Mechanoregulation of Osteoclastogenesis-Inducing Potentials of Fibrosarcoma Cell Line by Substrate Stiffness
Abstract
1. Introduction
2. Results
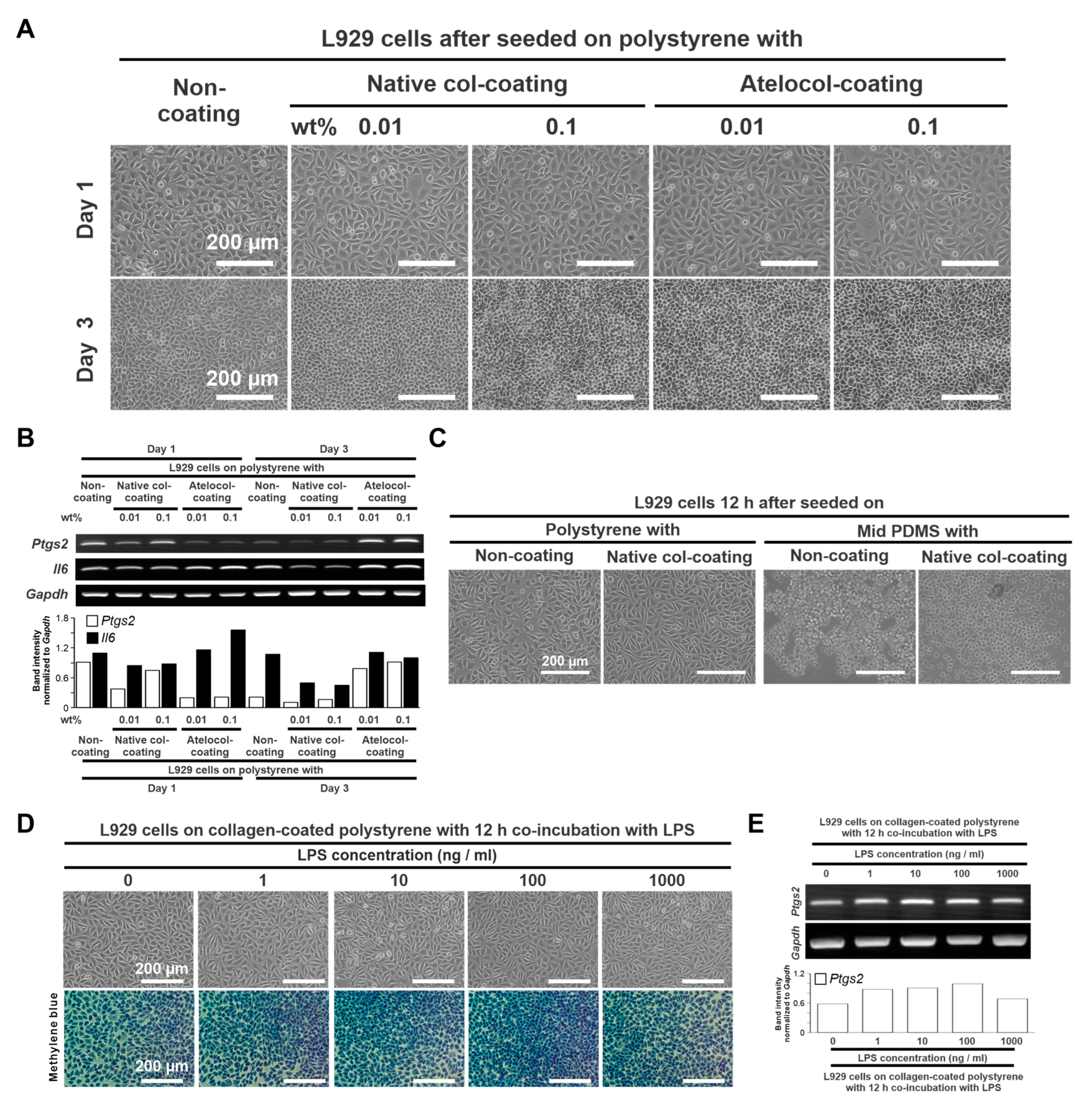
2.1. Determination of Culture Conditions
2.1.1. Collagen Coating Conditions on the PDMS Substrate
2.1.2. Concentration of an Inflammatory Ligand
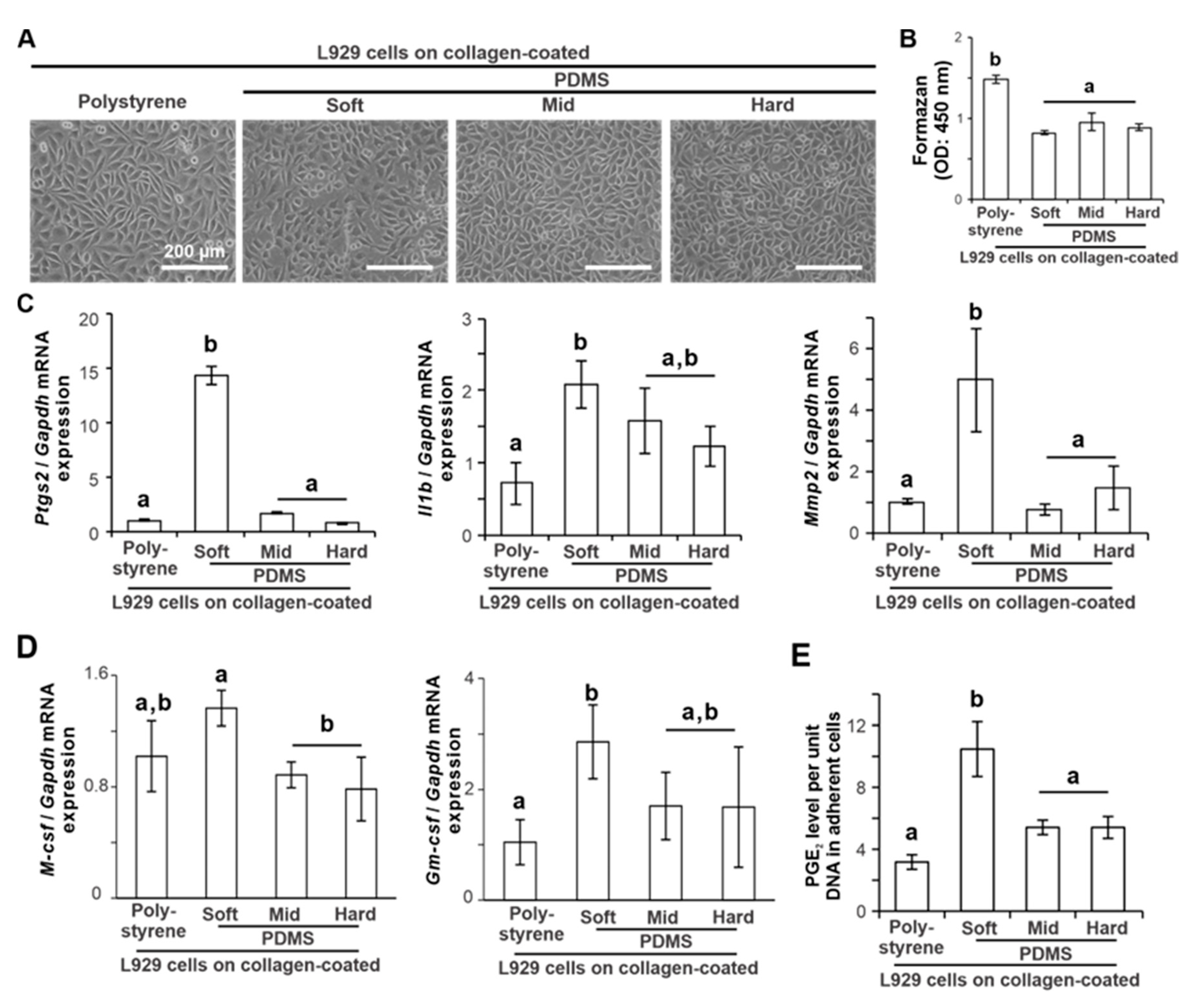
2.2. Effects of Substrate Stiffness on the Production of Proinflammatory Mediators of L929 Cells
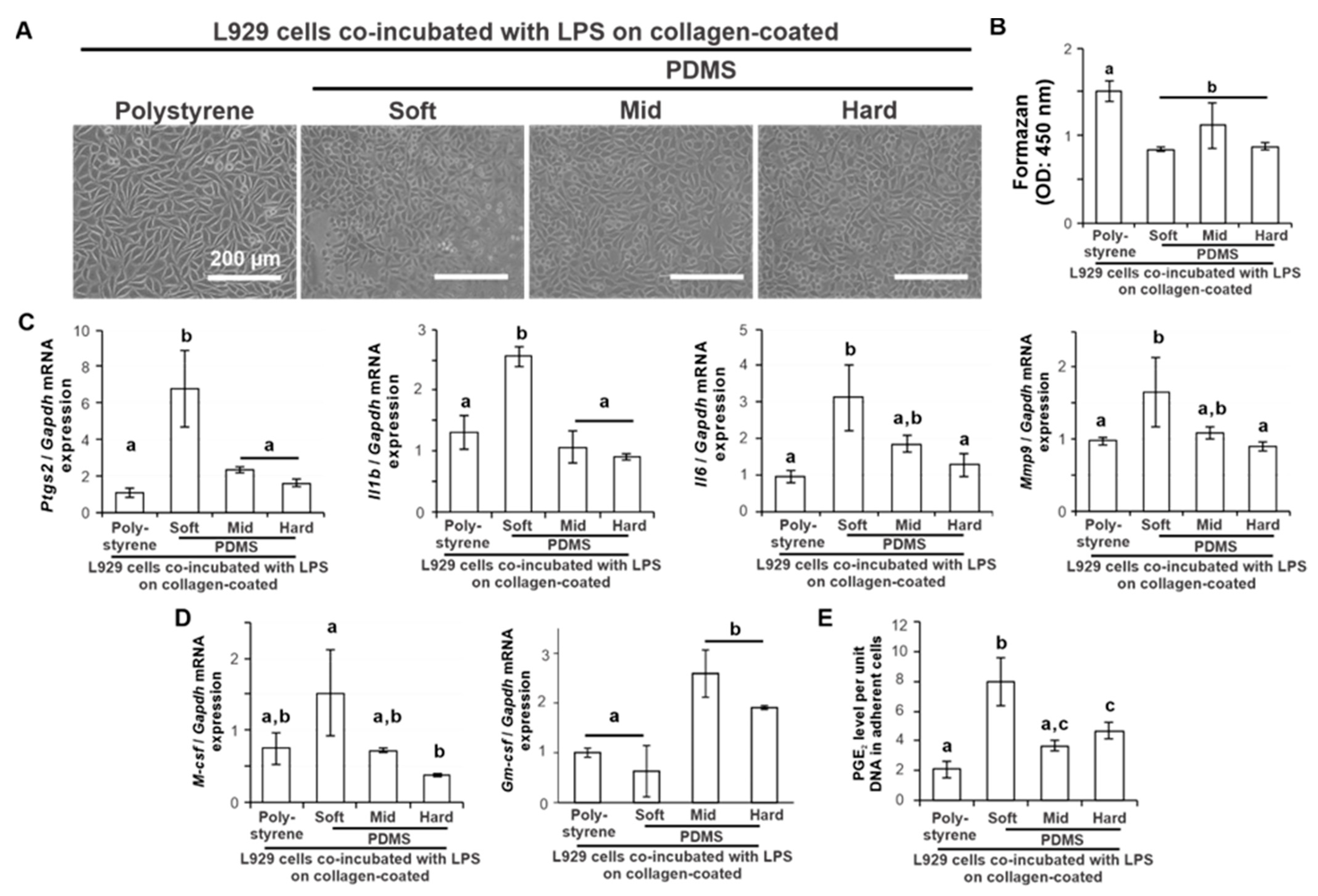
2.3. Effects of Substrate Stiffness on the Production of Proinflammatory Mediators of L929 Cells under Additional Ligand Stimulation
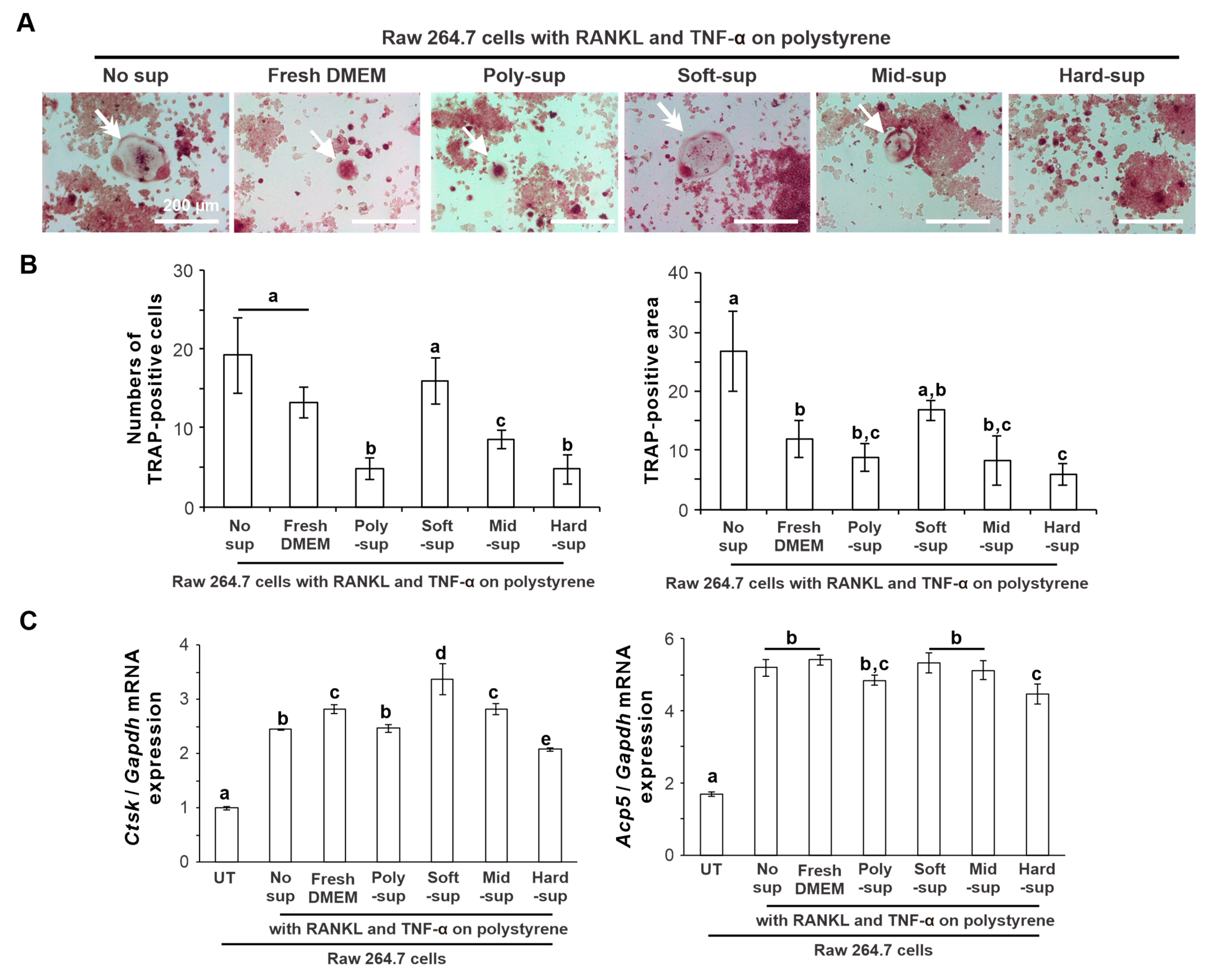
2.4. Effects of Substrate Stiffness on the Production of Osteoclastogenesis-Inducing Factor from L929 Cells
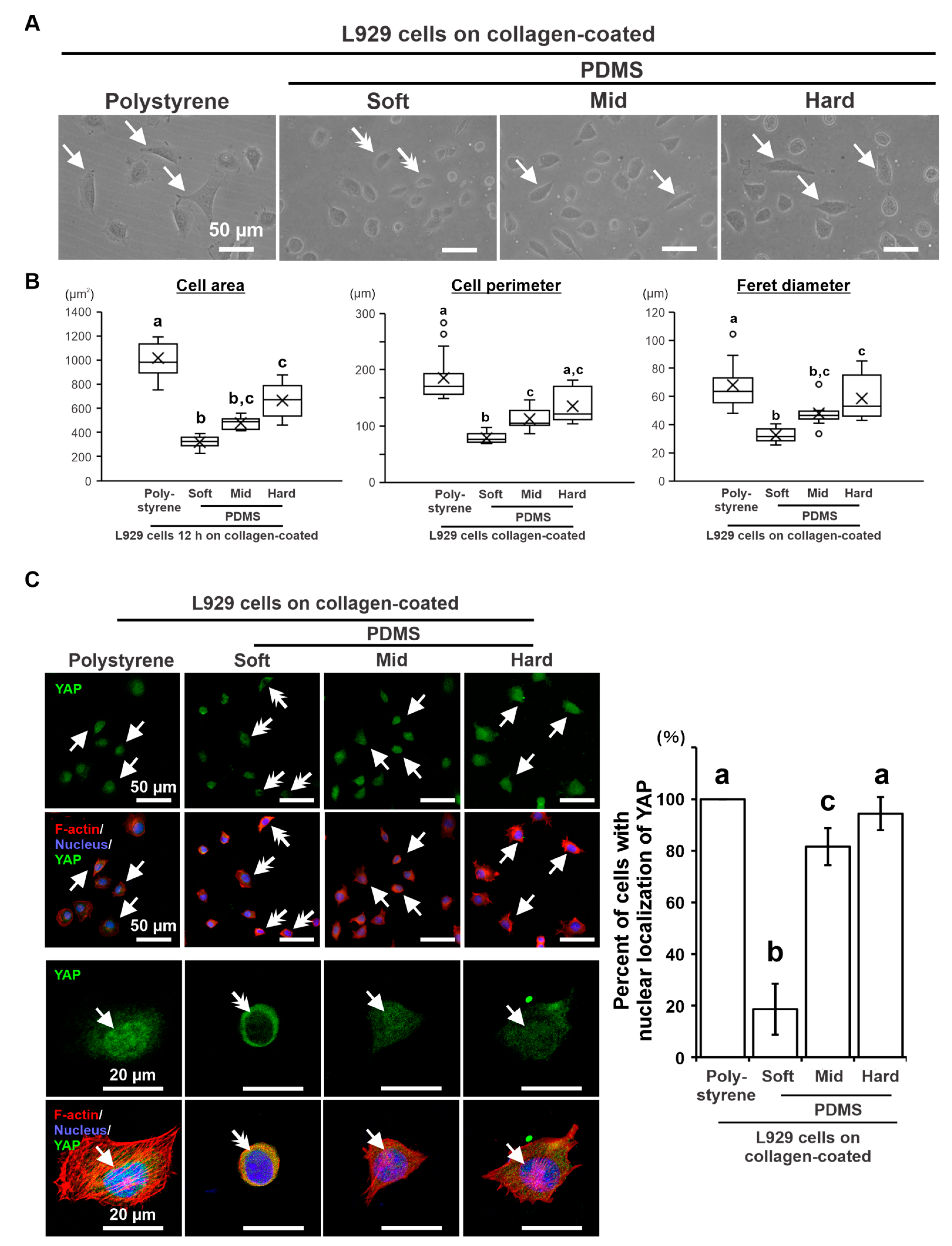
2.5. Effects of Substrate Stiffness on Cellular Mechanotransduction in L929 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of Stiffness-Controlled Substrates
4.2. L929 Fibroblast Cell Cultures
4.3. Culture Conditions for the Induction of a Proinflammatory Response
4.4. Mouse Osteoclast Precursor Cell Line Culture
4.5. Reverse Transcription–Polymerase Chain Reaction (RT–PCR)
4.6. Cell Viability and Attachment Assays
4.7. Cell Morphometry Analysis
4.8. Quantification of Prostaglandin E2
4.9. Immunofluorescence Staining and Analyses
4.10. TRAP Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Subedi, N.; Tel, J. Integrating Immunology and Microfluidics for Single Immune Cell Analysis. Front. Immunol. 2018, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, X.; Qiao, H.; Chen, P. Global Trends of Organoid and Organ-On-a-Chip in the Past Decade: A Bibliometric and Comparative Study. Tissue Eng. Part A 2020, 26, 656–671. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef]
- Gokaltun, A.; Kang, Y.B.A.; Yarmush, M.L.; Usta, O.B.; Asatekin, A. Simple Surface Modification of Poly(dimethylsiloxane) via Surface Segregating Smart Polymers for Biomicrofluidics. Sci. Rep. 2019, 9, 7377. [Google Scholar] [CrossRef]
- Thompson, C.L.; Fu, S.; Knight, M.M.; Thorpe, S.D. Mechanical Stimulation: A Crucial Element of Organ-on-Chip Models. Front. Bioeng. Biotechnol. 2020, 8, 602646. [Google Scholar] [CrossRef]
- Kondo, T.; Kanayama, K.; Egusa, H.; Nishimura, I. Current perspectives of residual ridge resorption: Pathological activation of oral barrier osteoclasts. J. Prosthodont. Res. 2023, 67, 12–22. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Goto, M.; Mochizuki, S.I.; Tsuda, E.; Morinaga, T.; Udagawa, N.; et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone 1999, 25, 109–113. [Google Scholar] [CrossRef]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef]
- Ladner, M.B.; Martin, G.A.; Noble, J.A.; Wittman, V.P.; Warren, M.K.; McGrogan, M.; Stanley, E.R. cDNA cloning and expression of murine macrophage colony-stimulating factor from L929 cells. Proc. Natl. Acad. Sci. USA 1988, 85, 6706–6710. [Google Scholar] [CrossRef]
- Jiang, L.; Qi, Y.; Kong, X.; Wang, R.; Qi, J.; Lin, F.; Cui, X.; Liu, Z. Activin A as a Novel Chemokine Induces Migration of L929 Fibroblasts by ERK Signaling in Microfluidic Devices. Front. Cell Dev. Biol. 2021, 9, 660316. [Google Scholar] [CrossRef] [PubMed]
- Yazdian Kashani, S.; Keshavarz Moraveji, M.; Bonakdar, S. Computational and experimental studies of a cell-imprinted-based integrated microfluidic device for biomedical applications. Sci. Rep. 2021, 11, 12130. [Google Scholar] [CrossRef] [PubMed]
- Heap, R.E.; Marin-Rubio, J.L.; Peltier, J.; Heunis, T.; Dannoura, A.; Moore, A.; Trost, M. Proteomics characterisation of the L929 cell supernatant and its role in BMDM differentiation. Life Sci. Alliance 2021, 4, e202000957. [Google Scholar] [CrossRef]
- De Brito Monteiro, L.; Davanzo, G.G.; de Aguiar, C.F.; Correa da Silva, F.; Andrade, J.R.; Campos Codo, A.; Silva Pereira, J.A.D.; Freitas, L.P.; Moraes-Vieira, P.M. M-CSF- and L929-derived macrophages present distinct metabolic profiles with similar inflammatory outcomes. Immunobiology 2020, 225, 151935. [Google Scholar] [CrossRef]
- Biela, S.A.; Su, Y.; Spatz, J.P.; Kemkemer, R. Different sensitivity of human endothelial cells, smooth muscle cells and fibroblasts to topography in the nano-micro range. Acta Biomater. 2009, 5, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.; Nagasaki, A.; Okawa, H.; Matsumoto, T.; Kondo, T.; Yatani, H.; Nishimura, I.; Egusa, H. Cyclic pressure-induced cytokines from gingival fibroblasts stimulate osteoclast activity: Clinical implications for alveolar bone loss in denture wearers. J. Prosthodont. Res. 2023, 67, 77–86. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, T.; Nakamura, N.; Watanabe, J.; Kartikasari, N.; He, X.; Tiskratok, W.; Yoshioka, H.; Shinno, H.; Egusa, H. Titanium Nanosurface with a Biomimetic Physical Microenvironment to Induce Endogenous Regeneration of the Periodontium. ACS Appl. Mater. Interfaces 2022, 14, 27703–27719. [Google Scholar] [CrossRef]
- Meli, V.S.; Atcha, H.; Veerasubramanian, P.K.; Nagalla, R.R.; Luu, T.U.; Chen, E.Y.; Guerrero-Juarez, C.F.; Yamaga, K.; Pandori, W.; Hsieh, J.Y.; et al. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 2020, 6, eabb8471. [Google Scholar] [CrossRef]
- Ligresti, G.; Caporarello, N.; Meridew, J.A.; Jones, D.L.; Tan, Q.; Choi, K.M.; Haak, A.J.; Aravamudhan, A.; Roden, A.C.; Prakash, Y.S.; et al. CBX5/G9a/H3K9me-mediated gene repression is essential to fibroblast activation during lung fibrosis. JCI Insight 2019, 4, e127111. [Google Scholar] [CrossRef]
- Haak, A.J.; Kostallari, E.; Sicard, D.; Ligresti, G.; Choi, K.M.; Caporarello, N.; Jones, D.L.; Tan, Q.; Meridew, J.; Diaz Espinosa, A.M.; et al. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci. Transl. Med. 2019, 11, eaau6296. [Google Scholar] [CrossRef]
- Schwager, S.C.; Bordeleau, F.; Zhang, J.; Antonyak, M.A.; Cerione, R.A.; Reinhart-King, C.A. Matrix stiffness regulates microvesicle-induced fibroblast activation. Am. J. Physiol. Cell Physiol. 2019, 317, C82–C92. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Tiskratok, W.; Yamada, M.; Watanabe, J.; Kartikasari, N.; Kimura, T.; Egusa, H. Substrate stiffness controls proinflammatory responses in human gingival fibroblasts. Sci. Rep. 2023, 13, 1358. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Chen, X.; Wang, Y.; Liu, M.N.; Yan, J.; Cao, L.; Wang, L.; Wang, Z.B. Atomic force acoustic microscopy reveals the influence of substrate stiffness and topography on cell behavior. Beilstein J. Nanotechnol. 2019, 10, 2329–2337. [Google Scholar] [CrossRef]
- Darnell, M.; Mooney, D.J. Leveraging advances in biology to design biomaterials. Nat. Mater. 2017, 16, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Al-Anati, L.; Essid, E.; Stenius, U.; Beuerlein, K.; Schuh, K.; Petzinger, E. Differential cell sensitivity between OTA and LPS upon releasing TNF-alpha. Toxins 2010, 2, 1279–1299. [Google Scholar] [CrossRef]
- Moraes, C.; Labuz, J.M.; Shao, Y.; Fu, J.; Takayama, S. Supersoft lithography: Candy-based fabrication of soft silicone microstructures. Lab Chip 2015, 15, 3760–3765. [Google Scholar] [CrossRef]
- Yan, X.Z.; van den Beucken, J.; Yuan, C.; Jansen, J.A.; Yang, F. Evaluation of polydimethylsiloxane-based substrates for in vitro culture of human periodontal ligament cells. J. Biomed. Mater. Res. A 2019, 107, 2796–2805. [Google Scholar] [CrossRef]
- Akther, F.; Yakob, S.B.; Nguyen, N.T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef]
- Chuah, Y.J.; Koh, Y.T.; Lim, K.; Menon, N.V.; Wu, Y.; Kang, Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Sci. Rep. 2015, 5, 18162. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.L.; Eriksen, H.; Risteli, L.; Niemi, S.; Mansell, J.; Gowen, M.; Risteli, J. Immunochemical characterization of assay for carboxyterminal telopeptide of human type I collagen: Loss of antigenicity by treatment with cathepsin K. Bone 2000, 26, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Chumbimuni-Torres, K.Y.; Coronado, R.E.; Mfuh, A.M.; Castro-Guerrero, C.; Silva, M.F.; Negrete, G.R.; Bizios, R.; Garcia, C.D. Adsorption of Proteins to Thin-Films of PDMS and Its Effect on the Adhesion of Human Endothelial Cells. RSC Adv. 2011, 1, 706–714. [Google Scholar] [CrossRef]
- Kadzinski, L.; Prokopowicz, M.; Jakobkiewicz-Banecka, J.; Gabig-Ciminska, M.; Lukasiak, J.; Banecki, B. Effect of silicone on the collagen fibrillogenesis and stability. J. Pharm. Sci. 2015, 104, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Nam, U.; Kim, S.; Park, J.; Jeon, J.S. Lipopolysaccharide-Induced Vascular Inflammation Model on Microfluidic Chip. Micromachines 2020, 11, 747. [Google Scholar] [CrossRef]
- Achterberg, V.F.; Buscemi, L.; Diekmann, H.; Smith-Clerc, J.; Schwengler, H.; Meister, J.J.; Wenck, H.; Gallinat, S.; Hinz, B. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Investig. Dermatol. 2014, 134, 1862–1872. [Google Scholar] [CrossRef]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2018, 9, 788. [Google Scholar] [CrossRef]
- Cao, Y.; Jansen, I.D.; Sprangers, S.; Stap, J.; Leenen, P.J.; Everts, V.; de Vries, T.J. IL-1beta differently stimulates proliferation and multinucleation of distinct mouse bone marrow osteoclast precursor subsets. J. Leukoc. Biol. 2016, 100, 513–523. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mizoguchi, T.; Take, I.; Kurihara, S.; Udagawa, N.; Takahashi, N. Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J. Biol. Chem. 2005, 280, 11395–11403. [Google Scholar] [CrossRef]
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131, jcs216267. [Google Scholar] [CrossRef]
- Rucavado, A.; Escalante, T.; Teixeira, C.F.; Fernandes, C.M.; Diaz, C.; Gutierrez, J.M. Increments in cytokines and matrix metalloproteinases in skeletal muscle after injection of tissue-damaging toxins from the venom of the snake Bothrops asper. Mediat. Inflamm. 2002, 11, 121–128. [Google Scholar] [CrossRef]
- Scheven, B.A.; Milne, J.S.; Robins, S.P. A novel culture system to generate osteoclasts and bone resorption using porcine bone marrow cells: Role of M-CSF. Biochem. Biophys. Res. Commun. 1997, 231, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, H.; Poh, P.S.; Machens, H.G.; Schilling, A.F. Hydrogels for Engineering of Perfusable Vascular Networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, T.; Geiger, B.; Bershadsky, A.D.; Kozlov, M.M. Focal adhesions as mechanosensors: A physical mechanism. Proc. Natl. Acad. Sci. USA 2005, 102, 12383–12388. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.; Grimm, R.; Kargar, S.; Howe, B.M.; Fritchie, K.; Frick, M.; Wenger, D.; Okuno, S.; Ehman, R.; McGee, K.; et al. Soft Tissue Sarcoma Stiffness and Perfusion Evaluation by MRE and DCE-MRI for Radiation Therapy Response Assessment: A Technical Feasibility Study. Biomed. Phys. Eng. Express 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Petzold, J.; Gentleman, E. Intrinsic Mechanical Cues and Their Impact on Stem Cells and Embryogenesis. Front. Cell Dev. Biol. 2021, 9, 761871. [Google Scholar] [CrossRef]
- Ruef, N.; Dolder, S.; Aeberli, D.; Seitz, M.; Balani, D.; Hofstetter, W. Granulocyte-macrophage colony-stimulating factor-dependent CD11c-positive cells differentiate into active osteoclasts. Bone 2017, 97, 267–277. [Google Scholar] [CrossRef]
- Zhao, B.; Ivashkiv, L.B. Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res. Ther. 2011, 13, 234. [Google Scholar] [CrossRef]
- Kartikasari, N.; Yamada, M.; Watanabe, J.; Tiskratok, W.; He, X.; Kamano, Y.; Egusa, H. Titanium surface with nanospikes tunes macrophage polarization to produce inhibitory factors for osteoclastogenesis through nanotopographic cues. Acta Biomater. 2022, 137, 316–330. [Google Scholar] [CrossRef]
- Rice, H.M.; Rossi, A.P.; Bradford, M.K.; Elkins, K.L.; De Pascalis, R. rM-CSF efficiently replaces L929 in generating mouse and rat bone marrow-derived macrophages for in vitro functional studies of immunity to intracellular bacteria. J. Immunol. Methods 2020, 477, 112693. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, N.; Yamada, M.; Watanabe, J.; Tiskratok, W.; He, X.; Egusa, H. Titania nanospikes activate macrophage phagocytosis by ligand-independent contact stimulation. Sci. Rep. 2022, 12, 12250. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, Z.H.; Deitch, E.A.; Davidson, M.T.; Szabo, C.; Vizi, E.S.; Hasko, G. Disruption of the actin cytoskeleton results in nuclear factor-kappaB activation and inflammatory mediator production in cultured human intestinal epithelial cells. J. Cell Physiol. 2004, 200, 71–81. [Google Scholar] [CrossRef]
- Kustermans, G.; El Benna, J.; Piette, J.; Legrand-Poels, S. Perturbation of actin dynamics induces NF-kappaB activation in myelomonocytic cells through an NADPH oxidase-dependent pathway. Biochem. J. 2005, 387 Pt 2, 531–540. [Google Scholar] [CrossRef]
- Connolly, J.M.; Rose, D.P. Quantitative differences in the effects of mouse and human epidermal growth factors on A431 human tumor cells. Cancer Lett. 1987, 37, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gersuk, G.; Hiraoka, A.; Marr, K.A. Human monocytes differentiate into macrophages under the influence of human KPB-M15 conditioned medium. J. Immunol. Methods 2005, 299, 99–106. [Google Scholar] [CrossRef]
- Nattasit, P.; Niibe, K.; Yamada, M.; Ohori-Morita, Y.; Limraksasin, P.; Tiskratok, W.; Yamamoto, M.; Egusa, H. Stiffness-Tunable Hydrogel-Sandwich Culture Modulates the YAP-Mediated Mechanoresponse in Induced-Pluripotent Stem Cell Embryoid Bodies and Augments Cardiomyocyte Differentiation. Macromol. Biosci. 2023, e2300021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiskratok, W.; Yamada, M.; Watanabe, J.; Pengyu, Q.; Kimura, T.; Egusa, H. Mechanoregulation of Osteoclastogenesis-Inducing Potentials of Fibrosarcoma Cell Line by Substrate Stiffness. Int. J. Mol. Sci. 2023, 24, 8959. https://doi.org/10.3390/ijms24108959
Tiskratok W, Yamada M, Watanabe J, Pengyu Q, Kimura T, Egusa H. Mechanoregulation of Osteoclastogenesis-Inducing Potentials of Fibrosarcoma Cell Line by Substrate Stiffness. International Journal of Molecular Sciences. 2023; 24(10):8959. https://doi.org/10.3390/ijms24108959
Chicago/Turabian StyleTiskratok, Watcharaphol, Masahiro Yamada, Jun Watanabe, Qu Pengyu, Tsuyoshi Kimura, and Hiroshi Egusa. 2023. "Mechanoregulation of Osteoclastogenesis-Inducing Potentials of Fibrosarcoma Cell Line by Substrate Stiffness" International Journal of Molecular Sciences 24, no. 10: 8959. https://doi.org/10.3390/ijms24108959
APA StyleTiskratok, W., Yamada, M., Watanabe, J., Pengyu, Q., Kimura, T., & Egusa, H. (2023). Mechanoregulation of Osteoclastogenesis-Inducing Potentials of Fibrosarcoma Cell Line by Substrate Stiffness. International Journal of Molecular Sciences, 24(10), 8959. https://doi.org/10.3390/ijms24108959





