Eosinophil Granule Proteins Involvement in Acute Appendicitis—An Allergic Disease?
Abstract
1. Introduction
2. Results
2.1. Patients
2.2. Hemogram and Appendicular Histology
- (1)
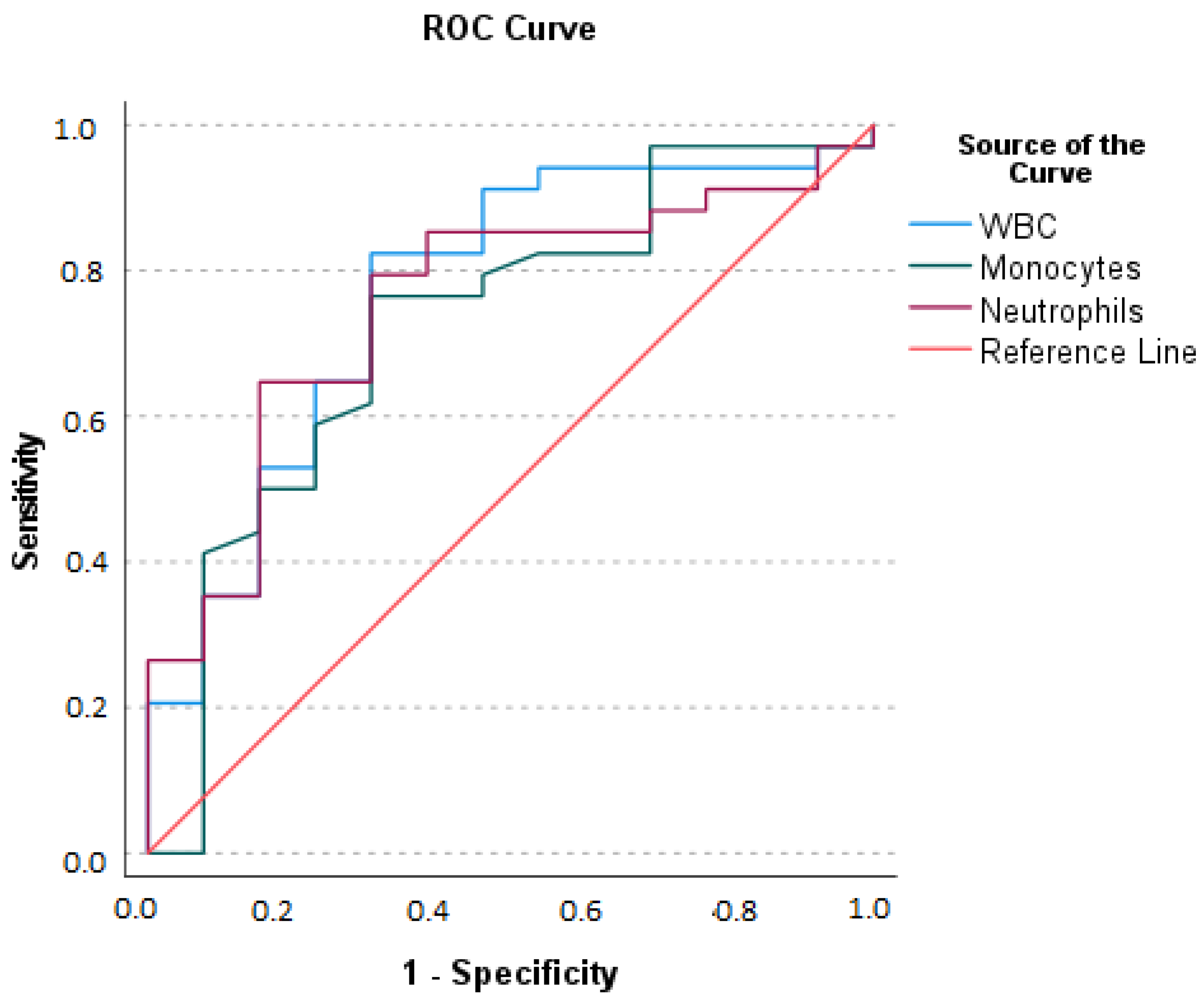
- The area Under the Curve (AUC) for WBC: optimal sum of sensitivity (0.824) and specificity (0.308) at a cut-off level of greater than 10.150 × 109/L is 0.767 (p = 0.001).
- (2)
- AUC for neutrophils: optimal sum of sensitivity (0.647) and specificity (0.154) at a cut-off level of greater than 9.360 × 109/L is 0.753 (p = 0.001); (3) AUC for monocytes: optimal sum of sensitivity (0.765) and specificity (0.308) at a cut-off level of greater than 0.72 × 109/L is 0.723 (p = 0.011).
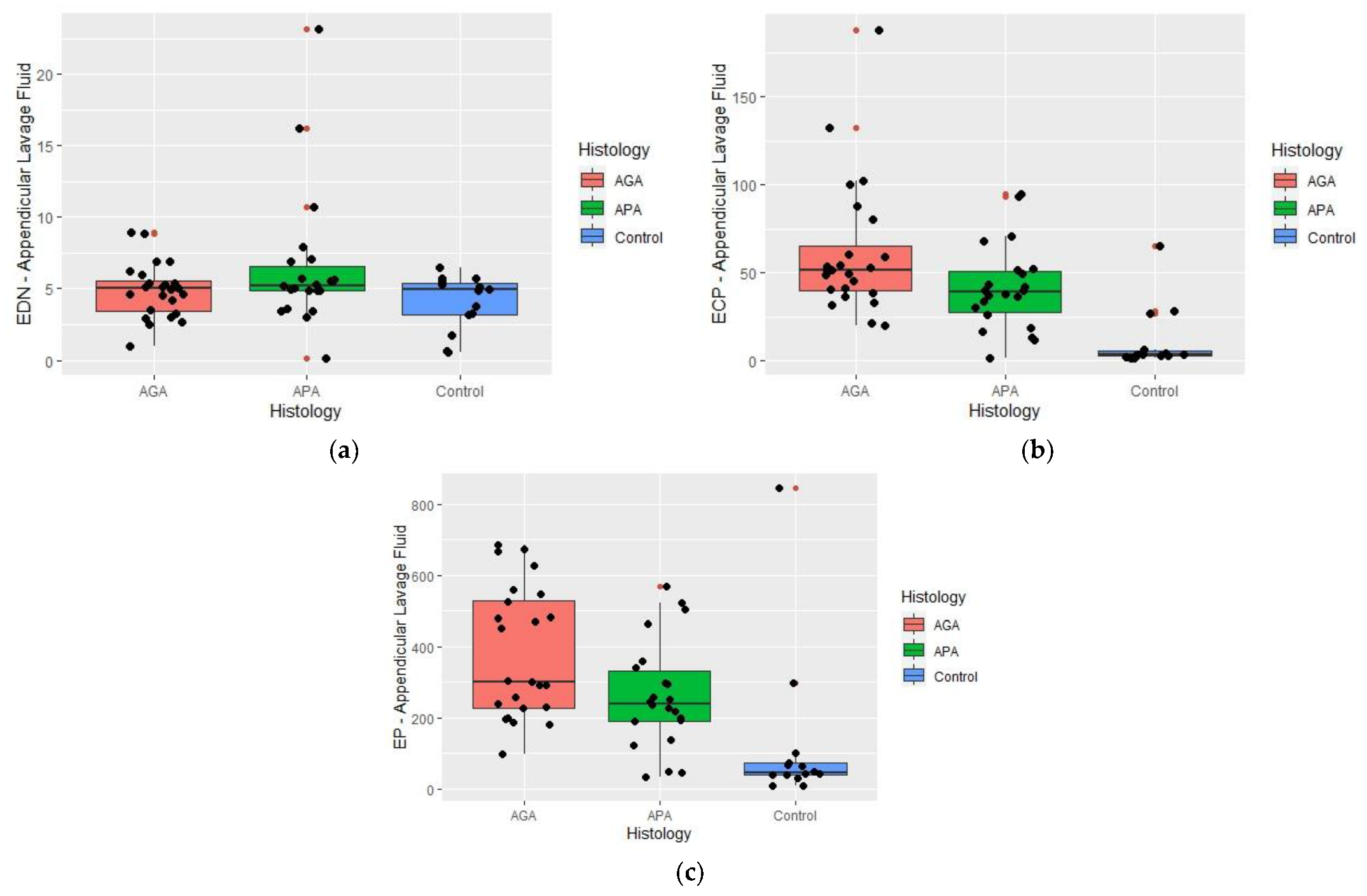
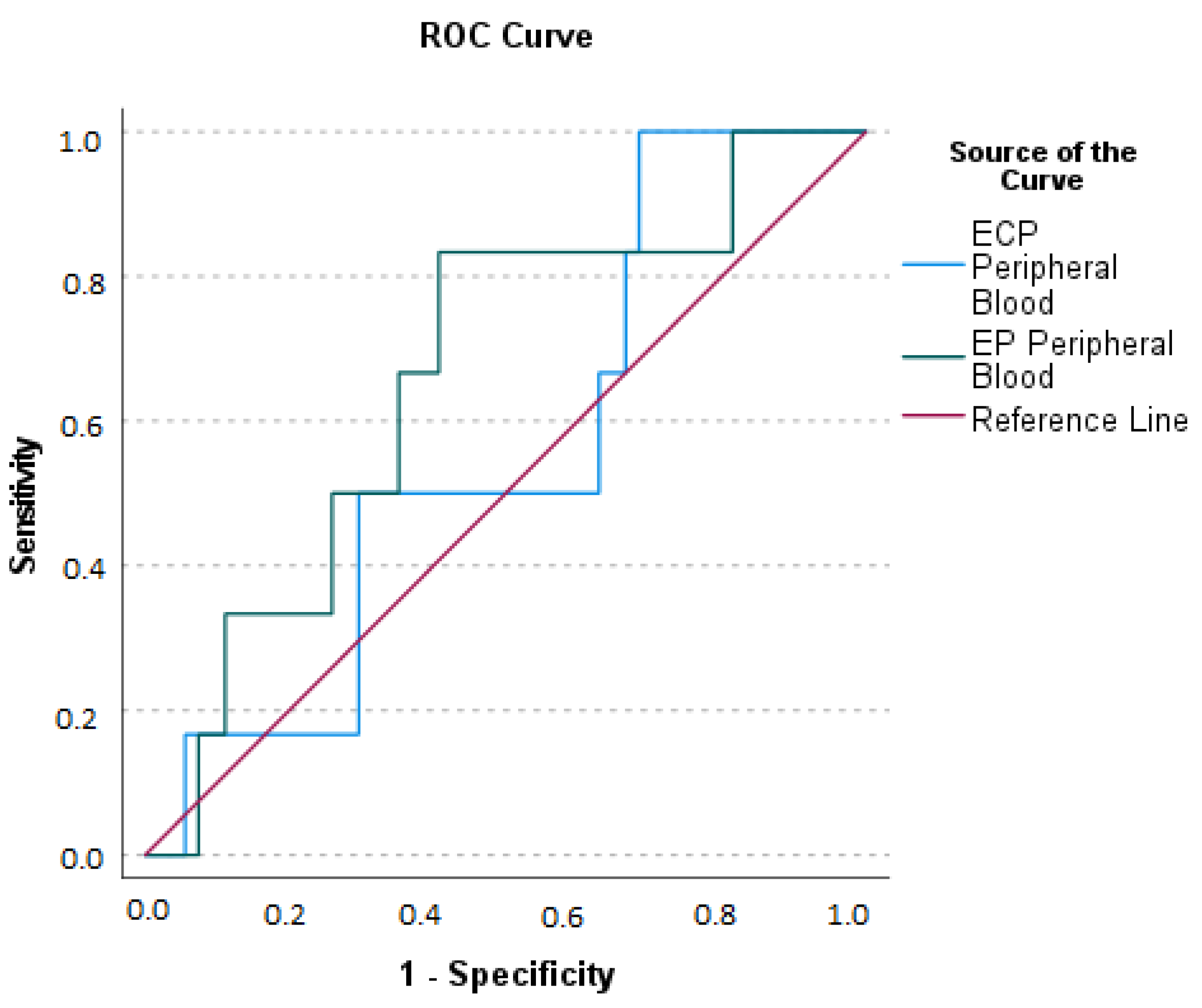
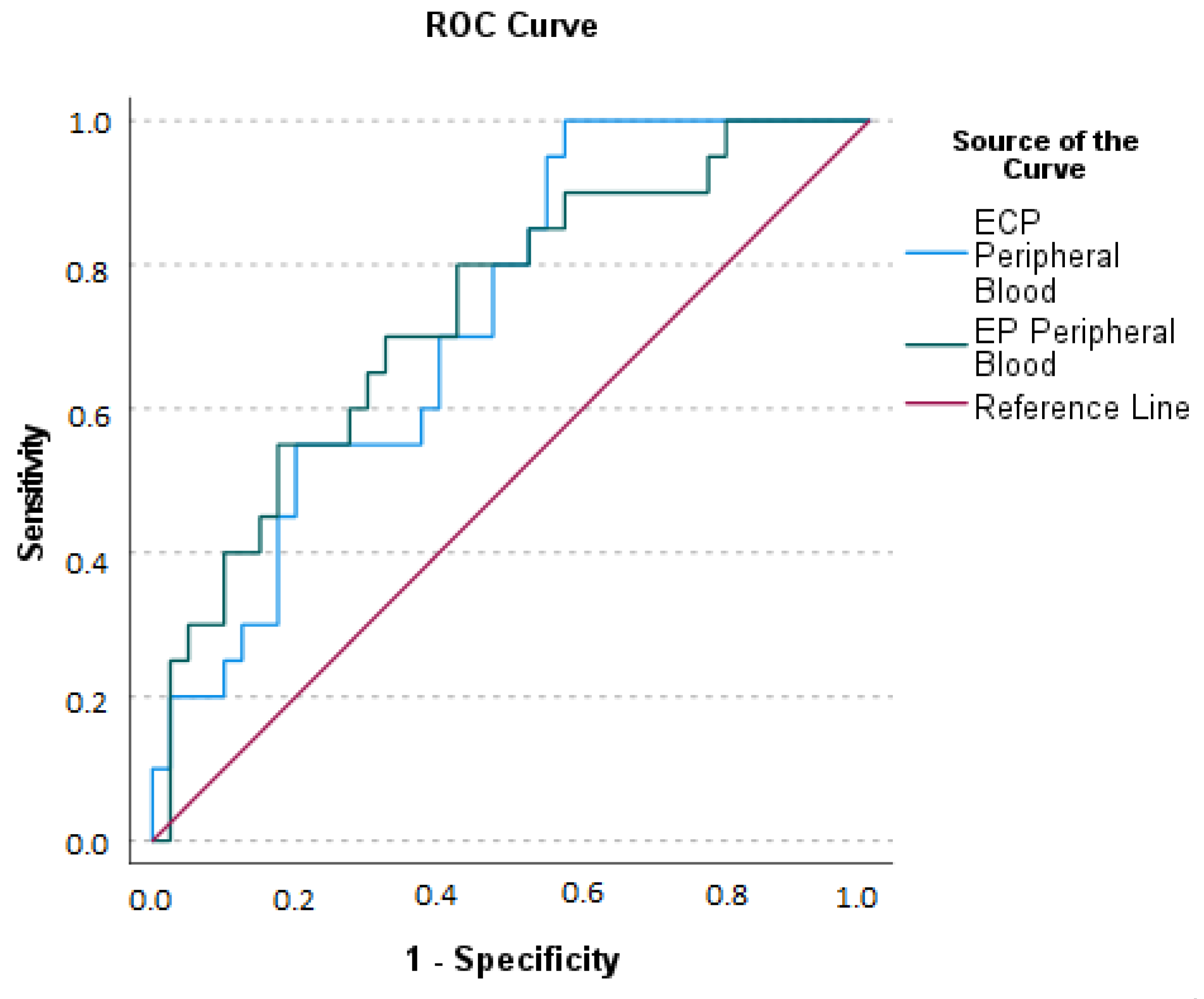
2.3. Appendicular Lavage Fluid (ALF) Eosinophil Derived Neurotoxin (EDN), Eosinophil Cationic Protein (ECP), and Eosinophil Peroxidase (EP) Concentrations
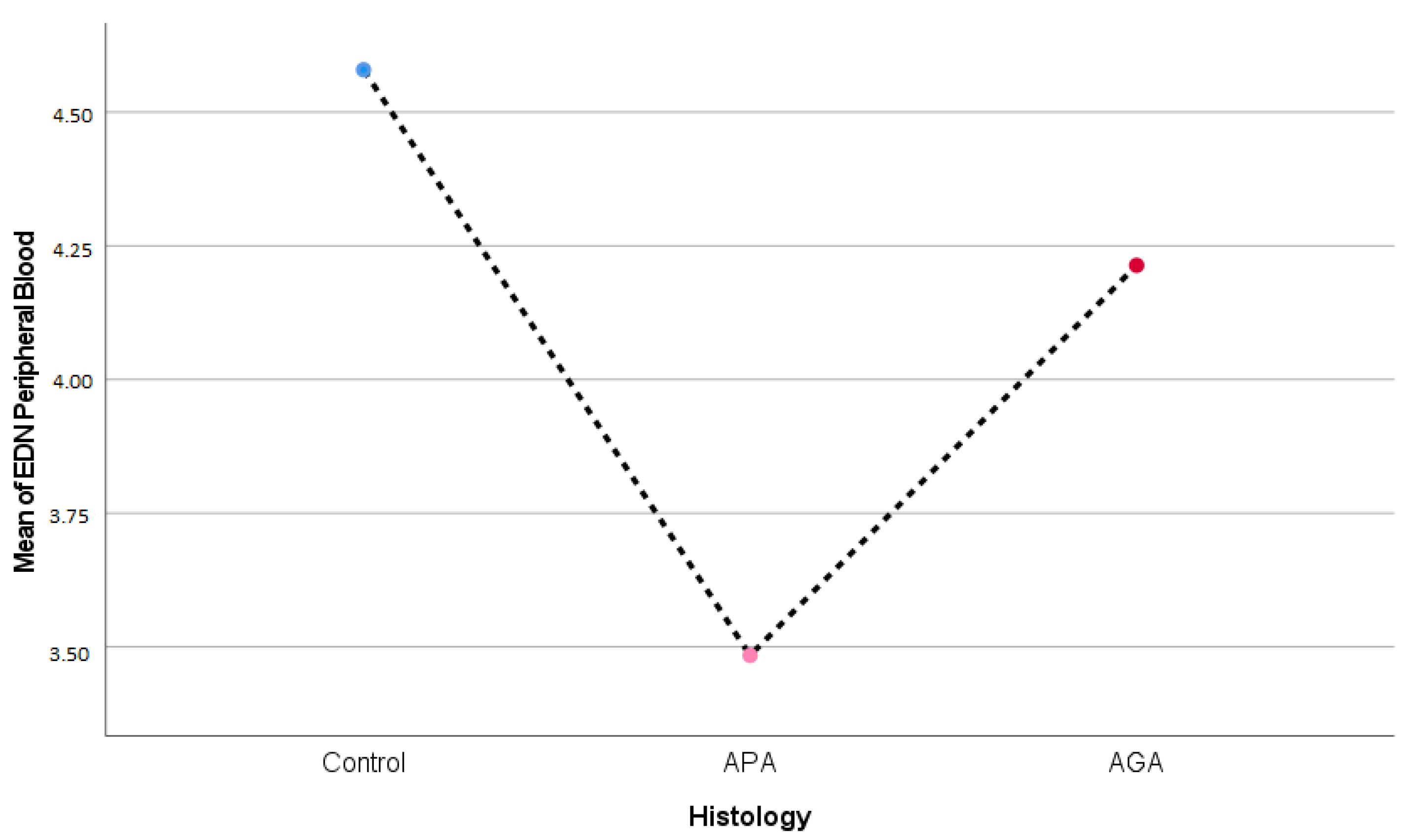
2.4. Serum Eosinophil Derived Neurotoxin (EDN), Eosinophil Cationic Protein (ECP), and Eosinophil Peroxidase (EP) Concentrations
2.5. Relationship between Eosinophil Derived Neurotoxin (EDN), Eosinophilic Cationic Protein (ECP), and Eosinophil Peroxidase (EP) Concentrations at Serum and Appendicular Lavage Fluid
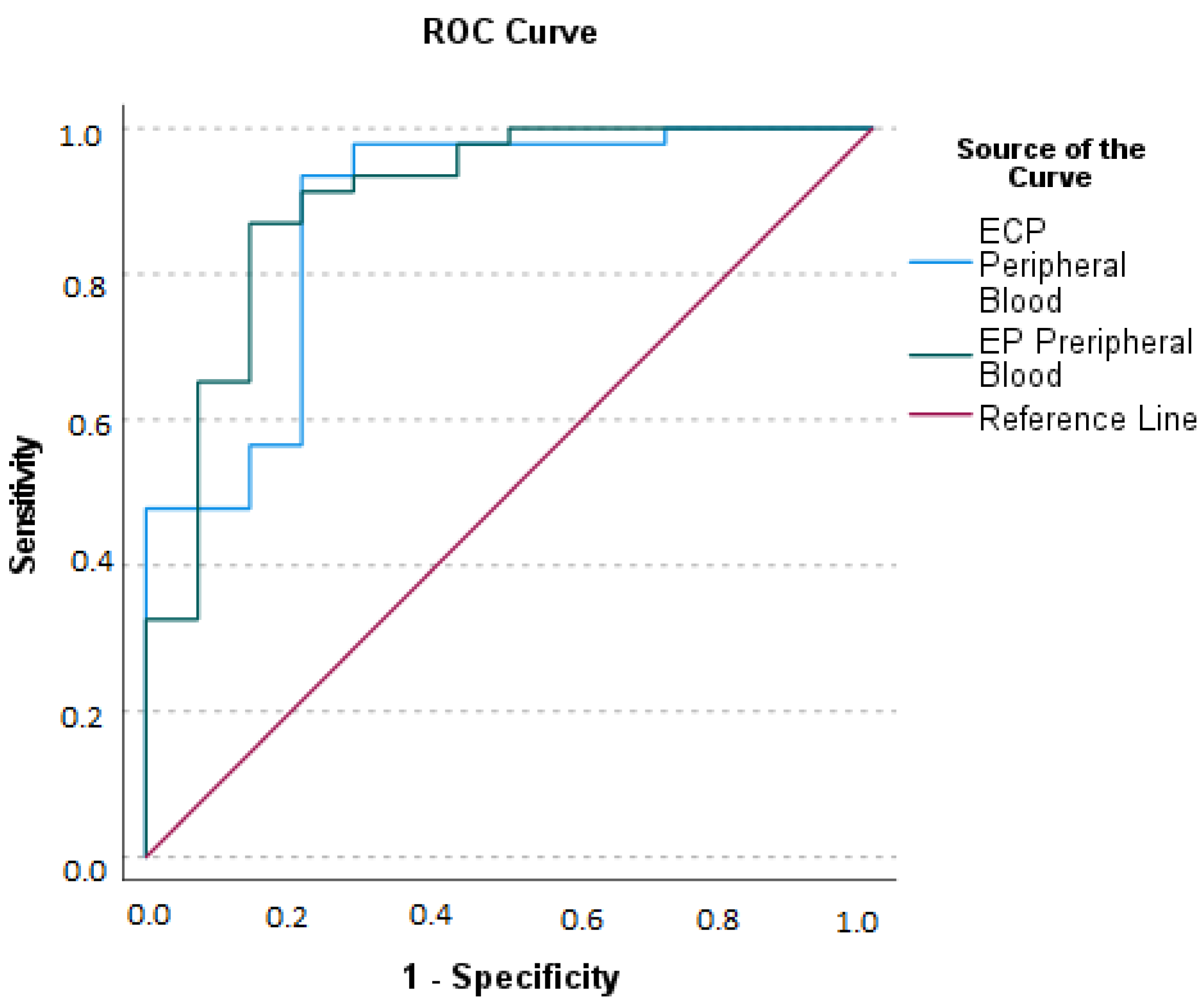
2.6. Relationship between Blood Eosinophils and Serum and Appendicular Lavage Fluid Eosinophil Derived Neurotoxin (EDN), Eosinophilic Cationic Protein (ECP), and Eosinophil Peroxidase (EP) Concentrations
2.7. Relationship between Serum Eosinophil Derived Neurotoxin (EDN), Eosinophilic Cationic Protein (ECP), Eosinophil Peroxidase (EP) Concentrations, and Acute Appendicitis Clinical Presentation
2.8. Relationship between Appendicular Lavage Fluid (ALF) Eosinophil Derived Neurotoxin (EDN), Eosinophilic Cationic Protein (ECP), Eosinophil Peroxidase (EP) Concentrations, and Acute Appendicitis Clinical Presentation
2.9. Other Data
3. Discussion
4. Materials and Methods
4.1. Aim
4.2. Patients and Study Design
4.3. Setting
4.4. Appendicular Lavage Fluid
4.5. Pathologic Analysis
4.6. Laboratory Proceedings
4.6.1. Hemogram
4.6.2. Eosinophil-Derived Neurotoxin (EDN), Eosinophilic Cationic Protein (ECP), and Eosinophil Peroxidase (EP) Determinations
4.7. Other Definitions
4.8. Ethics
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turanli, S.; Kiziltan, G. Did the COVID-19 Pandemic Cause a Delay in the Diagnosis of Acute Appendicitis? World J. Surg. 2021, 45, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Borges, F.; Costa, B.; Costa, P. A etiologia da apendicite aguda. Será a alergia o elo perdido? Uma revisão narrativa. The aetiology of acute appendicitis. Is allergy the missing link? A narrative review. Revista Portuguesa de Cirurgia, 13 March 2022. pp. 1–10; ISSN 2183-1165. Available online: https://revista.spcir.com/index.php/spcir/article/view/893 (accessed on 15 March 2023).
- Carvalho, N.; Barros, A.; Coelho, H.; Cóias, A.; Botelho, P.; Cismasiu, B.; Moita, L.; Costa, P. Increased IgE Deposition in Appendicular Tissue Specimens Is Compatible with a Type I Hypersensitivity Reaction in Acute Appendicitis. Mediators Inflamm. 2021, 2021, 4194859. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Carolino, E.; Coelho, H.; Cóias, A.; Trindade, M.; Vaz, J.; Cismasiu, B.; Moita, C.; Moita, L.; Costa, P.M. IL-5 Serum and Appendicular Lavage Fluid Concentrations Correlate with Eosinophilic Infiltration in the Appendicular Wall Supporting a Role for a Hypersensitivity Type I Reaction in Acute Appendicitis. Int. J. Mol. Sci. 2022, 23, 15086. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, S.; Munukka, E.; Kallonen, T.; Sippola, S.; Grönroos, J.; Haijanen, J.; Hakanen, A.J.; Salminen, P. Appendiceal microbiome in uncomplicated and complicated acute appendicitis: A prospective cohort study. PLoS ONE 2022, 17, e0276007. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, S.; Okayama, Y. Neuro-allergology: Mast cell-nerve cross-talk. Allergol. Int. 2022, 71, 288–293. [Google Scholar] [CrossRef]
- Salvo-Romero, E.; Rodiño-Janeiro, B.K.; Albert-Bayo, M.; Lobo, B.; Santos, J.; Farré, R.; Martinez, C.; Vicario, M. Eosinophils in the Gastrointestinal Tract: Key Contributors to Neuro-Immune Crosstalk and Potential Implications in Disorders of Brain-Gut Interaction. Cells 2022, 14, 1644. [Google Scholar] [CrossRef]
- Jenerowicz, D.; Czarnecka-Operacz, M.; Silny, W. Peripheral blood eosinophilia in atopic dermatitis. Acta Dermatovenerol. Alp. Panon. Adriat. 2007, 16, 47–52. [Google Scholar]
- Gudjonsdottir, J.; Roth, B.; Lovén, G.; Ohlsson, B.; Hagander, L.; Salö, M. An Evaluation of Serum IgE and Th2-Associated Interleukins in Children with Uncomplicated and Complicated Appendicitis. Front. Pediatr. 2022, 10, 884138. [Google Scholar] [CrossRef]
- Minderjahn, M.I.; Schädlich, D.; Radtke, J.; Rothe, K.; Reismann, M. Phlegmonous appendicitis in children is characterized by eosinophilia in white blood cell counts. World J. Pediatr. 2018, 14, 504–509. [Google Scholar] [CrossRef]
- Kim, C.K. Eosinophil-derived neurotoxin: A novel biomarker for diagnosis and monitoring of asthma. Korean J. Pediatr. 2013, 56, 8–12. [Google Scholar] [CrossRef]
- Bystrom, J.; Amin, K.; Bishop-Bailey, D. Analysing the eosinophil cationic protein—A clue to the function of the eosinophil granulocyte. Respir. Res. 2011, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Howarth, P.; Quirce, S.; Papi, A.; Israel, E.; Mallett, S.; Bates, S.; Yancey, S.; Albers, F.C.; Kwon, N. Eosinophil-derived neurotoxin and clinical outcomes with mepolizumab in severe eosinophilic asthma. Allergy 2020, 75, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Topic, R.Z.; Dodig, S. Eosinophil cationic protein--current concepts and controversies. Biochem. Med. 2011, 21, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Berti, A.; Cottini, M. The emerging roles of eosinophils: Implications for the targeted treatment of eosinophilic-associated inflammatory conditions. Curr. Res. Immunol. 2022, 3, 42–53. [Google Scholar] [CrossRef]
- Aravindan, K.P. Eosinophils in acute appendicitis: Possible significance. Ind. J. Pathol. Microbiol. 1997, 40, 491–498. [Google Scholar]
- Mohan, B.P.; Aravindan, K.P. Enterochromaffin Cells and Mast Cells in Acute Appendicitis. J. Lab. Physicians 2020, 12, 141–146. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Lukšić, B.; Ninčević, S.; Lukšić, B.; Polašek, O. Hyponatremia as a predictor of perforated acute appendicitis in pediatric population: A prospective study. J Pediatr. Surg. 2021, 56, 1816–1821. [Google Scholar] [CrossRef]
- Arredondo Montero, J.; Bardají Pascual, C.; Bronte Anaut, M.; López-Andrés, N.; Antona, G.; Martín-Calvo, N. Diagnostic performance of serum interleukin-6 in pediatric acute appendicitis: A systematic review. World J. Pediatr. 2022, 18, 91–99. [Google Scholar] [CrossRef]
- Tintor, G.; Jukić, M.; Šupe-Domić, D.; Jerončić, A.; Pogorelić, Z. Diagnostic Accuracy of Leucine-Rich_-2-Glycoprotein 1 as a Non-Invasive Salivary Biomarker in Pediatric Appendicitis. Int. J. Mol. Sci. 2023, 24, 6043. [Google Scholar] [CrossRef]
- Tintor, G.; Jukić, M.; Šupe-Domić, D.; Jerončić, A.; Pogorelić, Z. Diagnostic Utility of Serum Leucine-Rich_-2-Glycoprotein 1 for Acute Appendicitis in Children. J. Clin. Med. 2023, 12, 2455. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Lukšić, A.M.; Mihanović, J.; Đikić, D.; Balta, V. Hyperbilirubinemia as an Indicator of Perforated Acute Appendicitis in Pediatric Population: A Prospective Study. Surg. Infect. 2021, 10, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, L.; Feng, S.; Luo, J. Hyperfibrinogenemia and hyponatremia as predictors of perforated appendicitis in children: A retrospective cohort study. Int. J. Colorectal Dis. 2023, 38, 72. [Google Scholar] [CrossRef] [PubMed]
- Kakar, M.; Delorme, M.; Broks, R.; Asare, L.; Butnere, M.; Reinis, A.; Engelis, A.; Kroica, J.; Saxena, A.; Petersons, A. Determining acute complicated and uncomplicated appendicitis using serum and urine biomarkers: Interleukin-6 and neutrophil gelatinase-associated lipocalin. Pediatr. Surg. Int. 2020, 36, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.; Barros, A.; Coelho, H.O.; Moita, C.F.; Neves-Costa, A.; Pedroso, D.; Borges, F.C.; Moita, L.F.; Costa, P.M. A Th2 Cytokine Profile in Appendicular Lavage Fluid Suggests Allergy as a Possible Etiology for Acute Appendicitis. Mediators Inflamm. 2019, 2019, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Harlak, A.; Gulec, M.; Mentes, O.; Kilbas, Z.; Onguru, O.; Acikel, C.; Caliskaner, Z.; Erel, F. Atopy is a risk factor for acute appendicitis? A prospective clinical study. J. Gastrointest. Surg. 2008, 12, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.G.; Yin, X.; Swaminathan, A.; Eisenbarth, S.C. Antigen-Presenting Cells in Food Tolerance and Allergy. Front Immunol. 2021, 11, 616020. [Google Scholar] [CrossRef]
- Lönnkvist, K.; Hellman, C.; Lundahl, J.; Halldén, G.; Hedlin, G. Eosinophil markers in blood, serum, and urine for monitoring the clinical course in childhood asthma: Impact of budesonide treatment and withdrawal. J. Allergy Clin. Immunol. 2001, 107, 812–817. [Google Scholar] [CrossRef]
- Amoani, B.; Adu, B.; Frempong, M.T.; Sarkodie-Addo, T.; Nuvor, S.V.; Wilson, M.D.; Gyan, B. Levels of serum eosinophil cationic protein are associated with hookworm infection and intensity in endemic communities in Ghana. PLoS ONE 2019, 14, e0222382. [Google Scholar] [CrossRef]
- Koczera, P.; Martin, L.; Marx, G.; Schuerholz, T. The Ribonuclease A Superfamily in Humans: Canonical RNases as the Buttress of Innate Immunity. Int. J. Mol. Sci. 2016, 17, 1278. [Google Scholar] [CrossRef]
- Osmana, O.; Zohnea, E.R.A.; Magdyb, D.M.; Elminshawya, A.A. Eosinophilic cationic protein as a marker for detection of acute exacerbations of patients with chronic obstructive pulmonary disease. Egypt. J. Chest Dis. Tuberc. 2021, 70, 205–207. [Google Scholar] [CrossRef]
- Skjold-Ødegaard, B.; Søreide, K. The Diagnostic Differentiation Challenge in Acute Appendicitis: How to Distinguish between Uncomplicated and Complicated Appendicitis in Adults. Diagnostics 2022, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Reismann, J.; Romualdi, A.; Kiss, N.; Minderjahn, M.I.; Kallarackal, J.; Schad, M.; Reismann, M. Diagnosis and classification of pediatric acute appendicitis by artificial intelligence methods: An investigator-independent approach. PLoS ONE 2019, 14, e0222030. [Google Scholar] [CrossRef] [PubMed]
- Humes, D.J.; Simpson, J. Acute appendicitis. BMJ 2006, 333, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. A goodness-of-fit test for the multiple logistic regression model. Commun. Stat. 1980, 10, 1043–1069. [Google Scholar] [CrossRef]
- Komlósi, Z.I.; van de Veen, W.; Kovács, N.; Szűcs, G.; Sokolowska, M.; O'Mahony, L.; Akdis, M.; Akdis, C.A. Cellular and molecular mechanisms of allergic asthma. Mol. Aspects Med. 2022, 85, 100995. [Google Scholar] [CrossRef]
- Santosh, G.; Aravindan, K.P. Evidence for eosinophil degranulation in acute appendicitis. Indian J. Pathol. Microbiol. 2008, 51, 172–174. [Google Scholar] [CrossRef]
- Howell, E.C.; Dubina, E.D.; Lee, S.L. Perforation risk in pediatric appendicitis: Assessment and management. Pediatric Health Med. Ther. 2018, 9, 135–145. [Google Scholar] [CrossRef]
- Omari, A.H.; Khammash, M.R.; Qasaimeh, G.R.; Shammari, A.K.; Yaseen, M.K.; Hammori, S.K. Acute appendicitis in the elderly: Risk factors for perforation. World J. Emerg. Surg. 2014, 9, 6. [Google Scholar] [CrossRef]
- Mekakas, A.; Nagorni, E.-A.; Tablaridis, T. Complicated Appendicitis: A Surgical Controversy Concerning Risk Factors, Diagnostic Algorithm and Therapeutic Management. In Doubts, Problems and Certainties about Acute Appendicitis; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Rutten, B.; Young, S.; Rhedin, M.; Olsson, M.; Kurian, N.; Syed, F.; Beech, A.; Fidock, M.; Newbold, P.; Singh, D.; et al. Eosinophil-derived neurotoxin: A biologically and analytically attractive asthma biomarker. PLoS ONE 2021, 16, e0246627. [Google Scholar] [CrossRef]
- Psaltis, E.; Zaitoun, A.M.; Neal, K.R.; Lobo, D.N. Immunohistochemical Inflammation in Histologically Normal Appendices in Patients with Right Iliac Fossa Pain. World J. Surg. 2021, 12, 3592–3602. [Google Scholar] [CrossRef] [PubMed]
- Lamps, L.W. Beyond acute inflammation: A review of appendicitis and infections of the appendix. Diagn. Histopathol. 2008, 14, 68–77. [Google Scholar] [CrossRef]
- Arr, N.J. The pathology of acute appendicitis. Ann. Diagn. Pathol. 2000, 4, 46–58. [Google Scholar] [CrossRef]
- Kiss, N.; Minderjahn, M.; Reismann, J.; Svensson, J.; Wester, T.; Hauptmann, K.; Schad, M.; Kallarackal, J.; von Bernuth, H.; Reismann, M. Use of gene expression profiling to identify candidate genes for pretherapeutic patient classification in acute appendicitis. BJS Open 2021, 5, zraa045. [Google Scholar] [CrossRef]
- Mariage, M.; Sabbagh, C.; Grelpois, G.; Prevot, F.; Darmon, I.; Regimbeau, J.M. Surgeon’s Definition of Complicated Appendicitis: A Prospective Video Survey Study. Euroasian J. Hepatogastroenterol. 2019, 9, 1–4. [Google Scholar] [CrossRef]

| Control | APA | AGA | p-Value | |
|---|---|---|---|---|
| N (%) | 14 (23.3) | 22 (36.7) | 24 (40) | |
| Age (y) | 67.5 (30–78) | 48 (36–66) | 31 (23–55) | 0.044 * |
| Sex M/F | 9/5 (64.3/35.7) | 11/11 (50/50) | 12/12 (50/50) | 0.644 ** |
| Allergy N/Y | 12/1 (92.3/7.7) | 18/2 (90/10) | 20/1 (95.2/4.8) | 0.834 *** |
| BMI | 28.04 ± 4.76 | 24.57 ± 4.07 | 26.44 ± 4.540 | 0.298 **** |
| Control | APA | AGA | p-Value | |
|---|---|---|---|---|
| WBC | 10.10 ± 3.43 | 12.08 ± 2.93 | 15.97 ± 5.05 | <0.001 ** |
| Neutrophils | 6.73 (5.69–9.28) | 8.21 (7.20–12.46) | 11.50 (9.50–14.70) | 0.005 * |
| Lymphocytes | 1.29 (1.05–1.86) | 1.83 (1.63–2.42) | 1.37 (0.83–2.10) | 0.253 * |
| Basophils | 0.06 ± 0.02 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.051 ** |
| Eosinophils | 0.04 (0.01–0.21) | 0.14 (0.04–0.26) | 0.02 (0.00–0.07) | 0.052 * |
| Monocytes | 0.06 (0.04–0.07) | 0.03 (0.02–0.05) | 0.03 (0.02–0.06) | 0.034 * |
| Control | APA | AGA | p-Value | |
|---|---|---|---|---|
| EDN | 4.93 (3.20–5.43) | 5.23 (4.88–6.9) | 5.02 (3.55–5.65) | 0.256 * |
| ECP | 3.35 (2.60–6.10) | 38.85 (26.50–51.77) | 51.55 (39.55–70.09) | <0.001 * |
| EP | 45.55 (39.9–74.0) | 240.28 (191.2–341.3) | 302.5 (227.7–535.85) | <0.001 * |
| Control | APA | AGA | p-Value | |
|---|---|---|---|---|
| EDN | 4.58 ± 1.63 | 3.48 ± 1.13 | 4.21 ± 1.54 | 0.067 ** |
| ECP | 7.56 (4.76–11.13) | 39 (21.30–56.90) | 51.30 (20.25–62.59) | <0.001 * |
| EP | 38.20 (30.20–59.10) | 158.4 (111.09–222.1) | 235.27 (192.33–262.51) | <0.001 * |
| ECP (ALF) | EDN (PB) | EDN (ALF) | EP (PB) | EP (ALF) | |
|---|---|---|---|---|---|
| rS (p-Value) | |||||
| ECP (PB) | 0.767 ** (<0.001) | 0.137 (0.288) | 0.223 (0.082) | 0.676 ** (<0.001) | 0.544 ** (<0.001) |
| ECP (ALF) | 0.142 (0.271) | 0.128 (0.322) | 0.726 ** (<0.001) | 0.671 ** (<0.001) | |
| EDN (PB) | 0.087 (0.500) | 0.095 (0.462) | 0.158 (0.221) | ||
| EDN (ALF) | 0.150 (0.243) | 0.072 (0.581) | |||
| EP (PB) | 0.643 ** (<0.001) | ||||
| Eosinophils | |||
|---|---|---|---|
| EDN | PB | rS (p-value) | −0.274 (0.054) |
| ALF | −0.034 (0.812) | ||
| ECP | PB | rS (p-value) | −0.148 (0.304) |
| ALF | −0154 (0.287) | ||
| EP | PB | rS (p-value) | −0.0146 (0.311) |
| ALF | −0.217 (0.129) |
| EDN | p-Value | ECP | p-Value | EP | p-Value | ||
|---|---|---|---|---|---|---|---|
| Perforation | No | 4.35 (2.9–5.04) | 0.667 | 35.95 (8.4–58.7) | 0.005 | 168 (58.19–250.47) | 0.003 |
| Yes | 4.73 (3.9–5.09) | 35 (16.20–52.10) | 232.15 (191–264.2) | ||||
| Peritonitis | No | 4.15 (2.9–5.03) | 0.527 | 21.05 (7.84–46.2) | 0.005 | 132.02 (47.5–220.5) | 0.003 |
| Yes | 4.6 (3.05–5.14) | 51.30 (22.2–64.3) | 241.6 (168–278.35) | ||||
| Complicated | No | 3.14 (2.8–4.53) | 0.119 | 42.77 (16–59.07) | 0.586 | 164.93 ± 90.13 | 0.08 * |
| Yes | 4.6 (2.91–5.09) | 41.02 (21–61.20) | 213.55 ± 81.18 |
| EDN | p-Value * | ECP | p-Value | * EP | p-Value * | ||
|---|---|---|---|---|---|---|---|
| Perforation | No | 4.63 (2.43–5.6) | 0.538 | 4.92 (11.8–52.6) | 0.475 | 274.8 (68.5–463.4) | 0.961 |
| Yes | 5.14 (4.5–5.92) | 48.5 (31.9–54.5) | 276.4 (188.3–257.9) | ||||
| Peritonitis | No | 4.81 (3.3–5.5) | 0.259 | 31.06 (4.05–50.3) | 0.002 | 235.98 (50.1–322.4) | 0.021 |
| Yes | 4.6 (3.05–5.14) | 51.30 (22.2–64.3) | 241.6 (168–278.35) | ||||
| Complicated | No | 4.98 (3.5–5.5) | 0.854 | 38.75 (22.34–50.4) | 0.07 | 223.5 (159.5–330.3) | 0.059 |
| Yes | 5.08 (3.48–5.92) | 50.5 (36.5–60.09) | 292.4 (226–584.76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, N.; Carolino, E.; Coelho, H.; Barreira, A.L.; Moreira, L.; André, M.; Henriques, S.; Cardoso, C.; Moita, L.; Costa, P.M. Eosinophil Granule Proteins Involvement in Acute Appendicitis—An Allergic Disease? Int. J. Mol. Sci. 2023, 24, 9091. https://doi.org/10.3390/ijms24109091
Carvalho N, Carolino E, Coelho H, Barreira AL, Moreira L, André M, Henriques S, Cardoso C, Moita L, Costa PM. Eosinophil Granule Proteins Involvement in Acute Appendicitis—An Allergic Disease? International Journal of Molecular Sciences. 2023; 24(10):9091. https://doi.org/10.3390/ijms24109091
Chicago/Turabian StyleCarvalho, Nuno, Elisabete Carolino, Hélder Coelho, Ana Lúcia Barreira, Luísa Moreira, Margarida André, Susana Henriques, Carlos Cardoso, Luis Moita, and Paulo Matos Costa. 2023. "Eosinophil Granule Proteins Involvement in Acute Appendicitis—An Allergic Disease?" International Journal of Molecular Sciences 24, no. 10: 9091. https://doi.org/10.3390/ijms24109091
APA StyleCarvalho, N., Carolino, E., Coelho, H., Barreira, A. L., Moreira, L., André, M., Henriques, S., Cardoso, C., Moita, L., & Costa, P. M. (2023). Eosinophil Granule Proteins Involvement in Acute Appendicitis—An Allergic Disease? International Journal of Molecular Sciences, 24(10), 9091. https://doi.org/10.3390/ijms24109091







