Diabetic Neuropathy of the Retina and Inflammation: Perspectives
Abstract
1. Introduction
2. Historical Perspectives
2.1. Dyslipidemia and Hyperglycemia
2.2. Inflammation in Type 2 Diabetes
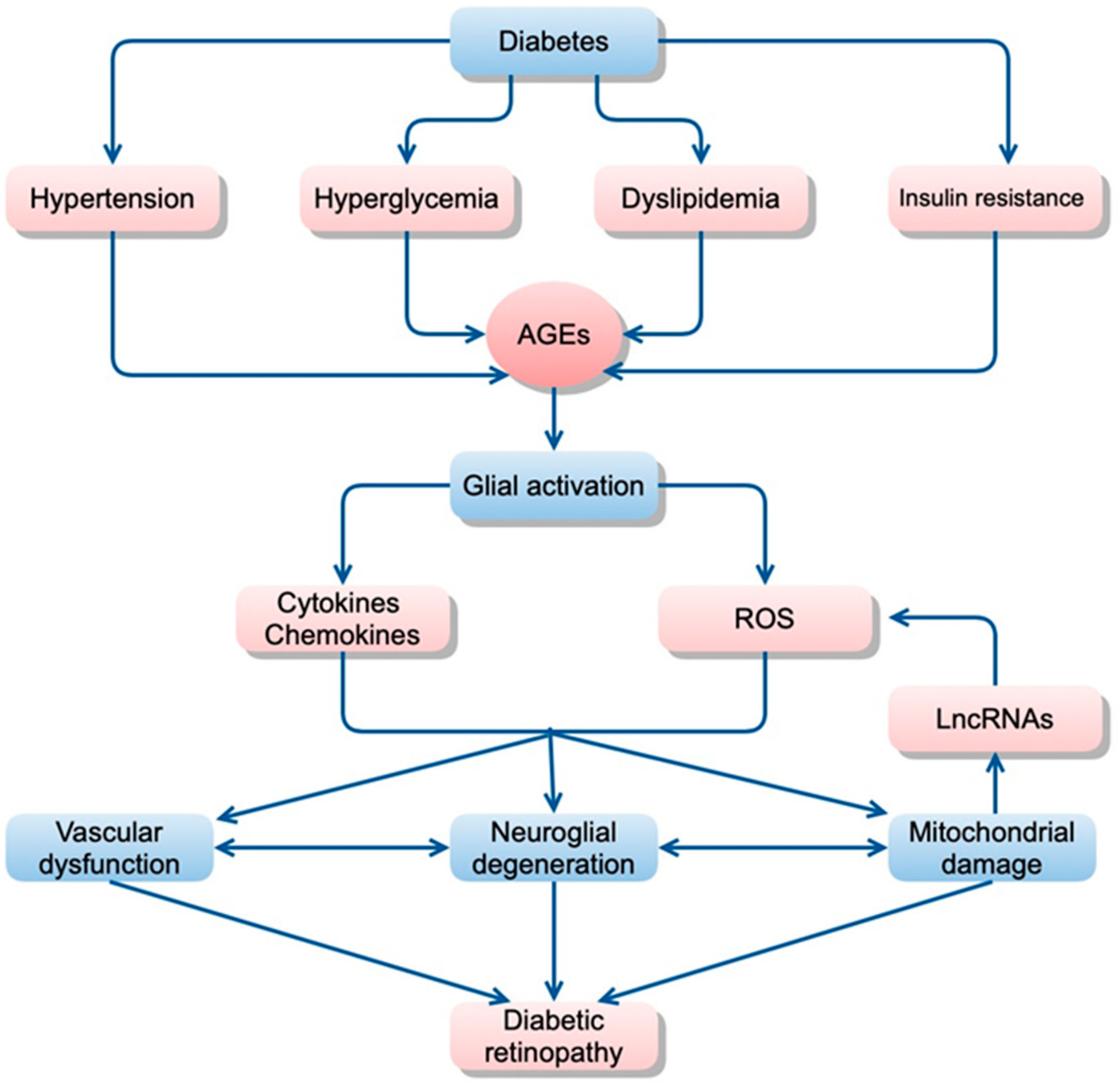
2.2.1. Role of Inflammation in Diabetic Retinopathy
2.2.2. Inflammatory Pathways in the Neurovascular Cells of Diabetic Retinopathy
2.3. Nutritional Consequences of Hyperglycemia
3. Future Perspectives for Treatment of Diabetes and Diabetic Retinopathy
3.1. Therapeutic Options of Anti-Inflammation for Diabetic Retinopathy
3.2. Anti-Inflammatory Therapies for Diabetic Macular Edema
3.3. Anti-Hyperlipidemic Therapies for Diabetic Retinopathy
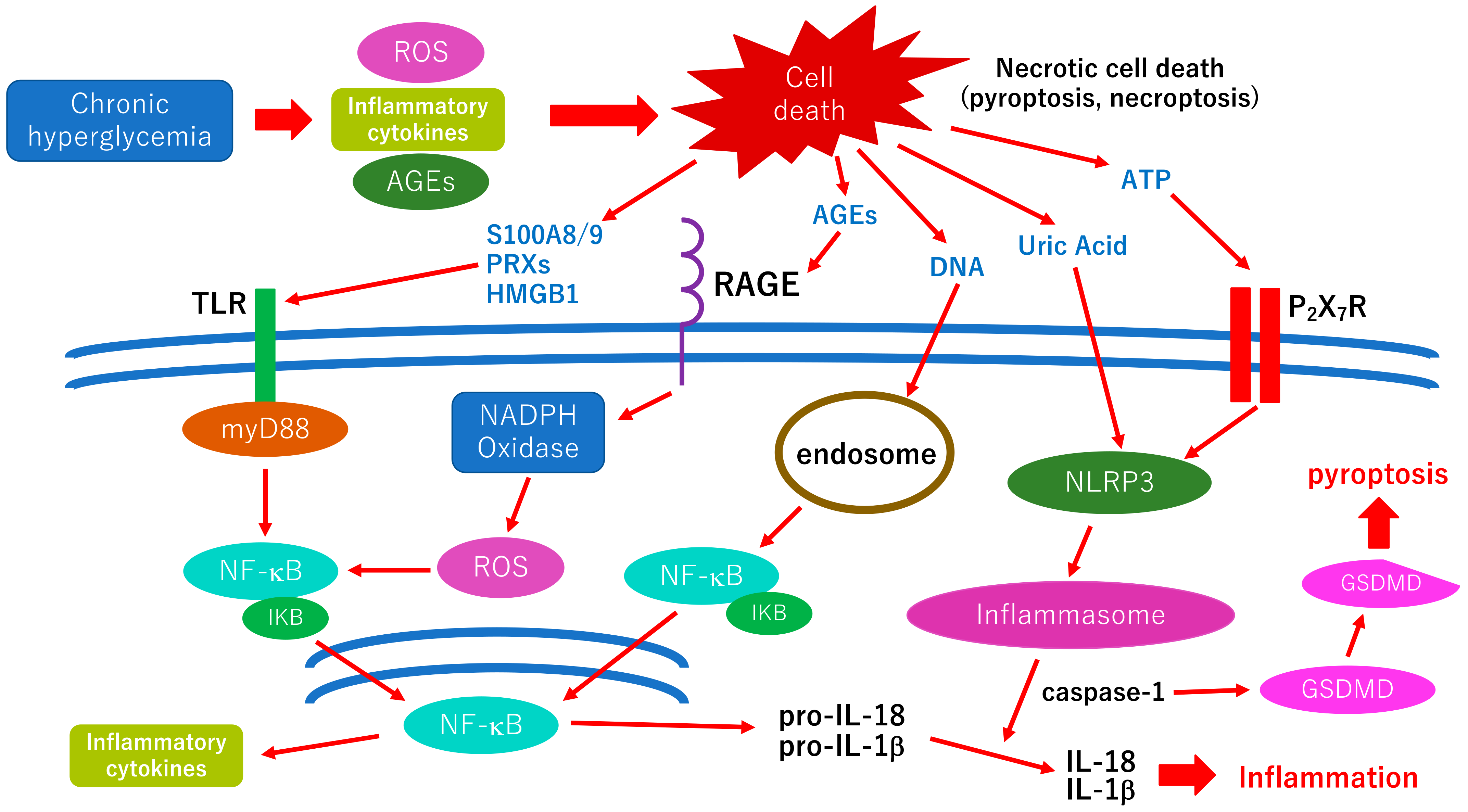
3.4. Pyroptosis; A New Therapeutic Target for the Treatment of Diabetic Retinopathy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inzucchi, S.E. Diagnosis of diabetes. N. Engl. J. Med. 2013, 368, 193. [Google Scholar] [CrossRef] [PubMed]
- Pasnoor, M.; Dimachkie, M.M.; Kluding, P.; Barohn, R.J. Diabetic neuropathy part 1: Overview and symmetric phenotypes. Neurol. Clin. 2013, 31, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 1990 to 2010. Diabetes Care 2016, 39, 1643–1649. [Google Scholar] [CrossRef]
- Yokoyama, H.; Araki, S.I.; Kawai, K.; Yamazaki, K.; Tomonaga, O.; Shirabe, S.I.; Maegawa, H. Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46). BMJ Open. Diabetes Res. Care 2018, 6, e000521. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Chan, J.C.; Malik, V.; Jia, W.; Kadowaki, T.; Yajnik, C.S.; Yoon, K.H.; Hu, F.B. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA 2009, 301, 2129–2140. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Kearns, C.E.; Schmidt, L.A.; Glantz, S.A. Sugar Industry and Coronary Heart Disease Research: A Historical Analysis of Internal Industry Documents. JAMA Intern. Med. 2016, 176, 1680–1685. [Google Scholar] [CrossRef]
- Anagnostis, P.; Paschou, S.A.; Goulis, D.G.; Athyros, V.G.; Karagiannis, A. Dietary management of dyslipidaemias. Is there any evidence for cardiovascular benefit? Maturitas 2018, 108, 45–52. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Veiraiah, A. Hyperglycemia, lipoprotein glycation, and vascular disease. Angiology 2005, 56, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Solovieff, N.; Dewan, A.; Walsh, K.M.; Puca, A.; Hartley, S.W.; Perls, T.T. Genetic signatures of exceptional longevity in humans. PLoS ONE 2012, 7, e29848. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Yamamoto, S. Neurite regeneration in advanced glycation end-products exposed adult rat retinas and regenerative effects of neurotrophin-4. Brain. Res. 2013, 1543, 33–45. [Google Scholar] [CrossRef]
- Salekeen, R.; Haider, A.N.; Akhter, F.; Billah, M.M.; Islam, M.E.; Didarul Islam, K.M. Lipid oxidation in pathophysiology of atherosclerosis: Current understanding and therapeutic strategies. Int. J. Cardiol. Cardiovasc. Risk. Prev. 2022, 14, 200143. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: Oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Bikbova, G.; Oshitari, T.; Baba, T.; Yamamoto, S. Mechanisms of Neuronal Cell Death in AGE-exposed Retinas—Research and Literature Review. Curr. Diabetes Rev. 2017, 13, 280–288. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The diabetes mellitus–atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.; Jha, J.C. Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology 2021, 10, 18. [Google Scholar] [CrossRef]
- Bubb, K.J.; Drummond, G.R.; Figtree, G.A. New opportunities for targeting redox dysregulation in cardiovascular disease. Cardiovasc. Res. 2020, 116, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.M.; Rodrigues, D.; Pereira, C.A.; Silva, J.F.; Alves, J.V.; Lobato, N.S.; Tostes, R.C. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front. Pharmacol. 2019, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, Diabetes and Atherosclerosis: Role of Inflammation and ROS-Redox-Sensitive Factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, W.; Yang, J.K.; Qin, M.Z. Proliferative diabetic retinopathy in patients with type 2 diabetes correlates with the presence of atherosclerosis cardiovascular disease. Diabetol. Metab. Syndr. 2021, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Castelblanco, E.; Granado-Casas, M.; Hernández, M.; Pinyol, M.; Correig, E.; Julve, J.; Rojo-López, M.I.; Alonso, N.; Avogaro, A.; Ortega, E.; et al. Diabetic retinopathy predicts cardiovascular disease independently of subclinical atherosclerosis in individuals with type 2 diabetes: A prospective cohort study. Front. Cardiovasc. Med. 2022, 9, 945421. [Google Scholar] [CrossRef]
- Williamson, R.T. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. Br. Med. J. 1901, 1, 760–762. [Google Scholar] [CrossRef]
- Reid, J.; Macdougall, A.I.; Andrews, M.M. Aspirin and diabetes mellitus. Br. Med. J. 1957, 2, 1071–1074. [Google Scholar] [CrossRef]
- Shulman, G.I. Unraveling the cellular mechanism of insulin resistance in humans: New insights from magnetic resonance spectroscopy. Physiology 2004, 19, 183–190. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Ogston, D.; McAndrew, G.M. Fibrinolysis in obesity. Lancet 1964, 2, 1205–1207. [Google Scholar] [CrossRef]
- Fearnley, G.R.; Vincent, C.T.; Chakrabarti, R. Reduction of blood fibrinolytic activity in diabetes mellitus by insulin. Lancet 1959, 2, 1067. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Shman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Ballantyne, C.M.; Couper, D.; Vigo, A.; Hoogeveen, R.; Folsom, A.R.; Heiss, G. Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes 2003, 52, 1799–1805. [Google Scholar] [CrossRef]
- Marques-Vidal, P.; Schmid, R.; Bochud, M.; Bastardot, F.; Von Känel, R.; Paccaud, F.; Glaus, J.; Preisig, M.; Waeber, G.; Vollenweider, P. Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes the CoLaus study. PLoS ONE 2012, 7, e51768. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart. J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef]
- Maachi, M.; Pieroni, L.; Bruckert, E.; Jardel, C.; Fellahi, S.; Hainque, B.; Capeau, J.; Bastard, J.P. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFalpha, leptin and IL-6 levels in obese women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 993–997. [Google Scholar] [CrossRef]
- Yan, Y.; Li, S.; Liu, Y.; Bazzano, L.; He, J.; Mi, J.; Chen, W. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: The Bogalusa Heart Study. Cardiovasc. Diabetol. 2019, 18, 109. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Thorand, B.; Löwel, H.; Schneider, A.; Kolb, H.; Meisinger, C.; Fröhlich, M.; Koenig, W. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: Results from the MONICA Augsburg cohort study, 1984–1998. Arch. Intern. Med. 2003, 163, 93–99. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Tousoulis, D. The molecular mechanisms of obesity paradox. Cardiovasc. Res. 2017, 113, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T.; Bikbova, G.; Yamamoto, S. Increased expression of phosphorylated c-Jun and phosphorylated c-Jun N-terminal kinase associated with neuronal cell death in diabetic and high glucose exposed rat retinas. Brain. Res. Bull 2014, 101, 18–25. [Google Scholar] [CrossRef]
- di Pino, A.; Defronzo, R.A. Insulin resistance and atherosclerosis: Implications for insulin-sensitizing agents. Endocr. Rev. 2019, 40, 1447–1467. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Saad, M.; Pettitt, D.; Mott, D.; Knowler, W.; Nelson, R.; Bennett, P. Sequential changes in serum insulin concentration during development of non-insulin-dependent diabetes. Lancet 1989, 1, 1356–1359. [Google Scholar] [CrossRef]
- Martin, B.C.; Warram, J.H.; Krolewski, A.S.; Soeldner, J.S.; Kahn, C.R.; Bergman, R.N. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: Results of a 25-year follow-up study. Lancet 1992, 340, 925–929. [Google Scholar] [CrossRef]
- Jallut, D.; Golay, A.; Munger, R.; Frascarolo, P.; Schutz, Y.; Jequier, E.; Felber, J.P. Impaired glucose tolerance and diabetes in obesity: A 6-year follow-up study of glucose metabolism. Metabolism 1990, 39, 1068–1075. [Google Scholar] [CrossRef]
- Tabák, A.G.; Jokela, M.; Akbaraly, T.N.; Brunner, E.J.; Kivimäki, M.; Witte, D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: An analysis from the Whitehall II study. Lancet 2009, 373, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Prigeon, R.L.; McCulloch, D.K.; Edward, J.B.; Richard, N.B.; Micheal, W.S.; James, L.N.; Kenneth, W.; James, C.B.; Jerry, P.P. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects Evidence for a hyperbolic function. Diabetes 1993, 42, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Wittmann, J.; Giaccari, A.; Knoflach, M.; Willeit, P.; Bozec, A.; Alexander, R.M.; Muscogiuri, G.; Sorice, G.P.; Kireva, T.; et al. Blockade of receptor activator of nuclear factor-kappaB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat. Med. 2013, 19, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Cai, D. Neuroinflammation in overnutrition-induced diseases. Vitam. Horm. 2013, 91, 195–218. [Google Scholar] [CrossRef]
- Varma, V.; Yao-Borengasser, A.; Rasouli, N.; Greg, T.; Nolen, P.B.; Starks, T.; Gurley, C.; Simpson, P.; Robert, E.M.; Philip, A.; et al. PetersonMuscle inflammatory response and insulin resistance: Synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1300–E1310. [Google Scholar] [CrossRef]
- Kampoli, A.M.; Tousoulis, D.; Briasoulis, A.; Latsios, G.; Papageorgiou, N.; Stefanadis, C. Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr. Pharm. Des. 2011, 17, 4147–4158. [Google Scholar] [CrossRef]
- Papaoikonomou, S.; Tousoulis, D.; Tentolouris, N.; Papadogiannis, D.; Miliou, A.; Hatzis, G.; Papageorgiou, N.; Antoniades, C.; Christodoulos, S. The role of C-reactive protein genetic variability in the onset of carotid artery disease and renal function impairment in patients with diabetes mellitus type 2. Int. J. Cardiol. 2013, 168, 4331–4332. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. N. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef]
- Taylor, R. Pathogenesis of type 2 diabetes: Tracing the reverse route from cure to cause. Diabetologia 2008, 51, 1781–1789. [Google Scholar] [CrossRef]
- Nikolajczyk, B.S.; Jagannathan-Bogdan, M.; Shin, H.; Gyurko, R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011, 12, 239–250. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Margaritis, M.; Coutinho, P.; Shirodaria, C.; Psarros, C.; Herdman, L.; Antoniades, C. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: The regulatory role of perivascular adipose tissue. Diabetes 2015, 64, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T. Advanced glycation end-products and diabetic neuropathy of the retina. Int. J. Mol. Sci. 2023, 24, 2927. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T. The pathogenesis and therapeutic approaches of diabetic neuropathy in the retina. Int. J. Mol. Sci. 2021, 22, 9050. [Google Scholar] [CrossRef]
- Gardner, T.W.; Davila, J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes. Arch. Clin. Exp. Ophthalmol. 2017, 255, 1–6. [Google Scholar] [CrossRef]
- Murugeswari, P.; Shukla, D.; Rajendran, A.; Kim, R.; Namperumalsamy, P.; Muthukkaruppan, V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales’ disease. Retina 2008, 28, 817–824. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Hernández, C.; Simó, R. Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediat. Inflamm. 2012, 2012, 872978. [Google Scholar] [CrossRef]
- Dal Monte, M.; Rezzola, S.; Cammalleri, M.; Belleri, M.; Locri, F.; Morbidelli, L.; Corsini, M.; Paganini, G.; Semeraro, F.; Cancarini, A.; et al. Anti-angiogenic effectiveness of the urokinase receptor-derived peptide UPARANT in a model of oxygen induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2392–2407. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- Demircan, N.; Safran, B.G.; Soylu, M.; Ozcan, A.A.; Sizmaz, S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye 2006, 20, 1366–1369. [Google Scholar] [CrossRef]
- Mizutani, M.; Gerhardinger, C.; Lorenzi, M. Muller cell changes in human diabetic retinopathy. Diabetes 1998, 47, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Asrar, A.M.; Dralands, L.; Missotten, L.; Al-Jadaan, I.A.; Geboes, K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Kowluru, R.A. Nuclear Genome-Encoded Long Noncoding RNAs and Mitochondrial Damage in Diabetic Retinopathy. Cells 2021, 10, 3271. [Google Scholar] [CrossRef]
- Alfaifi, M.; Beg, M.M.A.; Alshahrani, M.Y.; Ahmad, I.; Alkhathami, A.G.; Joshi, P.C.; Alshehri, O.M.; Alamri, A.M.; Verma, A.K. Circulating long non-coding RNAs NKILA, NEAT1, MALAT1, and MIAT expression and their association in type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2021, 9, e001821. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Zhang, M. Targeting Novel Regulated Cell Death: Pyroptosis, Necroptosis, and Ferroptosis in Diabetic Retinopathy. Front. Cell Dev. Biol. 2022, 10, 932886. [Google Scholar] [CrossRef]
- Young, V.R. Trace element biology: The knowledge base and its application for the nutrition of individuals and populations. J. Nutr. 2003, 133 (Suppl. 1), 1581S–1587S. [Google Scholar] [CrossRef]
- Uğurlu, V.; Binay, C.; Şimşek, E.; Bal, C. Cellular Trace Element Changes in Type 1 Diabetes Patients. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 180–186. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, C.; Yang, Z.; Zhang, W.; Niu, Y.; Li, X.; Qin, L.; Su, Q. Alterations of serum trace elements in patients with type 2 diabetes. J. Trace Elem. Med. Boil. 2017, 40, 91–96. [Google Scholar] [CrossRef]
- Siddiqui, K.; Bawazeer, N.; Joy, S.S. Variation in Macro and Trace Elements in Progression of Type 2 Diabetes. Sci. World J. 2014, 2014, 461591. [Google Scholar] [CrossRef]
- Badran, M.; Morsy, R.; Soliman, H.; Elnimr, T. Assessment of trace elements levels in patients with Type 2 diabetes using multivariate statistical analysis. J. Trace. Elements. Med. Boil. 2016, 33, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wolide, A.D.; Zawdie, B.; Alemayehu, T.; Tadesse, S. Association of trace metal elements with lipid profiles in type 2 diabetes mellitus patients: A cross sectional study. BMC Endocr. Disord. 2017, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, J.; Wang, W.; Hou, J.; Cheng, Y.; Fu, Y.; Xu, Z.; Cai, L. The beneficial effects of Zn on Akt-mediated insulin and cell survival signaling pathways in diabetes. J. Trace. Elements Med. Boil. 2018, 46, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, X.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, Z.; Qi, C.; Wang, T.; et al. Dietary Chromium Restriction of Pregnant Mice Changes the Methylation Status of Hepatic Genes Involved with Insulin Signaling in Adult Male Offspring. PLoS ONE 2017, 12, e0169889. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Sinitskii, A.; Popova, E.; Nemereshina, O.; Gatiatulina, E.; Skalnaya, M.G.; Skalny, A.V.; Nikonorov, A. Alteration of local adipose tissue trace element homeostasis as a possible mechanism of obesity-related insulin resistance. Med. Hypotheses 2015, 85, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Kashiv, Y.; Austin, J.R.; Lai, B.; Rose, V.; Vogt, S.; El-Muayed, M. Imaging trace element distributions in single organelles and subcellular features. Sci. Rep. 2016, 6, 21437. [Google Scholar] [CrossRef]
- Koekkoek, W.A.; Van Zanten, A.R. Antioxidant Vitamins and Trace Elements in Critical Illness. Nutr. Clin. Pract. 2016, 31, 457–474. [Google Scholar] [CrossRef]
- Praveena, S.P.; Pasula, S.; Sameera, K. Trace elements in diabetes mellitus. J. Clin. Diagn. Res. 2013, 7, 1863–1865. [Google Scholar] [CrossRef]
- Napoleão, A.; Fernandes, L.; Miranda, C.; Marum, A.P. Effects of Calorie Restriction on Health Span and Insulin Resistance: Classic Calorie Restriction Diet vs. Ketosis-Inducing Diet. Nutrients 2021, 13, 1302. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; De Lorenzo, A. Diet, Nutrition and Chronic Degenerative Diseases. Nutrients 2021, 13, 1372. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Fang, S. Therapeutic Strategies for Diabetes: Immune Modulation in Pancreatic β Cells. Front. Endocrinol. 2021, 12, 716692. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The potential for microRNA therapeutics and clinical research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef]
- Ding, X.; Sun, Z.; Guo, Y.; Tang, W.; Shu, Q.; Xu, G. Inhibition of NF-κB ameliorates aberrant retinal glia activation and inflammatory responses in streptozotocin-induced diabetic rats. Ann. Transl. Med. 2023, 11, 197. [Google Scholar] [CrossRef]
- Huang, Z.; Liang, J.; Chen, S.; Ng, T.K.; Brelén, M.E.; Liu, Q.; Yang, R.; Xie, B.; Ke, S.; Chen, W.; et al. RIP3-mediated microglial necroptosis promotes neuroinflammation and neurodegeneration in the early stages of diabetic retinopathy. Cell Death Dis. 2023, 14, 227. [Google Scholar] [CrossRef]
- Liao, L.; Chen, J.; Peng, S. hsa_circ_0000047 targeting miR-6720-5p/CYB5R2 axis alleviates inflammation and angiogenesis in diabetic retinopathy. Arch. Physiol. Biochem. 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dionysopoulou, S.; Wikstrom, P.; Bucolo, C.; Romano, G.L.; Micale, V.; Svensson, R.; Spyridakos, D.; Mastrodimou, N.; Georgakis, S.; Verginis, P.; et al. Topically administered NOX4 inhibitor, GLX7013114, is efficacious in treating the early pathological events of diabetic retinopathy. Diabetes 2023, 72, 638–652. [Google Scholar] [CrossRef]
- Jerome, J.R.; Deliyanti, D.; Suphapimol, V.; Kolkhof, P.; Wilkinson-Berka, J.L. Finerenone, a Non-Steroidal Mineralocorticoid Receptor Antagonist, Reduces Vascular Injury and Increases Regulatory T-Cells: Studies in Rodents with Diabetic and Neovascular Retinopathy. Int. J. Mol. Sci. 2023, 24, 2334. [Google Scholar] [CrossRef]
- Chen, Y.; Schlotterer, A.; Kurowski, L.; Li, L.; Dannehl, M.; Hammes, H.P.; Lin, J. miRNA-124 Prevents Rat Diabetic Retinopathy by Inhibiting the Microglial Inflammatory Response. Int. J. Mol. Sci. 2023, 24, 2291. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diab. Rep. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Catalani, E.; Silvestri, F.; Bongiorni, S.; Taddei, A.R.; Fanelli, G.; Rinalducci, S.; De Palma, C.; Perrotta, C.; Prantera, G.; Cervia, D. Retinal damage in a new model of hyperglycemia induced by high-sucrose diets. Pharmacol. Res. 2021, 166, 105488. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Yu, A.C.; Lau, L.T.; Lee, C.; Wu, L.M.; Zhu, X.; Tso, M.O. Minocycline inhibits LPS-induced retinal microglia activation. Neurochem. Int. 2005, 47, 152–158. [Google Scholar] [CrossRef]
- Wang, W.; Sidoli, S.; Zhang, W.; Wang, Q.; Wang, L.; Jensen, O.N.; Guo, L.; Zhao, X.; Zheng, L. Abnormal levels of histone methylation in the retinas of diabetic rats are reversed by minocycline treatment. Sci. Rep. 2017, 7, 45103. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.A.; O’Brien, P.D.; Hinder, L.M.; Hayes, J.M.; Mendelson, F.E.; Zhang, H.; Narayanan, S.; Abcouwer, S.F.; Brosius, F.C.; Pennathur, S.; et al. Differential effects of minocycline on microvascular complications in murine models of type 1 and type 2 diabetes. J. Transl. Sci. 2021, 7, 1000431. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.J.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.Y.; Cui, H.; Hashad, Y.; Whitcup, S.M.; et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014, 121, 1904–1940. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.; Alpay, A.; Ugurbas, S.H. Comparison of the efficacy of intravitreal Anti-VEGF versus intravitreal dexamethasone implant in treatment resistant diabetic Macular Edema. BMC Ophthalmol. 2023, 23, 97. [Google Scholar] [CrossRef]
- Tatsumi, T.; Oshitari, T.; Baba, T.; Takatsuna, Y.; Yamamoto, S. Effects of Switching from Anti-VEGF Treatment to Triamcinolone Acetonide in Eyes with Refractory Macular Edema Associated with Diabetic Retinopathy or Retinal Vein Occlusion. Biomed. Res. Int. 2020, 2020, 4529850. [Google Scholar] [CrossRef]
- Oshitari, T. Neurovascular impairment and therapeutic strategies in diabetic retinopathy. Int. J. Environ. Res. Public Health 2022, 19, 439. [Google Scholar] [CrossRef]
- Kandasamy, R.; Constantinou, M.; Rogers, S.L.; Sandhu, S.S.; Wickremasinghe, S.; Al-Qureshi, S.; Lim, L.L. Prospective randomised clinical trial of intravitreal bevacizumab versus triamcinolone in eyes with diabetic macular oedema undergoing cataract surgery: 6-month results. Br. J. Ophthalmol. 2019, 103, 1753–1758. [Google Scholar] [CrossRef]
- Fallico, M.; Lotery, A.; Maugeri, A.; Favara, G.; Barchitta, M.; Agodi, A.; Russo, A.; Longo, A.; Bonfiglio, V.; Avitabile, T.; et al. Intravitreal dexamethasone implant versus anti-vascular endothelial growth factor therapy combined with cataract surgery in patients with diabetic macular oedema: A systematic review with meta-analysis. Eye 2022, 36, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Jalali, J.A.; Johnston, T.P.; Koulen, P. Emerging Roles of Dyslipidemia and Hyperglycemia in Diabetic Retinopathy: Molecular Mechanisms and Clinical Perspectives. Front. Endocrinol. 2021, 12, 620045. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Lee, D.; Tsubota, K.; Kurihara, T. PPARα Agonist Oral Therapy in Diabetic Retinopathy. Biomedicines 2020, 8, 433. [Google Scholar] [CrossRef]
- Arai, T.; Kim, H.-J.; Chiba, H.; Matsumoto, A. Interaction of Fenofibrate and Fish Oil in Relation to Lipid Metabolism in Mice. J. Atheroscler. Thromb. 2009, 16, 283–291. [Google Scholar] [CrossRef]
- Bogdanov, P.; Hernández, C.; Corraliza, L.; Carvalho, A.R.; Simó, R. Effect of fenofibrate on retinal neurodegeneration in an experimental model of type 2 diabetes. Acta Diabetol. 2015, 52, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- ACCORD Study Group; Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [PubMed]
- Tomita, Y.; Ozawa, N.; Miwa, Y.; Ishida, A.; Ohta, M.; Tsubota, K.; Kurihara, T. Pemafibrate Prevents Retinal Pathological Neovascularization by Increasing FGF21 Level in a Murine Oxygen-Induced Retinopathy Model. Int. J. Mol. Sci. 2019, 20, 5878. [Google Scholar] [CrossRef]
- Meng, C.; Gu, C.; He, S.; Su, T.; Lhamo, T.; Draga, D.; Qiu, Q. Pyroptosis in the Retinal Neurovascular Unit: New Insights Into Diabetic Retinopathy. Front. Immunol. 2021, 12, 763092. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guo, X.L.; Xu, M.; Chen, J.L.; Wang, Y.F.; Xiao, Y.G.; Gao, A.S.; Zhang, L.C.; Liu, X.Z.; Wang, T.H. Network pharmacology mechanism of Scutellarin to inhibit RGC pyroptosis in diabetic retinopathy. Sci. Rep. 2023, 13, 6504. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Cai, Y.; Yang, Y.; Yuan, J.; Chen, Q. Inhibition of the pyroptosis-associated inflammasome pathway: The important potential mechanism of ginsenosides in ameliorating diabetes and its complications. Eur. J. Med. Chem. 2023, 253, 115336. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, S.; Chen, G.; He, S. MiR-200c-3p regulates pyroptosis by targeting SLC30A7 in diabetic retinopathy. Hum. Exp. Toxicol. 2022, 41, 9603271221099589. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, H.; Li, Q.; Zhao, S.; Gao, Y. MiR-192 attenuates high glucose-induced pyroptosis in retinal pigment epithelial cells via inflammasome modulation. Bioengineered 2022, 13, 10362–10372. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhao, S.; Li, C.; Tang, M.; Sun, T.; Zheng, Z. Transient receptor potential channel 6 knockdown prevents high glucose-induced Müller cell pyroptosis. Exp. Eye. Res. 2023, 227, 109381. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikbova, G.; Oshitari, T.; Bikbov, M. Diabetic Neuropathy of the Retina and Inflammation: Perspectives. Int. J. Mol. Sci. 2023, 24, 9166. https://doi.org/10.3390/ijms24119166
Bikbova G, Oshitari T, Bikbov M. Diabetic Neuropathy of the Retina and Inflammation: Perspectives. International Journal of Molecular Sciences. 2023; 24(11):9166. https://doi.org/10.3390/ijms24119166
Chicago/Turabian StyleBikbova, Guzel, Toshiyuki Oshitari, and Mukharram Bikbov. 2023. "Diabetic Neuropathy of the Retina and Inflammation: Perspectives" International Journal of Molecular Sciences 24, no. 11: 9166. https://doi.org/10.3390/ijms24119166
APA StyleBikbova, G., Oshitari, T., & Bikbov, M. (2023). Diabetic Neuropathy of the Retina and Inflammation: Perspectives. International Journal of Molecular Sciences, 24(11), 9166. https://doi.org/10.3390/ijms24119166





