Triphosphate Tunnel Metalloenzyme 2 Acts as a Downstream Factor of ABI4 in ABA-Mediated Seed Germination
Abstract
1. Introduction
2. Results
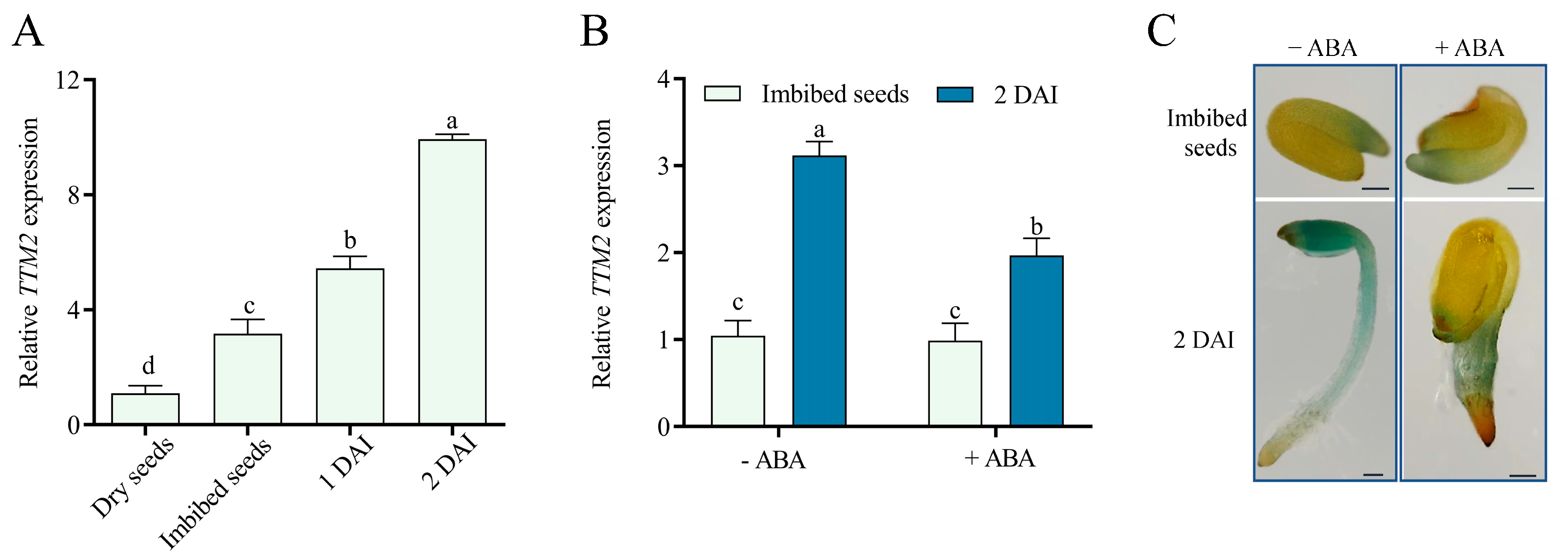
2.1. ABA Regulates TTM2 Expression during Seed Germination
2.2. Repression of TTM2 Expression Is Required for ABA-Mediated Inhibition of Seed Germination
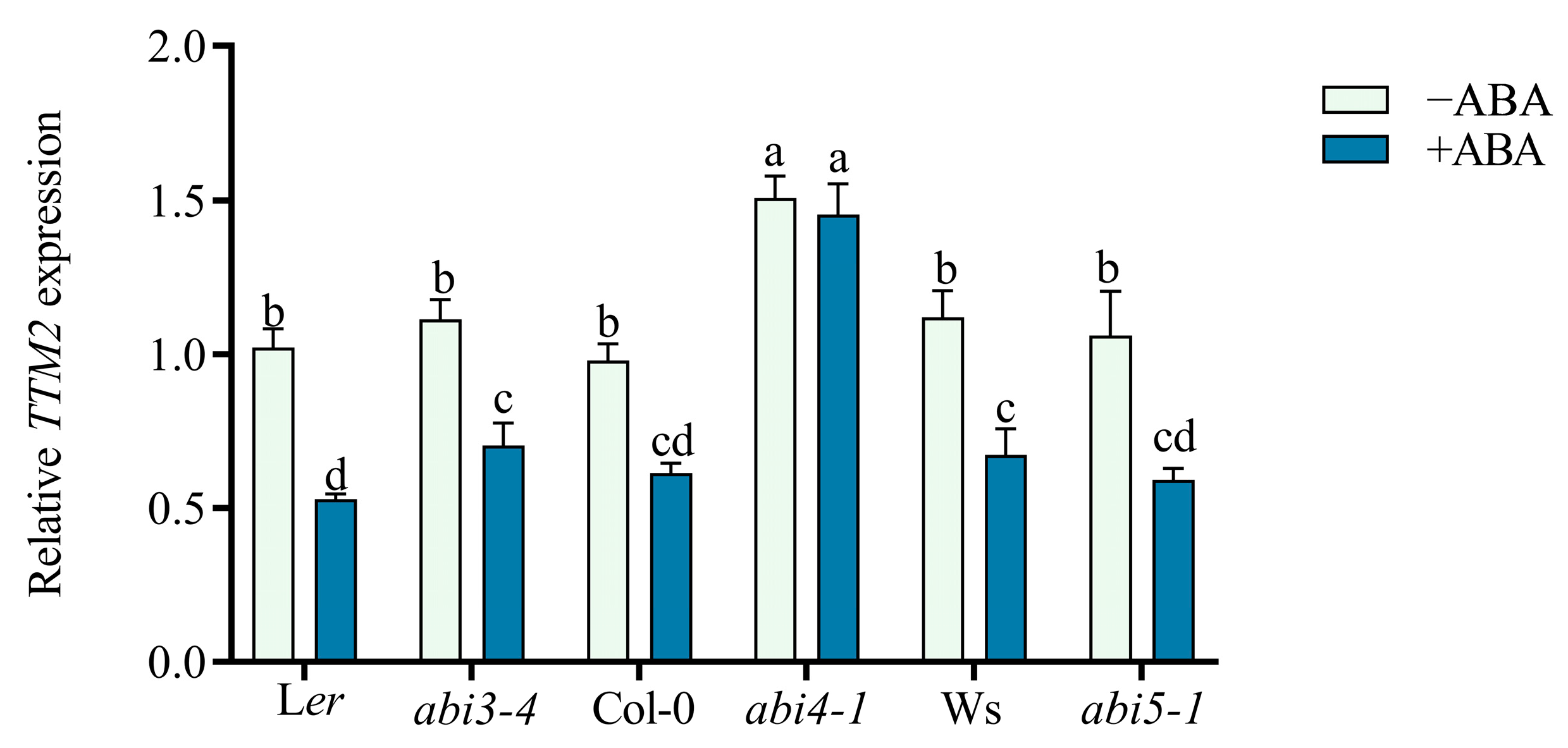
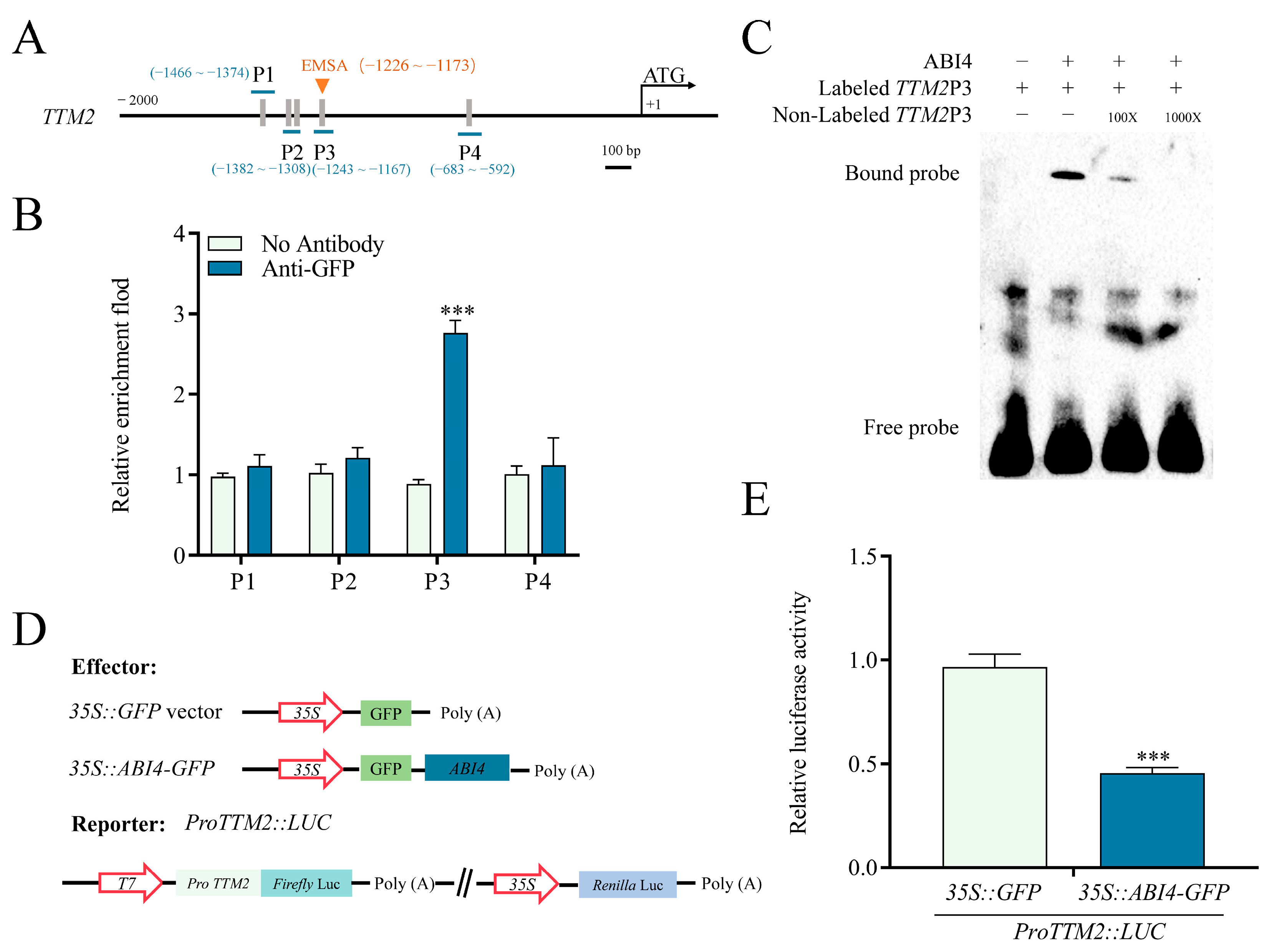
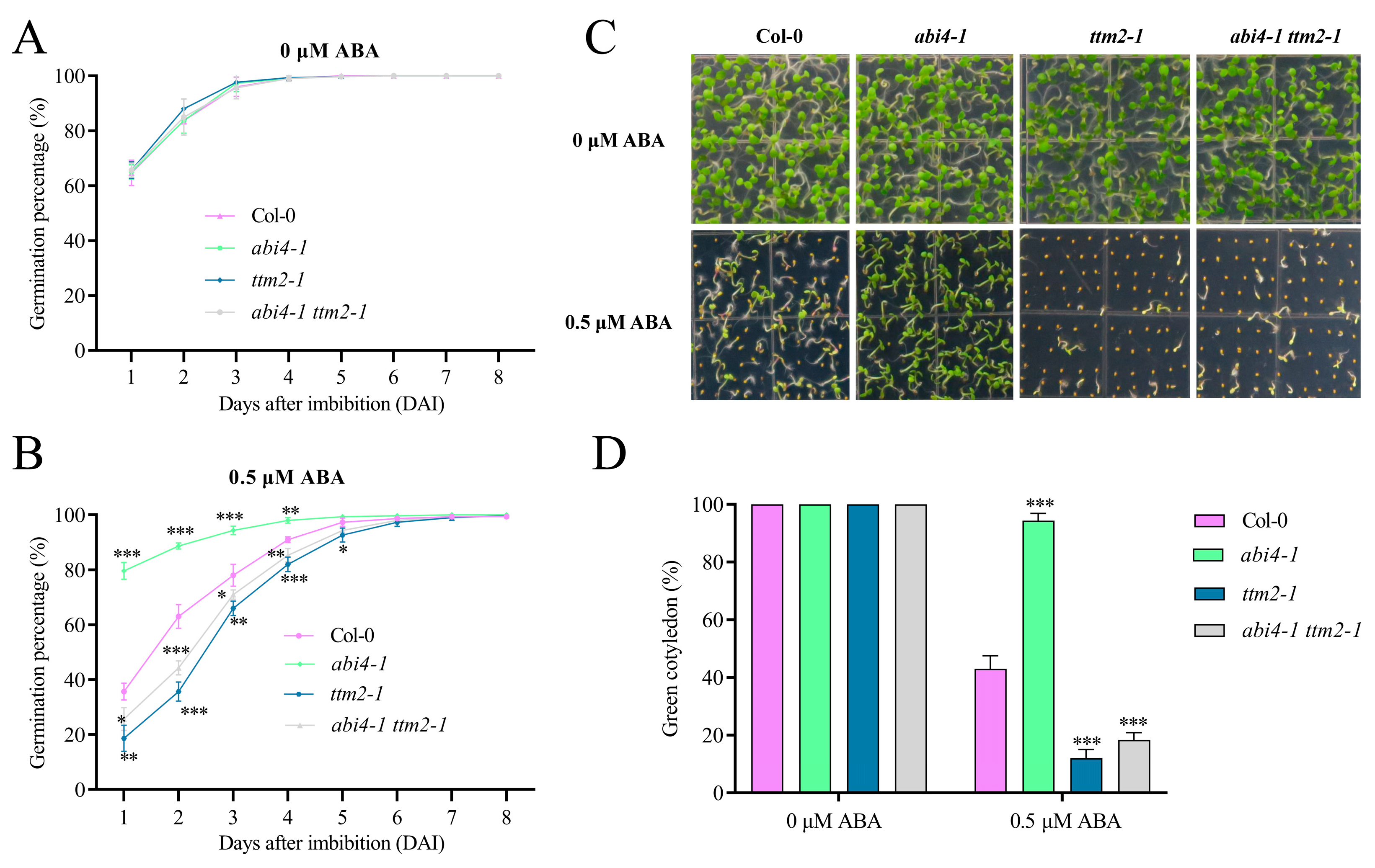
2.3. TTM2 Acts Downstream of ABI4 to Regulate Seed Germination
2.4. TTM1 Is Not Involved in ABA-Mediated Seed Germination and Early Seedling Development
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Production of Transgenic Plants
4.3. Determination of Germination Rate and Green Cotyledon
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Electrophoresis Mobility Shift Assay (EMSA)
4.6. Chromatin Immunoprecipitation (ChIP)-qPCR Assay
4.7. Dual Luciferase Assays
4.8. GUS Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finch-Savage, W.E.; Footitt, S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Neé, G.; Xiang, Y.; Soppe, W.J. The release of dormancy, a wake-up call for seeds to germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant. 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Shinomura, T.; Nagatani, A.; Chory, J.; Furuya, M. The lnduction of Seed Germination in Arabidopsis tbaliana 1s Regulated Principally by Phytochrome B and Secondarily by Phytochrome A. Plant Physiol. 1994, 104, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Lechowska, K.; Kubala, S.; Wojtyla, A.; Nowaczyk, G.; Quinet, M.; Lutts, S.; Garnczarska, M. New Insight on Water Status in Germinating Brassica napus Seeds in Relation to Priming-Improved Germination. Int. J. Mol. Sci. 2019, 20, 540. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.B.; Pires, S.N.; Ávila, G.E.; Silva, B.E.P.; Schmitz, V.N.; Deuner, C.; Da Silva Armesto, R.; Da Silva Moura, D.; Deuner, S. Application of vigor indexes to evaluate the cold tolerance in rice seeds germination conditioned in plant extract. Sci. Rep. 2021, 11, 11038. [Google Scholar] [CrossRef]
- Giraudat, J.L.A.J. Abscisic Acid Signal Transduction. Annu. Rev. Plant Physiol. 1998, 49, 199–222. [Google Scholar]
- Yang, L.; Jiang, Z.; Liu, S.; Lin, R. Interplay between REVEILLE1 and RGA-LIKE2 regulates seed dormancy and germination in Arabidopsis. New Phytol. 2020, 225, 1593–1605. [Google Scholar] [CrossRef]
- Grappin, P.; Bouinot, D.; Sotta, B.; Miginiac, E.; Jullien, M. Control of seed dormancy in Nicotiana plumbaginifolia: Post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 2000, 210, 279–285. [Google Scholar] [CrossRef]
- Zhao, H.; Nie, K.; Zhou, H.; Yan, X.; Zhan, Q.; Zheng, Y.; Song, C.P. ABI5 modulates seed germination via feedback regulation of the expression of the PYR/PYL/RCAR ABA receptor genes. New Phytol. 2020, 228, 596–608. [Google Scholar] [CrossRef]
- Li, Z.; Sheerin, D.J.; von Roepenack-Lahaye, E.; Stahl, M.; Hiltbrunner, A. The phytochrome interacting proteins ERF55 and ERF58 repress light-induced seed germination in Arabidopsis thaliana. Nat. Commun. 2022, 13, 1656. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Kuwahara, A.; Seo, M.; Kushiro, T.; Asami, T.; Hirai, N.; Kamiya, Y.; Koshiba, T.; Nambara, E. CYP707A1 and CYP707A2, Which Encode Abscisic Acid 8′-Hydroxylases, Are Indispensable for Proper Control of Seed Dormancy and Germination in Arabidopsis. Plant Physiol. 2006, 141, 97–107. [Google Scholar] [CrossRef]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.J.; Li, X.; Mou, Z.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin inhibits seed germination by crosstalk with abscisic acid, gibberellin, and auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef] [PubMed]
- Manfre, A.J.; Lanni, L.M.; Marcotte, W.R. The Arabidopsis Group 1 Late Embryogenesis Abundant Protein ATEM6 Is Required for Normal Seed Development. Plant Physiol. 2006, 140, 140–149. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wu, J.; Sun, X.; Dai, M. The Maize Clade A PP2C Phosphatases Play Critical Roles in Multiple Abiotic Stress Responses. Int. J. Mol. Sci. 2019, 20, 3573. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Waadt, R.; Schroeder, J.I. Evolution of Abscisic Acid Synthesis and Signaling Mechanisms. Curr. Biol. 2011, 21, R346–R355. [Google Scholar] [CrossRef]
- Koornneef, M.; Reuling, G.; Karssen, C.M. The isolation and characterization of abscisic acid-insensitive. Physiol. Plant. 1984, 61, 377–383. [Google Scholar] [CrossRef]
- Shu, K.; Zhang, H.; Wang, S.; Chen, M.; Wu, Y.; Tang, S.; Liu, C.; Feng, Y.; Cao, X.; Xie, Q. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in arabidopsis. PLoS Genet. 2013, 9, e1003577. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Somerville, C.R. Three Classes of Abscisic Acid (ABA)-lnsensitive Mutations of Arabidopsis Define Genes that Control Overlapping Subsets of ABA Responses. Plant Physiol. 1990, 3, 1172–1179. [Google Scholar] [CrossRef]
- Acevedo-Hernández, G.J.; León, P.; Herrera-Estrella, L.R. Sugar and ABA responsiveness of a minimal RBCS light-responsive unit is mediated by direct binding of ABI4. Plant J. 2005, 43, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mao, Y.; Duan, X.; Zhou, H.; Lai, D.; Zhang, Y.; Shen, W. Arabidopsis HY1-Modulated Stomatal Movement: An Integrative Hub Is Functionally Associated with ABI4 in Dehydration-Induced ABA Responsiveness. Plant Physiol. 2016, 170, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Kakan, X.; Yu, Y.; Li, S.; Li, X.; Huang, R.; Wang, J. Ascorbic acid modulation by ABI4 transcriptional repression of VTC2 in the salt tolerance of Arabidopsis. BMC Plant Biol. 2021, 21, 112. [Google Scholar] [CrossRef]
- Shu, K.; Chen, Q.; Wu, Y.; Liu, R.; Zhang, H.; Wang, S.; Tang, S.; Yang, W.; Xie, Q. ABSCISIC ACID-INSENSITIVE 4 negatively regulates flowering through directly promoting Arabidopsis Flowering Locus C transcription. J. Exp. Bot. 2015, 67, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.Z.; Chen, Y.; Wang, C.; Kong, Y.H.; Wu, W.H.; Chen, Y.F. Arabidopsis RAV1 transcription factor, phosphorylated by SnRK2 kinases, regulates the expression ofABI3,ABI 4, and ABI5 during seed germination and early seedling development. Plant J. 2014, 80, 654–668. [Google Scholar] [CrossRef]
- Chandrasekaran, U.; Luo, X.; Zhou, W.; Shu, K. Multifaceted Signaling Networks Mediated by Abscisic Acid Insensitive 4. Plant. Commun. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Xiao, S.; Jiang, L.; Wang, C.; Ow, D.W. Arabidopsis OXS3 family proteins repress ABA signaling through interactions with AFP1 in the regulation ofABI4 expression. J. Exp. Bot. 2021, 72, 5721–5734. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, X.; Duan, Y.; Li, S.; Wang, Y.; Rehman, A.U.; He, J.; Zhang, J.; Hua, D.; Yang, L.; et al. The Arabidopsis Nodulin Homeobox Factor AtNDX Interacts with AtRING1A/B and Negatively Regulates Abscisic Acid Signaling. Plant Cell 2020, 32, 703–721. [Google Scholar] [CrossRef]
- Zhao, Y.; Ai, X.; Wang, M.; Xiao, L.; Xia, G. A putative pyruvate transporter TaBASS2 positively regulates salinity tolerance in wheat via modulation of ABI4 expression. BMC Plant Biol. 2016, 16, 109. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, C.; Ye, Q.; Wu, W.; Chen, Y. Arabidopsis WRKY6 Transcription Factor Acts as a Positive Regulator of Abscisic Acid Signaling during Seed Germination and Early Seedling Development. PLoS Genet. 2016, 12, e1005833. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.G.; Yoon, S.; Kim, H.U.; Seo, P.J. The Arabidopsis MYB96 Transcription Factor Is a Positive Regulator ofABSCISIC ACID-INSENSITIVE4 in the Control of Seed Germination. Plant Physiol. 2015, 168, 677–689. [Google Scholar] [CrossRef]
- Greco, M.; Chiappetta, A.; Bruno, L.; Bitonti, M.B. In Posidonia oceanica cadmium induces changes in DNA methylation and chromatin patterning. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar] [CrossRef]
- Bossi, F.; Cordoba, E.; Dupré, P.; Mendoza, M.S.; Román, C.S.; León, P. The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J. 2009, 59, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Wind, J.J.; Peviani, A.; Snel, B.; Hanson, J.; Smeekens, S.C. ABI4: Versatile activator and repressor. Trends Plant Sci. 2013, 18, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Tatematsu, K.; Yano, R.; Preston, J.; Kitamura, S.; Takahashi, H.; McCourt, P.; Kamiya, Y.; Nambara, E. CHOTTO1, a Double AP2 Domain Protein of Arabidopsis thaliana, Regulates Germination and Seedling Growth Under Excess Supply of Glucose and Nitrate. Plant Cell Physiol. 2009, 50, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Aravind, L. The catalytic domains of thiamine triphosphatase and CyaB-like adenylyl cyclase define a novel superfamily of domains that bind organic phosphates. BMC Genom. 2002, 3, 33. [Google Scholar] [CrossRef]
- Ung, H.; Karia, P.; Ebine, K.; Ueda, T.; Yoshioka, K.; Moeder, W. Triphosphate Tunnel Metalloenzyme Function in Senescence Highlights a Biological Diversification of This Protein Superfamily. Plant Physiol. 2017, 175, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Ung, H.; Moeder, W.; Yoshioka, K. Arabidopsis Triphosphate Tunnel Metalloenzyme2 Is a Negative Regulator of the Salicylic Acid-Mediated Feedback Amplification Loop for Defense Responses. Plant Physiol. 2014, 166, 1009–1021. [Google Scholar] [CrossRef]
- Moeder, W.; Garcia-Petit, C.; Ung, H.; Fucile, G.; Samuel, M.A.; Christendat, D.; Yoshioka, K. Crystal structure and biochemical analyses reveal that the Arabidopsis triphosphate tunnel metalloenzyme AtTTM3 is a tripolyphosphatase involved in root development. Plant J. 2013, 76, 615–626. [Google Scholar] [CrossRef]
- Karia, P.; Yoshioka, K.; Moeder, W. Multiple phosphorylation events of the mitochondrial membrane protein TTM1 regulate cell death during senescence. Plant J. 2021, 108, 766–780. [Google Scholar] [CrossRef]
- Klepikova, A.V.; Kasianov, A.S.; Gerasimov, E.S.; Logacheva, M.D.; Penin, A.A. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. 2016, 88, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, S.; Lu, M.; Zhang, Y.; Li, J.; Wang, W.; Wang, P.; Zhang, J.; Hu, Z.; Li, L.; et al. Biosynthesis of DHGA12 and its roles in Arabidopsis seedling establishment. Nat. Commun. 2019, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Gubler, F.; AMillar, A.; VJacobsen, J. Dormancy release, ABA and pre-harvest sprouting Frank Gubler1,2, Anthony A Millar2 and John V Jacobsen1. Curr. Opin. Plant Biol. 2005, 8, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Zheng, Y. Integration of ABA, GA, and light signaling in seed germination through the regulation of ABI5. Front. Plant Sci. 2022, 13, 1000803. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The Role and Regulation of ABI5 (ABA-Insensitive 5) in Plant Development, Abiotic Stress Responses and Phytohormone Crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Söderman, E.M.E.M.; Brocard, I.M.I.M.; Lynch, T.J.T.J.; Finkelstein, R.R.R.R. Regulation and Function of the Arabidopsis ABA-insensitive4 Gene in Seed and Abscisic Acid Response Signaling Networks1. Plant Physiol. 2000, 124, 1752–1765. [Google Scholar] [CrossRef]
- Hossain, A.; Teixeira Da Silva, J.A.; Lozovskaya, M.V.; Zvolinsky, V.P. High temperature combined with drought affect rainfed spring wheat and barley in South-Eastern Russia: I. Phenology and growth. Saudi J. Biol. Sci. 2012, 19, 473–487. [Google Scholar] [CrossRef]
- Boter, M.; Calleja-Cabrera, J.; Carrera-Castaño, G.; Wagner, G.; Hatzig, S.V.; Snowdon, R.J.; Legoahec, L.; Bianchetti, G.; Bouchereau, A.; Nesi, N.; et al. An Integrative Approach to Analyze Seed Germination in Brassica napus. Front. Plant Sci. 2019, 10, 1342. [Google Scholar] [CrossRef]
- Xu, Z.; Kim, D.H.; Hwang, I. ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep. 2013, 32, 807–813. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Shu, K.; Chen, Q.; Wu, Y.; Liu, R.; Zhang, H.; Wang, P.; Li, Y.; Wang, S.; Tang, S.; Liu, C.; et al. ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. Plant J. 2016, 85, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, X.; Song, L.; An, C. ABI4 Activates DGAT1 Expression in Arabidopsis Seedlings during Nitrogen Deficiency. Plant Physiol. 2011, 156, 873–883. [Google Scholar] [CrossRef]
- Nambara, E.; Suzuki, M.; Abrams, S.; McCarty, D.R.; Kamiya, Y.; McCourt, P. A Screen for Genes That Function in Abscisic Acid Signaling in Arabidopsis thaliana. Genetics 2002, 161, 1247–1255. [Google Scholar] [CrossRef]
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Lim, J.; Mittler, R.; Chory, J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007, 316, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, J.; Zheng, C.; Yang, Y.; Wang, L.; Zhang, R.; Ren, X.; Wei, S.; Aziz, U.; Du, J.; et al. Abscisic acid inhibits primary root growth by impairing ABI4-mediated cell cycle and auxin biosynthesis. Plant Physiol. 2022, 191, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yu, D. Arabidopsis WRKY2 transcription factor mediates seed germination and postgermination arrest of development by abscisic acid. BMC Plant Biol. 2009, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, Z.; Li, C.; Jiang, J.; Zhou, Y.; Wang, R.; Wang, Q.; Ni, C.; Liang, Q.; Chen, H.; et al. RSM1, an Arabidopsis MYB protein, interacts with HY5/HYH to modulate seed germination and seedling development in response to abscisic acid and salinity. PLoS Genet. 2018, 14, e1007839. [Google Scholar] [CrossRef]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012, 10, 2–11. [Google Scholar] [CrossRef]
- Lorenzo-Orts, L.; Witthoeft, J.; Deforges, J.; Martinez, J.; Loubéry, S.; Placzek, A.; Poirier, Y.; Hothorn, L.A.; Jaillais, Y.; Hothorn, M. Concerted expression of a cell cycle regulator and a metabolic enzyme from a bicistronic transcript in plants. Nat. Plants 2019, 5, 184–193. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Wang, M.L.; Lynch, T.J.; Rao, S.; Goodman, H.M. The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell. 1998, 10, 1043–1054. [Google Scholar] [CrossRef]
- Giraudat, J.; Hauge, B.M.; Valon, C.; Smalle, J.; Parcy, F.; Goodman, H.M. lsolation of the Arabidopsis AB13 Gene by Positional Cloning. Plant Cell 1992, 19, 1251–1261. [Google Scholar]
- Hong, L.; Yan, D.; Liu, W.; Chen, H.; Lu, Y. TIME FOR COFFEE controls root meristem size by changes in auxin accumulation in Arabidopsis. J. Exp. Bot. 2014, 65, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, M.; Ye, Q.; Wu, X.; Wu, W.; Chen, Y. Abscisic Acid Modulates Seed Germination via ABA INSENSITIVE5-Mediated PHOSPHATE1. Plant Physiol. 2017, 175, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Xu, H.; Zhang, Q.; Zhang, L.; Lu, Y. The COP1 Target SHI-RELATED SEQUENCE5 Directly Activates Photomorphogenesis-Promoting Genes. Plant Cell 2018, 30, 2368–2382. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.-R.; Li, T.-T.; Wang, S.-J.; Lu, Y.-T.; Yuan, T.-T. Triphosphate Tunnel Metalloenzyme 2 Acts as a Downstream Factor of ABI4 in ABA-Mediated Seed Germination. Int. J. Mol. Sci. 2023, 24, 8994. https://doi.org/10.3390/ijms24108994
Feng Y-R, Li T-T, Wang S-J, Lu Y-T, Yuan T-T. Triphosphate Tunnel Metalloenzyme 2 Acts as a Downstream Factor of ABI4 in ABA-Mediated Seed Germination. International Journal of Molecular Sciences. 2023; 24(10):8994. https://doi.org/10.3390/ijms24108994
Chicago/Turabian StyleFeng, Yu-Rui, Ting-Ting Li, Shi-Jia Wang, Ying-Tang Lu, and Ting-Ting Yuan. 2023. "Triphosphate Tunnel Metalloenzyme 2 Acts as a Downstream Factor of ABI4 in ABA-Mediated Seed Germination" International Journal of Molecular Sciences 24, no. 10: 8994. https://doi.org/10.3390/ijms24108994
APA StyleFeng, Y.-R., Li, T.-T., Wang, S.-J., Lu, Y.-T., & Yuan, T.-T. (2023). Triphosphate Tunnel Metalloenzyme 2 Acts as a Downstream Factor of ABI4 in ABA-Mediated Seed Germination. International Journal of Molecular Sciences, 24(10), 8994. https://doi.org/10.3390/ijms24108994





