–14835. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J. MicroRNA-21-3p Engineered Umbilical Cord Stem Cell-Derived Exosomes Inhibit Tendon Adhesion. J. Inflamm. Res. 2020, 13, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Liu, Z.; Wu, S.; Chen, X.; You, M.; Li, Y.; Yang, F.; Zhang, S.; Lai, Y.; Liu, P.; et al. Pro-angiognetic and pro-osteogenic effects of human umbilical cord mesenchymal stem cell-derived exosomal miR-21-5p in osteonecrosis of the femoral head. Cell Death Discov. 2022, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, J.; Chen, P.; Lin, L.; Luo, Y.; Ma, X.; Lin, J.; Shen, Y.; Zhang, L. Exosomal miR-22-3p from human umbilical cord blood-derived mesenchymal stem cells protects against lipopolysaccharid-induced acute lung injury. Life Sci. 2021, 269, 119004. [Google Scholar] [CrossRef]
- Dong, C.; Chen, M.; Cai, B.; Zhang, C.; Xiao, G.; Luo, W. Mesenchymal Stem Cell-Derived Exosomes Improved Cerebral Infarction via Transferring miR-23a-3p to Activate Microglia. NeuroMol. Med. 2022, 24, 290–298. [Google Scholar] [CrossRef]
- Song, Y.; Wang, B.; Zhu, X.; Hu, J.; Sun, J.; Xuan, J.; Ge, Z. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol. Toxicol. 2021, 37, 51–64. [Google Scholar] [CrossRef]
- Hu, H.; Dong, L.; Bu, Z.; Shen, Y.; Luo, J.; Zhang, H.; Zhao, S.; Lv, F.; Liu, Z. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J. Extracell. Vesicles 2020, 9, 1778883. [Google Scholar] [CrossRef]
- Wang, W.; Ji, Z.; Yuan, C.; Yang, Y. Mechanism of Human Umbilical Cord Mesenchymal Stem Cells Derived-Extracellular Vesicle in Cerebral Ischemia-Reperfusion Injury. Neurochem. Res. 2021, 46, 455–467. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Knockout of beta-2 microglobulin reduces stem cell-induced immune rejection and enhances ischaemic hindlimb repair via exosome/miR-24/Bim pathway. J. Cell. Mol. Med. 2020, 24, 695–710. [Google Scholar] [CrossRef]
- Li, G.; Xiao, L.; Qin, H.; Zhuang, Q.; Zhang, W.; Liu, L.; Di, C.; Zhang, Y. Exosomes-carried microRNA-26b-5p regulates microglia M1 polarization after cerebral ischemia/reperfusion. Cell Cycle 2020, 19, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, B.; Guo, Q. MiR-26b-5p-modified hUB-MSCs derived exosomes attenuate early brain injury during subarachnoid hemorrhage via MAT2A-mediated the p38 MAPK/STAT3 signaling pathway. Brain Res. Bull. 2021, 175, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xi, Z.; Chen, G.; Liu, K.; Ma, R. Extracellular vesicle-carried microRNA-27b derived from mesenchymal stem cells accelerates cutaneous wound healing via E3 ubiquitin ligase ITCH. J. Cell. Mol. Med. 2020, 24, 11254–11271. [Google Scholar] [CrossRef]
- Han, Q.; Wang, S.; Chen, D.; Gan, D.; Wang, T. Exosomes derived from human umbilical cord mesenchymal stem cells reduce tendon injuries via the miR-27b-3p/ARHGAP5/RhoA signaling pathway. Acta Biochim. Biophys. Sin. 2022, 54, 232–242. [Google Scholar] [CrossRef]
- Su, W.H.; Wang, C.J.; Hung, Y.Y.; Lu, C.W.; Ou, C.Y.; Tseng, S.H.; Tsai, C.C.; Kao, Y.T.; Chuang, P.C. MicroRNA-29a Exhibited Pro-Angiogenic and Anti-Fibrotic Features to Intensify Human Umbilical Cord Mesenchymal Stem Cells-Renovated Perfusion Recovery and Preventing against Fibrosis from Skeletal Muscle Ischemic Injury. Int. J. Mol. Sci. 2019, 20, 5859. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, J.; Xiong, H.; Cui, H.; Ning, J.; Wang, S.; Ouyang, X.; Qian, Y. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J. Nanobiotechnology 2021, 19, 169. [Google Scholar] [CrossRef]
- Xiao, X.; Li, W.; Rong, D.; Xu, Z.; Zhang, Z.; Ye, H.; Xie, L.; Wu, Y.; Zhang, Y.; Wang, X. Human umbilical cord mesenchymal stem cells-derived extracellular vesicles facilitate the repair of spinal cord injury via the miR-29b-3p/PTEN/Akt/mTOR axis. Cell Death Discov. 2021, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, W.; Xu, Z.; Sun, Z.; Ye, H.; Wu, Y.; Zhang, Y.; Xie, L.; Jiang, D.; Jia, R.; et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells reduce lipopolysaccharide-induced spinal cord injury neuronal apoptosis by mediating miR-29b-3p/PTEN. Connect. Tissue Res. 2022, 63, 634–649. [Google Scholar] [CrossRef]
- Yan, L.; Liu, G.; Wu, X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin. Transl. Med. 2021, 11, e255. [Google Scholar] [CrossRef]
- Shi, Q.; Sun, B.; Wang, D.; Zhu, Y.; Zhao, X.; Yang, X.; Zhang, Y. Circ6401, a novel circular RNA, is implicated in repair of the damaged endometrium by Wharton’s jelly-derived mesenchymal stem cells through regulation of the miR-29b-1-5p/RAP1B axis. Stem Cell Res. Ther. 2020, 11, 520. [Google Scholar] [CrossRef]
- Chen, W.X.; Zhou, J.; Zhou, S.S.; Zhang, Y.D.; Ji, T.Y.; Zhang, X.L.; Wang, S.M.; Du, T.; Ding, D.G. Microvesicles derived from human Wharton’s jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR-100. Stem Cell Res. Ther. 2020, 11, 113. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.; Xu, H.; Huang, J.; Shen, Y.; Chen, F.; Luo, M. Exosomes of Human Umbilical Cord MSCs Protect Against Hypoxia/Reoxygenation-Induced Pyroptosis of Cardiomyocytes via the miRNA-100-5p/FOXO3/NLRP3 Pathway. Front. Bioeng. Biotechnol. 2020, 8, 615850. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Cai, Z.; Zhou, Q.; Li, L.; Fu, P. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol. Int. 2021, 45, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Zhang, L.; Li, Q.; Li, Y.; Ding, F.H.; Li, X. hUCB-MSC derived exosomal miR-124 promotes rat liver regeneration after partial hepatectomy via downregulating Foxg1. Life Sci. 2021, 265, 118821. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhao, Y.Y.; Sun, L.; Shi, Y.; Li, Z.Q.; Zhao, X.D.; Xu, C.G.; Ji, H.G.; Wang, M.; Xu, W.R.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells improve myocardial repair via upregulation of Smad7. Int. J. Mol. Med. 2018, 41, 3063–3072. [Google Scholar] [CrossRef]
- Cao, J.Y.; Wang, B.; Tang, T.T.; Wen, Y.; Li, Z.L.; Feng, S.T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.L.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Xu, Y.; Yin, X.M.; Lin, F.Y. Exosomes Derived from miR-126-modified MSCs Promote Angiogenesis and Neurogenesis and Attenuate Apoptosis after Spinal Cord Injury in Rats. Neuroscience 2020, 424, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Sun, L.; Zhao, P.; Liu, Y.; Zhang, J.; Zhang, Y.; Hong, Y.; Zhu, Y.; Lu, Y.; Zhao, W.; et al. Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. J. Nanobiotechnology 2021, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhu, J.; Ma, Q.; Zhao, Y.; Wang, Y.; Hu, X.; Chen, J.; Zhu, W.; Han, Z.; Yu, H. Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res. Ther. 2020, 11, 273. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, F.; Wan, X.; Liu, S.; Fu, L.; Mo, J.; Meng, X.; Mo, Z. Hyaluronic Acid Facilitates Angiogenesis of Endothelial Colony Forming Cell Combining With Mesenchymal Stem Cell via CD44/ MicroRNA-139-5p Pathway. Front. Bioeng. Biotechnol. 2022, 10, 794037. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, L.; Chen, D.; Fan, P.; Yu, H. Exosomal microRNA-140-3p from human umbilical cord mesenchymal stem cells attenuates joint injury of rats with rheumatoid arthritis by silencing SGK1. Mol. Med. 2022, 28, 36. [Google Scholar] [CrossRef]
- Geng, Y.; Chen, J.; Alahdal, M.; Chang, C.; Duan, L.; Zhu, W.; Mou, L.; Xiong, J.; Wang, M.; Wang, D. Intra-articular injection of hUC-MSCs expressing miR-140-5p induces cartilage self-repairing in the rat osteoarthritis. J. Bone Miner. Metab. 2020, 38, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Zhang, Q. Transfer of lncRNA UCA1 by hUCMSCs-derived exosomes protects against hypoxia/reoxygenation injury through impairing miR-143-targeted degradation of Bcl-2. Aging 2021, 13, 5967–5985. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Jiang, T.; Zhang, W.; Xie, W.; Tang, X.; Zhang, J. Human umbilical cord-derived mesenchymal stem cells enhanced HK-2 cell autophagy through MicroRNA-145 by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Cell Res. 2019, 378, 198–205. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, X.; Zhang, R.; Xie, Y.; Feng, Z.; Li, F.; Han, J.; Sun, H.; Ouyang, Q.; Hua, S.; et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 2021, 13, 3060–3079. [Google Scholar] [CrossRef]
- Lai, X.; Wang, Y.; Wang, X.; Liu, B.; Rong, L. miR-146a-5p-modified hUCMSC-derived exosomes facilitate spinal cord function recovery by targeting neurotoxic astrocytes. Stem Cell Res. Ther. 2022, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, Y.; Li, Y.; Liu, W.; Yin, L.; Yin, S.; Ji, C.; Hu, Y.; Wang, Q.; Zhou, X.; et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol. Lett. 2020, 42, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Xiu, C.; Zheng, H.; Jiang, M.; Li, J.; Zhou, Y.; Mu, L.; Liu, W. MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN. Int. J. Stem Cells 2022, 15, 359–371. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Dao, H.H.; Duong, C.M.; Nguyen, X.H.; Hoang, D.H.; Do, X.H.; Truong, T.Q.; Nguyen, T.D.; Nguyen, L.T.; Than, U.T.T. Cytokine-primed umbilical cord mesenchymal stem cells enhanced therapeutic effects of extracellular vesicles on osteoarthritic chondrocytes. Front. Immunol. 2022, 13, 1041592. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Yang, J.; Yu, Y.; Chai, J.; Wang, L.; Ma, L.; Yin, H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine 2016, 8, 72–82. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, L.; Zou, S.; Feng, Y.; Miao, X.; Huang, L. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying MicroRNA-181c-5p Promote BMP2-Induced Repair of Cartilage Injury through Inhibition of SMAD7 Expression. Stem Cells Int. 2022, 2022, 1157498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Chen, L.; Shen, C.; Xiao, Z.; Xu, R.; Wang, J.; Luo, Y. HucMSCs-Derived miR-206-Knockdown Exosomes Contribute to Neuroprotection in Subarachnoid Hemorrhage Induced Early Brain Injury by Targeting BDNF. Neuroscience 2019, 417, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liao, M.; Liu, R.; Zhang, Q.; Zhang, S.; He, Y.; Jin, J.; Zhang, P.; Zhou, L. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles loaded with miR-223 ameliorate myocardial infarction through P53/S100A9 axis. Genomics 2022, 114, 110319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, L. circPTP4A2-miR-330-5p-PDK2 Signaling Facilitates In Vivo Survival of HuMSCs on SF-SIS Scaffolds and Improves the Repair of Damaged Endometrium. Oxidative Med. Cell. Longev. 2022, 2022, 2818433. [Google Scholar] [CrossRef]
- Jiang, S.; Tian, G.; Yang, Z.; Gao, X.; Wang, F.; Li, J.; Tian, Z.; Huang, B.; Wei, F.; Sang, X.; et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 2021, 6, 2711–2728. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yi, X.; Lv, H. MicroRNA-377-3p released by mesenchymal stem cell exosomes ameliorates lipopolysaccharide-induced acute lung injury by targeting RPTOR to induce autophagy. Cell Death Diseas 2020, 11, 657. [Google Scholar] [CrossRef]
- Han, J.; Yang, S.; Hao, X.; Zhang, B.; Zhang, H.; Xin, C.; Hao, Y. Extracellular Vesicle-Derived microRNA-410 From Mesenchymal Stem Cells Protects Against Neonatal Hypoxia-Ischemia Brain Damage Through an HDAC1-Dependent EGR2/Bcl2 Axis. Front. Cell Dev. Biol. 2020, 8, 579236. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Liang, C.; Liu, B.; Ding, F.; Wang, Y.; Zhu, B.; Zhao, R.; Yu, X.Y.; Li, Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics 2020, 10, 6728–6742. [Google Scholar] [CrossRef]
- Liu, J.; Xing, F.; Fu, Q.; He, B.; Jia, Z.; Du, J.; Li, Y.; Zhang, X.; Chen, X. hUC-MSCs exosomal miR-451 alleviated acute lung injury by modulating macrophage M2 polarization via regulating MIF-PI3K-AKT signaling pathway. Environ. Toxicol. 2022, 37, 2819–2831. [Google Scholar] [CrossRef]
- Shao, M.; Xu, Q.; Wu, Z.; Chen, Y.; Shu, Y.; Cao, X.; Chen, M.; Zhang, B.; Zhou, Y.; Yao, R.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res. Ther. 2020, 11, 37. [Google Scholar] [CrossRef]
- Sun, D.; Jiang, Z.; Chen, Y.; Shang, D.; Miao, P.; Gao, J. MiR-455-5p upregulation in umbilical cord mesenchymal stem cells attenuates endometrial injury and promotes repair of damaged endometrium via Janus kinase/signal transducer and activator of transcription 3 signaling. Bioengineered 2021, 12, 12891–12904. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, S.; Lin, C.; Cheng, X.; Meng, Z. Mesenchymal stem cell conditioned medium attenuates oxidative stress injury in hepatocytes partly by regulating the miR-486-5p/PIM1 axis and the TGF-β/Smad pathway. Bioengineered 2021, 12, 6434–6447. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Zhang, W.; Liu, M.; Deng, C. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosome Repairs Endometrial Epithelial Cells Injury Induced by Hypoxia via Regulating miR-663a/CDKN2A Axis. Oxidative Med. Cell. Longev. 2022, 2022, 3082969. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Zhu, B.; et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C 2019, 99, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shen, X.; Yan, C.; Xiong, W.; Ma, Z.; Tan, Z.; Wang, J.; Li, Y.; Liu, J.; Duan, A.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with METTL3 to reduce m6A of NLRP3 in macrophage. Stem Cell Res. Ther. 2022, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Liu, L.; Chen, J.; Liu, F. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle 2019, 18, 3491–3501. [Google Scholar] [CrossRef]
- Xie, K.; Liu, L.; Chen, J.; Liu, F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life 2019, 71, 2020–2030. [Google Scholar] [CrossRef]
- Yang, B.C.; Kuang, M.J.; Kang, J.Y.; Zhao, J.; Ma, J.X.; Ma, X.L. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem. Biophys. Res. Commun. 2020, 524, 883–889. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, D.; Ding, X.; Yang, X.; Zhang, Y. Exosome-shuttled miR-7162-3p from human umbilical cord derived mesenchymal stem cells repair endometrial stromal cell injury by restricting APOL6. Arch. Biochem. Biophys. 2021, 707, 108887. [Google Scholar] [CrossRef]
- Liu, J.S.; Du, J.; Cheng, X.; Zhang, X.Z.; Li, Y.; Chen, X.L. Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. J. Chin. Med.Assoc. JCMA 2019, 82, 895–901. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Wu, D.; Liu, B.; Wang, N.; Rong, L. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res. Ther. 2021, 12, 117. [Google Scholar] [CrossRef]
- Li, W.; Jin, L.Y.; Cui, Y.B.; Xie, N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int. Immunopharmacol. 2021, 90, 107010. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, L.; Shen, H.; Lu, J.; Zou, Q.; Huang, C.; Li, H.; Huang, B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells 2020, 38, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tian, N.; Li, S.; Li, K.; Guo, H.; Zhang, H.; Jin, H.; An, M.; Yu, X. Extracellular vesicles secreted from mesenchymal stem cells exert anti-apoptotic and anti-inflammatory effects via transmitting microRNA-18b in rats with diabetic retinopathy. Int. Immunopharmacol. 2021, 101, 108234. [Google Scholar] [CrossRef]
- Yang, Z.; Shan, N.; Deng, Q.; Wang, Y.; Hou, Y.; Mei, J.; Wu, Z. Extracellular vesicle-derived microRNA-18b ameliorates preeclampsia by enhancing trophoblast proliferation and migration via Notch2/TIM3/mTORC1 axis. J. Cell. Mol. Med. 2021, 25, 4583–4595. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ren, J.; Li, J.; Wang, D.; Wang, Y.; Qin, T.; Li, X.; Zhang, G.; Li, C.; Wang, Y. Extracellular vesicles derived from umbilical cord mesenchymal stromal cells alleviate pulmonary fibrosis by means of transforming growth factor-β signaling inhibition. Stem Cell Res. Ther. 2021, 12, 230. [Google Scholar] [CrossRef]
- Cai, J.H.; Sun, Y.T.; Bao, S. HucMSCs-exosomes containing miR-21 promoted estrogen production in ovarian granulosa cells via LATS1-mediated phosphorylation of LOXL2 and YAP. Gen. Comp. Endocrinol. 2022, 321–322, 114015. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef]
- Du, X.; Li, H.; Han, X.; Ma, W. Mesenchymal stem cells-derived exosomal miR-24-3p ameliorates non-alcohol fatty liver disease by targeting Keap-1. Biochem. Biophys. Res. Commun. 2022, 637, 331–340. [Google Scholar] [CrossRef]
- Yuan, X.; Li, T.; Shi, L.; Miao, J.; Guo, Y.; Chen, Y. Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Mol. Med. 2021, 27, 91. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Liu, Z.; Gong, Y.; Zheng, Z. Human umbilical cord mesenchymal stem cell-derived exosomal miR-27b attenuates subretinal fibrosis via suppressing epithelial-mesenchymal transition by targeting HOXC6. Stem Cell Res. Ther. 2021, 12, 24. [Google Scholar] [CrossRef]
- Gao, T.; Cao, Y. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying MicroRNA-29a Improves Ovarian Function of Mice with Primary Ovarian Insufficiency by Targeting HMG-Box Transcription Factor/Wnt/β-Catenin Signaling. Dis. Markers 2022, 2022, 5045873. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Berenstein, P.; Kronfeld, N.; Morgoulis, D.; Ziv-Av, A.; Goldstein, H.; Kazimirsky, G.; Cazacu, S.; Meir, R.; Popovtzer, R.; et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials 2018, 174, 67–78. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Z.; Yao, T.; Huang, L.; Gan, J.; Lv, H.; Chen, J. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells relieves diabetic retinopathy through a microRNA-30c-5p-dependent mechanism. Diabetes Res. Clin. Pract. 2022, 190, 109861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, F.; Lu, J. microRNA-100 shuttled by human umbilical cord MSC-secreted extracellular vesicles induces endometriosis by inhibiting HS3ST2. Cell. Signal. 2023, 102, 110532. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yu, F.; Luo, X.; Chen, S. Human Umbilical Cord Mesenchymal Stem Cells Improve Premature Ovarian Failure through Cell Apoptosis of miR-100-5p/NOX4/NLRP3. BioMed Res. Int. 2022, 2022, 3862122. [Google Scholar] [CrossRef]
- Li, N.; Gao, Z.; Zhao, L.; Du, B.; Ma, B.; Nian, H.; Wei, R. MSC-Derived Small Extracellular Vesicles Attenuate Autoimmune Dacryoadenitis by Promoting M2 Macrophage Polarization and Inducing Tregs via miR-100-5p. Front. Immunol. 2022, 13, 888949. [Google Scholar] [CrossRef]
- Zhong, Z.; Tian, Y.; Luo, X.; Zou, J.; Wu, L.; Tian, J. Extracellular Vesicles Derived From Human Umbilical Cord Mesenchymal Stem Cells Protect Against DOX-Induced Heart Failure Through the miR-100-5p/NOX4 Pathway. Front. Bioeng. Biotechnol. 2021, 9, 703241. [Google Scholar] [CrossRef]
- Gao, H.; Yu, Z.; Li, Y.; Wang, X. miR-100-5p in human umbilical cord mesenchymal stem cell-derived exosomes mediates eosinophilic inflammation to alleviate atherosclerosis via the FZD5/Wnt/β-catenin pathway. Acta Biochim. Biophys. Sin. 2021, 53, 1166–1176. [Google Scholar] [CrossRef]
- Cui, J.; Chen, X.; Lin, S.; Li, L.; Fan, J.; Hou, H.; Li, P. MiR-101-containing extracellular vesicles bind to BRD4 and enhance proliferation and migration of trophoblasts in preeclampsia. Stem Cell Res. Ther. 2020, 11, 231. [Google Scholar] [CrossRef]
- Oh, S.J.; Lee, E.N.; Park, J.H.; Lee, J.K.; Cho, G.J.; Park, I.H.; Shin, O.S. Anti-Viral Activities of Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Against Human Respiratory Viruses. Front. Cell. Infect. Microbiol. 2022, 12, 850744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes Derived From Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Investig. Ophthalmol. Vis. Sci. 2019, 60, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Liu, L. miR-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res. Ther. 2022, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, S. SDF-1α Facilitates Mesenchymal Stem Cells to Induce Regulatory B Cell Differentiation from Patients with Immune Thrombocytopenia. Stem Cells Int. 2021, 2021, 3254488. [Google Scholar] [CrossRef]
- Wang, D.; Na, Q.; Song, G.Y.; Wang, L. Human umbilical cord mesenchymal stem cell-derived exosome-mediated transfer of microRNA-133b boosts trophoblast cell proliferation, migration and invasion in preeclampsia by restricting SGK1. Cell Cycle 2020, 19, 1869–1883. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, T.; Xia, Q.; Tian, J.; Yang, J. microRNA-140-5p from human umbilical cord mesenchymal stem cells-released exosomes suppresses preeclampsia development. Funct. Integr. Genom. 2022, 22, 813–824. [Google Scholar] [CrossRef]
- Kwak, J.; Choi, S.J.; Oh, W.; Yang, Y.S.; Jeon, H.B. Cobalt Chloride Enhances the Anti-Inflammatory Potency of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells through the ERK-HIF-1α-MicroRNA-146a-Mediated Signaling Pathway. Stem Cells Int. 2018, 2018, 4978763. [Google Scholar] [CrossRef]
- Wang, J.; Pei, B.; Yan, J.; Xu, X.; Fang, A.N.; Ocansey, D.K.W.; Zhang, X.; Qian, H. hucMSC-Derived Exosomes Alleviate the Deterioration of Colitis via the miR-146a/SUMO1 Axis. Mol. Pharm. 2022, 19, 484–493. [Google Scholar] [CrossRef]
- Liang, Y.C.; Wu, Y.P.; Li, X.D.; Chen, S.H.; Ye, X.J.; Xue, X.Y. TNF-α-induced exosomal miR-146a mediates mesenchymal stem cell-dependent suppression of urethral stricture. J. Cell. Physiol. 2019, 234, 23243–23255. [Google Scholar] [CrossRef]
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221. [Google Scholar] [CrossRef]
- Hua, T.; Yang, M.; Song, H.; Kong, E.; Deng, M.; Li, Y.; Li, J.; Liu, Z.; Fu, H.; Wang, Y.; et al. Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis. J. Nanobiotechnology 2022, 20, 324. [Google Scholar] [CrossRef]
- Chen, X.; Su, C.; Wei, Q.; Sun, H.; Xie, J.; Nong, G. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Diffuse Alveolar Hemorrhage Associated with Systemic Lupus Erythematosus in Mice by Promoting M2 Macrophage Polarization via the microRNA-146a-5p/NOTCH1 Axis. Immunol. Investig. 2022, 51, 1975–1993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Le, X.; Zheng, S.; Zhang, K.; He, J.; Liu, M.; Tu, C.; Rao, W.; Du, H.; Ouyang, Y.; et al. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res. Ther. 2022, 13, 171. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Y.; Zheng, T.; Pu, Y.; Ma, Y.; Qi, X.; Zhang, W.; Xue, F.; Shan, Z.; Liu, J.; et al. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell Res. Ther. 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Spinosa, M.; Lu, G.; Su, G.; Bontha, S.V.; Gehrau, R.; Salmon, M.D.; Smith, J.R.; Weiss, M.L.; Mas, V.R.; Upchurch, G.R., Jr.; et al. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEBJ. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, fj201701138RR. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Rong, C.; Gu, T.; Li, H.; Wu, L.; Zhuansun, X.; Zhao, X.; Xiao, Z.; Kuang, Y.; Xu, S.; et al. Mesenchymal stem cells improve liver fibrosis and protect hepatocytes by promoting microRNA-148a-5p-mediated inhibition of Notch signaling pathway. Stem Cell Res. Ther. 2022, 13, 354. [Google Scholar] [CrossRef]
- Li, D.; Lu, Z.; Li, X.; Xu, Z.; Jiang, J.; Zheng, Z.; Jia, J.; Lin, S. Human umbilical cord mesenchymal stem cells facilitate the up-regulation of miR-153-3p, whereby attenuating MGO-induced peritoneal fibrosis in rats. J. Cell. Mol. Med. 2018, 22, 3452–3463. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Duan, M.; Dou, Y.; Feng, Y.; Nan, N.; Zhang, W. MiR-153-3p induces immune dysregulation by inhibiting PELI1 expression in umbilical cord-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Autoimmunity 2020, 53, 201–209. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, P.; Yuan, L.; Chhetri, R.K.; Guo, Y.; Deng, D. Effects of human umbilical cord mesenchymal stem cells on inflammatory factors and miR-181a in T lymphocytes from patients with systemic lupus erythematosus. Lupus 2020, 29, 126–135. [Google Scholar] [CrossRef]
- Zhou, C.; Wan, S.; Zhao, X.; Gu, S.; Pei, J.; Wu, Y.; Han, Z.; Che, R.; Hua, X. Exosomal miR-195 in hUC-MSCs alleviates hypoxia-induced damage of trophoblast cells through tissue factor pathway inhibitor 2. Curr. Res. Transl. Med. 2022, 70, 103352. [Google Scholar] [CrossRef]
- Bi, Z.M.; Zhou, Q.F.; Geng, Y.; Zhang, H.M. Human umbilical cord mesenchymal stem cells ameliorate experimental cirrhosis through activation of keratinocyte growth factor by suppressing microRNA-199. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4905–4912. [Google Scholar] [PubMed]
- Cheng, T.; Ding, S.; Liu, S.; Li, Y.; Sun, L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics 2021, 11, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, X.; Fang, A.; Yan, J.; Kofi Wiredu Ocansey, D.; Zhang, X.; Mao, F. HucMSC-Ex carrying miR-203a-3p.2 ameliorates colitis through the suppression of caspase11/4-induced macrophage pyroptosis. Int. Immunopharmacol. 2022, 110, 108925. [Google Scholar] [CrossRef]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, W.; Wang, W.; Song, A.; Mukama, O.; Deng, S.; Han, X.; De Dieu Habimana, J.; Peng, K.; Ni, B.; et al. MicroRNA-206 down-regulated human umbilical cord mesenchymal stem cells alleviate cognitive decline in D-galactose-induced aging mice. Cell Death Discov. 2022, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, J.; Shi, P.; Su, D.; Cheng, X.; Yi, W.; Yan, J.; Chen, H.; Cheng, F. Small Extracellular Vesicles Derived From MSCs Have Immunomodulatory Effects to Enhance Delivery of ASO-210 for Psoriasis Treatment. Front. Cell Dev. Biol. 2022, 10, 842813. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, N.; Liu, Y.; Zhang, W.; Li, X.; Wang, Y.; Zheng, R.; Zhang, Y. Mesenchymal stem cell exosome-derived miR-223 alleviates acute graft-versus-host disease via reducing the migration of donor T cells. Stem Cell Res. Ther. 2021, 12, 153. [Google Scholar] [CrossRef]
- Liu, L.; Yin, H.; Hao, X.; Song, H.; Chai, J.; Duan, H.; Chang, Y.; Yang, L.; Wu, Y.; Han, S.; et al. Down-Regulation of miR-301a-3p Reduces Burn-Induced Vascular Endothelial Apoptosis by potentiating hMSC-Secreted IGF-1 and PI3K/Akt/FOXO3a Pathway. iScience 2020, 23, 101383. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, J.; Kofi Wiredu Ocansey, D.; Lu, B.; Wan, A.; Chen, X.; Zhang, X.; Qiu, W.; Mao, F. Exosomes derived from human umbilical cord mesenchymal stem cells regulate lymphangiogenesis via the miR-302d-3p/VEGFR3/AKT axis to ameliorate inflammatory bowel disease. Int. Immunopharmacol. 2022, 110, 109066. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, J.; Cai, X.; Xu, Z.; Wang, J.; Ocansey, D.K.W.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; et al. HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve inflammatory bowel disease in mice. Clin. Transl. Med. 2020, 10, e113. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhong, Z.; Zhang, Y.; Tan, H.; Deng, B.; Meng, G. Human umbilical cord mesenchymal stem cell-derived exosomal miR-335-5p attenuates the inflammation and tubular epithelial-myofibroblast transdifferentiation of renal tubular epithelial cells by reducing ADAM19 protein levels. Stem Cell Res. Ther. 2022, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Chen, Q.; Ding, H.; Li, H. MicroRNA-342-3p loaded by human umbilical cord mesenchymal stem cells-derived exosomes attenuates deep vein thrombosis by downregulating EDNRA. J. Thromb. Thrombolysis 2022, 54, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, F.; Hou, X.; Xu, H.; Tang, D. Ameliorating role of microRNA-378 carried by umbilical cord mesenchymal stem cells-released extracellular vesicles in mesangial proliferative glomerulonephritis. Cell Commun. Signal. CCS 2022, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, Z.Y.; Yuan, J.T.; Ocansey, D.K.W.; Tu, Q.; Zhang, X.; Qian, H.; Xu, W.R.; Qiu, W.; Mao, F. hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis. Stem Cell Res. Ther. 2021, 12, 416. [Google Scholar] [CrossRef]
- Zhou, Q.; Gu, T. Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Hepatic Stellate Cell Activation and Liver Fibrosis by Upregulating MicroRNA-455-3p through Suppression of p21-Activated Kinase-2. BioMed Res. Int. 2021, 2021, 6685605. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Jeong, J.B.; Oh, S.J. Wharton’s Jelly-Derived Mesenchymal Stem Cells Reduce Fibrosis in a Mouse Model of Duchenne Muscular Dystrophy by Upregulating microRNA 499. Biomedicines 2021, 9, 1089. [Google Scholar] [CrossRef]
- Cheng, L.; Yu, P.; Li, F.; Jiang, X.; Jiao, X.; Shen, Y. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum. Cell 2021, 34, 1697–1708. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, D.; Wang, S.; Lin, H.; Wang, Y.; Xu, W. Exosomal microRNA-1246 from human umbilical cord mesenchymal stem cells potentiates myocardial angiogenesis in chronic heart failure. Cell Biol. Int. 2021, 45, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Y.; Ye, Y.; Wu, R.; Li, W.; Yao, C.; Wang, S. Human umbilical cord mesenchymal stem cell-derived exosomal microRNA-148a-3p inhibits neointimal hyperplasia by targeting Serpine1. Arch. Biochem. Biophys. 2022, 719, 109155. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res. Ther. 2021, 12, 434. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wang, T.; Wang, Z.-X.; Huang, K.-P.; Zhang, Y.-W.; Wang, G.-L.; Zhang, H.-J.; Chen, Z.-H.; Wang, C.-Y.; Zhang, J.-X.; et al. Hypoxic ucMSC-secreted exosomal miR-125b promotes endothelial cell survival and migration during wound healing by targeting TP53INP1. Mol. Ther.-Nucleic Acids 2021, 26, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yan, G.; Huang, H.; Zheng, M.; Ma, K.; Cui, X.; Lu, D.; Zheng, L.; Zhu, B.; Cheng, J.; et al. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnology 2022, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Tang, S.; Gao, H.; Zhang, H.; Chen, C.; Fang, Z.; Peng, G.; Weng, H.; Chen, A.; Zhang, C.; et al. Wharton’s jelly mesenchymal stem cell-derived small extracellular vesicles as natural nanoparticles to attenuate cartilage injury via microRNA regulation. Int. J. Pharm. 2022, 623, 121952. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Tao, X.; Shen, X. Human umbilical cord mesenchymal stem cell-derived exosomes inhibit migration and invasion of breast cancer cells via miR-21-5p/ZNF367 pathway. Breast Cancer 2021, 28, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, Y.; Qu, Y.; Liu, L.; Li, H. Exosomes Derived From MicroRNA-148b-3p-Overexpressing Human Umbilical Cord Mesenchymal Stem Cells Restrain Breast Cancer Progression. Front. Oncol. 2019, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Khazaei-Poul, Y.; Shojaei, S.; Koochaki, A.; Ghanbarian, H.; Mohammadi-Yeganeh, S. Evaluating the influence of Human Umbilical Cord Mesenchymal Stem Cells-derived exosomes loaded with miR-3182 on metastatic performance of Triple Negative Breast Cancer cells. Life Sci. 2021, 286, 120015. [Google Scholar] [CrossRef]
- Khazaei-Poul, Y.; Mirmotalebisohi, S.A.; Zali, H.; Molavi, Z.; Mohammadi-Yeganeh, S. Identification of miR-3182 and miR-3143 target genes involved in the cell cycle as a novel approach in TNBC treatment: A systems biology approach. Chem. Biol. Drug Des. 2023, 101, 662–677. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Zhao, L.; Chen, X.; Lin, Z.; Zhang, L.; Li, Z. miR-224-5p Carried by Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes Regulates Autophagy in Breast Cancer Cells via HOXA5. Front. Cell Dev. Biol. 2021, 9, 679185. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, X.; Li, Y.; Yan, P.; Zhang, H. Human umbilical cord blood mesenchymal stem cells-derived exosomal microRNA-503-3p inhibits progression of human endometrial cancer cells through downregulating MEST. Cancer Gene Ther. 2022, 29, 1130–1139. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.L.; Yao, J.L.; Wang, K. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a. Stem Cells Int. 2019, 2019, 8108576. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wu, D.; Li, M.; Zhao, F.; Ren, M.; Cai, Y.; Dou, J. IL-21-secreting hUCMSCs combined with miR-200c inhibit tumor growth and metastasis via repression of Wnt/β-catenin signaling and epithelial-mesenchymal transition in epithelial ovarian cancer. OncoTargets Ther. 2018, 11, 2037–2050. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, J.; Chen, M.; Chen, F.; Tu, W. Exosomal microRNA-146a derived from mesenchymal stem cells increases the sensitivity of ovarian cancer cells to docetaxel and taxane via a LAMC2-mediated PI3K/Akt axis. Int. J. Mol. Med. 2020, 46, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Finniss, S.; Cazacu, S.; Bucris, E.; Ziv-Av, A.; Xiang, C.; Bobbitt, K.; Rempel, S.A.; Hasselbach, L.; Mikkelsen, T.; et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget 2013, 4, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Ghahremani, M.H.; Soleimani, M. Delivery of Exogenous miR-124 to Glioblastoma Multiform Cells by Wharton’s Jelly Mesenchymal Stem Cells Decreases Cell Proliferation and Migration, and Confers Chemosensitivity. Stem Cell Rev. Rep. 2018, 14, 236–246. [Google Scholar] [CrossRef]
- Hao, S.C.; Ma, H.; Niu, Z.F.; Sun, S.Y.; Zou, Y.R.; Xia, H.C. hUC-MSCs secreted exosomes inhibit the glioma cell progression through PTENP1/miR-10a-5p/PTEN pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10013–10023. [Google Scholar] [CrossRef]
- Li, N.; Wang, B. Suppressive effects of umbilical cord mesenchymal stem cell-derived exosomal miR-15a-5p on the progression of cholangiocarcinoma by inhibiting CHEK1 expression. Cell Death Discov. 2022, 8, 205. [Google Scholar] [CrossRef]
- Ma, H.; Weng, F.; Wang, L.; Tong, X.; Yao, Y.; Li, H. Extracellular vesicle-mediated delivery of miR-127-3p inhibits the proliferation and invasion of choriocarcinoma cells by targeting ITGA6. Exp. Cell Res. 2022, 414, 113098. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J. MicroRNA-320a-containing exosomes from human umbilical cord mesenchymal stem cells curtail proliferation and metastasis in lung cancer by binding to SOX4. J. Recept. Signal Transduct. Res. 2022, 42, 268–278. [Google Scholar] [CrossRef]
- Dong, L.; Pu, Y.; Zhang, L.; Qi, Q.; Xu, L.; Li, W.; Wei, C.; Wang, X.; Zhou, S.; Zhu, J.; et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018, 9, 218. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, M.; Tang, Q. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-181a retards nasopharyngeal carcinoma development by mediating KDM5C. J. Cancer Res. Clin. Oncol. 2021, 147, 2867–2877. [Google Scholar] [CrossRef]
- Chen, M.; Xia, Z.; Deng, J. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles carrying miR-655-3p inhibit the development of esophageal cancer by regulating the expression of HIF-1α via a LMO4/HDAC2-dependent mechanism. Cell Biol. Toxicol. 2022. [Google Scholar] [CrossRef]
- He, Z.; Li, W.; Zheng, T.; Liu, D.; Zhao, S. Human umbilical cord mesenchymal stem cells-derived exosomes deliver microRNA-375 to downregulate ENAH and thus retard esophageal squamous cell carcinoma progression. J. Exp. Clin. CancerRes. CR 2020, 39, 140. [Google Scholar] [CrossRef]
- Qu, M.; Li, J.; Hong, Z.; Jia, F.; He, Y.; Yuan, L. The role of human umbilical cord mesenchymal stem cells-derived exosomal microRNA-431-5p in survival and prognosis of colorectal cancer patients. Mutagenesis 2022, 37, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Chen, W.; Li, T.; Chen, X.; Liu, B. microRNA-6785-5p-loaded human umbilical cord mesenchymal stem cells-derived exosomes suppress angiogenesis and metastasis in gastric cancer via INHBA. Life Sci. 2021, 284, 119222. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ding, X.; Zhou, L.; Zhang, L.; Yang, X. Mesenchymal stem cells-derived exosomal microRNA-139-5p restrains tumorigenesis in bladder cancer by targeting PRC1. Oncogene 2021, 40, 246–261. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, Y.; Xu, X.; Qi, X.; Liu, J.; Pu, Y.; Zhang, S.; Gao, X.; Luo, X.; Li, M.; et al. MiR-30c-5p loss-induced PELI1 accumulation regulates cell proliferation and migration via activating PI3K/AKT pathway in papillary thyroid carcinoma. J. Transl. Med. 2022, 20, 20. [Google Scholar] [CrossRef]
- Gan, J.; Liu, S.; Zhang, Y.; He, L.; Bai, L.; Liao, R.; Zhao, J.; Guo, M.; Jiang, W.; Li, J.; et al. MicroRNA-375 is a therapeutic target for castration-resistant prostate cancer through the PTPN4/STAT3 axis. Exp. Mol. Med. 2022, 54, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lai, Y.; Cao, L.; Li, Y.; Chen, G.; Chen, L.; Weng, H.; Chen, T.; Wang, L.; Ye, Y. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-451a represses epithelial-mesenchymal transition of hepatocellular carcinoma cells by inhibiting ADAM10. RNA Biol. 2021, 18, 1408–1423. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, F.; Sun, H.; Wang, Y.; Liu, S.; Wu, Y.; Cui, Q.; Mei, W.; Li, F. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019, 442, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhong, P.; Zhang, J.; Chen, X.; Chen, J.; Lin, T.; Wu, Q. Human umbilical cord-mesenchymal stem cells-derived exosomes carrying microRNA-15a-5p possess therapeutic effects on Wilms tumor via regulating septin 2. Bioengineered 2022, 13, 6136–6149. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhang, M.; Cheng, H.; Mai, H.; Yi, M.; Xu, H.; Yuan, X.; Liu, S.; Wen, F. HucMSC exosomes promoted imatinib-induced apoptosis in K562-R cells via a miR-145a-5p/USP6/GLS1 axis. Cell Death Dis. 2022, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Mei, W.; Zheng, Z.; Cao, F.; Liang, K.; Jia, Y.; Wang, Y.; Liu, D.; Li, J.; Li, F. Exosomes secreted from human umbilical cord mesenchymal stem cells promote pancreatic ductal adenocarcinoma growth by transferring miR-100-5p. Tissue Cell 2021, 73, 101623. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Vuong, C.K.; Ngo, N.H.; Yamashita, T.; Ye, X.; Futamura, Y.; Fukushige, M.; Obata-Yasuoka, M.; Hamada, H.; Osaka, M.; et al. Extracellular vesicles derived from Wharton’s Jelly mesenchymal stem cells inhibit the tumor environment via the miR-125b/HIF1α signaling pathway. Sci. Rep. 2022, 12, 13550. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, H.; Chen, X.; Chen, S.; Yu, L.; Wang, C.; Liu, Y.; Zhang, K.; Wu, L.; Han, Z.C.; et al. The delivery of hsa-miR-11401 by extracellular vesicles can relieve doxorubicin-induced mesenchymal stem cell apoptosis. Stem Cell Res. Ther. 2021, 12, 77. [Google Scholar] [CrossRef]
- Joerger-Messerli, M.S.; Oppliger, B.; Spinelli, M.; Thomi, G.; di Salvo, I.; Schneider, P.; Schoeberlein, A. Extracellular Vesicles Derived from Wharton’s Jelly Mesenchymal Stem Cells Prevent and Resolve Programmed Cell Death Mediated by Perinatal Hypoxia-Ischemia in Neuronal Cells. Cell Transplant. 2018, 27, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Chen, Y.A. Multiplexed Molecular Imaging Strategy Integrated with RNA Sequencing in the Assessment of the Therapeutic Effect of Wharton’s Jelly Mesenchymal Stem Cell-Derived Extracellular Vesicles for Osteoporosis. Int. J. Nanomed. 2021, 16, 7813–7830. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Zhang, Y.; Zhang, H.; Liu, W.; Zhang, N.; Zhang, X.; Zhou, G.; Wu, L.; Hua, K.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate growth of VK2 vaginal epithelial cells through MicroRNAs in vitro. Hum. Reprod. 2019, 34, 248–260. [Google Scholar] [CrossRef]
- Yang, K.; Li, D.; Wang, M.; Xu, Z.; Chen, X.; Liu, Q.; Sun, W.; Li, J.; Gong, Y.; Liu, D.; et al. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2019, 10, 358. [Google Scholar] [CrossRef]
- Motawi, T.M.K.; Sabry, D.; Maurice, N.W.; Rizk, S.M. Role of mesenchymal stem cells exosomes derived microRNAs; miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and evaluation. Arch. Biochem. Biophys. 2018, 659, 13–21. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Xu, B.; He, Y.; Liu, W.; Li, J.; Zhang, S.; Lin, X.; Su, D.; Wu, T.; et al. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol.Ther. J. Am. Soc. Gene Ther. 2020, 28, 1200–1213. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Park, J.; Park, S.; You, S.; Song, G. Anti-inflammatory effects of mesenchymal stem cell-derived exosomal microRNA-146a-5p and microRNA-548e-5p on human trophoblast cells. Mol. Hum. Reprod. 2019, 25, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, J.; Deng, Z.; Qian, W. Exosomes derived from human umbilical cord mesenchymal stem cells protect against papain-induced emphysema by preventing apoptosis through activating VEGF-VEGFR2-mediated AKT and MEK/ERK pathways in rats. Regen. Ther. 2022, 21, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Karaoz, E. Can Wharton jelly derived or adipose tissue derived mesenchymal stem cell can be a treatment option for duchenne muscular dystrophy? Answers as transcriptomic aspect. Am. J. Stem Cells 2020, 9, 57–67. [Google Scholar] [PubMed]
- Fong, C.Y.; Tam, K.; Cheyyatraivendran, S.; Gan, S.U.; Gauthaman, K.; Arunmozhiarasi, A.; Jeyaseelan, K.; Choolani, M.; Biswas, A.; Bongso, A. Erratum: Human Wharton’s Jelly Stem Cells and Its Conditioned Medium Enhance Healing of Excisional and Diabetic Wounds. J. Cell. Biochem. 2017, 118, 3016. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, X.; Li, L. Biogenesis and function of extracellular miRNAs. ExRNA 2019, 1, 38. [Google Scholar] [CrossRef]
- Stepanov, G.; Zhuravlev, E.; Shender, V.; Nushtaeva, A.; Balakhonova, E.; Mozhaeva, E.; Kasakin, M.; Koval, V.; Lomzov, A.; Pavlyukov, M.; et al. Nucleotide Modifications Decrease Innate Immune Response Induced by Synthetic Analogs of snRNAs and snoRNAs. Genes 2018, 9, 531. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, Y.W.; Brewer, G.; Jing, Q. MicroRNA degradation and turnover: Regulating the regulators. RNA 2012, 3, 593–600. [Google Scholar] [CrossRef]
- Biscans, A.; Coles, A.; Haraszti, R.; Echeverria, D.; Hassler, M.; Osborn, M.; Khvorova, A. Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo. Nucleic Acids Res. 2019, 47, 1082–1096. [Google Scholar] [CrossRef]
- Suter, S.R.; Ball-Jones, A.; Mumbleau, M.M.; Valenzuela, R.; Ibarra-Soza, J.; Owens, H.; Fisher, A.J.; Beal, P.A. Controlling miRNA-like off-target effects of an siRNA with nucleobase modifications. Org. Biomol. Chem. 2017, 15, 10029–10036. [Google Scholar] [CrossRef]
- Yu, H.R.; Huang, L.H.; Li, S.C. Roles of microRNA in the immature immune system of neonates. Cancer Lett. 2018, 433, 99–106. [Google Scholar] [CrossRef]
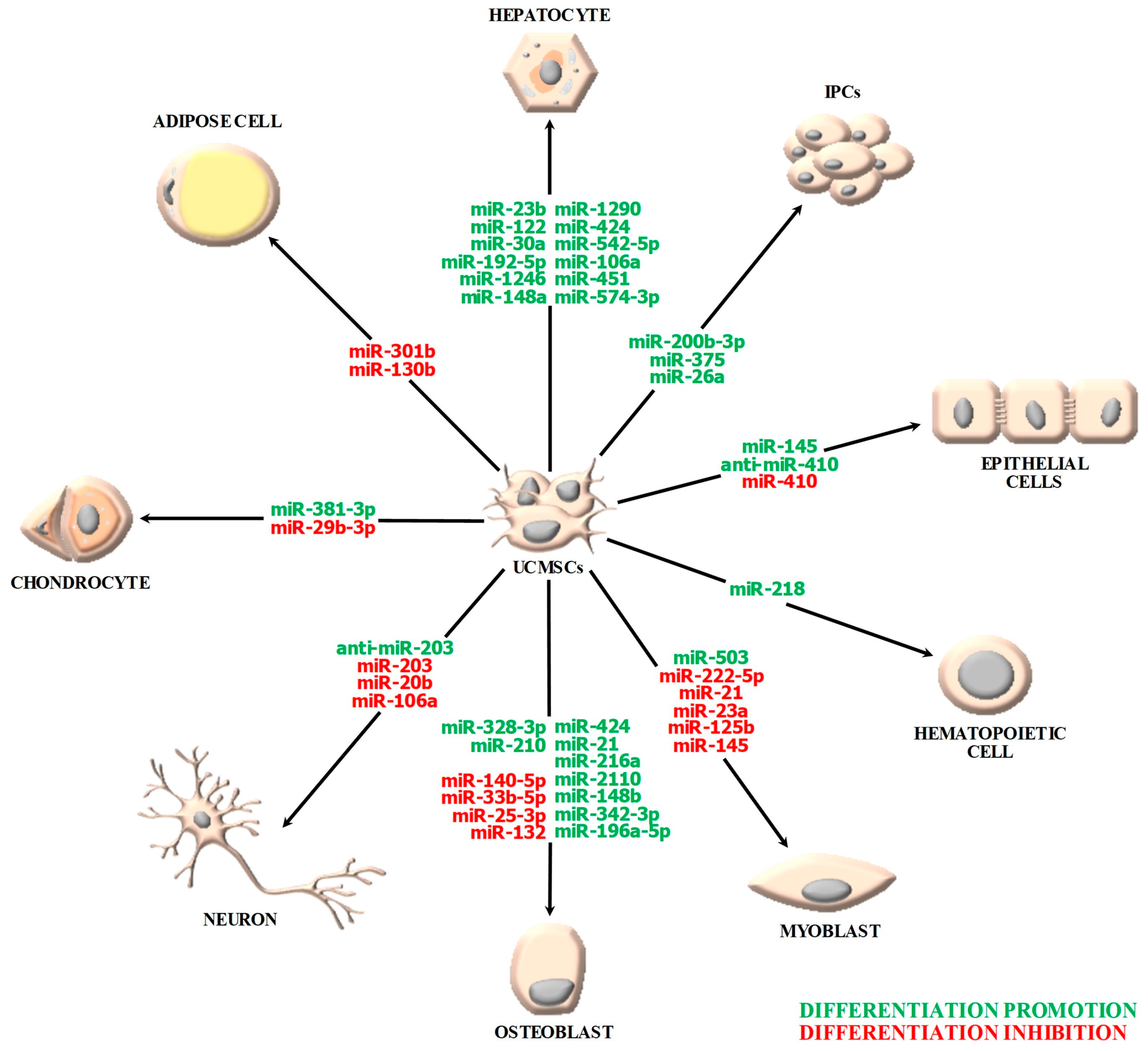
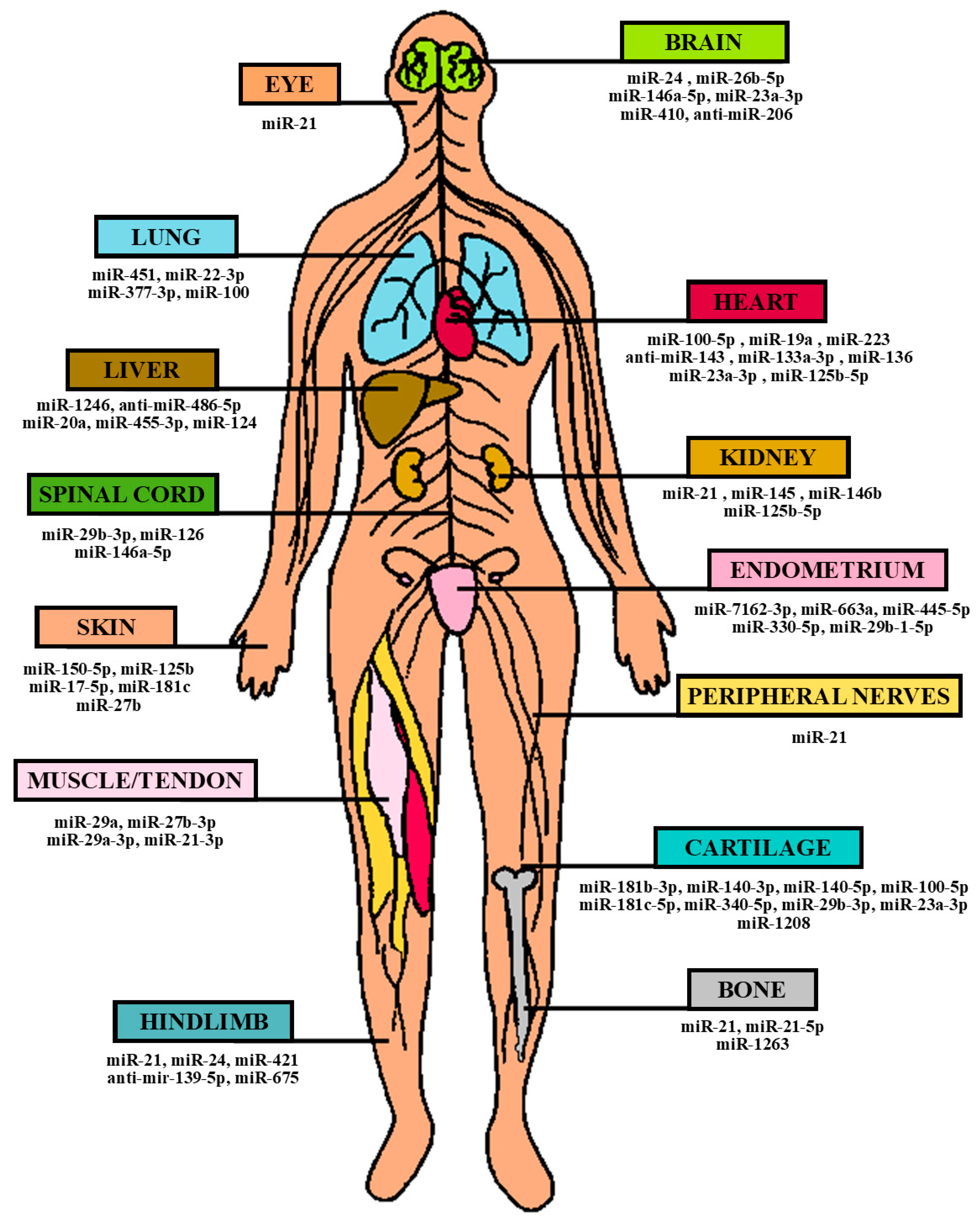
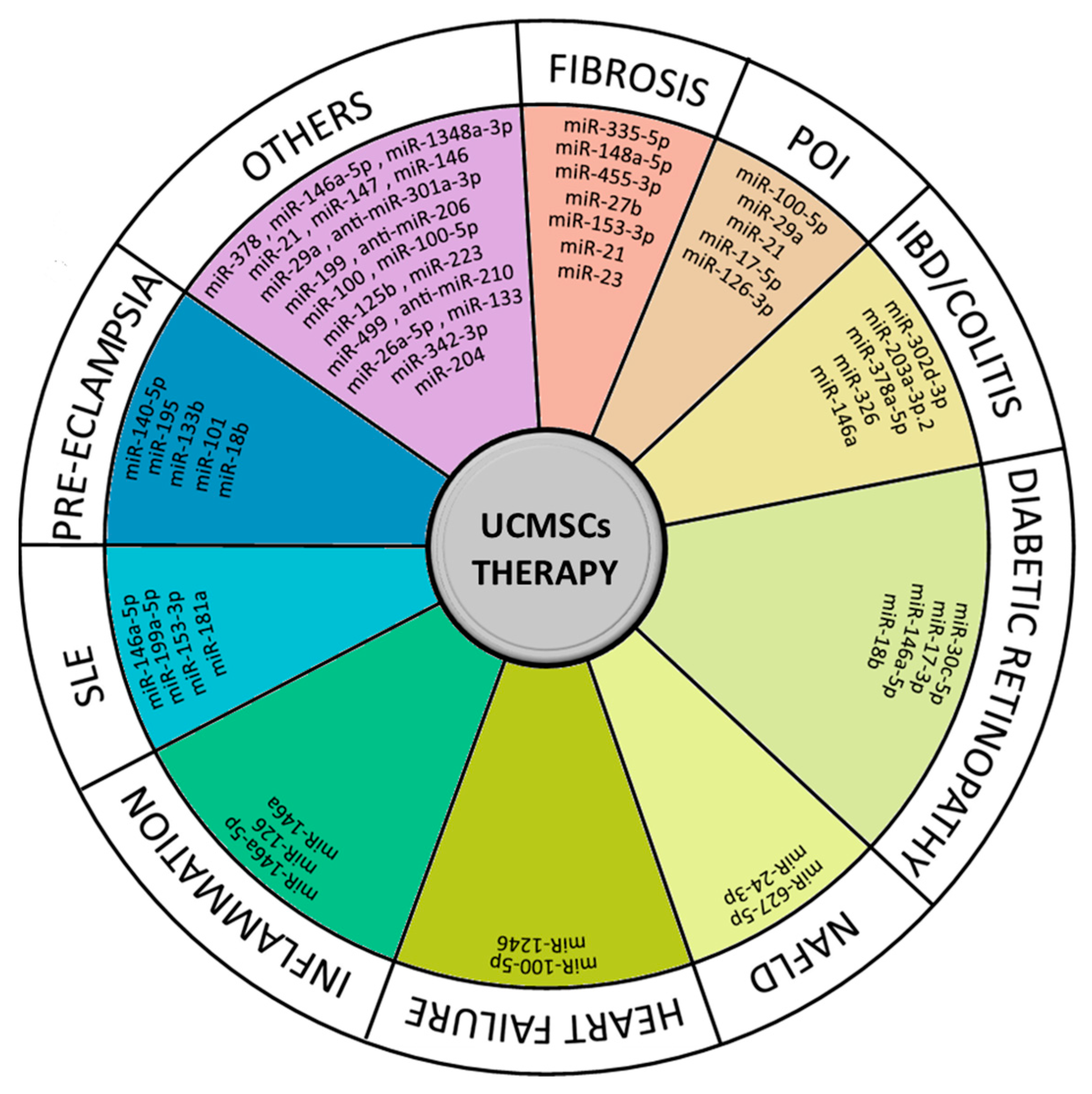
| miRNA | Tissue Origin | Target (Gene/Pathway) | Target Regulation | miRNA Function | Reference |
|---|---|---|---|---|---|
| miR-424 | WJMSCs | BMP2 | Up | Osteogenic | [26] |
| miR-140-5p | UCMSCs, ATMSCs, BMMSCs | BMP2 | Down | Anti-Osteogenic | [27] |
| miR-342-3p | UCMSCs | Sufu/Shh | Down/Up | Osteogenic | [28] |
| miR-196a-5p | WJMSCs | SERPINB2 | Down | Osteogenic | [29] |
| miR-21 | UCMSCs | KLF12/Wnt/β-catenin | Down/Up | Osteogenic | [30] |
| miR-21 | UCMSCs | PI3K/β-catenin | Up | Osteogenic | [31] |
| miR-2110 | UCMSCs (Exosomes) | (TNF) | (Up) | Osteogenic | [32] |
| miR-328-3p | UCMSCs (Exosomes) | CHRD | Down | Osteogenic | [32] |
| miR-25-3p | UCMSCs | SMAD5/(Dlx5/OSX) | Down/(Down) | Anti-Osteogenic | [33] |
| miR-33b-5p | UCMSCs | Wnt10b/(Dlx5/OSX) | Down/(Down) | Anti-Osteogenic | [33] |
| miR-132 | UCMSCs | Osterix/Wnt/β-catenin | Down/Down | Anti-Osteogenic | [34] |
| miR-216a | UCMSCs, ATMSCs | c-Cbl/PI3K/AKT | Down/Up | Osteogenic | [35] |
| miR-122-5p | UCMSCs | SOX11, VIM, (CUTL1, CAT1) | Down | Hepatogenic | [36] |
| miR-106a, miR-574-3p, miR-451 | UCMSCs | Sox17, FoxA2, AFP | Up | Hepatogenic | [37] |
| miR-20b, miR-106a | WJMSCs | Ngn2, MAP2, TUBB3 | Down | Anti-Neurogenic | [38] |
| miR-203 | UCBMSCs | DKK1, CRX, NRL | Down | Anti-Neurogenic | [39] |
| miR-29b-3p | UCMSCs | SOX9 | Down | Anti-Chondrogenic | [40] |
| miR-381 | UCMSCs (sEVs) | SOX9/TAOK1 | Up/Down | Chondrogenic | [41] |
| miR-410 | UCBMSCs | MITF, LRAT, RPE65, Bestrophin, EMMPRIN | Down | Anti-Epithelial | [42] |
| miR-145 | UCMSCs | TGFβRII | Down | Epithelial | [43] |
| miR-200b-3p | UCMSCs | ZEB2 | Down | IPCs Differentiation | [44] |
| miR-375, miR-26a | N-UCMSCs (chicken) | MTPN, SOX6, BHLHE22, CCND1 | Down | IPCs Differentiation | [45] |
| miR-218 | UCMSCs | MITF, (HoxB4, NF-Ya) | Down, (Up) | Hematopoietic | [46] |
| miR-301b/miR-130b | UCMSCs, ATMSCs, BMMSCs | PPARγ | Down | Anti-Adipogenic | [47] |
| miR-21, miR-23a, miR-125b, miR-145 | UCMSCs (Exosomes) | TGF-β2, TGF-βR2, SMAD2 | Down | Anti-Myofibroblastic | [48] |
| miR-503-5p | UCMSCs | SMAD7 | Down | Myogenetic | [49] |
| miR-222-5p | UCMSCs | ROCK2, αSMA | Down | Anti-Myogenetic | [49] |
| miRNA | Tissue Origin | Vehicle Type | Target (Gene/Pathway) | Function | Clinical Application | Reference |
|---|---|---|---|---|---|---|
| miR-17-5p | UCMSCs | EVs | PTEN/AKT/HIF-1α/VEGF | Angiogenesis/Proliferation/Migration/ Tube Formation Promotion | Diabetic Wound Healing | [64] |
| miR-19a | UCMSCs | Exosomes | SOX6, AKT/JNK3/caspase-3 | Hypoxic Damage Reduction | AMI | [65] |
| miR-20a | UCMSCs | Exosomes | Beclin-I, FAS, Caspase-3, mTOR, P62, LC3II | Apoptosis/Autophagy Inhibition | Hepatic IRI | [66] |
| miR-21 | UCMSCs | MVs | PDCD4 | Apoptosis Inhibition | Renal IRI | [67] |
| UCBMSCs | - | CHIP/HIF-1α | Proliferation/Migration/Angiogenesis/ Neovascularization Promotion | CLI | [68] | |
| UCMSCs | EVs | PI3K/AKT | Proliferation Promotion | Peripheral Nerve Injury | [69] | |
| UCMSCs | EVs | PTEN/PI3K/Akt | Proliferation/Migration Promotion | Corneal Wound Healing | [70] | |
| WJMSCs | Exosomes | PTEN/Akt | Angiogenesis/Osteogenesis/Cell Survival Promotion | ONFH | [71] | |
| UCMSCs | Exosomes | NOTCH1/DLL4 | Angiogenesis Promotion | Bone Regeneration | [72] | |
| miR-21-3p | UCMSCs | Exosomes | p65, COX2 | Fibrosis/Inflammation Inhibition | Tendon Injury | [73] |
| miR-21-5p | UCMSCs | Exosomes | SOX5, EZH2 | Angiogenesis/Osteogenesis Promotion | ONFH | [74] |
| miR-22-3p | UCBMSCs | Exosomes | FZD6 | Inflammation/Oxidative Stress/Apoptosis Suppression, Proliferation Promotion | Acute Lung Injury | [75] |
| miR-23a-3p | UCBMSCs | Exosomes | - | M2 Macrophage Polarization Promotion, Microglia Activation Inhibition | Cerebral Infarction | [76] |
| UCBMSCs | Exosomes | DMT1 | Ferroptosis Inhibition | AMI | [77] | |
| UCMSCs | EVs | PTEN/AKT | Differentiation/Proliferation/Migration Promotion | Cartilage Regeneration | [78] | |
| miR-24 | UCMSCs | EVs | AQP4/P38 MAPK/ERK1/2/P13K/AKT | Apoptosis/Inflammation Inhibition, Proliferation/Migration Promotion | Cerebral IRI | [79] |
| UCMSCs | Exosomes | Bim | Immune Rejection Prevention | Ischemic Hindlimb Injury | [80] | |
| miR-26b-5p | UCMSCs | Exosomes | CH25H-/TLR | Microglia M1 Polarization Inhibition | Cerebral IRI | [81] |
| UCMSCs | Exosomes | MAT2A/MAPK/STAT3 | Apoptosis/Inflammatory Inhibition | EBI | [82] | |
| miR-27b | UCMSCs | EVs | ITCH/JUNB/IRE1α | Proliferation/Migration Promotion | Skin Wound Healing | [83] |
| miR-27b-3p | UCMSCs | Exosomes | ARHGAP5/RhoA | Proliferation/Invasion Promotion | Tendon Injury | [84] |
| miR-29a | WJMSCs | - | TIMP-2 | Angiogenesis, Blood Perfusion Promotion | Skeletal Muscle Ischemic Injury | [85] |
| miR-29a-3p | UCMSCs | Exosomes | PTEN/mTOR/TGF-β1 | Differentiation/Healing Promotion | Tendon Injury | [86] |
| miR-29b-3p | UCMSCs | EVs | PTEN/Akt/mTOR | Necrosis Reduction, Nerve Function Promotion | SCI | [87] |
| UCMSCs | EVs | PTEN/PI3K/AKT | Neuronal Apoptosis Inhibition | SCI | [88] | |
| UCMSCs | Exosomes | FoxO3 | Apoptosis/Senescence Promotion, Migration/Inflammation Inhibition | Cartilage Defect | [89] | |
| miR-29b-1-5p | WJMSCs | - | RAP1B/VEGF | Angiogenesis Promotion/Tissue Repair | Damaged Endometrium | [90] |
| miR-100 | WJMSCs | MVs | mTOR | Autophagy Promotion | Acute Lung Injury | [91] |
| miR-100-5p | UCMSCs | Exosomes | FOXO3, NLRP | Ιnflammasome/Cytokine Activation Suppression, Pyroptosis Inhibition | AMI | [92] |
| UCMSCs | Exosomes | NOX4 | ROS Production/Apoptosis Inhibition | Osteoarthritis | [93] | |
| miR-124 | UCBMSCs | Exosomes | Foxg1 | Proliferation/Regeneration Promotion, Injury Inhibition | Liver Regeneration | [94] |
| miR-125b | UCMSCs | Exosomes | TP53INP1 | Cell Survival/Migration Promotion, Apoptosis Inhibition | Skin Wound Healing | [75] |
| miR-125b-5p | UCMSCs | Exosomes | SMAD7 | Hypoxic Injury/Apoptosis Promotion | AMI | [95] |
| UCMSCs | Exosomes | p53 | Cell cycle arrest/Apoptosis Inhibition | Ischemic AKI | [96] | |
| miR-126 | UCMSCs | Exosomes | SPRED1, PIK3R2 | Neurogenesis/Angiogenesis Promotion, Apoptosis Inhibition | SCI | [97] |
| miR-133a-3p | UCMSCs | MIF-Exosomes | AKT/VEGF | Angiogenesis/Proliferation Promotion, Apoptosis/Fibrosis Inhibition | AMI | [98] |
| miR-136 | UCMSCs | Exosomes | Apaf1 | Apoptosis/Fibrosis/Senescence Suppression, Angiogenesis Promotion | Age-associated MI | [99] |
| anti-miR-139-5p | UCBMSCs/UCMSCs | - | - | Proliferation/Migration/Angiogenesis Promotion | Peripheral Arterial Disease (Limb Ischemia) | [100] |
| miR-140-3p | UCMSCs | EVs | SGK1 | Inflammation/Oxidative Stress/Fibrosis Suppression | Rheumatoid Arthritis | [101] |
| miR-140-5p | UCMSCs | - | - | Chondrogenesis/Cartilage Self-Repair Promotion | Osteoarthritis | [102] |
| anti-miR-143 | UCMSCs | Exosomes | Bcl-2/Beclin-1 | Apoptosis/Autophagy Inhibition | Cardiac IRI | [103] |
| miR-145 | UCMSCs | - | PI3K/AKT/mTOR | Autophagy Promotion | AKI | [104] |
| miR-146a-5p | UCMSCs | Exosomes | IRAK1/TRAF6 | Neuroinflammation Suppression | Ischemic Stroke | [105] |
| UCMSCs | Exosomes | Traf6/Irak1/NFκB | Neurotoxic Astrocytes Reduction | SCI | [106] | |
| miR-146b | UCMSCs | - | IRAK1/NF-κB | Apoptosis/Inflammation Inhibition | Sepsis-associated AKI | [107] |
| miR-150-5p | UCMSCs | Exosomes | PTEN/PI3K/Akt | Growth/Migration Promotion, Apoptosis Inhibition | Skin Wound Healing | [108] |
| miR-181b-3p | UCMSCs | EVs | - | Chondrocyte Proliferation/Cartilage Regeneration Inhibition | Osteoarthritis | [109] |
| miR-181c | WJMSCs | Exosomes | TLR4/NF-κB/p65 | Burn-induced Inflammation/Macrophage Inflammation Suppression | Burn Injury | [110] |
| miR-181c-5p | UCMSCs | EVs | SMAD7 | Proliferation/Migration/Chondrogenesis Promotion | Cartilage Injury | [111] |
| anti-miR-206 | UCMSCs | Exosomes | BDNF/TrkB/CREB | Apoptosis Inhibition | EBI | [112] |
| miR-223 | UCMSCs | EVs | P53/S100A9 | Angiogenesis Promotion, Inflammation/Fibrosis Suppression | MI | [113] |
| miR-330-5p | UCMSCs | SF-SIS Scaffold | CircPTP4A2, PDK2 | Mitochondrial Metabolism Impairment, Fibrosis Promotion | Endometrial Hypoxic Injury | [114] |
| miR-340-5p | WJMSCs | Exosomes | IL4 | Osteochondral Regeneration | Articular Cartilage Defect | [115] |
| miR-377-3p | UCMSCs | Exosomes | RPTOR | Autophagy Promotion | Acute Lung Injury | [116] |
| miR-410 | UCMSCs | EVs | HDAC1/EGR2/Bcl2 | Apoptosis Inhibition | HIBD | [117] |
| miR-421 | UCMSCs | Exosomes | FOXO3a | Pyroptosis/Ischemic Injury Promotion | Acute Lower Limb Ischemic Injury | [118] |
| miR-451 | UCMSCs | Exosomes | TLR4/NF-κB, MIF/PI3K/Akt | Inflammation Suppression, M2 Macrophage Polarization Activation | Acute Lung Injury | [119] |
| miR-455-3p | UCMSCs | Exosomes | PIK3r1/IL-6 | Macrophages Activation Inhibition, Inflammatory Cytokine Suppression | Acute Liver Injury | [120] |
| miR-455-5p | UCMSCs | - | SOCS3/JAK/STAT3 | Cell Cycle/Proliferation Promotion, Endometrial Glands Increase, Fibrosis Suppression | Endometrial Injury | [121] |
| anti-miR-486-5p | UCBMSCs | - | PIM1/TGF-β/Smad | Apoptosis/Inflammation Suppression, Proliferation Promotion | Oxidative Stress Injury | [122] |
| miR-663a | UCMSCs | Exosomes | CDKN2A | Proliferation Promotion, Apoptosis/Migration/EMT Inhibition | Endometrial Hypoxic Injury | [123] |
| miR-675 | UCMSCs | Exosomes | p21, TGF-β1 | Blood Perfusion Promotion, Senescence Inhibition | Ischemia-induced Vascular Dysfunction | [124] |
| miR-1208 | UCMSCs | EVs | METTLE3 | Proliferation/Migration Promotion, Apoptosis/Inflammation Inhibition | Osteoarthritis | [125] |
| miR-1246 | UCBMSCs | Exosomes | GSK3β-Wnt/β-catenin | Apoptosis Inhibition | Hepatic IRI | [126] |
| UCMSCs | Exosomes | IL-6/gp130/STAT3 | Th17/Treg Balance Regulation | Hepatic IRI | [127] | |
| miR-1263 | UCMSCs | Exosomes | Mob1/Hippo | Apoptosis Inhibition | Disuse Osteoporosis | [128] |
| miR-7162-3p | UCMSCs | Exosomes | APOL6 | Apoptosis Inhibition, Cell Repair/Regeneration | Endometrial Injury | [129] |
| miRNAs | Tissue Origin | Vehicle Type | Target (Gene/Pathway) | Function | Clinical Application | Reference |
|---|---|---|---|---|---|---|
| miR-199a-3p/145-5p | UCMSCs | Exosomes | Cblb, Cbl, NGF/TrkA | Neuronal Differentiation Promotion | SCI | [131] |
| miR-let-7a, miR-let-7e | WJMSCs | EVs | Casp3 | Apoptosis Inhibition | Hypoxic-ischemic Brain Injury | [225] |
| miR-21-5p, miR-125b-5p | UCBMSCs | Exosomes | TGF-βI, TGF-βII | Wound Closure Acceleration, Scar Formation Inhibition, Skin/Nerve/Vessel Regeneration | Skin Wound Healing | [190] |
| miR-21, miR-29, miR-221, let-7a | WJMSCs | EVs | BMP, PI3K/AKT | Osteogenic Differentiation/Osteoblast Activity Promotion | Osteoporosis | [226] |
| miR-122-5p, miR-148a-3p, miR-486-5p, miR-let-7a-5p, miR-100-5p | UCMSCs | EVs | PI3K/Akt | Cartilage Degradation/Inflammation Suppression, M2 Macrophage Polarization Promotion | Osteoarthritis | [192] |
| miR-92b-3p, miR-32-5p, let-7b-5p, miR-19a-3p, miR-19b-3p | WJMSCs | Exosomes | DKK3 | Osteochondral Regeneration | Articular Cartilage Defect | [115] |
| miR-23a-3p, miR-221-3p, miR-23b-3p, miR-141-3p, miR-144-3p, miR-200a-3p, miR-454-3p, miR-23c, miR-320b | WJMSCs | Exosomes | CXCL12 | Osteochondral Regeneration | Articular Cartilage Defect | [115] |
| miR-374a-5p, miR-495-3p, miR-323a-3p | WJMSCs | Exosomes | CCL2 | Osteochondral Regeneration | Articular Cartilage Defect | [115] |
| miR-19b-3p, miR-185-5p | WJMSCs | Exosomes | POSTN | Osteochondral Regeneration | Articular Cartilage Defect | [115] |
| let-7e-5p, miR-423-5p, miR-199a-3p, miR-125b-5p, miR-142-3p, miR-92a-3p | WJMSCs | EVs | ECM-receptor interaction, NOTCH, (STAT3, IGF1R) | Proliferation/Migration/M2 Infiltration Promotion, Homeostasis Maintenance, Injury Degradation Suppression | Osteoarthritis | [193] |
| miR-100, miR-146a, (miR-21, miR-221, miR-143) | UCMSCs | Exosomes | - | Cell Cycle/Proliferation Promotion, Apoptosis Inhibition | Vaginal Reconstruction | [227] |
| miR-135b-5p, miR-499a-3p | UCMSCs | Exosomes | MEF2C | Angiogenesis Promotion | Tissue Regeneration | [228] |
| miR-136, miR-494, miR-495 | UCMSCs | Exosomes | - | Biomarkers (Diagnosis, Evaluation) | Pre-Eclampsia | [229] |
| miR-21, miR-146a-5p | UCMSCs | Exosomes | PI3K/mTOR | Fertility Recovery, Oocyte Production Promotion | Premature Ovarian Insufficiency | [230] |
| miR-146a-5p, miR-548e-5p | UCMSCs | Exosomes | NF-κB, AKT, MAPK | Inflammation Suppression, Proliferation/Migration Promotion | Inflammatory Diseases/Preterm Birth | [231] |
| miR-17 Superfamily | WJMSCs, BMMSCs | Exosomes | (BMPR2) | Proliferation Promotion | Pulmonary Hypertension | [174] |
| miR-10a-5p, miR-146a-5 | UCMSCs | Exosomes | VEGF-VEGFR2/AKT, MEK/ERK | Apoptosis Inhibition | Chronic Obstructive Pulmonary Disease | [232] |
| miR-24, miR-199a-5p | WJMSCs | Exosomes | - | Muscle Regeneration | Duchenne Muscular Dystrophy | [233] |
| miR-124, miR-145 | Bone Marrow/Adipose Tissue/Placenta/Umbilical Cord MSCs | - | SCP-1, Sox2 | Migration/Cell Self-Renewal Inhibition | Glioma | [203] |
| let-7a-2-3p, let-7d, let-7e, let-7f, let-7f-1-3p, let-7g, let-7i, let-7i-3p, miR-100, miR-106a, miR-106b, miR-125a-3p, miR-125a-5p, miR-126, miR-146a, miR-17, miR-181a, miR-18b, miR-196a-3p, miR-19a, miR-19b, miR-200b, miR-20a, miR-20b, miR-21, miR-210, miR-25, miR-27a-5p, miR-29b, miR-302a, miR-302b-5p, miR-302c-5p, miR-30c, miR-31, miR-32, miR-335, miR-34a, miR-374a, miR-378 miR-3915, miR-3924, miR-601, miR-622, miR-920, miR-92a, miR-93, miR-98 | WJMSCs | - | - | Wound Healing Promotion | Excisional/Diabetic Wounds | [234] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomaidou, A.C.; Goulielmaki, M.; Tsintarakis, A.; Zoumpourlis, P.; Toya, M.; Christodoulou, I.; Zoumpourlis, V. miRNA-Guided Regulation of Mesenchymal Stem Cells Derived from the Umbilical Cord: Paving the Way for Stem-Cell Based Regeneration and Therapy. Int. J. Mol. Sci. 2023, 24, 9189. https://doi.org/10.3390/ijms24119189
Thomaidou AC, Goulielmaki M, Tsintarakis A, Zoumpourlis P, Toya M, Christodoulou I, Zoumpourlis V. miRNA-Guided Regulation of Mesenchymal Stem Cells Derived from the Umbilical Cord: Paving the Way for Stem-Cell Based Regeneration and Therapy. International Journal of Molecular Sciences. 2023; 24(11):9189. https://doi.org/10.3390/ijms24119189
Chicago/Turabian StyleThomaidou, Arsinoe C., Maria Goulielmaki, Antonis Tsintarakis, Panagiotis Zoumpourlis, Marialena Toya, Ioannis Christodoulou, and Vassilis Zoumpourlis. 2023. "miRNA-Guided Regulation of Mesenchymal Stem Cells Derived from the Umbilical Cord: Paving the Way for Stem-Cell Based Regeneration and Therapy" International Journal of Molecular Sciences 24, no. 11: 9189. https://doi.org/10.3390/ijms24119189
APA StyleThomaidou, A. C., Goulielmaki, M., Tsintarakis, A., Zoumpourlis, P., Toya, M., Christodoulou, I., & Zoumpourlis, V. (2023). miRNA-Guided Regulation of Mesenchymal Stem Cells Derived from the Umbilical Cord: Paving the Way for Stem-Cell Based Regeneration and Therapy. International Journal of Molecular Sciences, 24(11), 9189. https://doi.org/10.3390/ijms24119189






