Revisiting Fur Regulon Leads to a Comprehensive Understanding of Iron and Fur Regulation
Abstract
1. Introduction
2. Results
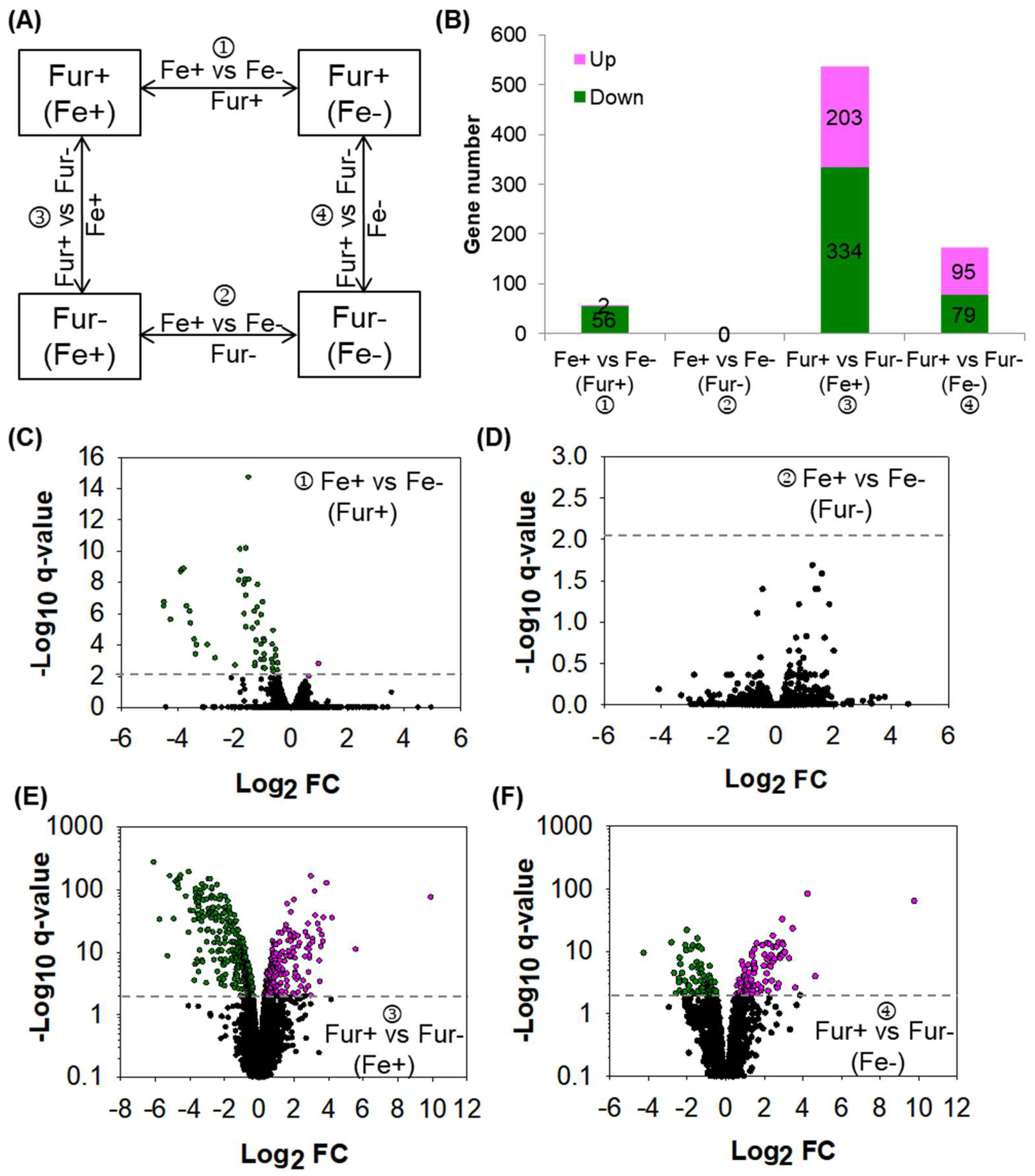
2.1. Fur Is the Sole Regulatory Factor Responding to Iron
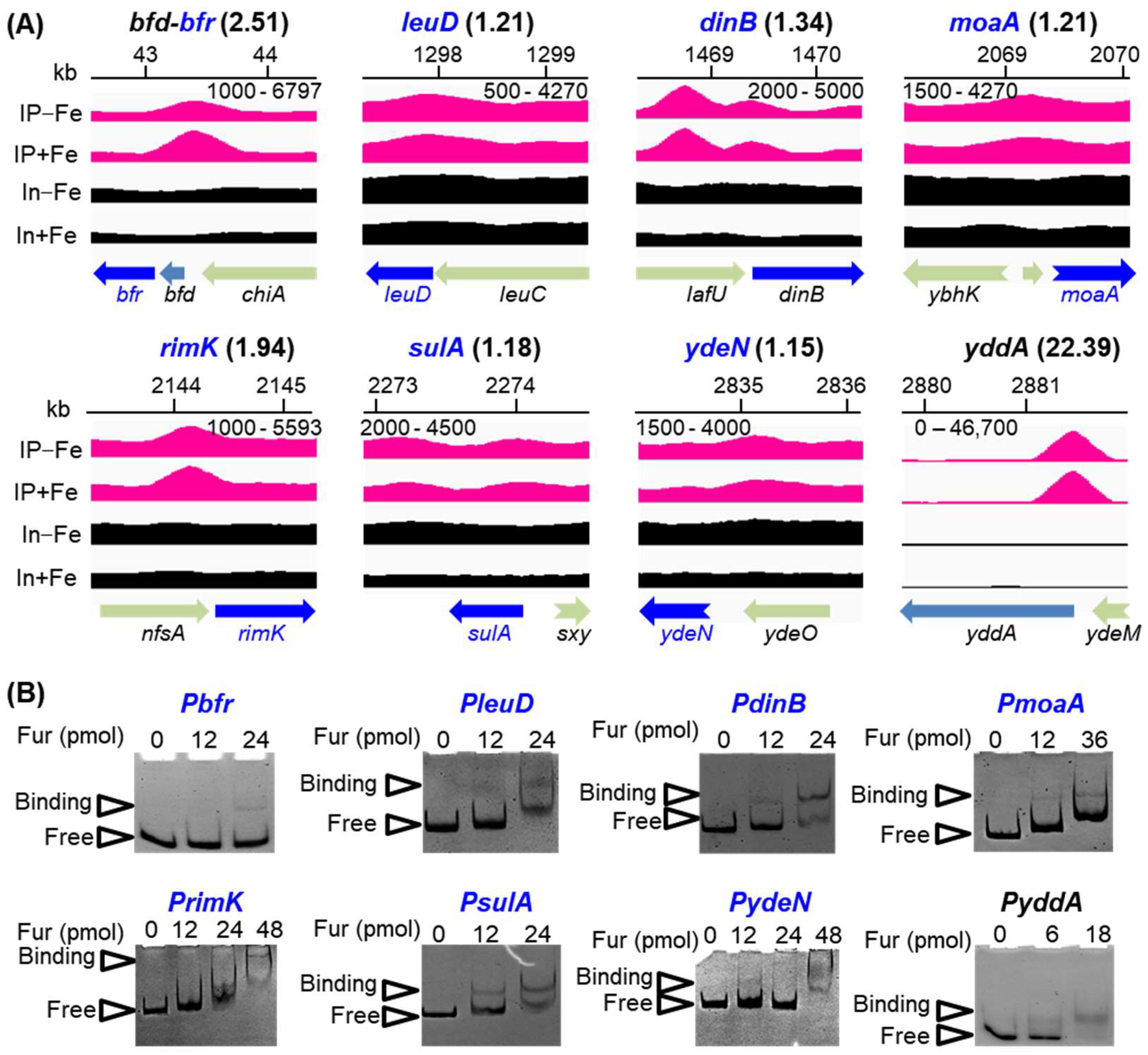
2.2. Fur Regulon Is Expanded by Combined Analysis of ChIP-seq and RNA-seq
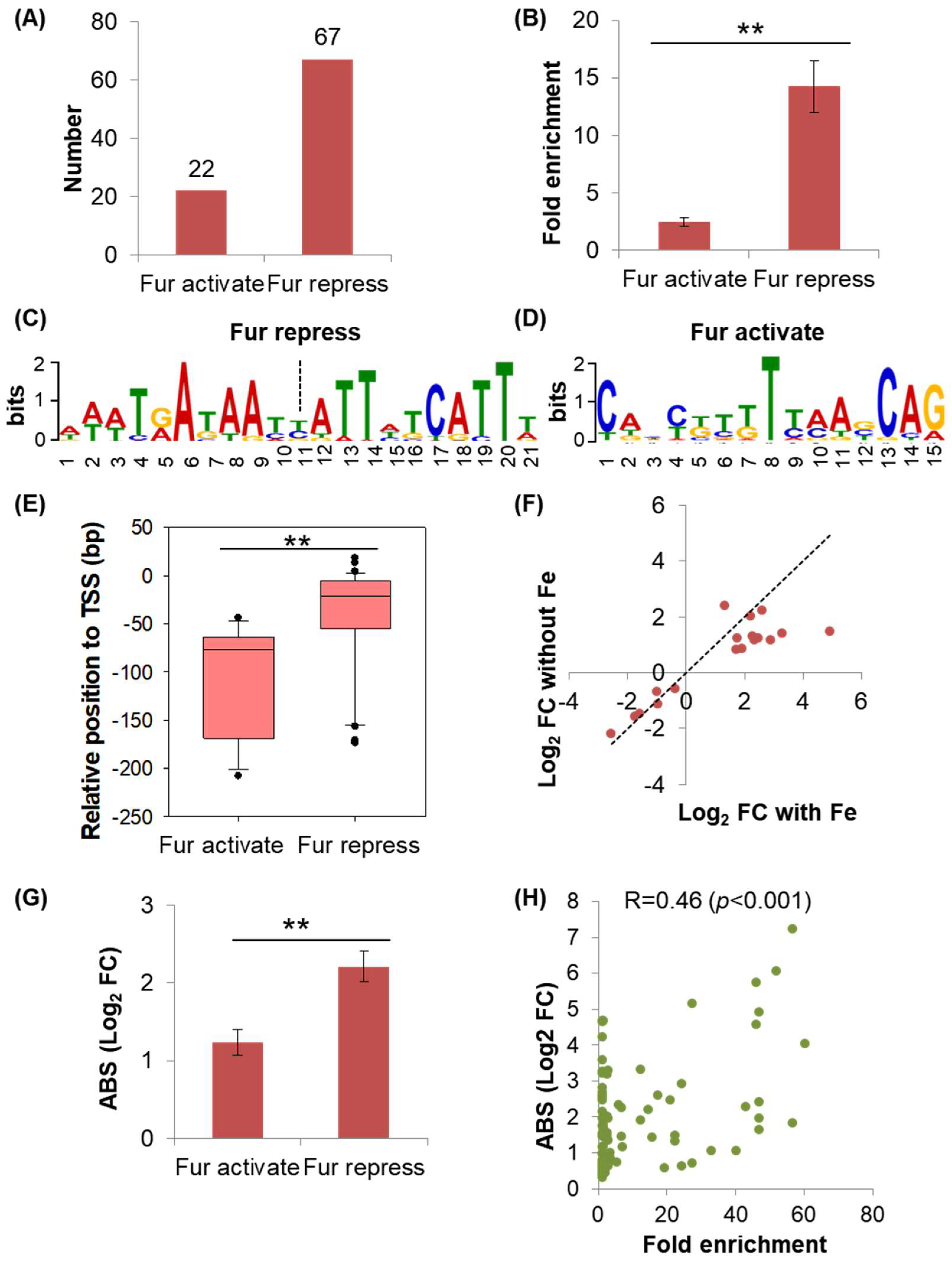
2.3. Discrepancies Existed between the Regulations of Fur on the Genes under Its Direct Repression and Activation
2.4. The Regulatory Role of Fur Is Far beyond Maintaining Iron Homeostasis
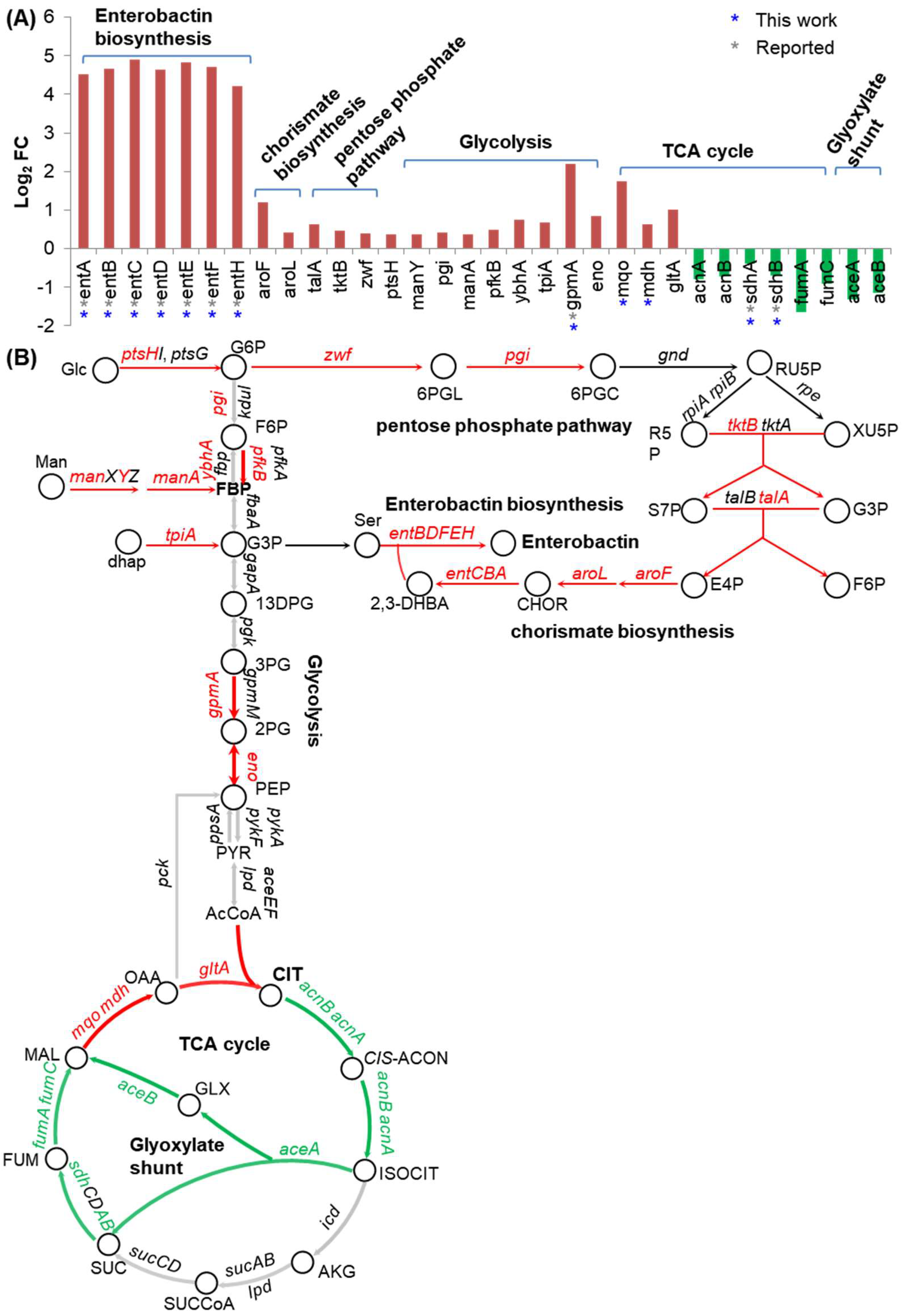
2.5. Fur Coordinates Iron and Carbon Metabolism
2.6. Fur Activates Anaerobic Respiration but Represses Aerobic Respiration
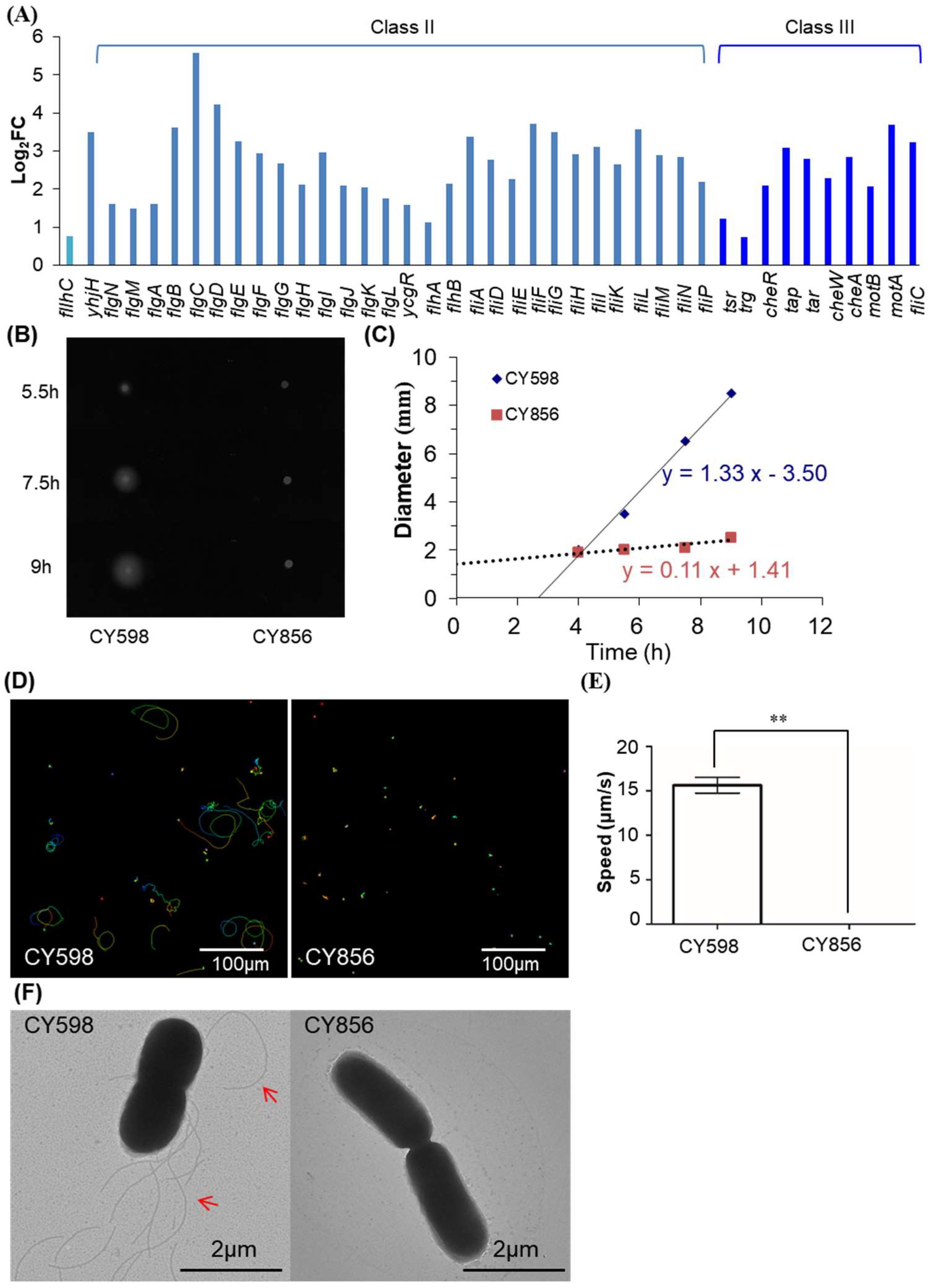
2.7. Fur Activates the Expression of Motility Genes
3. Discussion
4. Materials and Methods
4.1. Construction of Bacterial Strains
4.2. RNA-seq Assay and Analysis of Transcriptome Data
4.3. ChIP-seq Assay and Analysis of ChIP-seq Data
4.4. Overexpression and Purification of Fur
4.5. EMSA of Fur
4.6. Real Time Quantitative PCR (RT-qPCR)
4.7. Swimming Motility Assay on Soft Agar Plate
4.8. Time-Lapse Microscopy and Trajectory Analysis of Single-Cell Motility
4.9. Flagella Observation under Transmission Electron Microscope
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McHugh, J.P.; Rodríguez-Quiñones, F.; Abdul-Tehrani, H.; Svistunenko, D.A.; Poole, R.K.; Cooper, C.E.; Andrews, S.C. Global Iron-dependent Gene Regulation in Escherichia coli: A new mechanism for iron homeostasis. J. Biol. Chem. 2003, 278, 29478–29486. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Kim, D.; Latif, H.; O’brien, E.J.; Szubin, R.; Palsson, B.O. Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nat. Commun. 2014, 5, 4910. [Google Scholar] [CrossRef]
- Beauchene, N.A.; Myers, K.S.; Chung, D.; Park, D.M.; Weisnicht, A.M.; Keleş, S.; Kiley, P.J. Impact of Anaerobiosis on Expression of the Iron-Responsive Fur and RyhB Regulons. mBio 2015, 6, e01947-15. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rojas, A.; Kim, J.J.; Johnston, P.R.; Makarova, O.; Eravci, M.; Weise, C.; Hengge, R.; Rolff, J. Non-Lethal exposure to H2O2 boosts bacterial survival and evolvability against oxidative stress. PLoS Genet. 2020, 16, e1008649. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA Damage by Hydrogen Peroxide through the Fenton Reaction In Vivo and In Vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Zhou, Y.; Imlay, J.A. During Oxidative Stress the Clp Proteins of Escherichia coli Ensure that Iron Pools Remain Sufficient to Reactivate Oxidized Metalloenzymes. J. Bacteriol. 2020, 202, e00235-20. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-Stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Ju, X.; Fang, X.; Xiao, Y.; Li, B.; Shi, R.; Wei, C.; You, C. Small RNA GcvB Regulates Oxidative Stress Response of Escherichia coli. Antioxidants 2021, 10, 1774. [Google Scholar] [CrossRef]
- Zeng, J.; Hong, Y.; Zhao, N.; Liu, Q.; Zhu, W.; Xiao, L.; Wang, W.; Chen, M.; Hong, S.; Wu, L.; et al. A broadly applicable, stress-mediated bacterial death pathway regulated by the phosphotransferase system (PTS) and the cAMP-Crp cascade. Proc. Natl. Acad. Sci. USA 2022, 119, e2118566119. [Google Scholar] [CrossRef]
- Carpenter, B.M.; Whitmire, J.M.; Merrell, D.S. This is not your mother’s repressor: The complex role of fur in pathogenesis. Infect. Immun. 2009, 77, 2590–2601. [Google Scholar] [CrossRef]
- Butcher, J.; Sarvan, S.; Brunzelle, J.S.; Couture, J.-F.; Stintzi, A. Structure and regulon of Campylobacter jejuni ferric uptake regulator Fur define apo-Fur regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 10047–10052. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.M.; Gilbreath, J.J.; Pich, O.Q.; McKelvey, A.M.; Maynard, E.L.; Li, Z.-Z.; Merrell, D.S. Identification and Characterization of Novel Helicobacter pylori apo-Fur-Regulated Target Genes. J. Bacteriol. 2013, 195, 5526–5539. [Google Scholar] [CrossRef] [PubMed]
- Young, G.M.; Postle, K. Repression of tonB transcription during anaerobic growth requires Fur binding at the promoter and a second factor binding upstream. Mol. Microbiol. 1994, 11, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Escolar, L.; Perez-Martin, J.; de Lorenzo, V. Coordinated repression in vitro of the divergent fepA-fes promoters of Escherichia coli by the iron uptake regulation (Fur) protein. J. Bacteriol. 1998, 180, 2579–2582. [Google Scholar] [CrossRef]
- Chen, Z.; Lewis, K.A.; Shultzaberger, R.K.; Lyakhov, I.G.; Zheng, M.; Doan, B.; Storz, G.; Schneider, T. Discovery of Fur binding site clusters in Escherichia coli by information theory models. Nucleic Acids Res. 2007, 35, 6762–6777. [Google Scholar] [CrossRef]
- Nandal, A.; Huggins, C.C.; Woodhall, M.R.; McHugh, J.; Rodriguez-Quinones, F.; Quail, M.A.; Guest, J.R.; Andrews, S.C. Induction of the ferritin gene (ftnA) of Escherichia coli by Fe(2+)-Fur is mediated by reversal of H-NS silencing and is RyhB independent. Mol. Microbiol. 2010, 75, 637–657. [Google Scholar] [CrossRef]
- Yu, C.; Genco, C.A. Fur-Mediated Activation of Gene Transcription in the Human Pathogen Neisseria gonorrhoeae. J. Bacteriol. 2012, 194, 1730–1742. [Google Scholar] [CrossRef]
- Masse, E.; Gottesman, S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 4620–4625. [Google Scholar] [CrossRef]
- Porcheron, G.; Dozois, C.M. Interplay between iron homeostasis and virulence: Fur and RyhB as major regulators of bacterial pathogenicity. Vet. Microbiol. 2015, 179, 2–14. [Google Scholar] [CrossRef]
- Stojiljkovic, I.; Baumler, A.; Hantke, K. Fur Regulon in Gram-negative Bacteria: Identification and Characterization of New Iron-regulated Escherichia coli Genes by a Fur Titration Assay. J. Mol. Biol. 1994, 236, 531–545. [Google Scholar] [CrossRef]
- Vassinova, N.; Kozyrev, D. A method for direct cloning of Fur-regulated genes: Identification of seven new Fur-regulated loci in Escherichia coli. Microbiology 2000, 146, 3171–3182. [Google Scholar] [CrossRef] [PubMed]
- Keseler, I.M.; Mackie, A.; Santos-Zavaleta, A.; Billington, R.; Bonavides-Martinez, C.; Caspi, R.; Fulcher, C.; Gama-Castro, S.; Kothari, A.; Krummenacker, M.; et al. The EcoCyc database: Reflecting new knowledge about Escherichia coli K-12. Nucleic Acids Res. 2017, 45, D543–D550. [Google Scholar] [CrossRef] [PubMed]
- Santos-Zavaleta, A.; Salgado, H.; Gama-Castro, S.; Sanchez-Perez, M.; Gomez-Romero, L.; Ledezma-Tejeida, D.; Garcia-Sotelo, J.S.; Alquicira-Hernandez, K.; Muniz-Rascado, L.J.; Pena-Loredo, P.; et al. RegulonDB v 10.5: Tackling challenges to unify classic and high throughput knowledge of gene regulation in E. coli K-12. Nucleic Acids Res. 2019, 47, D212–D220. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, Q.; Xiao, Y.; Fang, X.; Shi, R.; Fu, C.; Danchin, A.; You, C. Deciphering global gene expression and regulation strategy in Escherichia coli during carbon limitation. Microb. Biotechnol. 2018, 12, 360–376. [Google Scholar] [CrossRef]
- Pan, Q.; Li, Z.; Ju, X.; Hou, C.; Xiao, Y.; Shi, R.; Fu, C.; Danchin, A.; You, C. Escherichia coli segments its controls on carbon-dependent gene expression into global and specific regulations. Microb. Biotechnol. 2021, 14, 1084–1106. [Google Scholar] [CrossRef]
- Solomon, M.J.; Larsen, P.L.; Varshavsky, A. Mapping proteinDNA interactions in vivo with formaldehyde: Evidence that histone H4 is retained on a highly transcribed gene. Cell 1988, 53, 937–947. [Google Scholar] [CrossRef]
- Bonocora, R.P.; Wade, J.T. ChIP-seq for genome-scale analysis of bacterial DNA-binding proteins. Methods Mol. Biol. 2015, 1276, 327–340. [Google Scholar]
- Kroner, G.M.; Wolfe, M.B.; Freddolino, P.L. Escherichia coli Lrp Regulates One-Third of the Genome via Direct, Cooperative, and Indirect Routes. J. Bacteriol. 2019, 201, e00411-18. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37 (Suppl. S2), w202–w208. [Google Scholar] [CrossRef]
- Wade, J.T. Mapping Transcription Regulatory Networks with ChIP-seq and RNA-seq. Adv. Exp. Med. Biol. 2015, 883, 119–134. [Google Scholar]
- Street, L.A.; Morao, A.K.; Winterkorn, L.H.; Jiao, C.-Y.; Albritton, S.E.; Sadic, M.; Kramer, M.; Ercan, S. Binding of an X-Specific Condensin Correlates with a Reduction in Active Histone Modifications at Gene Regulatory Elements. Genetics 2019, 212, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.N.; Kong, X.; Parkin, M.C.; Cammack, R.; Hider, R.C. Iron(iii) citrate speciation in aqueous solution. Dalton Trans. 2009, 40, 8616–8625. [Google Scholar] [CrossRef] [PubMed]
- Frawley, E.R.; Crouch, M.-L.V.; Bingham-Ramos, L.K.; Robbins, H.F.; Wang, W.; Wright, G.D.; Fang, F.C. Iron and citrate export by a major facilitator superfamily pump regulates metabolism and stress resistance in Salmonella Typhimurium. Proc. Natl. Acad. Sci. USA 2013, 110, 12054–12059. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hou, C.; Ju, X.; Feng, Y.; Ye, Z.-Q.; Xiao, Y.; Gu, M.; Fu, C.; Wei, C.; You, C. Gain of Spontaneous clpX Mutations Boosting Motility via Adaption to Environments in Escherichia coli. Front. Bioeng. Biotechnol. 2021, 9, 772397. [Google Scholar] [CrossRef]
- Kurabayashi, K.; Agata, T.; Asano, H.; Tomita, H.; Hirakawa, H. Fur Represses Adhesion to, Invasion of, and Intracellular Bacterial Community Formation within Bladder Epithelial Cells and Motility in Uropathogenic Escherichia coli. Infect. Immun. 2016, 84, 3220–3231. [Google Scholar] [CrossRef]
- Niu, L.; Cai, W.; Cheng, X.; Li, Z.; Ruan, J.; Li, F.; Qi, K.; Tu, J. Fur Protein Regulates the Motility of Avian Pathogenic Escherichia coli AE17 through Promoter Regions of the Flagella Key Genes flhD. Front. Veter. Sci. 2022, 9, 854916. [Google Scholar] [CrossRef]
- Lyons, E.; Freeling, M.; Kustu, S.; Inwood, W. Using genomic sequencing for classical genetics in E. coli K12. PLoS ONE 2011, 6, e16717. [Google Scholar] [CrossRef]
- Wang, X.; Wood, T.K. IS5 inserts upstream of the master motility operon flhDC in a quasi-Lamarckian way. ISME J. 2011, 5, 1517–1525. [Google Scholar] [CrossRef]
- Fahrner, K.A.; Berg, H.C. Mutations That Stimulate flhDC Expression in Escherichia coli K-12. J. Bacteriol. 2015, 197, 3087–3096. [Google Scholar] [CrossRef]
- Zhang, Z.; Kukita, C.; Humayun, M.Z.; Saier, M.H. Environment-directed activation of the Escherichia coli flhDC operon by transposons. Microbiology 2017, 163, 554–569. [Google Scholar] [CrossRef]
- Shimizu, K. Metabolic Regulation and Coordination of the Metabolism in Bacteria in Response to a Variety of Growth Conditions. Adv. Biochem. Eng. Biotechnol. 2015, 155, 1–54. [Google Scholar] [CrossRef]
- Zhang, Z.; Gosset, G.; Barabote, R.; Gonzalez, C.S.; Cuevas, W.A.; Saier, M.H., Jr. Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J. Bacteriol. 2005, 187, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; De Lay, N.R. Target recognition by RNase E RNA-binding domain AR2 drives sRNA decay in the absence of PNPase. Proc. Natl. Acad. Sci. USA 2022, 119, e2208022119. [Google Scholar] [CrossRef] [PubMed]
- Beauchene, N.A.; Mettert, E.L.; Moore, L.J.; Keles, S.; Willey, E.R.; Kiley, P.J. O2 availability impacts iron homeostasis in Escherichia coli. Proc. Natl. Acad. Sci. USA 2017, 114, 12261–12266. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, T.; Takaya, A.; Isogai, E.; Yamamoto, T. Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol. Microbiol. 2003, 48, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Josenhans, C.; Suerbaum, S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 2002, 291, 605–614. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Genet. 2017, 16, 91–102. [Google Scholar] [CrossRef]
- You, C.; Okano, H.; Hui, S.; Zhang, Z.; Kim, M.; Gunderson, C.W.; Wang, Y.-P.; Lenz, P.; Yan, D.; Hwa, T. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 2013, 500, 301–306. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Landt, S.G.; Marinov, G.K.; Kundaje, A.; Kheradpour, P.; Pauli, F.; Batzoglou, S.; Bernstein, B.E.; Bickel, P.; Brown, J.B.; Cayting, P.; et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012, 22, 1813–1831. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows—Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.; Liu, L.; Ju, X.; Xiao, Y.; Li, B.; You, C. Revisiting Fur Regulon Leads to a Comprehensive Understanding of Iron and Fur Regulation. Int. J. Mol. Sci. 2023, 24, 9078. https://doi.org/10.3390/ijms24109078
Hou C, Liu L, Ju X, Xiao Y, Li B, You C. Revisiting Fur Regulon Leads to a Comprehensive Understanding of Iron and Fur Regulation. International Journal of Molecular Sciences. 2023; 24(10):9078. https://doi.org/10.3390/ijms24109078
Chicago/Turabian StyleHou, Chaofan, Lin Liu, Xian Ju, Yunzhu Xiao, Bingyu Li, and Conghui You. 2023. "Revisiting Fur Regulon Leads to a Comprehensive Understanding of Iron and Fur Regulation" International Journal of Molecular Sciences 24, no. 10: 9078. https://doi.org/10.3390/ijms24109078
APA StyleHou, C., Liu, L., Ju, X., Xiao, Y., Li, B., & You, C. (2023). Revisiting Fur Regulon Leads to a Comprehensive Understanding of Iron and Fur Regulation. International Journal of Molecular Sciences, 24(10), 9078. https://doi.org/10.3390/ijms24109078




