The Essential Role of Light-Induced Autophagy in the Inner Choroid/Outer Retinal Neurovascular Unit in Baseline Conditions and Degeneration
Abstract
1. Introduction
1.1. General Relevance of Light-Induced Autophagy in the Outer Retina/Inner Choroid Neurovascular Unit
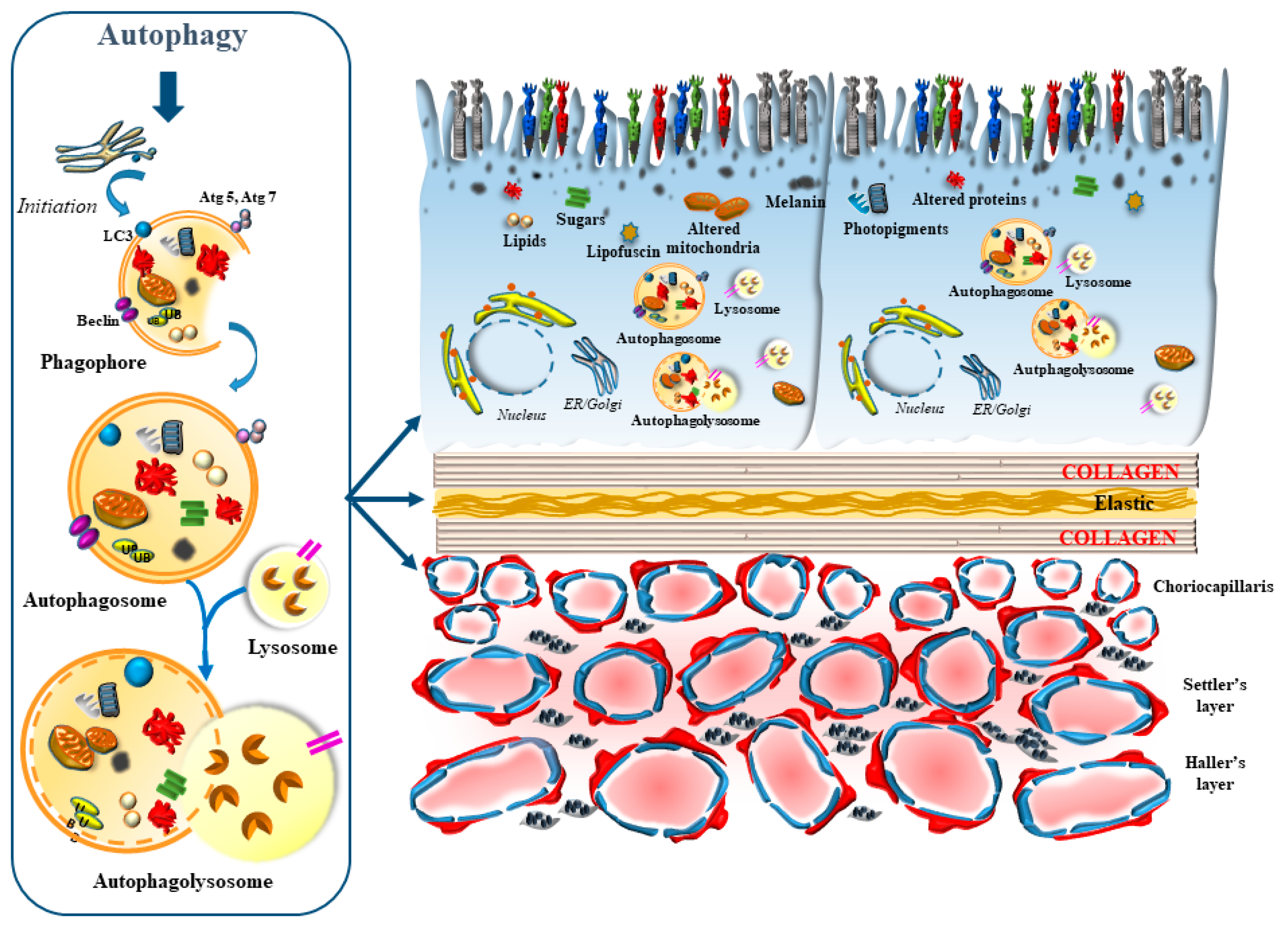
1.2. The Occurrence of Specific Autophagy-Related Structures within Baseline and Degenerating Retina
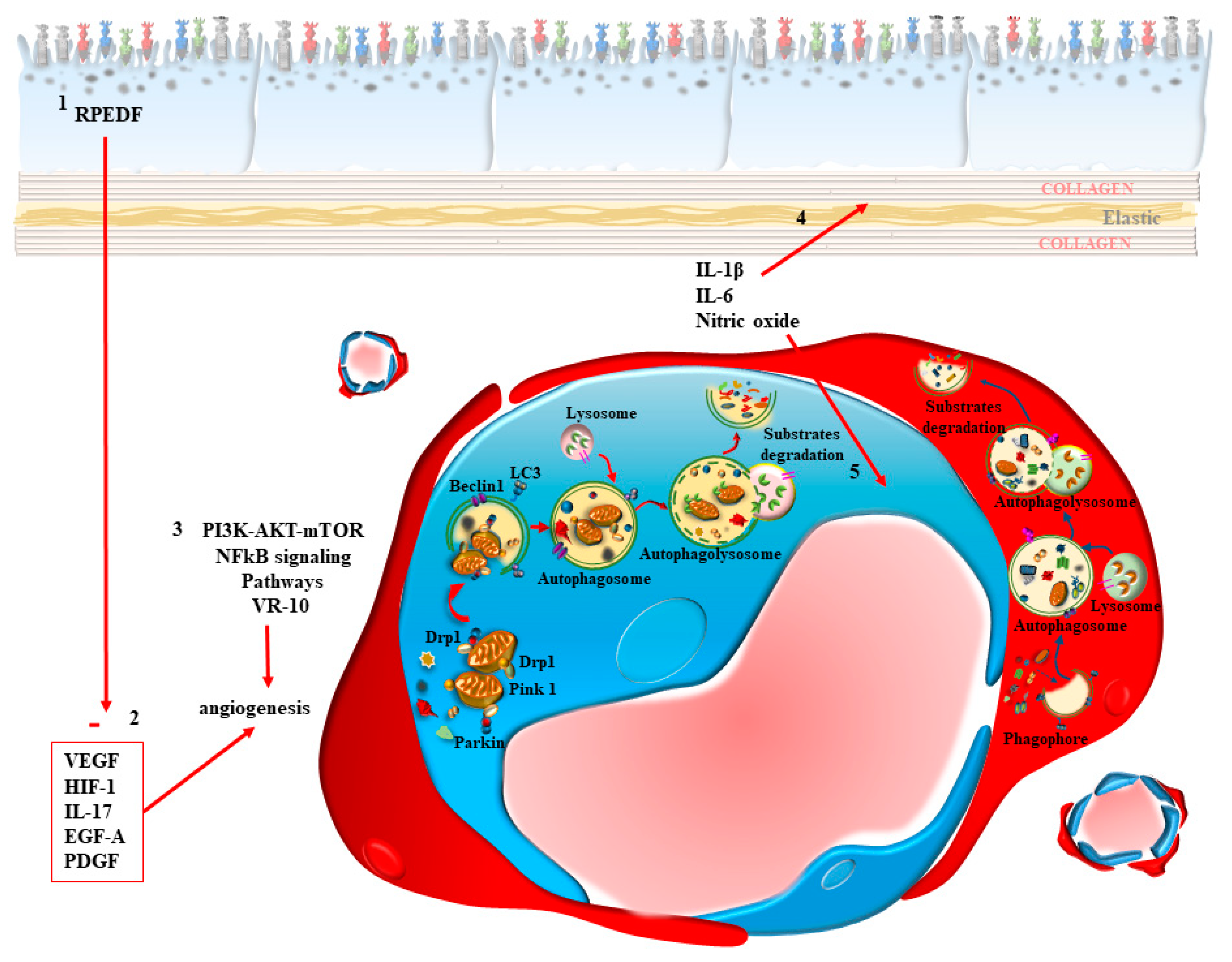
1.3. The Synergism of Autophagy in the Outer Retina/Inner Choroid
2. Interdependency of Various Targets of Autophagy within RPE and CC in the Retinal Neurovascular Unit
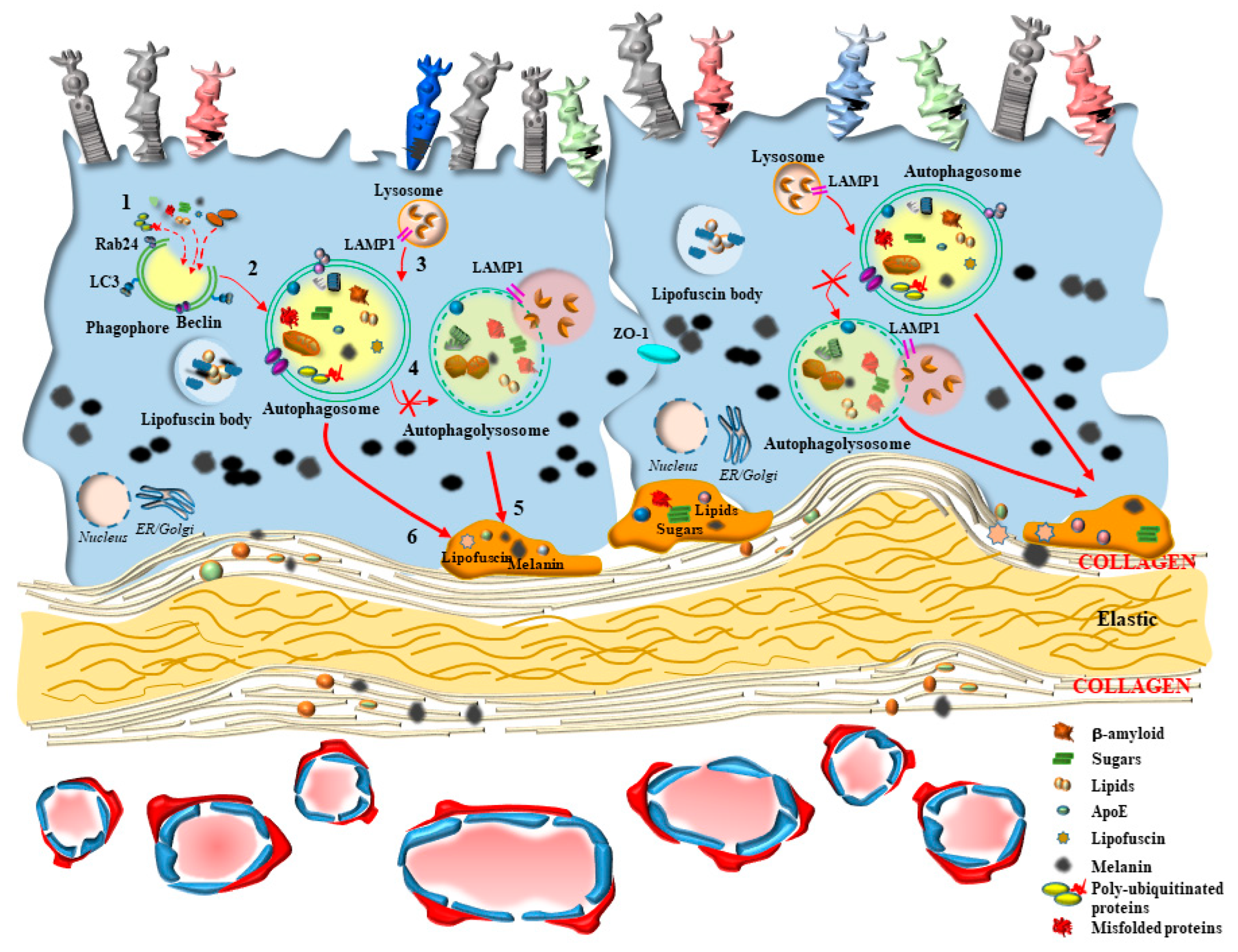
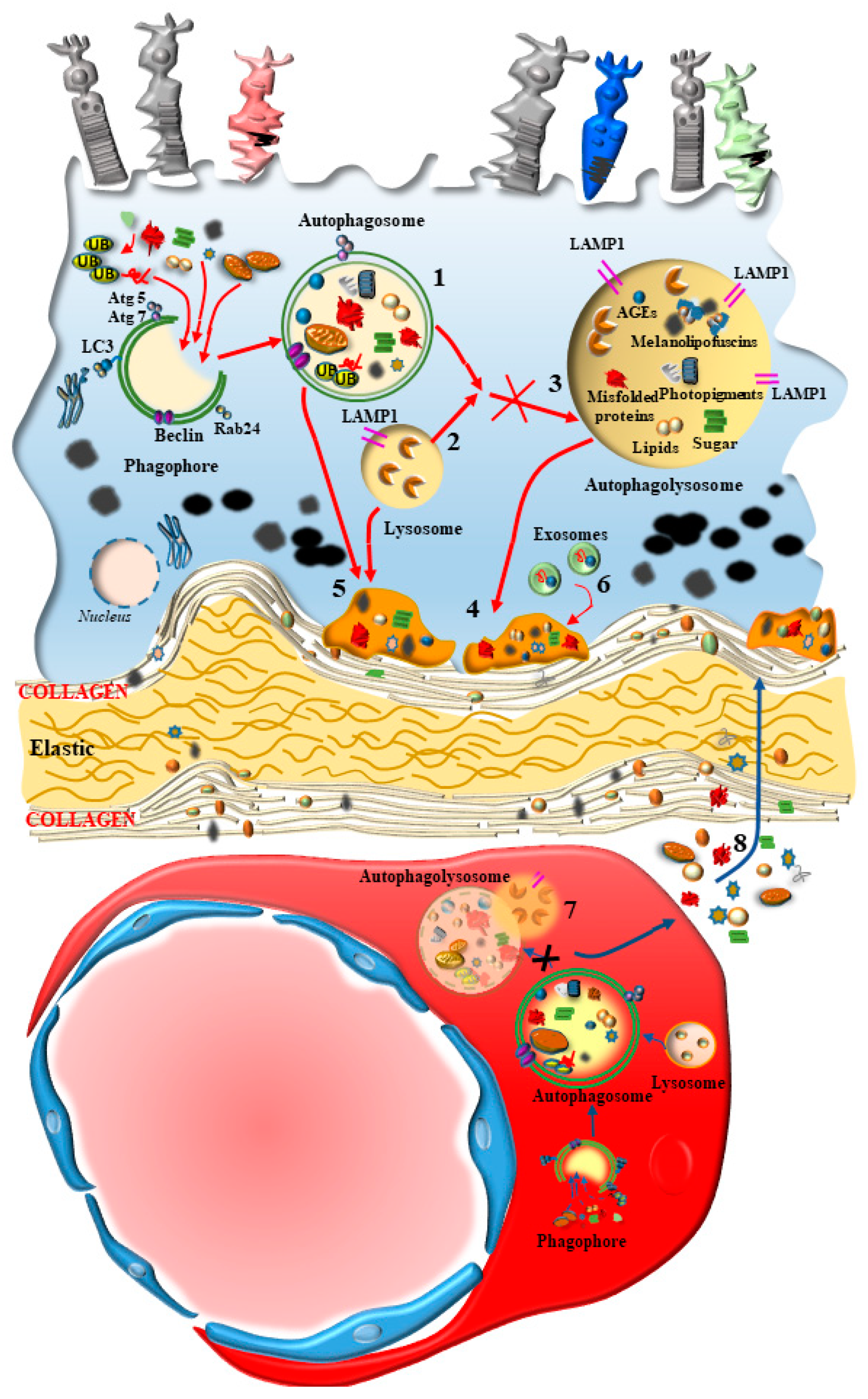
2.1. A Focus on RPE
2.2. A Focus on Endothelial Cells
2.3. A Focus on Pericytes
2.4. A Focus on BM
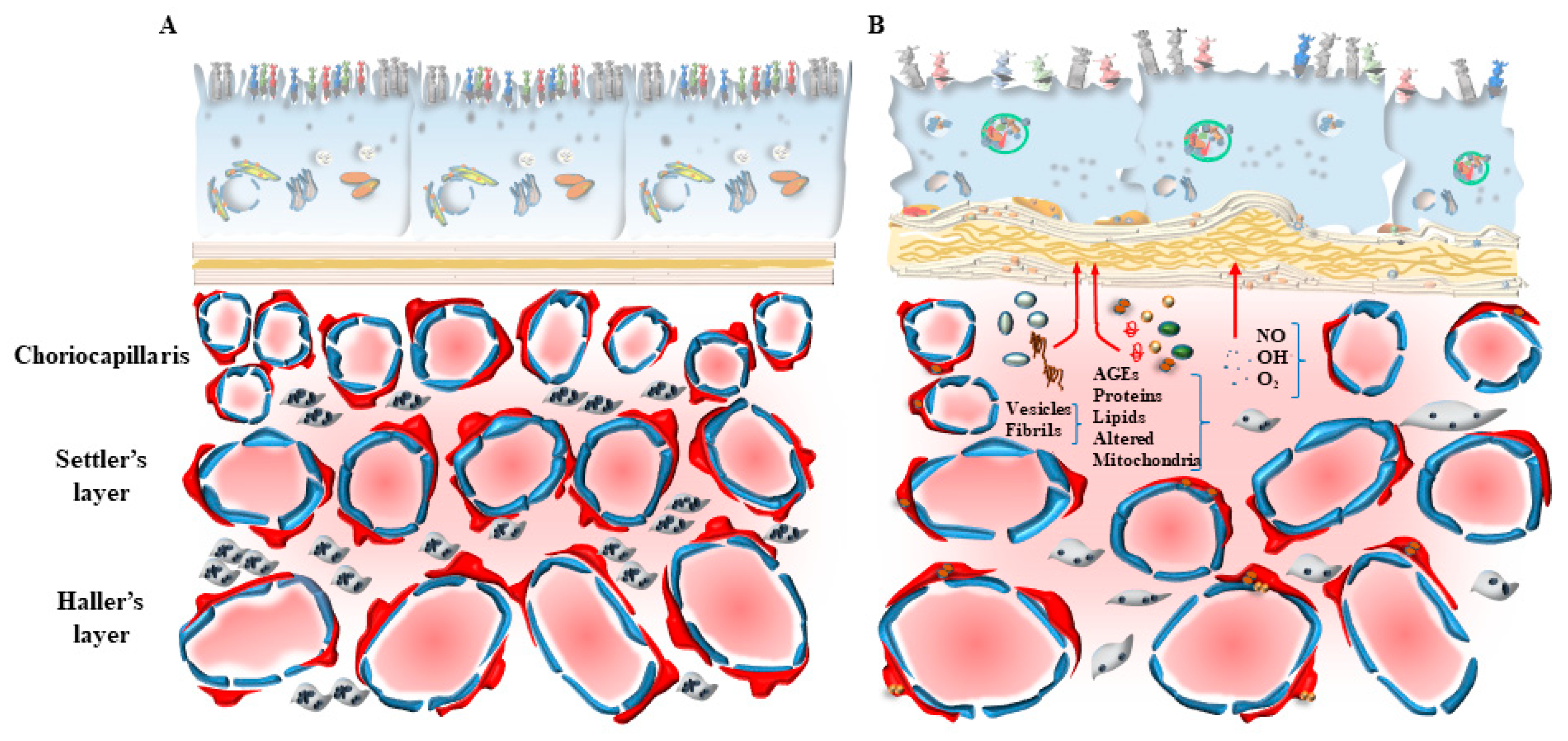
3. Dysfunctional Autophagy in the Inner Choroid/Outer Retina Is Mostly Relevant for AMD
4. Dysfunctional Autophagy within CC in AMD
5. The Loss of Autophagy within RPE Cells in AMD
6. An Autophagy Defect within RPE May Early Induce a Visual Defect Rather than Later Neuropathology
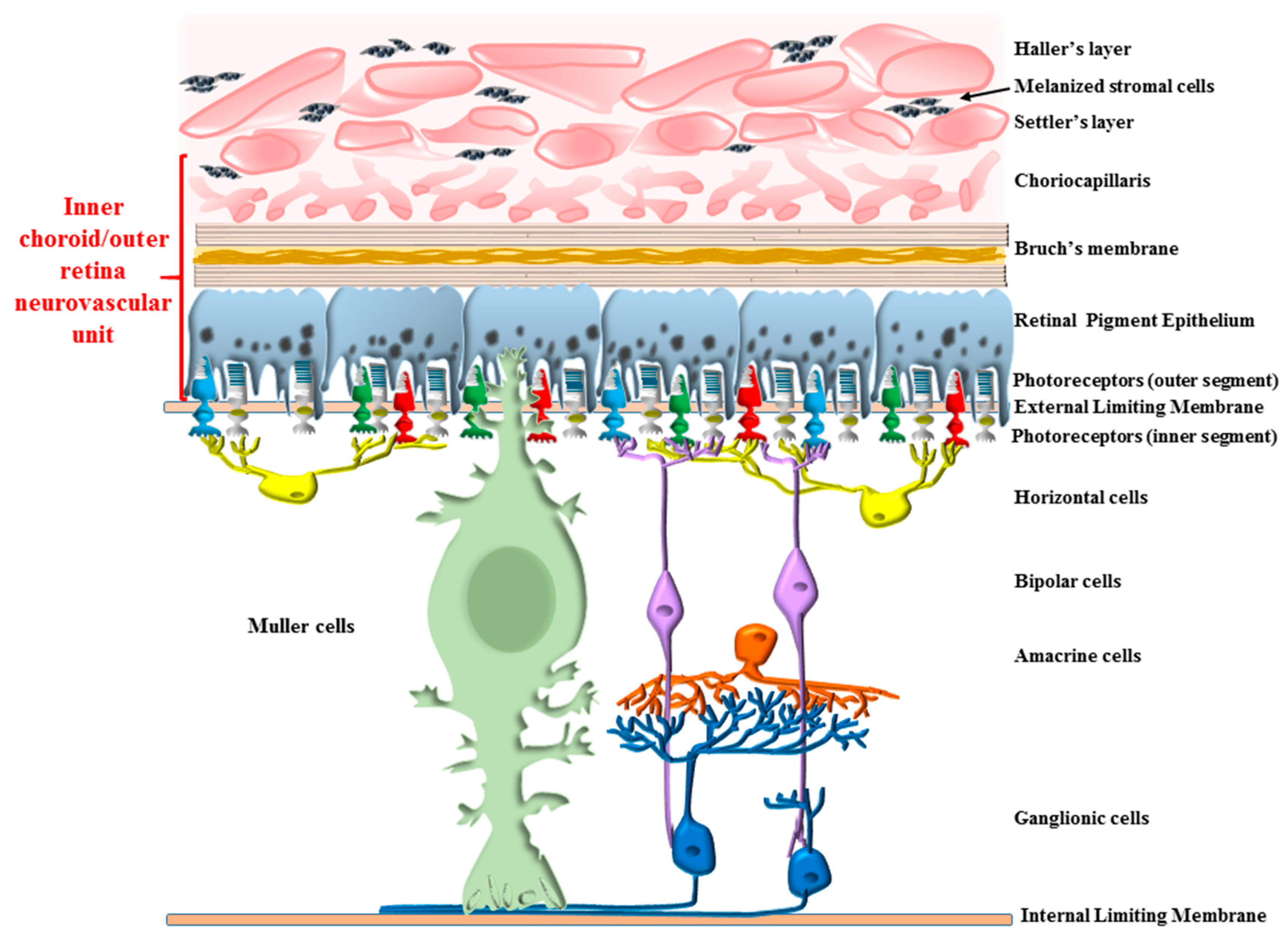
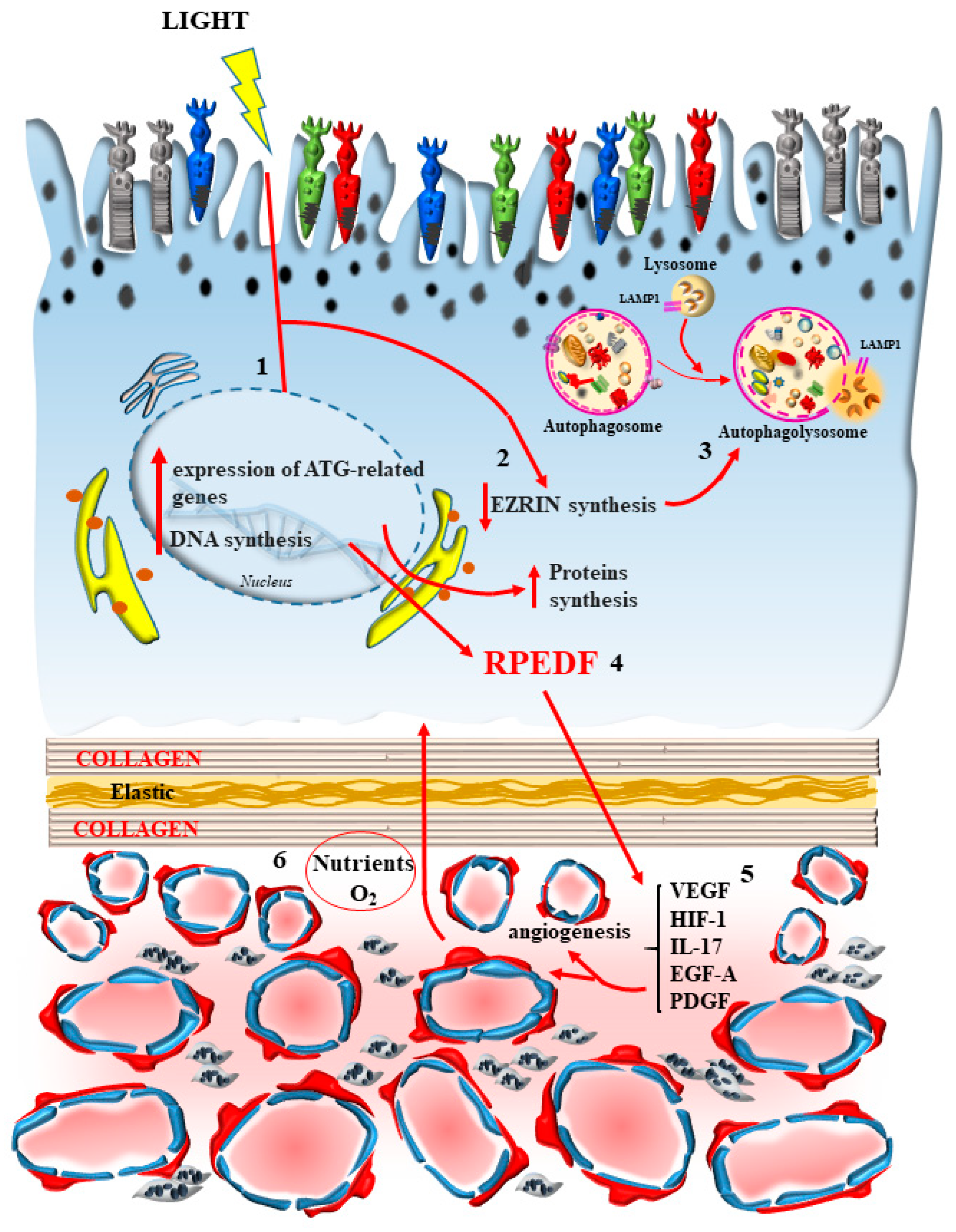
7. The Novel Concept of “Inner Choroid/Outer Retina Neurovascular Unit”
7.1. Connecting Photoreceptor Stimulation with Metabolic Activity and Blood Supply
7.2. The Stimulating Effects of Light on Autophagy
7.3. The Other Side of the Coin Provided by Light Stimulation
8. Downstream of Outer Retina
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramachandra Rao, S.; Fliesler, S.J. Monitoring basal autophagy in the retina utilizing CAG-mRFP-EGFP-MAP1LC3B reporter mouse: Technical and biological considerations. Autophagy 2022, 18, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cross, S.D.; Stanton, J.B.; Marmorstein, A.D.; Le, Y.Z.; Marmorstein, L.Y. Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7. Mol. Vis. 2017, 23, 228–241. [Google Scholar] [PubMed]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [Google Scholar] [CrossRef]
- Shinojima, N.; Yokoyama, T.; Kondo, Y.; Kondo, S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy 2007, 3, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, J.F.; Hsiao, C.Y.; Chang, H.H.; Li, H.J.; Chang, H.H.; Lee, G.A.; Hung, C.F. Lutein Induces Autophagy via Beclin-1 Upregulation in IEC-6 Rat Intestinal Epithelial Cells. Am. J. Chin. Med. 2017, 45, 1273–1291. [Google Scholar] [CrossRef]
- Comerota, M.M.; Tumurbaatar, B.; Krishnan, B.; Kayed, R.; Taglialatela, G. Near Infrared Light Treatment Reduces Synaptic Levels of Toxic Tau Oligomers in Two Transgenic Mouse Models of Human Tauopathies. Mol. Neurobiol. 2019, 56, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Ryskalin, L.; Polzella, M.; Frati, A.; Fornai, F. Phytochemicals Bridging Autophagy Induction and Alpha-Synuclein Degradation in Parkinsonism. Int. J. Mol. Sci. 2019, 20, 3274. [Google Scholar] [CrossRef]
- Munia, I.; Gafray, L.; Bringer, M.A.; Goldschmidt, P.; Proukhnitzky, L.; Jacquemot, N.; Cercy, C.; Ramchani Ben Otman, K.; Errera, M.H.; Ranchon-Cole, I. Cytoprotective Effects of Natural Highly Bio-Available Vegetable Derivatives on Human-Derived Retinal Cells. Nutrients 2020, 12, 879. [Google Scholar] [CrossRef]
- Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Lazzeri, G.; Frati, A.; Fornai, F. The Multi-Faceted Effect of Curcumin in Glioblastoma from Rescuing Cell Clearance to Autophagy-Independent Effects. Molecules 2020, 25, 4839. [Google Scholar] [CrossRef]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Bumah, V.V.; Biagioni, F.; Busceti, C.L.; Puglisi-Allegra, S.; Fornai, F. The neurobiology of nutraceuticals combined with light exposure, a case report in the course of retinal degeneration. Arch. Ital. Biol. 2021, 159, 134–150. [Google Scholar] [CrossRef]
- Ryskalin, L.; Puglisi-Allegra, S.; Lazzeri, G.; Biagioni, F.; Busceti, C.L.; Balestrini, L.; Fornasiero, A.; Leone, S.; Pompili, E.; Ferrucci, M.; et al. Neuroprotective Effects of Curcumin in Methamphetamine-Induced Toxicity. Molecules 2021, 26, 2493. [Google Scholar] [CrossRef] [PubMed]
- Stefenon, L.; Boasquevisque, M.; Garcez, A.S.; de Araújo, V.C.; Soares, A.B.; Santos-Silva, A.R.; Sperandio, F.; Brod, J.M.M.; Sperandio, M. Autophagy upregulation may explain inhibition of oral carcinoma in situ by photobiomodulation in vitro. J. Photochem. Photobiol. B Biol. 2021, 221, 112245. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Khoo, B.Y.; Ong, M.T.; Yoong, I.C.K.; Sreeramanan, S. In vitro anti-breast cancer studies of LED red light therapy through autophagy. Breast Cancer 2021, 28, 60–66. [Google Scholar] [CrossRef]
- Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1852. [Google Scholar] [CrossRef]
- Dlamini, M.B.; Bao, S.; Gao, Z.; Mei, J.; Ge, H.; Jiang, L.; Geng, C.; Li, Q.; Shi, X.; Liu, Y.; et al. Curcumin attenuates Cr (VI)-induced cell growth and migration by targeting autophagy-dependent reprogrammed metabolism. J. Biochem. Mol. Toxicol. 2022, 4, e23193. [Google Scholar] [CrossRef]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Zivkovic, A.M.; Yiu, G.; Hackman, R.M. Potential roles of dietary zeaxanthin and lutein in macular health and function. Nutr. Rev. 2022, 81, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Berti, C.; Scaffidi, E.; Lazzeri, G.; Bumah, V.V.; Ruffoli, R.; Biagioni, F.; Busceti, C.L.; Puglisi-Allegra, S.; Fornai, F. Combined pulses of light and sound in the retina with nutraceuticals may enhance the recovery of foveal holes. Arch. Ital. Biol. 2022, 160, 1–19. [Google Scholar] [CrossRef]
- Jin, Q.H.; Hu, X.J.; Zhao, H.Y. Curcumin activates autophagy and attenuates high glucose-induced apoptosis in HUVECs through the ROS/NF-κB signaling pathway. Exp. Ther. Med. 2022, 24, 596. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Błasiak, J.; Niittykoski, M.; Kinnunen, K.; Kauppinen, A.; Salminen, A.; Kaarniranta, K. DNA damage response and autophagy in the degeneration of retinal pigment epithelial cells-Implications for age-related macular degeneration (AMD). Ageing Res. Rev. 2017, 36, 64–77. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Querques, G.; Rosenfeld, P.J.; Cavallero, E.; Borrelli, E.; Corvi, F.; Querques, L.; Bandello, F.M.; Zarbin, M.A. Treatment of dry age-related macular degeneration. Ophthalmic Res. 2014, 52, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bales, K.L.; Gross, A.K. Aberrant protein trafficking in retinal degenerations: The initial phase of retinal remodeling. Exp. Eye Res. 2016, 150, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Arcella, A.; Biagioni, F.; Oliva, A.M.; Bucci, D.; Frati, A.; Esposito, V.; Cantore, G.; Giangaspero, F.; Fornai, F. Rapamycin inhibits the growth of glioblastoma. Brain Res. 2013, 1495, 37–51. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Kroemer, G. Autophagy for the avoidance of neurodegeneration. Genes Dev. 2009, 23, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Sethna, S.; Scott, P.A.; Giese, A.P.J.; Duncan, T.; Jian, X.; Riazuddin, S.; Randazzo, P.A.; Redmond, T.M.; Bernstein, S.L.; Riazuddin, S.; et al. CIB2 regulates mTORC1 signaling and is essential for autophagy and visual function. Nat. Commun. 2021, 12, 3906. [Google Scholar] [CrossRef]
- Yefimova, M.G. Myelinosome organelles in pathological retinas: Ubiquitous presence and dual role in ocular proteostasis maintenance. Neural Regen. Res. 2023, 18, 1009–1016. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Sinha, D.; Kaarniranta, K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog. Retin. Eye Res. 2016, 51, 69–89. [Google Scholar] [CrossRef]
- Vessey, K.A.; Jobling, A.I.; Tran, M.X.; Wang, A.Y.; Greferath, U.; Fletcher, E.L. Treatments targeting autophagy ameliorate the age-related macular degeneration phenotype in mice lacking APOE (apolipoprotein E). Autophagy 2022, 18, 2368–2384. [Google Scholar] [CrossRef]
- Nag, T.C.; Gorla, S.; Kumari, C.; Roy, T.S. Aging of the human choriocapillaris: Evidence that early pericyte damage can trigger endothelial changes. Exp. Eye Res. 2021, 212, 108771. [Google Scholar] [CrossRef]
- Lengyel, I.; Tufail, A.; Hosaini, H.A.; Luthert, P.; Bird, A.C.; Jeffery, G. Association of drusen deposition with choroidal intercapillary pillars in the aging human eye. Investig. Opthalmol. Vis. Sci. 2004, 45, 2886–2892. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.F.; Johnson, M.N.; Faidley, E.A.; Skeie, J.M.; Huang, J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Brunk, U.T. Lipofuscin. Int. J. Biochem. Cell Biol. 2004, 36, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, E.; Schutt, F.; Krohne, T.U.; Holz, F.G.; Kopitz, J. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Investig. Opthalmol. Vis. Sci. 2007, 48, 1342–1347. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Salminen, A.; Eskelinen, E.-L.; Kopitz, J. Heat-shock proteins as gatekeepers, of proteolytic pathways: Implications for age-related macular degeneration. Ageing Res. Rev. 2009, 8, 128–139. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhu, L.; Bao, X.; Xie, T.H.; Cai, J.; Zou, J.; Wang, W.; Gu, S.; Li, Y.; Li, H.Y.; et al. Inhibition of Drp1 ameliorates diabetic retinopathy by regulating mitochondrial homeostasis. Exp. Eye Res. 2022, 220, 109095. [Google Scholar] [CrossRef]
- Edwards, M.; Lutty, G.A. Bruch’s Membrane and the Choroid in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2021, 1256, 89–119. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, H.; Zhang, Y.F.; Zhou, Z.; Wu, S. MicroRNA-29 enhances autophagy and cleanses exogenous mutant αB-crystallin in retinal pigment epithelial cells. Exp. Cell Res. 2019, 374, 231–248. [Google Scholar] [CrossRef]
- Liu, J.; Copland, D.A.; Theodoropoulou, S.; Chiu, H.A.; Barba, M.D.; Mak, K.W.; Mack, M.; Nicholson, L.B.; Dick, A.D. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci. Rep. 2016, 6, 20639. [Google Scholar] [CrossRef]
- Pinelli, R.; Biagioni, F.; Limanaqi, F.; Bertelli, M.; Scaffidi, E.; Polzella, M.; Busceti, C.L.; Fornai, F. A Re-Appraisal of Pathogenic Mechanisms Bridging Wet and Dry Age-Related Macular Degeneration Leads to Reconsider a Role for Phytochemicals. Int. J. Mol. Sci. 2020, 21, 5563. [Google Scholar] [CrossRef]
- Du, J.H.; Li, X.; Li, R.; Cheng, B.X.; Kuerbanjiang, M.; Ma, L. Role of Autophagy in Angiogenesis Induced by a High-Glucose Condition in RF/6A Cells. Ophthalmologica 2017, 237, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Busceti, C.L.; Biagioni, F.; Fornai, F. Exosomes and alpha-synuclein within retina from autophagy to protein spreading in neurodegeneration. Arch. Ital. Biol. 2021, 159, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.T.; Niittykoski, M.; Salminen, A.; Kaarniranta, K. Maturation of autophagosomes and endosomes: A key role for Rab7. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 503–510. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Amadio, M.; Viiri, J.; Pascale, A.; Salminen, A.; Kaarniranta, K. Clearance of misfolded and aggregated proteins by aggrephagy and implication for aggregation diseases. Ageing Res. Rev. 2014, 18, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Tokarz, P.; Koskela, A.; Paterno, J.; Blasiak, J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol. Toxicol. 2017, 33, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef]
- Ishibashi, T.; Murata, T.; Hangai, M.; Nagai, R.; Horiuchi, S.; Lopez, P.F.; Hinton, D.R.; Ryan, S.J. Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol. 1998, 116, 1629–1632. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef]
- Fang, J.; Ouyang, M.; Qu, Y.; Wang, M.; Huang, X.; Lan, J.; Lai, W.; Xu, Q. Advanced Glycation End Products Promote Melanogenesis by Activating NLRP3 Inflammasome in Human Dermal Fibroblasts. J. Investig. Dermatol. 2022, 142, 2591–2602. [Google Scholar] [CrossRef]
- Spraul, C.W.; Lang, G.E.; Grossniklaus, H.E.; Lang, G.K. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv. Ophthalmol. 1999, 44, S10–S32. [Google Scholar] [CrossRef]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Yang, C.M.; Yang, C.H. Protective Effect of Astaxanthin on Blue Light Light-Emitting Diode-Induced Retinal Cell Damage via Free Radical Scavenging and Activation of PI3K/Akt/Nrf2 Pathway in 661W Cell Model. Mar. Drugs. 2020, 18, 387. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. Interplay between reactive oxygen species and autophagy in the course of age-related macular degeneration. EXCLI J. 2020, 19, 1353–1371. [Google Scholar] [CrossRef]
- Cheng, K.C.; Hsu, Y.T.; Liu, W.; Huang, H.L.; Chen, L.Y.; He, C.X.; Sheu, S.J.; Chen, K.J.; Lee, P.Y.; Lin, Y.H.; et al. The Role of Oxidative Stress and Autophagy in Blue-Light-Induced Damage to the Retinal Pigment Epithelium in Zebrafish In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 1338. [Google Scholar] [CrossRef]
- Intartaglia, D.; Giamundo, G.; Conte, I. Autophagy in the retinal pigment epithelium: A new vision and future challenges. FEBS J. 2022, 289, 7199–7212. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Biagioni, F.; Scaffidi, E.; Vakunseth Bumah, V.; Busceti, C.L.; Puglisi-Allegra, S.; Lazzeri, G.; Fornai, F. The potential effects of nutrients and light on autophagy-mediated visual function and clearance of retinal aggregates. Arch. Ital. Biol. 2022, 160, 115–135. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, X.; Zhang, D.; Wen, Y.; Zhang, L.; Xia, Y.; Chen, J.; Xie, C.; Zhu, H.; Tong, J.; et al. b Long-term blue light exposure impairs mitochondrial dynamics in the retina in light-induced retinal degeneration in vivo and in vitro. J. Photochem. Photobiol. B Biol. 2023, 240, 112654. [Google Scholar] [CrossRef]
- Zou, G.P.; Wang, T.; Xiao, J.X.; Wang, X.Y.; Jiang, L.P.; Tou, F.F.; Chen, Z.P.; Qu, X.H.; Han, X.J. Lactate protects against oxidative stress-induced retinal degeneration by activating autophagy. Free. Radic. Biol. Med. 2023, 194, 209–219. [Google Scholar] [CrossRef]
- Notomi, S.; Ishihara, K.; Efstathiou, N.E.; Lee, J.J.; Hisatomi, T.; Tachibana, T.; Konstantinou, E.K.; Ueta, T.; Murakami, Y.; Maidana, D.E.; et al. Genetic LAMP2 deficiency accelerates the age-associated formation of basal laminar deposits in the retina. Proc. Natl. Acad. Sci. USA 2019, 116, 23724–23734. [Google Scholar] [CrossRef]
- Lee, J.J.; Ishihara, K.; Notomi, S.; Efstathiou, N.E.; Ueta, T.; Maidana, D.; Chen, X.; Iesato, Y.; Caligiana, A.; Vavvas, D.G. Lysosome-associated membrane protein-2 deficiency increases the risk of reactive oxygen species-induced ferroptosis in retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 2020, 521, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Santo, M.; Conte, I. Emerging Lysosomal Functions for Photoreceptor Cell Homeostasis and Survival. Cells 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, B.; Defoe, D.M.; Besharse, J.C. Membrane turnover in rod photoreceptors: Ensheathment and phagocytosis of outer segment distal tips by pseudopodia of the retinal pigment epithelium. Proc. R. Soc. London. Ser. B Boil. Sci. 1987, 230, 339–354. [Google Scholar] [CrossRef]
- Kim, J.Y.; Zhao, H.; Martinez, J.; Doggett, T.A.; Kolesnikov, A.V.; Tang, P.H.; Ablonczy, Z.; Chan, C.C.; Zhou, Z.; Green, D.R.; et al. Noncanonical autophagy promotes the visual cycle. Cell 2013, 154, 365–376. [Google Scholar] [CrossRef]
- Kwon, W.; Freeman, S.A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef]
- Ferguson, T.A.; Green, D.R. Autophagy and phagocytosis converge for better vision. Autophagy 2014, 10, 165–167. [Google Scholar] [CrossRef]
- Frost, L.S.; Lopes, V.S.; Bragin, A.; Reyes-Reveles, J.; Brancato, J.; Cohen, A.; Mitchell, C.H.; Williams, D.S.; Boesze-Battaglia, K. The Contribution of Melanoregulin to Microtubule-Associated Protein 1 Light Chain 3 (LC3) Associated Phagocytosis in Retinal Pigment Epithelium. Mol. Neurobiol. 2015, 52, 1135–1151. [Google Scholar] [CrossRef]
- Muniz-Feliciano, L.; Doggett, T.A.; Zhou, Z.; Ferguson, T.A. RUBCN/rubicon and EGFR regulate lysosomal degradative processes in the retinal pigment epithelium (RPE) of the eye. Autophagy 2017, 13, 2072–2085. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1898–2005. [Google Scholar] [CrossRef]
- Boyer, N.P.; Tang, P.H.; Higbee, D.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Lipofuscin and A2E accumulate with age in the retinal pigment epithelium of Nrl-/- mice. Photochem. Photobiol. 2012, 88, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Hyttinen, J.; Ryhänen, T.; Viiri, J.; Paimela, T.; Toropainen, E.; Sorri, I.; Salminen, A. Mechanisms of protein aggregation in the retinal pigment epithelial cells. Front. Biosci. 2010, 2, 1374–1384. [Google Scholar] [CrossRef]
- Blazer, A.; Qian, Y.; Schlegel, M.P.; Algasas, H.; Buyon, J.P.; Cadwell, K.; Cammer, M.; Heffron, S.P.; Liang, F.X.; Mehta-Lee, S.; et al. APOL1 variant-expressing endothelial cells exhibit autophagic dysfunction and mitochondrial stress. Front. Genet. 2022, 13, 769936. [Google Scholar] [CrossRef]
- Lenzi, P.; Marongiu, R.; Falleni, A.; Gelmetti, V.; Busceti, C.L.; Michiorri, S.; Valente, E.M.; Fornai, F. A subcellular analysis of genetic modulation of PINK1 on mitochondrial alterations, autophagy and cell death. Arch. Ital. Biol. 2012, 150, 194–217. [Google Scholar] [CrossRef] [PubMed]
- Liegl, R.; Koenig, S.; Siedlecki, J.; Haritoglou, C.; Kampik, A.; Kernt, M. Temsirolimus inhibits proliferation and migration in retinal pigment epithelial and endothelial cells via mTOR inhibition and decreases VEGF and PDGF expression. PLoS ONE 2014, 9, e88203. [Google Scholar] [CrossRef]
- Sghaier, R.; Perus, M.; Cornebise, C.; Courtaut, F.; Scagliarini, A.; Olmiere, C.; Aires, V.; Hermetet, F.; Delmas, D. Resvega, a Nutraceutical Preparation, Affects NFκB Pathway and Prolongs the Anti-VEGF Effect of Bevacizumab in Undifferentiated ARPE-19 Retina Cells. Int. J. Mol. Sci. 2022, 23, 11704. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, C.; Xu, Y.; Tan, H.Y.; Chen, H.; Feng, Y. Berberine improves insulin-induced diabetic retinopathy through exclusively suppressing Akt/mTOR-mediated HIF-1α/VEGF activation in retina endothelial cells. Int. J. Biol. Sci. 2021, 17, 4316–4326. [Google Scholar] [CrossRef]
- Asani, B.; Siedlecki, J.; Wertheimer, C.; Liegl, R.; Wolf, A.; Ohlmann, A.; Priglinger, S.; Priglinger, C. Anti-angiogenic properties of rapamycin on human retinal pericytes in an in vitro model of neovascular AMD via inhibition of the mTOR pathway. BMC Ophthalmol. 2022, 22, 138. [Google Scholar] [CrossRef]
- Xia, W.; Li, C.; Chen, Q.; Huang, J.; Zhao, Z.; Liu, P.; Xu, K.; Li, L.; Hu, F.; Zhang, S.; et al. Intravenous route to choroidal neovascularization by macrophage-disguised nanocarriers for mTOR modulation. Acta Pharm. Sin. B 2022, 12, 2506–2521. [Google Scholar] [CrossRef]
- Tian, R.; Deng, A.; Pang, X.; Chen, Y.; Gao, Y.; Liu, H.; Hu, Z. VR-10 polypeptide interacts with CD36 to induce cell apoptosis and autophagy in choroid-retinal endothelial cells: Identification of VR-10 as putative novel therapeutic agent for choroid neovascularization (CNV) treatment. Peptides 2022, 157, 170868. [Google Scholar] [CrossRef]
- Xie, L.; Ji, X.; Tu, Y.; Wang, K.; Zhu, L.; Zeng, X.; Wang, X.; Zhang, J.; Zhu, M. MLN4924 inhibits hedgehog signaling pathway and activates autophagy to alleviate mouse laser-induced choroidal neovascularization lesion. Biomed. Pharmacother. 2020, 130, 110654. [Google Scholar] [CrossRef]
- Curcio, C.A.; Millican, C.L. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch. Ophthalmol. 1999, 117, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Kim, H.R.; Jung, J.; Byeon, S.H.; Kim, S.S.; Kim, M. Bilateral Macular Choroidal Abnormalities with Drusenoid Deposits in Patients with Unilateral Peripheral Exudative Hemorrhagic Chorio-retinopathy. Retina 2023, 43, 120–129. [Google Scholar] [CrossRef]
- Ma, J.; Teng, Y.; Huang, Y.; Tao, X.; Fan, Y. Autophagy plays an essential role in ultraviolet radiation-driven skin photoaging. Front. Pharmacol. 2022, 13, 864331. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Z.; Wang, H.; Zhang, D.; Han, T.; Chen, H.; Chen, J.; Chen, Z.; Xie, Y.; Wang, L.; et al. Melatonin promotes peripheral nerve repair through Parkin-mediated mitophagy. Free. Radic. Biol. Med. 2022, 185, 52–66. [Google Scholar] [CrossRef]
- Ning, R.; Li, Y.; Du, Z.; Li, T.; Sun, Q.; Lin, L.; Xu, Q.; Duan, J.; Sun, Z. The mitochondria-targeted antioxidant MitoQ attenuated PM2.5-induced vascular fibrosis via regulating mitophagy. Redox Biol. 2021, 46, 102113. [Google Scholar] [CrossRef]
- Gross-Jendroska, M.; Lui, G.M.; Song, M.K.; Stern, R. Retinal pigment epithelium-stromal interactions modulate hyaluronic acid deposition. Investig. Opthalmol. Vis. Sci. 1992, 33, 3394–3399. [Google Scholar]
- Congdon, N.; O’Colmain, B.; Klaver, C.C.; Klein, R.; Muñoz, B.; Friedman, D.S.; Kempen, J.; Taylor, H.R.; Mitchell, P. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef]
- Pascolini, D.; Mariotti, S.P.; Pokharel, G.P.; Pararajasegaram, R.; Etya’ale, D.; Négrel, A.D.; Resniko, S. 2002 global update of available data on visual impairment: A compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004, 11, 67–115. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: Asystematic review and meta-analysis. Lancet Glob. Health 2014, 2, CE106–CE116. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The natural history and prognosis of neovascular age-related macular degeneration: A systematic review of the literature and meta-analysis. Ophthalmology 2008, 115, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Fulceri, F.; Busceti, C.L.; Biagioni, F.; Fornai, F. Measurement of drusen and their correlation with visual symptoms in patients affected by age-related macular degeneration. Arch. Ital. Biol. 2020, 158, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Flati, V.; Delle Monache, S.; Lozzi, L.; Passacantando, M.; Maccarone, R. Nanoceria Particles Are an Eligible Candidate to Prevent Age-Related Macular Degeneration by Inhibiting Retinal Pigment Epithelium Cell Death and Autophagy Alterations. Cells 2020, 9, 1617. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef]
- Jaadane, I.; Villalpando Rodriguez, G.E.; Boulenguez, P.; Chahory, S.; Carré, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Effects of white light-emitting diode (LED) exposure on retinal pigment epithelium in vivo. J. Cell Mol. Med. 2017, 12, 3453–3466. [Google Scholar] [CrossRef]
- Hall, H.; Ma, J.; Shekhar, S.; Leon-Salas, W.D.; Weake, V.M. Blue light induces a neuroprotective gene expression program in Drosophila photoreceptors. BMC Neurosci. 2018, 19, 43. [Google Scholar] [CrossRef]
- Tao, J.X.; Zhou, W.C.; Zhu, X.G. Mitochondria as Potential Targets and Initiators of the Blue Light Hazard to the Retina. Oxidative Med. Cell. Longev. 2019, 2019, 6435364. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Light-Emitting Diodes (LEDS): Implications for Safety. Health Phys. 2020, 118, 549–561. [Google Scholar] [CrossRef]
- Otsu, W.; Ishida, K.; Nakamura, S.; Shimazawa, M.; Tsusaki, H.; Hara, H. Blue light-emitting diode irradiation promotes transcription factor EB-mediated lysosome biogenesis and lysosomal cell death in murine photoreceptor-derived cells. Biochem. Biophys. Res. Commun. 2020, 526, 479–484. [Google Scholar] [CrossRef]
- Lin, Y.H.; Sheu, S.J.; Liu, W.; Hsu, Y.T.; He, C.X.; Wu, C.Y.; Chen, K.J.; Lee, P.Y.; Chiu, C.C.; Cheng, K.C. Retinal protective effect of curcumin metabolite hexahydrocurcumin against blue light-induced RPE damage. Phytomedicine 2023, 110, 154606. [Google Scholar] [CrossRef]
- Kang, Q.; Dai, H.; Jiang, S.; Yu, L. Advanced glycation end products in diabetic retinopathy and phytochemical therapy. Front. Nutr. 2022, 9, 1037186. [Google Scholar] [CrossRef] [PubMed]
- Takkar, B.; Sheemar, A.; Jayasudha, R.; Soni, D.; Narayanan, R.; Venkatesh, P.; Shivaji, S.; Das, T. Unconventional avenues to decelerate diabetic retinopathy. Surv. Ophthalmol. 2022, 67, 1574–1592. [Google Scholar] [CrossRef]
- Lin, J.B.; Halawa, O.A.; Husain, D.; Miller, J.W.; Vavvas, D.G. Dyslipidemia in age-related macular degeneration. Eye 2022, 36, 312–318. [Google Scholar] [CrossRef]
- Pinheiro, R.L.; Marques, J.P.; Murta, J.N. “Lipoid” Macular Edema in Familial Hypertriglyceridemia and Retinal Dystrophy. Ophthalmol. Retin. 2023, 7, 188. [Google Scholar] [CrossRef]
- Campos, M.M.; Abu-Asab, M.S. Loss of endothelial planar cell polarity and cellular mclearance mechanisms in age-related macular degeneration. Ultrastruct. Pathol. 2017, 41, 312–319. [Google Scholar] [CrossRef]
- Torisu, K.; Singh, K.K.; Torisu, T.; Lovren, F.; Liu, J.; Pan, Y.; Quan, A.; Ramadan, A.; Al-Omran, M.; Pankova, N.; et al. Intact endothelial autophagy is required to maintain vascular lipid homeostasis. Aging Cell 2016, 15, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.C. Autophagy and Stem Cells: Self-Eating for Self-Renewal. Front. Cell Dev. Biol. 2020, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chu, Y.; Mowery, J.; Konkel, B.; Galli, S.; Theos, A.C.; Golestaneh, N. Pgc-1α repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice. Dis. Model. Mech. 2018, 11, dmm032698. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, N.; Chu, Y.; Xiao, Y.Y.; Stoleru, G.L.; Theos, A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017, 8, e2537. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef]
- Wen, R.H.; Stanar, P.; Tam, B.; Moritz, O.L. Autophagy in Xenopus laevis rod photoreceptors is independently regulated by phototransduction and misfolded RHO P23H. Autophagy 2019, 15, 1970–1989. [Google Scholar] [CrossRef]
- Intartaglia, D.; Giamundo, G.; Naso, F.; Nusco, E.; Di Giulio, S.; Salierno, F.G.; Polishchuk, E.; Conte, I. Induction of Autophagy Promotes Clearance of RHOP23H Aggregates and Protects from Retinal Degeneration. Front. Aging Neurosci. 2022, 14, 878958. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Satyanarayana, G.; Liu, T.; Wang, L.; Wang, J.; Cheng, J.; Itoh, K.; Sharma, A.; Bhutto, I.; et al. Mitophagy initiates retrograde mitochondrial-nuclear signaling to guide retinal pigment cell heterogeneity. Autophagy 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Wang, N.; Wei, L.; Liu, D.; Zhang, Q.; Xia, X.; Ding, L.; Xiong, S. Identification and Validation of Autophagy-Related Genes in Diabetic Retinopathy. Front. Endocrinol. 2022, 13, 867600. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.C.; Lee, J.; John, J.M.; Gonzalez-Lima, F. Neuroprotective effects of near infrared light in an in vivo model of mitochondrial optic neuropathy. J. Neurosci. 2008, 28, 13511–13521. [Google Scholar] [CrossRef]
- Rojas, J.C.; Gonzalaz-Lima, F. Low level light therapy of the eye and brain. Eye Brain. 2011, 3, 49–67. [Google Scholar] [CrossRef]
- Tata, D.B.; Waynant, R.W. Laser therapy: A review of its mechanism of action and potential medical applications. Laser Photonics Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, H.J.; Ham, M.; Choi, D.H.; Lee, T.R.; Shin, D.W. Amber Light (590 nm) Induces the Breakdown of Lipid Droplets through Autophagy-Related Lysosomal Degradation in Differentiated Adipocytes. Sci. Rep. 2016, 6, 28476. [Google Scholar] [CrossRef]
- Ren, C.; Hu, W.; Wei, Q.; Cai, W.; Jin, H.; Yu, D.; Liu, C.; Shen, T.; Zhu, M.; Liang, X.; et al. MicroRNA-27a Promotes Oxidative-Induced RPE Cell Death through Targeting FOXO1. Biomed. Res. Int. 2021, 2021, 6666506. [Google Scholar] [CrossRef] [PubMed]
- Abdouh, M.; Lu, M.; Chen, Y.; Goyeneche, A.; Burnier, J.V.; Burnier, M.N., Jr. Filtering blue light mitigates the deleterious effects induced by the oxidative stress in human retinal pigment epithelial cells. Exp. Eye Res. 2022, 217, 108978. [Google Scholar] [CrossRef] [PubMed]
- Subirada, P.V.; Vaglienti, M.V.; Joray, M.B.; Paz, M.C.; Barcelona, P.F.; Sánchez, M.C. Rapamycin and Resveratrol Modulate the Gliotic and Pro-Angiogenic Response in Müller Glial Cells Under Hypoxia. Front. Cell Dev. Biol. 2022, 10, 855178. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, X.; Zhu, M.; Du, J.; Xu, J.; Qin, X.; Xu, X.; Song, E. Epigallocatechin-3-gallate stimulates autophagy and reduces apoptosis levels in retinal Müller cells under high-glucose conditions. Exp. Cell Res. 2019, 380, 149–158. [Google Scholar] [CrossRef]
| Patient 1 | Patient 2 | |
|---|---|---|
| BCVA * | 20/32 | 20/40 |
| Drusenoid area | 22.00 mm2 | 1.32 mm2 |
| Central thickness | 255 μm | 216 μm |
| Metamorphopsia | 0.4° | 0.5° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinelli, R.; Ferrucci, M.; Berti, C.; Biagioni, F.; Scaffidi, E.; Bumah, V.V.; Busceti, C.L.; Lenzi, P.; Lazzeri, G.; Fornai, F. The Essential Role of Light-Induced Autophagy in the Inner Choroid/Outer Retinal Neurovascular Unit in Baseline Conditions and Degeneration. Int. J. Mol. Sci. 2023, 24, 8979. https://doi.org/10.3390/ijms24108979
Pinelli R, Ferrucci M, Berti C, Biagioni F, Scaffidi E, Bumah VV, Busceti CL, Lenzi P, Lazzeri G, Fornai F. The Essential Role of Light-Induced Autophagy in the Inner Choroid/Outer Retinal Neurovascular Unit in Baseline Conditions and Degeneration. International Journal of Molecular Sciences. 2023; 24(10):8979. https://doi.org/10.3390/ijms24108979
Chicago/Turabian StylePinelli, Roberto, Michela Ferrucci, Caterina Berti, Francesca Biagioni, Elena Scaffidi, Violet Vakunseth Bumah, Carla L. Busceti, Paola Lenzi, Gloria Lazzeri, and Francesco Fornai. 2023. "The Essential Role of Light-Induced Autophagy in the Inner Choroid/Outer Retinal Neurovascular Unit in Baseline Conditions and Degeneration" International Journal of Molecular Sciences 24, no. 10: 8979. https://doi.org/10.3390/ijms24108979
APA StylePinelli, R., Ferrucci, M., Berti, C., Biagioni, F., Scaffidi, E., Bumah, V. V., Busceti, C. L., Lenzi, P., Lazzeri, G., & Fornai, F. (2023). The Essential Role of Light-Induced Autophagy in the Inner Choroid/Outer Retinal Neurovascular Unit in Baseline Conditions and Degeneration. International Journal of Molecular Sciences, 24(10), 8979. https://doi.org/10.3390/ijms24108979










