Mitochondria in Human Fertility and Infertility
Abstract
1. Introduction
2. Roles of Mitochondria in Cell Metabolism
3. Roles of Mitochondria in Germ Cells
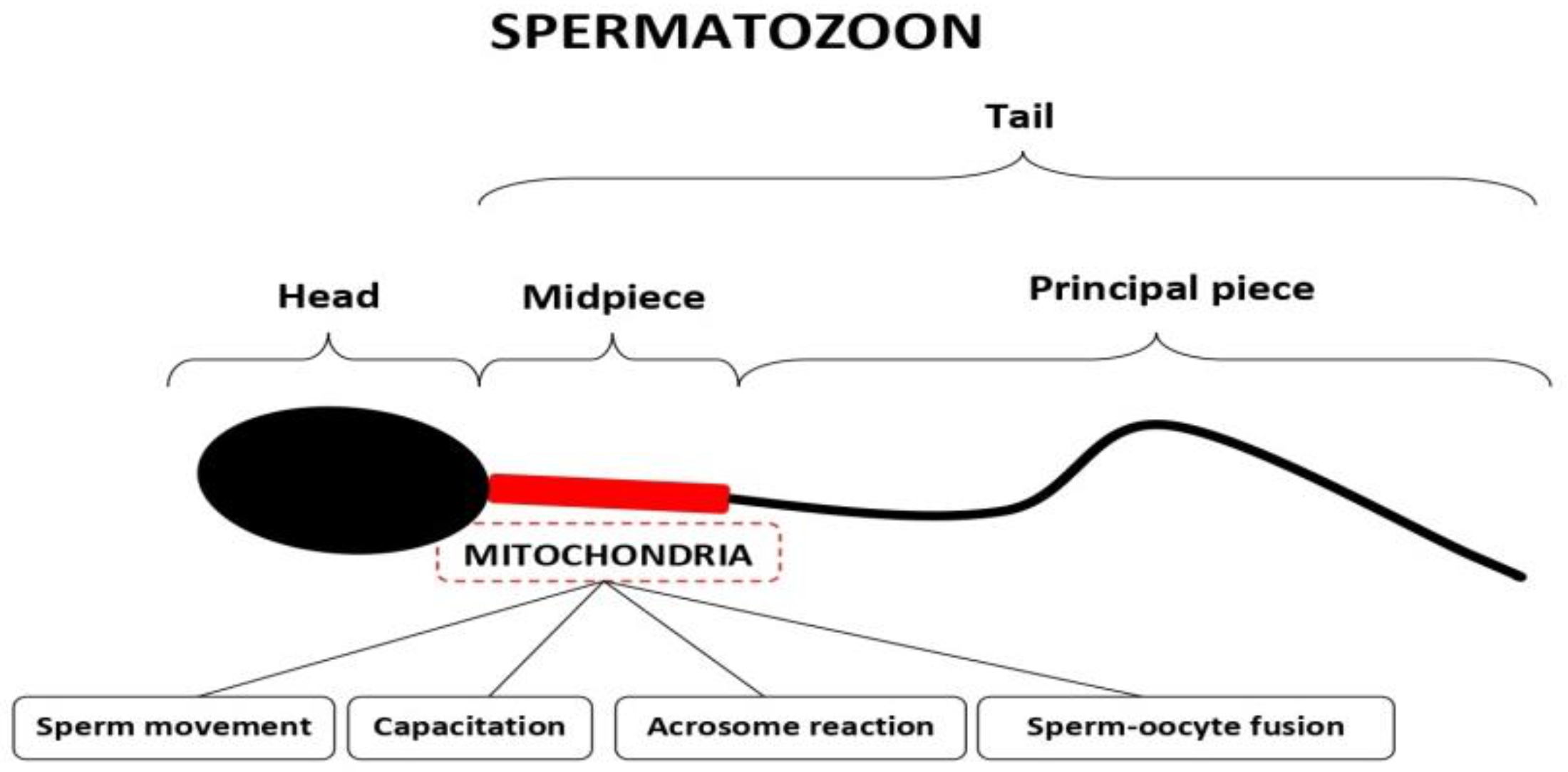
3.1. Mitochondria in Spermatozoa
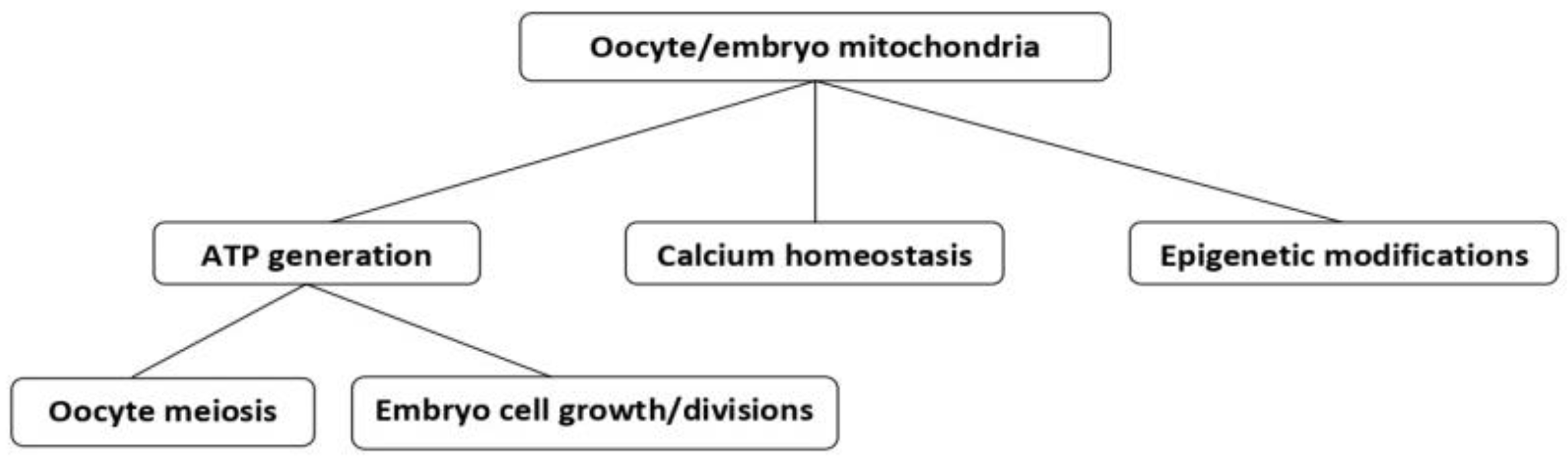
3.2. Mitochondria in Oocytes
4. The Role of Mitochondria in Embryos
5. Mitochondrial Therapies for Human Infertility
5.1. Mitochondrial Replacement Therapies
5.2. Mitochondrial Genome Editing
5.3. Currently Available Conservative Therapies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Boguenet, M.; Bouet, P.E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Update 2021, 27, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–556. [Google Scholar] [CrossRef]
- Sousa, M.; Barros, A.; Silva, J.; Tesarik, J. Developmental changes in calcium content of ultrastructurally distinct subcellular compartments of preimplantation human embryos. Mol. Hum. Reprod. 1997, 3, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Hoshi, K.; Shoda, T.; Mabuchi, T. Spermatozoon and mitochondrial DNA. Reprod. Med. Biol. 2002, 1, 41–47. [Google Scholar] [CrossRef]
- Reik, W.; Dean, W.; Walter, J. Epigenetic reprogramming in mammalian development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef]
- Matilainen, O.; Quirós, P.M.; Auwerx, J. Mitochondria and epigenetics—Crosstalk in homeostasis and stress. Trends Cell Biol. 2017, 27, 453–463. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxid. Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.M.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Xu, J.; Cao, X.; Zhang, D.; Liu, M.; Liu, S.; Dong, X.; Shi, H. Mitochondrial dysfunction in cumulus cells is related to decreased reproductive capacity in advanced-age women. Fertil. Steril. 2022, 118, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, M.; Jiang, Z.; Seli, E. Mitochondrial dysfunction and ovarian aging. Am. J. Reprod. Immunol. 2017, 77, e12651. [Google Scholar] [CrossRef] [PubMed]
- Sreerangaraja Urs, D.B.; Wu, W.H.; Komrskova, K.; Postlerova, P.; Lin, Y.F.; Tzeng, C.R.; Kao, S.H. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int. J. Mol. Sci. 2020, 21, 3592. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Galán-Lázaro, M.; Mendoza-Tesarik, R. Ovarian Aging: Molecular Mechanisms and Medical Management. Int. J. Mol. Sci. 2021, 22, 1371. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nuevo, A.; Torres-Sanchez, A.; Duran, J.M.; De Guirior, C.; Martínez-Zamora, M.A.; Böke, E. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 2022, 607, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Podolak, A.; Woclawek-Potocka, I.; Lukaszuk, K. The role of mitochondria in human fertility and early embryo development: What can we learn for clinical application of assessing and improving mitochondrial DNA? Cells 2022, 11, 797. [Google Scholar] [CrossRef]
- Holt, I.J.; Harding, A.E.; Petty, R.K.; Morgan-Hughes, J.A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am. J. Hum. Genet. 1990, 46, 428–433. [Google Scholar]
- Zhang, J.; Liu, H.; Luo, S.; Lu, Z.; Chávez-Badiola, A.; Liu, Z.; Yang, M.; Merhi, Z.; Silber, S.J.; Munné, S.; et al. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online 2017, 34, 361–368. [Google Scholar] [CrossRef]
- Falk, M.J.; Sondheimer, N. Mitochondrial genetic diseases. Curr. Opin. Pediatr. 2010, 22, 711–716. [Google Scholar] [CrossRef]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef]
- Barnes, R.B.; Rosenfield, R.L.; Namnoum, A.; Layman, L.C. Effect of follicle-stimulating hormone on ovarian androgen production in a woman with isolated follicle-stimulating hormone deficiency. N. Engl. J. Med. 2000, 343, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza, C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: Relationship to oocyte developmental potential. J. Clin. Endocrinol. Metab. 1995, 80, 1438–1443. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C. Direct non-genomic effects of follicular steroids on maturing human oocytes: Oestrogen versus androgen antagonism. Hum. Reprod. Update 1997, 3, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Scott, R.; Schimmel, T.; Levron, J.; Willadsen, S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 1997, 350, 186–187. [Google Scholar] [CrossRef]
- Tesarik, J.; Nagy, Z.P.; Sousa, M.; Mendoza, C.; Abdelmassih, R. Fertilizable oocytes reconstructed from patient’s somatic cell nuclei and donor ooplasts. Reprod. Biomed. Online 2001, 2, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Barritt, J.A.; Brenner, C.A.; Malter, H.E.; Cohen, J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum. Reprod 2001, 16, 513–516. [Google Scholar] [CrossRef]
- Tesarik, J. Purifying selection on mitochondrial DNA in maturing oocytes: Implication for mitochondrial replacement therapy. Hum. Reprod. 2017, 32, 1948–1950. [Google Scholar] [CrossRef]
- Tesarik, A. Forty years of in vitro fertilisation: A history of continuous expansion. In 40 Years after In Vitro Fertilisation. State of the Art and New Challenges; Tesarik, J., Ed.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019; pp. 1–24. [Google Scholar]
- Costa-Borges, N.; Nikitos, E.; Späth, K.; Miguel-Escalada, I.; Ma, H.; Rink, K.; Coudereau, C.; Darby, H.; Koski, A.; Van Dyken, C.; et al. First pilot study of maternal spindle transfer for the treatment of repeated in vitro fertilization failures in couples with idiopathic infertility. Fertil. Steril. 2023, 12, S0015-0282(23)00136-X. [Google Scholar] [CrossRef]
- Morimoto, Y.; Gamage, U.S.K.; Yamochi, T.; Saeki, N.; Morimoto, N.; Yamanaka, M.; Koike, A.; Miyamoto, Y.; Tanaka, K.; Fukuda, A.; et al. Mitochondrial transfer into human oocytes improved embryo quality and clinical outcomes in recurrent pregnancy failure cases. Int. J. Mol. Sci. 2023, 24, 2738. [Google Scholar] [CrossRef]
- Fu, L.; Luo, Y.-X.; Liu, Y.; Liu, H.; Li, H.-Z.; Yu, Y. Potential of Mitochondrial Genome Editing for Human Fertility Health. Front. Genet. 2021, 12, 673951. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesarik, J.; Mendoza-Tesarik, R. Mitochondria in Human Fertility and Infertility. Int. J. Mol. Sci. 2023, 24, 8950. https://doi.org/10.3390/ijms24108950
Tesarik J, Mendoza-Tesarik R. Mitochondria in Human Fertility and Infertility. International Journal of Molecular Sciences. 2023; 24(10):8950. https://doi.org/10.3390/ijms24108950
Chicago/Turabian StyleTesarik, Jan, and Raquel Mendoza-Tesarik. 2023. "Mitochondria in Human Fertility and Infertility" International Journal of Molecular Sciences 24, no. 10: 8950. https://doi.org/10.3390/ijms24108950
APA StyleTesarik, J., & Mendoza-Tesarik, R. (2023). Mitochondria in Human Fertility and Infertility. International Journal of Molecular Sciences, 24(10), 8950. https://doi.org/10.3390/ijms24108950






