Cooperation of Complement MASP-1 with Other Proinflammatory Factors to Enhance the Activation of Endothelial Cells
Abstract
1. Introduction
2. Results
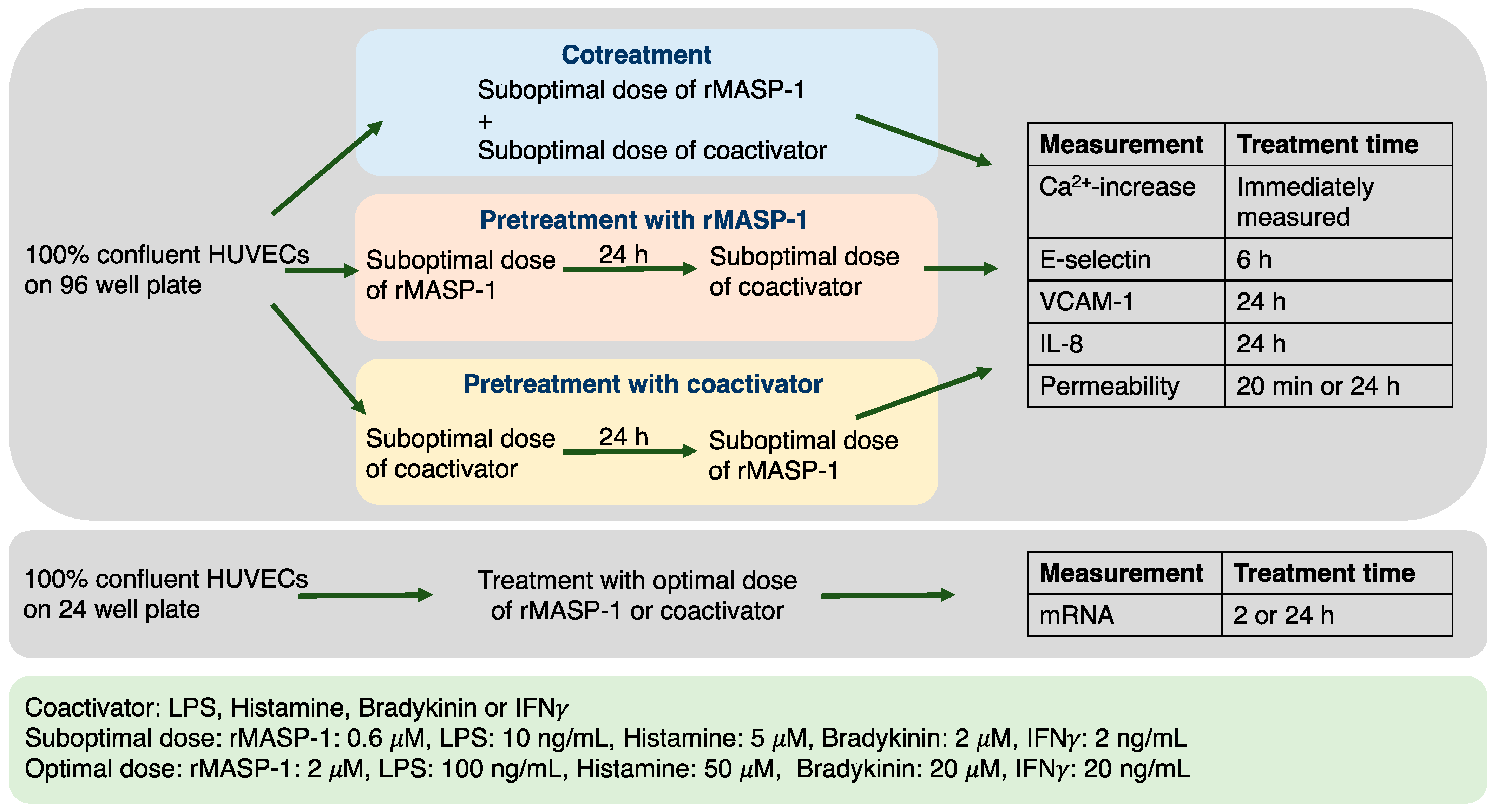
2.1. Experimental Setup
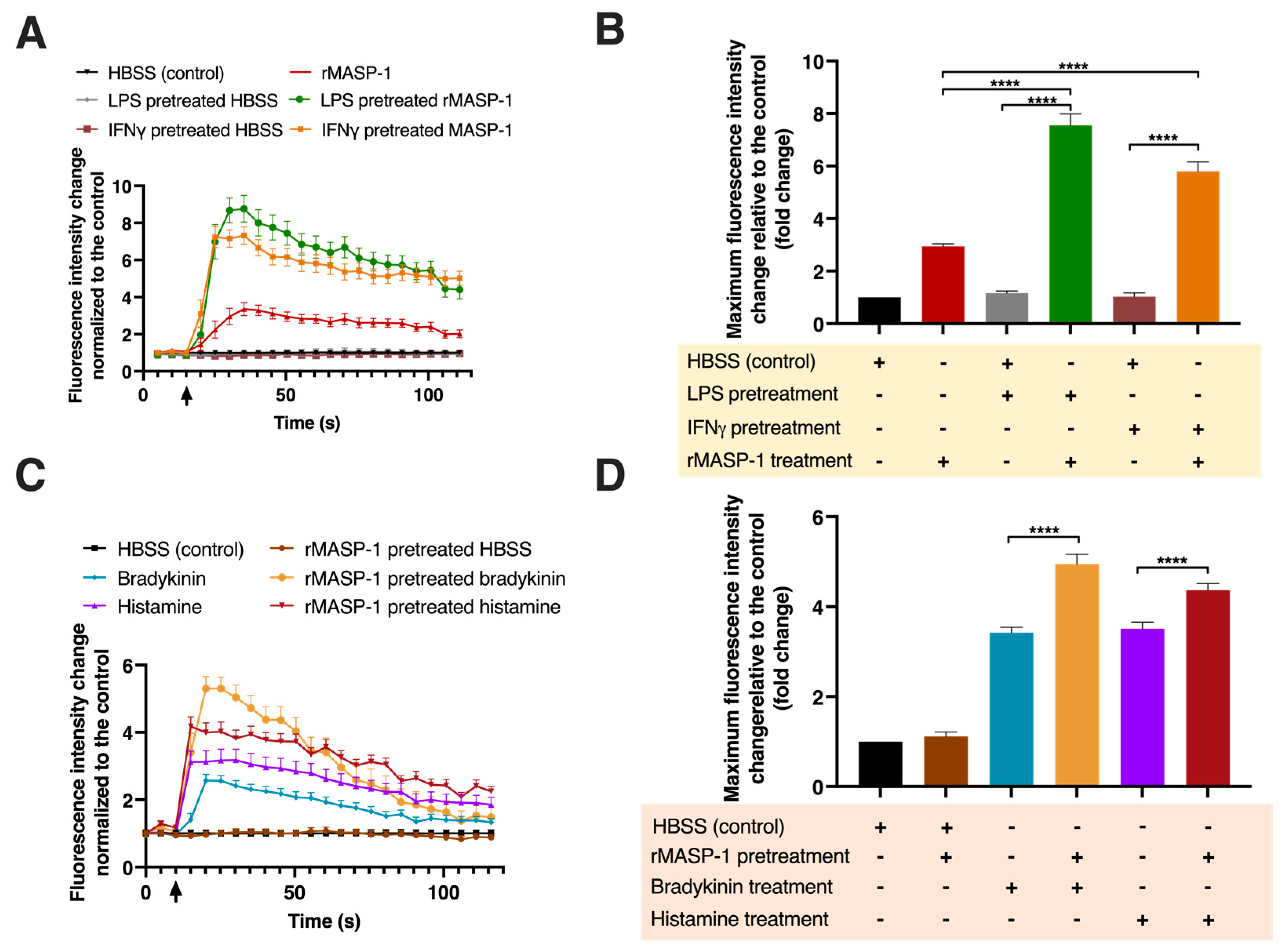
2.2. rMASP-1 Cooperates with LPS, IFNγ, Bradykinin, and Histamine to Induce Intracellular Ca2+ Mobilization
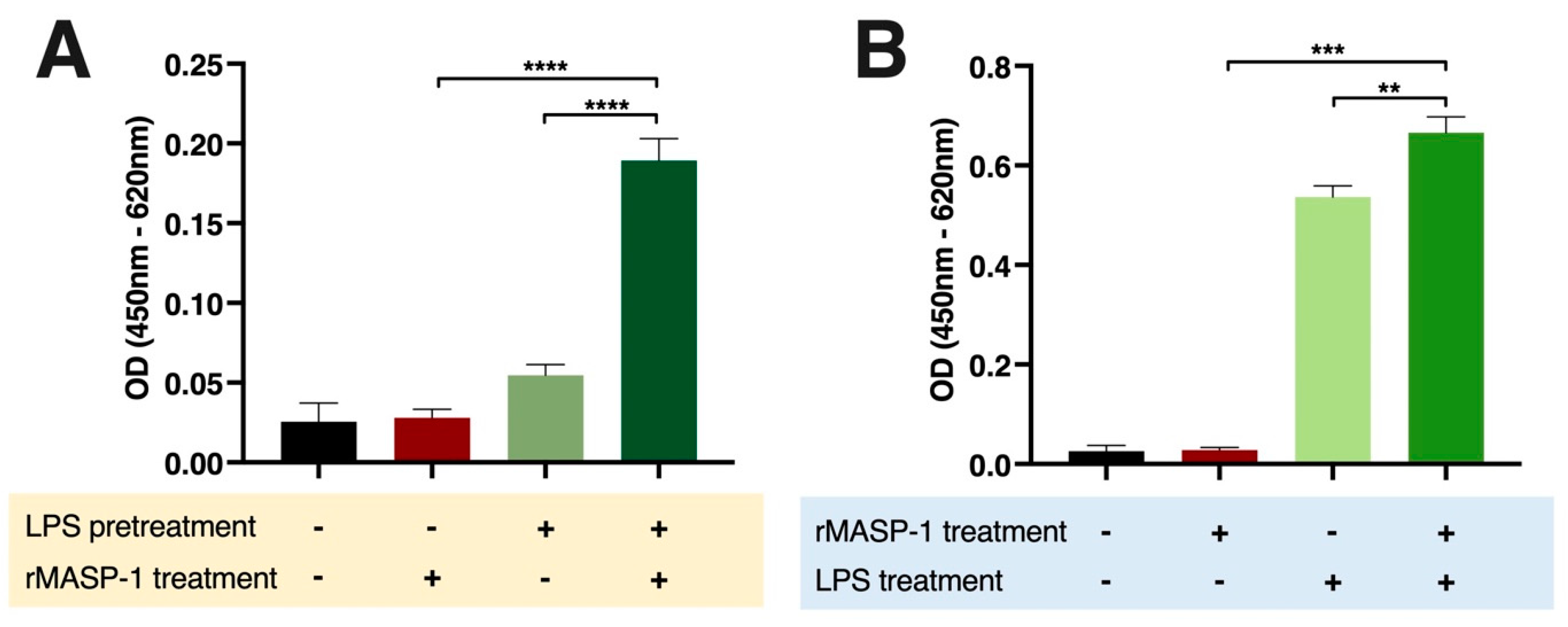
2.3. rMASP-1 Cooperates with LPS in the Induction of E-selectin Expression
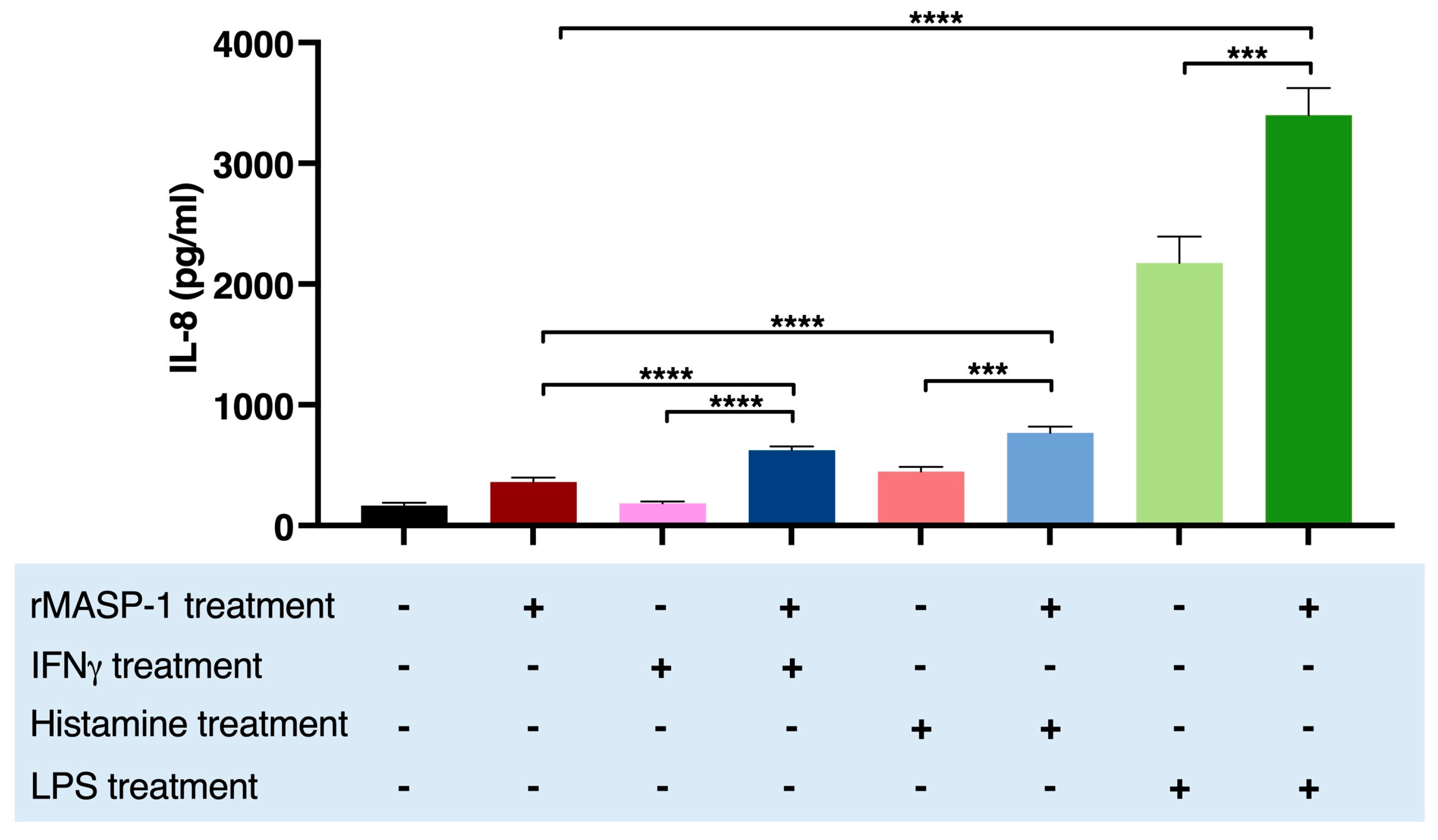
2.4. rMASP-1 Cooperates with LPS, IFNγ, and Histamine in the Induction of IL-8 Production
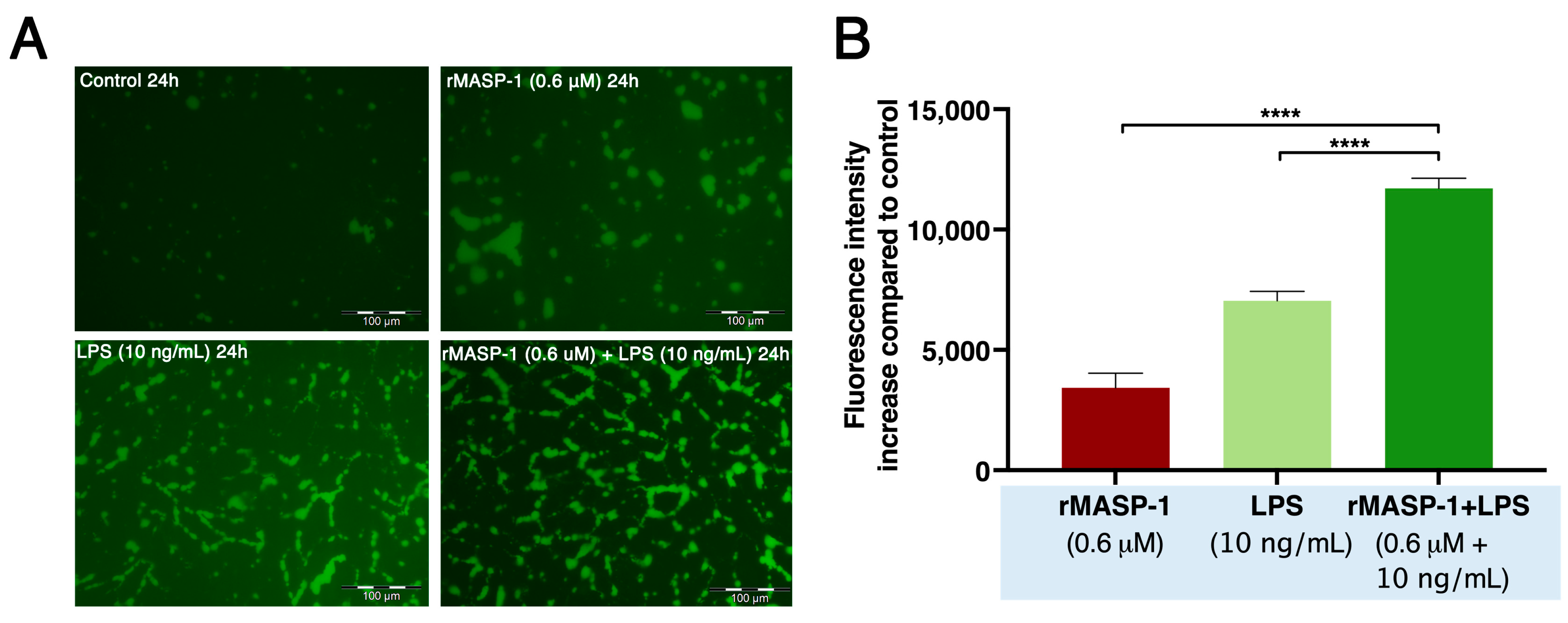
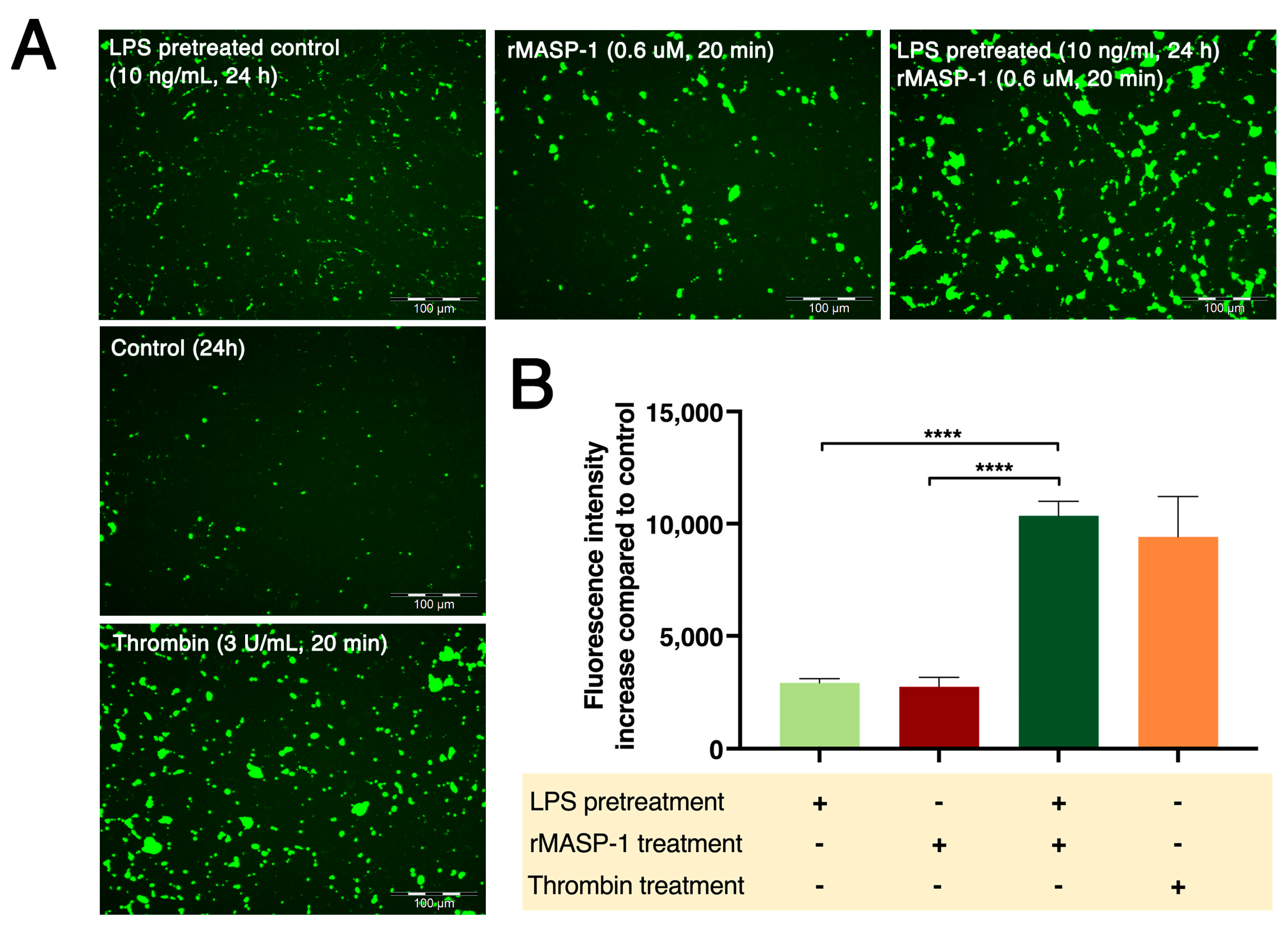
2.5. rMASP-1 and LPS Cooperates in Endothelial Permeability Induction
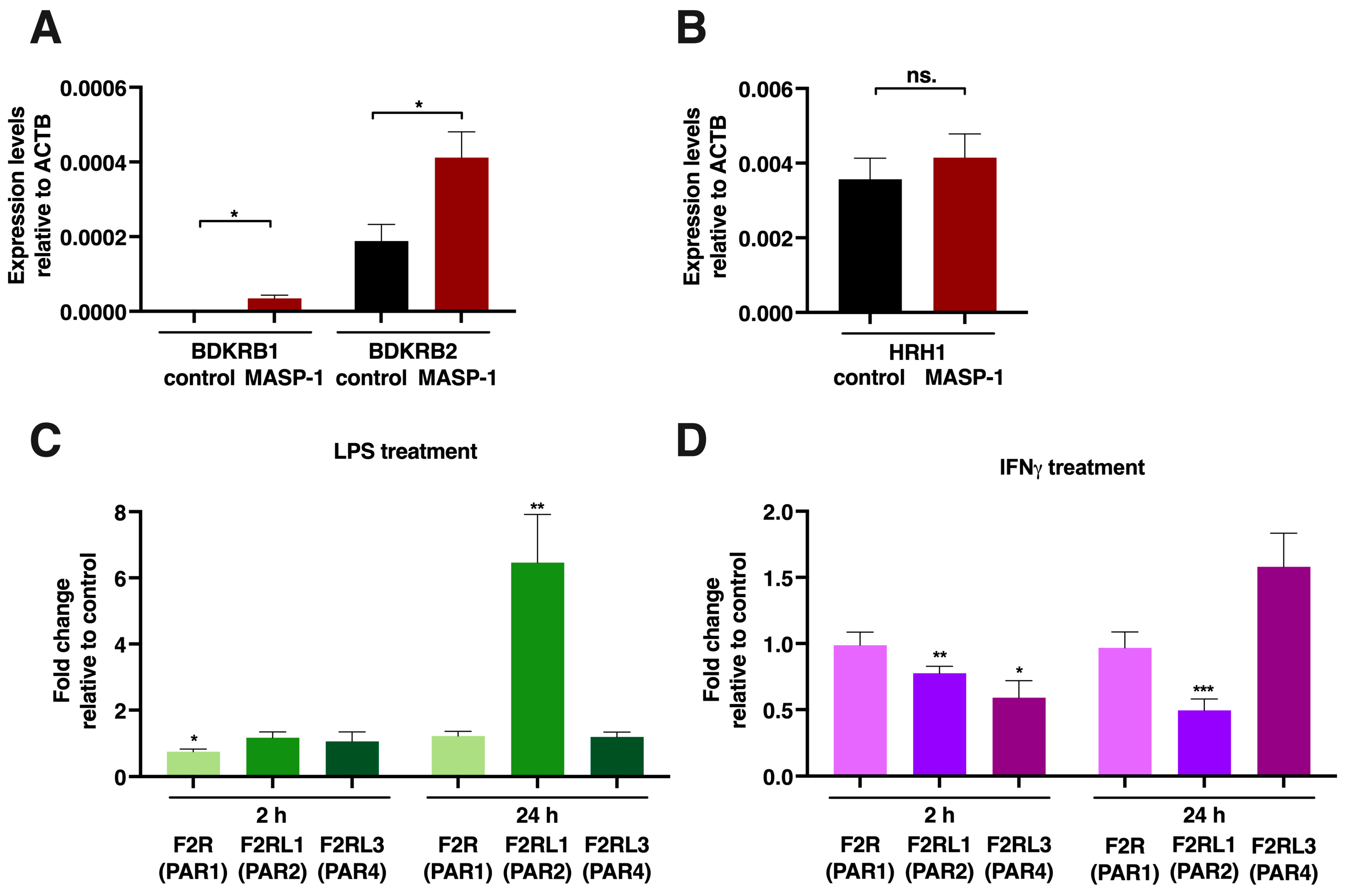
2.6. mRNA Measurements for Receptor Expression
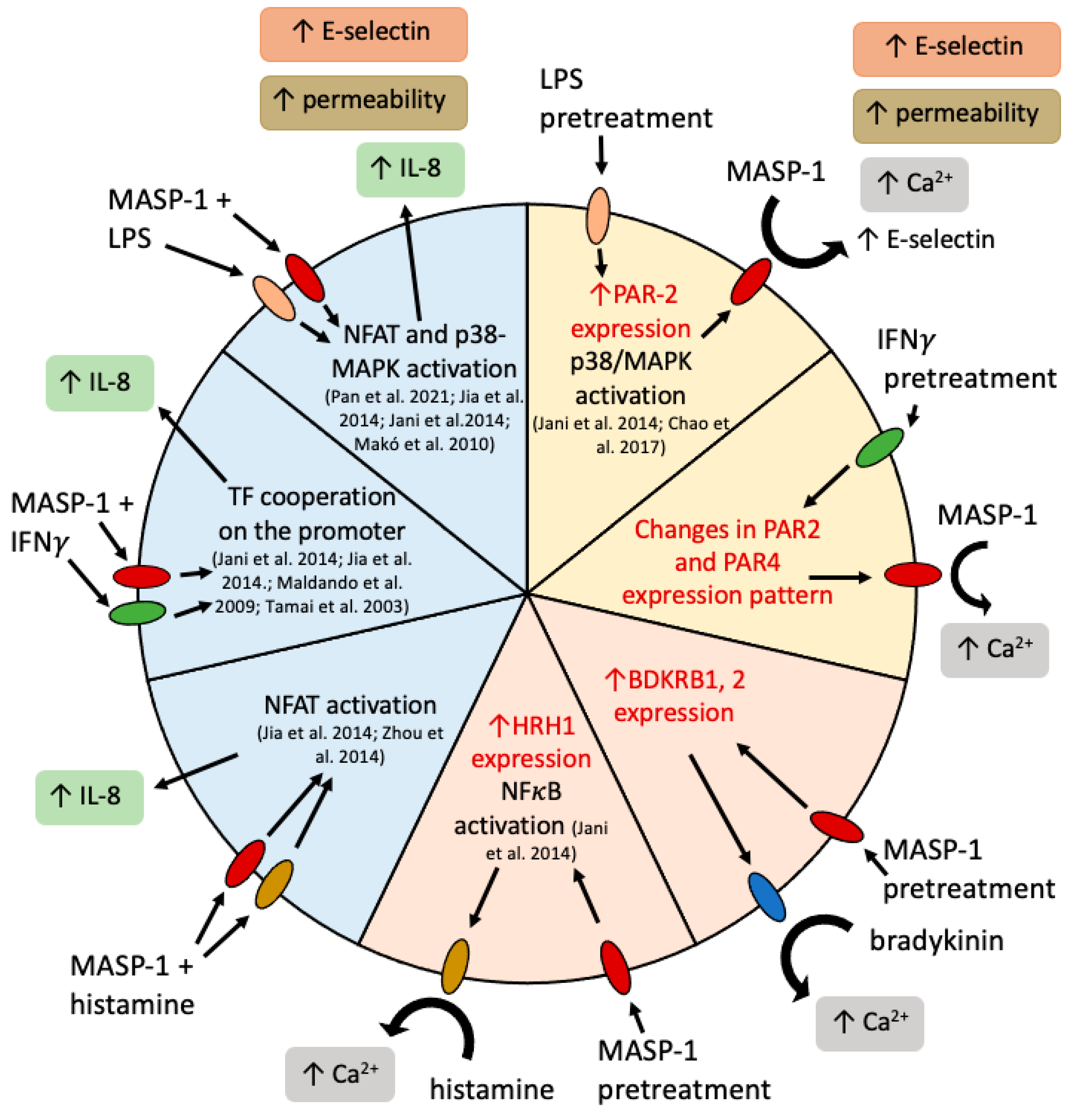
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation and Culturing of Human Umbilical Vein Endothelial Cells (HUVECs)
4.3. Intracellular Ca2+ Mobilization Assay
4.4. Measurement of E-Selectin and VCAM-1 Expression by Cell-Based ELISA
4.5. Measurement of IL-8 Cytokine Production by Sandwich ELISA
4.6. Permeability Measurement
4.7. RNA Purification and Quantitative Real-Time PCR
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Héja, D.; Harmat, V.; Fodor, K.; Wilmanns, M.; Dobó, J.; Kékesi, K.A.; Závodszky, P.; Gál, P.; Pál, G. Monospecific Inhibitors Show That Both Mannan-binding Lectin-associated Serine Protease-1 (MASP-1) and -2 Are Essential for Lectin Pathway Activation and Reveal Structural Plasticity of MASP-2. J. Biol. Chem. 2012, 287, 20290–20300. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.L.; Brandt, J.; Andrieu, J.-P.; Nielsen, C.; Jensen, P.H.; Holmskov, U.; Jorgensen, T.J.D.; Palarasah, Y.; Thielens, N.M.; Hansen, S. Heteromeric Complexes of Native Collectin Kidney 1 and Collectin Liver 1 Are Found in the Circulation with MASPs and Activate the Complement System. J. Immunol. 2013, 191, 6117–6127. [Google Scholar] [CrossRef]
- Dobó, J.; Schroeder, V.; Jenny, L.; Cervenak, L.; Závodszky, P.; Gál, P. Multiple roles of complement MASP-1 at the interface of innate immune response and coagulation. Mol. Immunol. 2014, 61, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Megyeri, M.; Makó, V.; Beinrohr, L.; Doleschall, Z.; Prohászka, Z.; Cervenak, L.; Závodszky, P.; Gál, P. Complement Protease MASP-1 Activates Human Endothelial Cells: PAR4 Activation Is a Link between Complement and Endothelial Function. J. Immunol. 2009, 183, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Schwaner, E.; Németh, Z.; Jani, P.K.; Kajdácsi, E.; Debreczeni, M.L.; Doleschall, Z.; Dobó, J.; Gál, P.; Rigó, J.; András, K.; et al. Transcriptome analysis of inflammation-related gene expression in endothelial cells activated by complement MASP-1. Sci. Rep. 2017, 7, 10462. [Google Scholar] [CrossRef]
- Jani, P.K.; Kajdácsi, E.; Megyeri, M.; Dobó, J.; Doleschall, Z.; Futosi, K.; Timar, C.; Mócsai, A.; Makó, V.; Gál, P.; et al. MASP-1 Induces a Unique Cytokine Pattern in Endothelial Cells: A Novel Link between Complement System and Neutrophil Granulocytes. PLoS ONE 2014, 9, e87104. [Google Scholar] [CrossRef]
- Jani, P.K.; Schwaner, E.; Kajdácsi, E.; Debreczeni, M.L.; Ungai-Salánki, R.; Dobó, J.; Doleschall, Z.; Rigó, J.; Geiszt, M.; Szabó, B.; et al. Complement MASP-1 enhances adhesion between endothelial cells and neutrophils by up-regulating E-selectin expression. Mol. Immunol. 2016, 75, 38–47. [Google Scholar] [CrossRef]
- Debreczeni, M.L.; Németh, Z.; Kajdácsi, E.; Schwaner, E.; Makó, V.; Masszi, A.; Doleschall, Z.; Rigó, J.; Walter, F.R.; Deli, M.A.; et al. MASP-1 Increases Endothelial Permeability. Front. Immunol. 2019, 10, 991. [Google Scholar] [CrossRef]
- Fujihara, M.; Muroi, M.; Tanamoto, K.-I.; Suzuki, T.; Azuma, H.; Ikeda, H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: Roles of the receptor complex. Pharmacol. Ther. 2003, 100, 171–194. [Google Scholar] [CrossRef]
- De Vries, H.E.; Blom-Roosemalen, M.C.; De Boer, A.G.; Van Berkel, T.J.; Breimer, D.D.; Kuiper, J. Effect of endotoxin on permeability of bovine cerebral endothelial cell layers in vitro. Experiment 1996, 277, 1418–1423. [Google Scholar]
- Park, H.S.; Chun, J.N.; Jung, H.Y.; Choi, C.; Bae, Y.S. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc. Res. 2006, 72, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.D.; Goldblum, S.E. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L899–L914. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.E.; Ganellin, C.R. Histamine and its receptors. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S127–S135. [Google Scholar] [CrossRef]
- Seifert, R.; Strasser, A.; Schneider, E.H.; Neumann, D.; Dove, S.; Buschauer, A. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol. Sci. 2012, 34, 33–58. [Google Scholar] [CrossRef]
- Mikelis, C.M.; Simaan, M.; Ando, K.; Fukuhara, S.; Sakurai, A.; Amornphimoltham, P.; Masedunskas, A.; Weigert, R.; Chavakis, T.; Adams, R.H.; et al. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat. Commun. 2015, 6, 6725. [Google Scholar] [CrossRef]
- Dobó, J.; Major, B.; Kékesi, K.A.; Szabó, I.; Megyeri, M.; Hajela, K.; Juhász, G.; Závodszky, P.; Gál, P. Cleavage of Kininogen and Subsequent Bradykinin Release by the Complement Component: Mannose-Binding Lectin-Associated Serine Protease (MASP)-1. PLoS ONE 2011, 6, e20036. [Google Scholar] [CrossRef]
- Hall, J.M. Bradykinin receptors. Gen. Pharmacol. Vasc. Syst. 1997, 28, 1–6. [Google Scholar] [CrossRef]
- Fox, R.H.; Goldsmith, R.; Kidd, D.J.; Lewis, G.P. Bradykinin as a vasodilator in man. J. Physiol. 1961, 157, 589–602. [Google Scholar] [CrossRef]
- Farmer, P.J.; Bernier, S.G.; Lepage, A.; Guillemette, G.; Regoli, M.; Sirois, P. Permeability of endothelial monolayers to albumin is increased by bradykinin and inhibited by prostaglandins. Am. J. Physiol. Cell. Mol. Physiol. 2001, 280, L732–L738. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Heesterbeek, D.A.; Angelier, M.L.; Harrison, R.A.; Rooijakkers, S.H. Complement and Bacterial Infections: From Molecular Mechanisms to Therapeutic Applications. J. Innate Immun. 2018, 10, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Stoermer, K.A.; Morrison, T.E. Complement and viral pathogenesis. Virology 2011, 411, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Speth, C.; Rambach, G.; Würzner, R.; Lass-Flörl, C. Complement and fungal pathogens: An update. Mycoses 2008, 51, 477–496. [Google Scholar] [CrossRef]
- Csuka, D.; Veszeli, N.; Varga, L.; Prohászka, Z.; Farkas, H. The role of the complement system in hereditary angioedema. Mol. Immunol. 2017, 89, 59–68. [Google Scholar] [CrossRef]
- Gerard, N.P.; Gerard, C. Complement in allergy and asthma. Curr. Opin. Immunol. 2002, 14, 705–708. [Google Scholar] [CrossRef]
- Mhatre, M.V.; Potter, J.A.; Lockwood, C.J.; Krikun, G.; Abrahams, V.M. Thrombin Augments LPS-Induced Human Endometrial Endothelial Cell Inflammation via PAR1 Activation. Am. J. Reprod. Immunol. 2016, 76, 29–37. [Google Scholar] [CrossRef]
- Makó, V.; Czúcz, J.; Weiszhár, Z.; Herczenik, E.; Matkó, J.; Prohászka, Z.; Cervenak, L. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. Cytom. Part A 2010, 77, 962–970. [Google Scholar] [CrossRef]
- Dubrovskyi, O.; Birukova, A.A.; Birukov, K.G. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab. Investig. 2013, 93, 254–263. [Google Scholar] [CrossRef]
- Altrogge, L.M.; Monard, D. An Assay for High-Sensitivity Detection of Thrombin Activity and Determination of Proteases Activating or Inactivating Protease-Activated Receptors. Anal. Biochem. 2000, 277, 33–45. [Google Scholar] [CrossRef]
- Ostrowska, E.; Reiser, G. Protease-activated receptor (PAR)-induced interleukin-8 production in airway epithelial cells requires activation of MAP kinases p44/42 and JNK. Biochem. Biophys. Res. Commun. 2008, 366, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Rallabhandi, P.; Nhu, Q.M.; Toshchakov, V.Y.; Piao, W.; Medvedev, A.E.; Hollenberg, M.D.; Fasano, A.; Vogel, S.N. Analysis of Proteinase-activated Receptor 2 and TLR4 Signal Transduction: A Novel Paradigm for Receptor Cooperativity. J. Biol. Chem. 2008, 283, 24314–24325. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-Y.; Ladd, A.V.; Biswal, M.R.; Valapala, M. Role of Nuclear Factor of Activated T Cells (NFAT) Pathway in Regulating Autophagy and Inflammation in Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 8684. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, H.; Cao, X.; Yin, Y.; Zhang, B. Activation of TRPV1 mediates thymic stromal lymphopoietin release via the Ca2+/NFAT pathway in airway epithelial cells. FEBS Lett. 2014, 588, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.-H.; Chen, P.-Y.; Hao, W.-R.; Chiang, W.-P.; Cheng, T.-H.; Loh, S.-H.; Leung, Y.-M.; Liu, J.-C.; Chen, J.-J.; Sung, L.-C. Lipopolysaccharide pretreatment increases protease-activated receptor-2 expression and monocyte chemoattractant protein-1 secretion in vascular endothelial cells. J. Biomed. Sci. 2017, 24, 85. [Google Scholar] [CrossRef]
- Beck, G.; Yard, B.A.; Breedijk, A.J.; Van Ackern, K.; Van Der Woude, F.J. Release of CXC-chemokines by human lung microvascular endothelial cells (LMVEC) compared with macrovascular umbilical vein endothelial cells. Clin. Exp. Immunol. 1999, 118, 298–303. [Google Scholar] [CrossRef]
- Suk, K.; Cha, S. Thrombin-Induced Interleukin-8 Production and Its Regulation by Interferon-Gamma and Prostaglandin E2 in Human Monocytic U937 Cells. Immunol. Lett. 1999, 67, 223–227. [Google Scholar] [CrossRef]
- Maldonado-Pérez, D.; Brown, P.; Morgan, K.; Millar, R.P.; Thompson, E.A.; Jabbour, H.N. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochim. Biophys. Acta 2009, 1793, 1315–1324. [Google Scholar] [CrossRef]
- Tamai, R.; Sugawara, S.; Takeuchi, O.; Akira, S.; Takada, H. Synergistic Effects of Lipopolysaccharide and Interferon-Gamma in Inducing Interleukin-8 Production in Human Monocytic Thp-1 Cells Is Accompanied by up-Regulation of Cd14, Toll-Like Receptor 4, Md-2 and Myd88 Expression. J. Endotoxin Res. 2003, 9, 145–153. [Google Scholar] [CrossRef]
- Zhou, M.-H.; Zheng, H.; Si, H.; Jin, Y.; Peng, J.M.; He, L.; Zhou, Y.; Muñoz-Garay, C.; Zawieja, D.C.; Kuo, L.; et al. Stromal Interaction Molecule 1 (STIM1) and Orai1 Mediate Histamine-evoked Calcium Entry and Nuclear Factor of Activated T-cells (NFAT) Signaling in Human Umbilical Vein Endothelial Cells. J. Biol. Chem. 2014, 289, 29446–29456. [Google Scholar] [CrossRef]
- Watanabe, K.; Tanaka, H.; Wen, F.; Yoshida, M. Effect of cytokines on thrombin-stimulated increases in intracellular calcium and PGI2 production by cultured human umbilical vein endothelial cells. Cell. Signal. 1996, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Dobó, J.; Harmat, V.; Beinrohr, L.; Sebestyén, E.; Závodszky, P.; Gál, P. MASP-1, a Promiscuous Complement Protease: Structure of Its Catalytic Region Reveals the Basis of Its Broad Specificity. J. Immunol. 2009, 183, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Megyeri, M.; Harmat, V.; Major, B.; Végh, A.; Balczer, J.; Héja, D.; Szilágyi, K.; Datz, D.; Pál, G.; Závodszky, P.; et al. Quantitative Characterization of the Activation Steps of Mannan-binding Lectin (MBL)-associated Serine Proteases (MASPs) Points to the Central Role of MASP-1 in the Initiation of the Complement Lectin Pathway. J. Biol. Chem. 2013, 288, 8922–8934. [Google Scholar] [CrossRef] [PubMed]
- Megyeri, M.; Jani, P.K.; Kajdácsi, E.; Dobó, J.; Schwaner, E.; Major, B.; Rigó, J.; Závodszky, P.; Thiel, S.; Cervenak, L.; et al. Serum MASP-1 in complex with MBL activates endothelial cells. Mol. Immunol. 2014, 59, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Oroszlán, M.; Herczenik, E.; Rugonfalvi-Kiss, S.; Roos, A.; Nauta, A.J.; Daha, M.R.; Gombos, I.; Karádi, I.; Romics, L.; Prohászka, Z.; et al. Proinflammatory changes in human umbilical cord vein endothelial cells can be induced neither by native nor by modified CRP. Int. Immunol. 2006, 18, 871–878. [Google Scholar] [CrossRef]
| Interaction | |||||||
|---|---|---|---|---|---|---|---|
| Pretreatment | Treatment | Ca2+-Mobilization | Permeability | E-Selectin | VCAM-1 | IL-8 | |
| Cotreatment | rMASP-1 + LPS | ↑ | ↑ | ↑ | |||
| rMASP-1 + Histamine | ↑ | ||||||
| rMASP-1 + IFNγ | ↑ | ||||||
| rMASP-1 + Bradykinin | |||||||
| Pretreatment with rMASP-1 | rMASP-1 | LPS | |||||
| rMASP-1 | Histamine | ↑ | |||||
| rMASP-1 | IFNγ | ||||||
| rMASP-1 | Bradykinin | ↑ | |||||
| Pretreatment with coactivator | LPS | rMASP-1 | ↑ | ↑ | ↑ | ||
| Histamine | rMASP-1 | ||||||
| IFNγ | rMASP-1 | ↑ | |||||
| Bradykinin | rMASP-1 | ||||||
| Gene Name | Sequence | ||
|---|---|---|---|
| β-actin (ACTB) | forward | 5′-ATCAAGATCATTGCTCCTCCTGA-3′ | |
| reverse | 5′-AAGGGTGTAACGCAACTAAGTCA-3′ | ||
| B1 bradykinin receptor (BDKRB1) | forward | 5′-CACAGAGTGCTGCCAACATTTAT-3′ | |
| reverse | 5′-ACTGGTTCCAGATATTCTCTGCC-3′ | ||
| B2 bradykinin receptor (BDKRB2) | forward | 5′-TCTGAGTCCAAATGTTCTCTCCC-3′ | |
| reverse | 5′-AGGACAAAGATGTTCTCTAGGGTG-3′ | ||
| Histamine H1 receptor (HRH1) | forward | 5′-GTCTTCATCCTGTGCATTGATCG-3′ | |
| reverse | 5′-AAGTCTGTCTCACACTTGTCCTC-3′ | ||
| Proteinase-activated receptor 1 (F2R) | forward | 5′-CTGTGTACACCGGAGTGTTTGT-3′ | |
| reverse | 5′-AGTAAAATGCTGCAGTGACGAA-3′ | ||
| Proteinase-activated receptor 2 (F2RL1) | forward | 5′-AAGAGGGCCATCAAACTCATT-3′ | |
| reverse | 5′-GTTCTTTGCATGATCCCTGAA-3′ | ||
| Proteinase-activated receptor 4 (F2RL3) | forward | 5′-ACCATGCTGCTGATGAACCT-3′ | |
| reverse | 5′-AGCACTGAGCCATACATGTGAC-3′ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, Z.; Debreczeni, M.L.; Kajdácsi, E.; Dobó, J.; Gál, P.; Cervenak, L. Cooperation of Complement MASP-1 with Other Proinflammatory Factors to Enhance the Activation of Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 9181. https://doi.org/10.3390/ijms24119181
Németh Z, Debreczeni ML, Kajdácsi E, Dobó J, Gál P, Cervenak L. Cooperation of Complement MASP-1 with Other Proinflammatory Factors to Enhance the Activation of Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(11):9181. https://doi.org/10.3390/ijms24119181
Chicago/Turabian StyleNémeth, Zsuzsanna, Márta L. Debreczeni, Erika Kajdácsi, József Dobó, Péter Gál, and László Cervenak. 2023. "Cooperation of Complement MASP-1 with Other Proinflammatory Factors to Enhance the Activation of Endothelial Cells" International Journal of Molecular Sciences 24, no. 11: 9181. https://doi.org/10.3390/ijms24119181
APA StyleNémeth, Z., Debreczeni, M. L., Kajdácsi, E., Dobó, J., Gál, P., & Cervenak, L. (2023). Cooperation of Complement MASP-1 with Other Proinflammatory Factors to Enhance the Activation of Endothelial Cells. International Journal of Molecular Sciences, 24(11), 9181. https://doi.org/10.3390/ijms24119181





