Synthesis, Antimicrobial, and Antibiofilm Activities of Some Novel 7-Methoxyquinoline Derivatives Bearing Sulfonamide Moiety against Urinary Tract Infection-Causing Pathogenic Microbes
Abstract
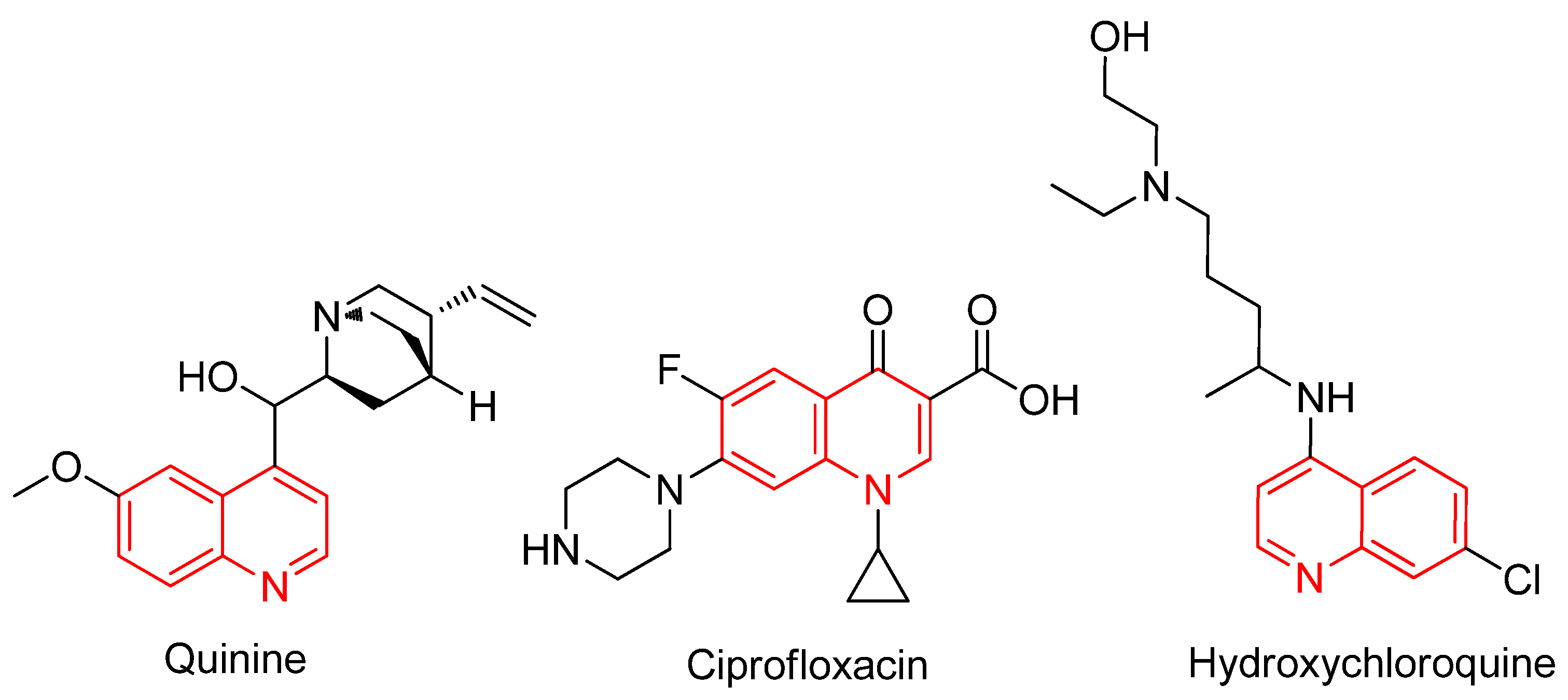
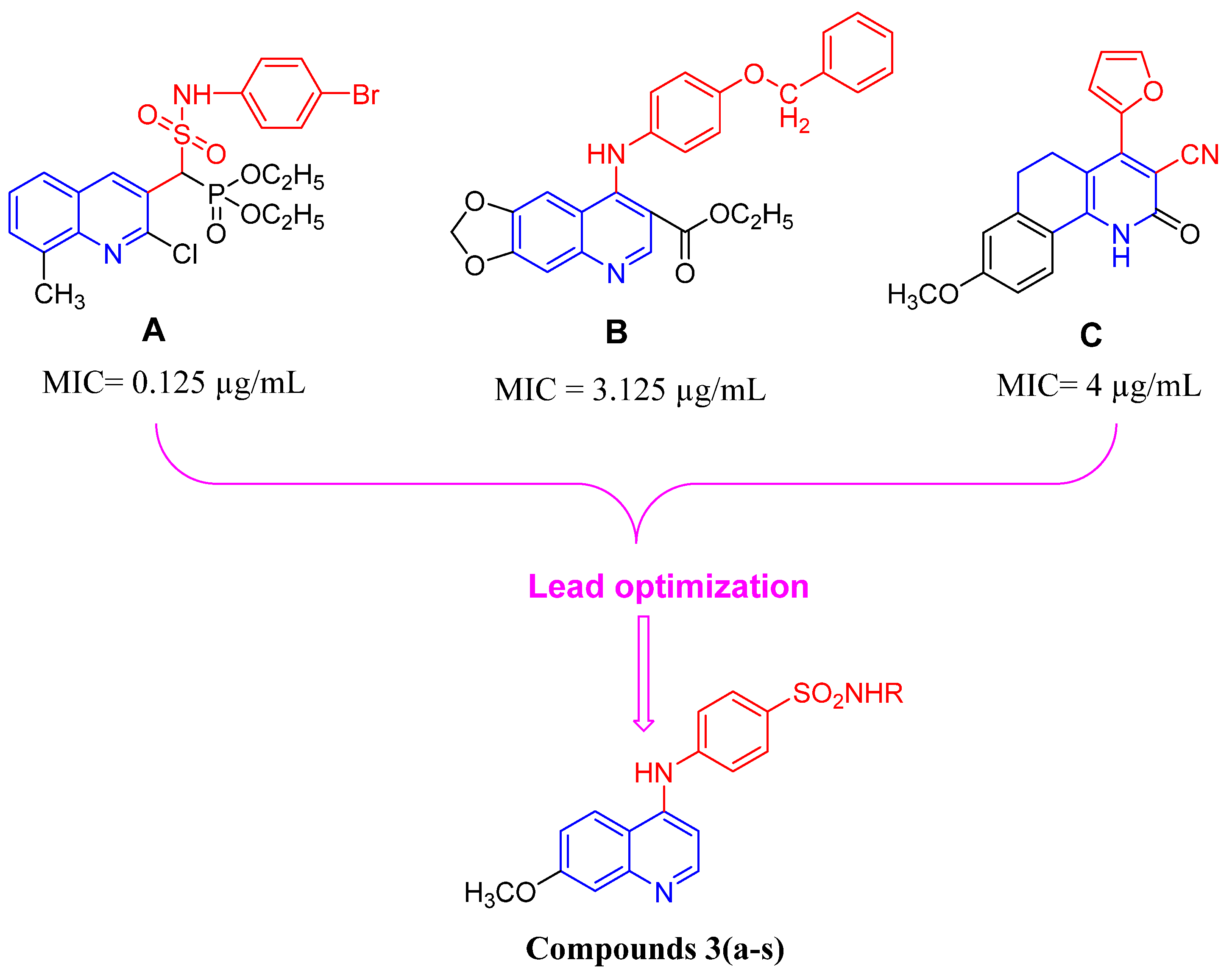
1. Introduction
2. Results and Discussion
2.1. Chemistry
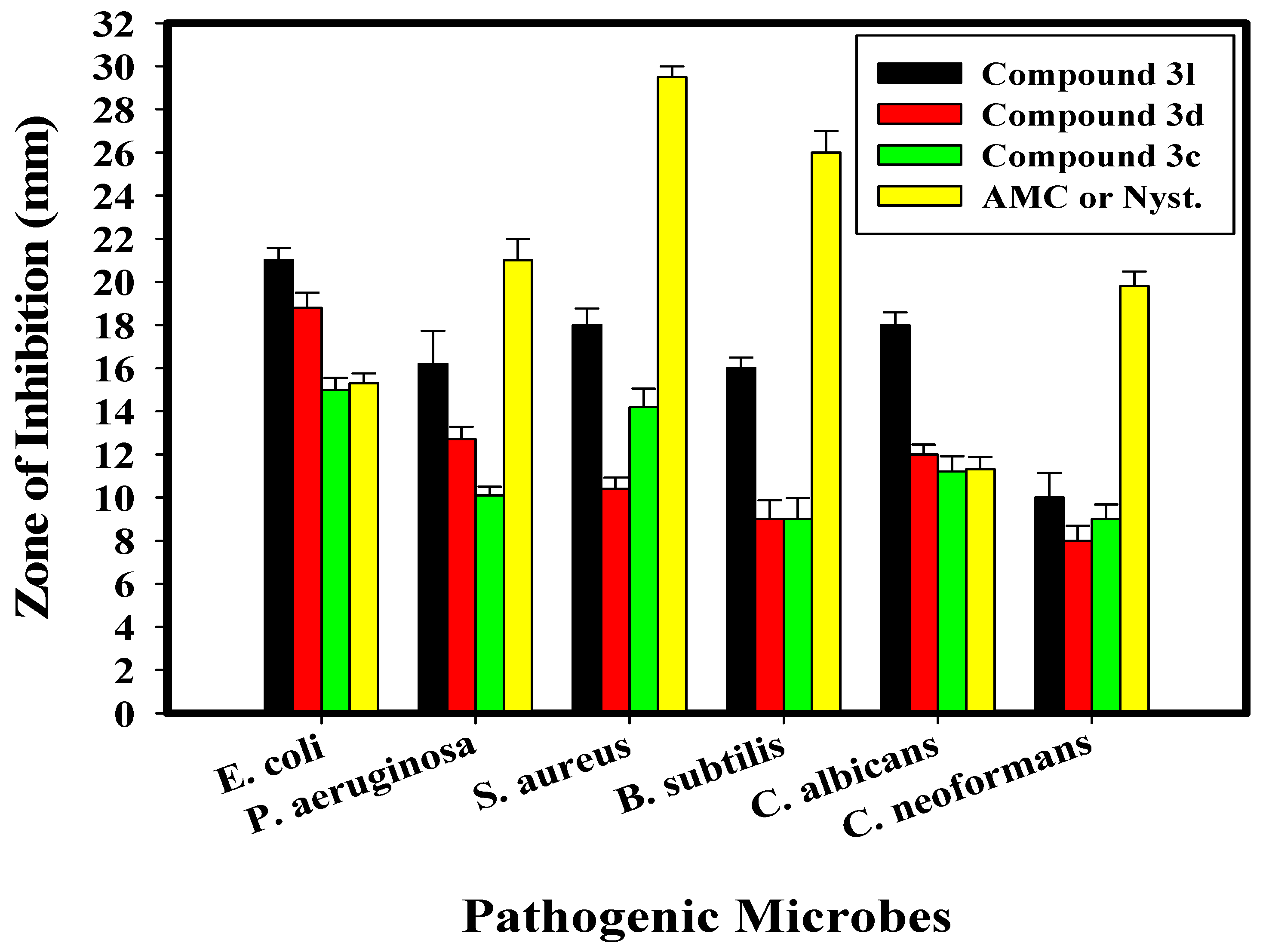
2.2. Antimicrobial Activity of the Newly Synthesized Compounds
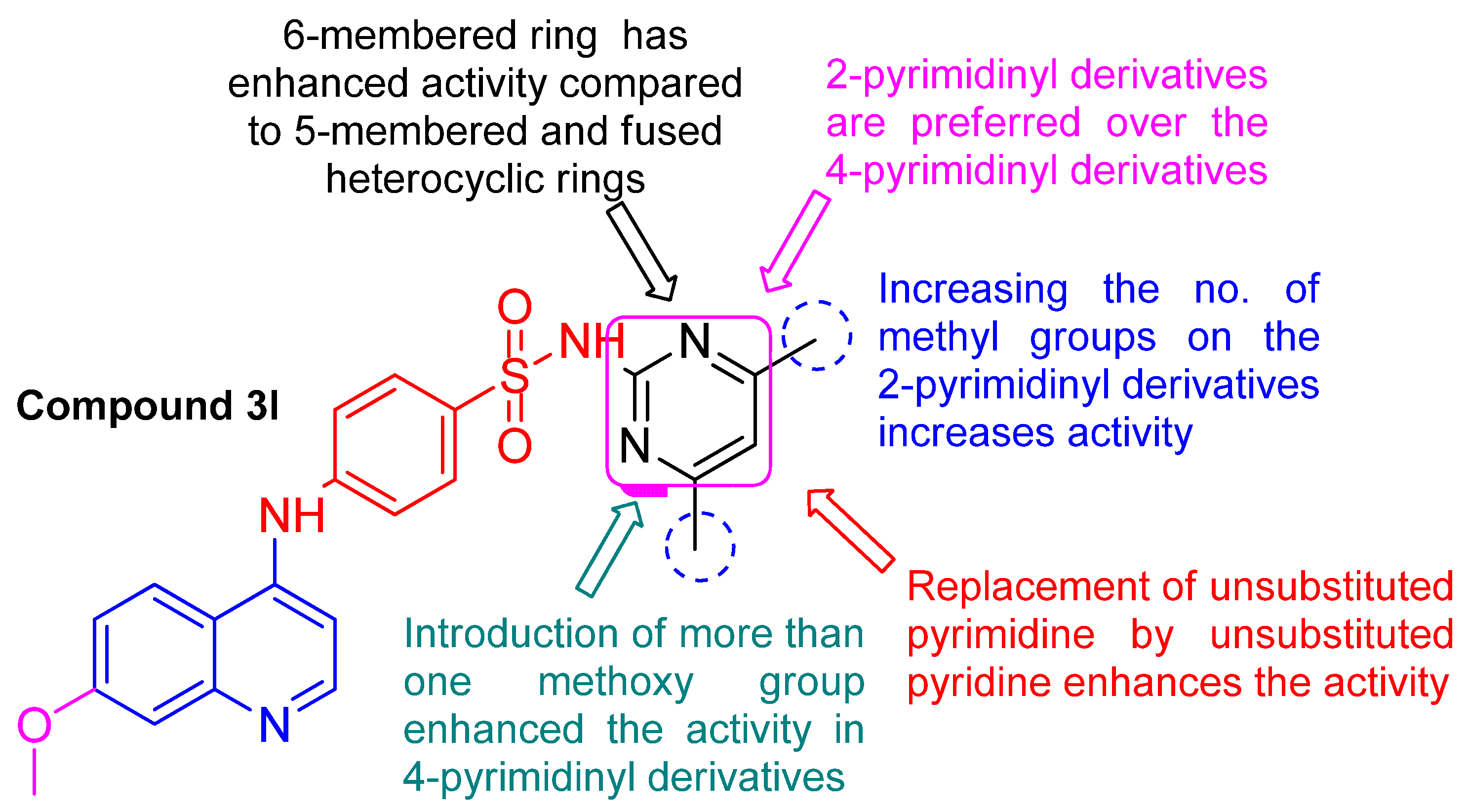
2.3. Structure Activity Relationship (SAR) Study
- Regarding the open chain derivatives 3(a–c):
- Regarding the heterocyclic aromatic derivatives 3(d–s):
- Regarding the 6-membered heterocyclic derivatives 3d, 3e, 3h, 3k, 3l, 3m, 3n, 3q, and 3r:
- The 2-pyrimidinyl derivatives 3e, 3h, 3l, and 3m:
- The 4-pyrimidinyl derivatives 3k, 3n, and 3q:
- The 5-membered ring derivatives 3f, 3g, 3i, 3j, and 3s:
- Regarding the fused heterocyclic derivatives 3o and 3p:
2.4. Antibiofilm Potential of Compound 3l
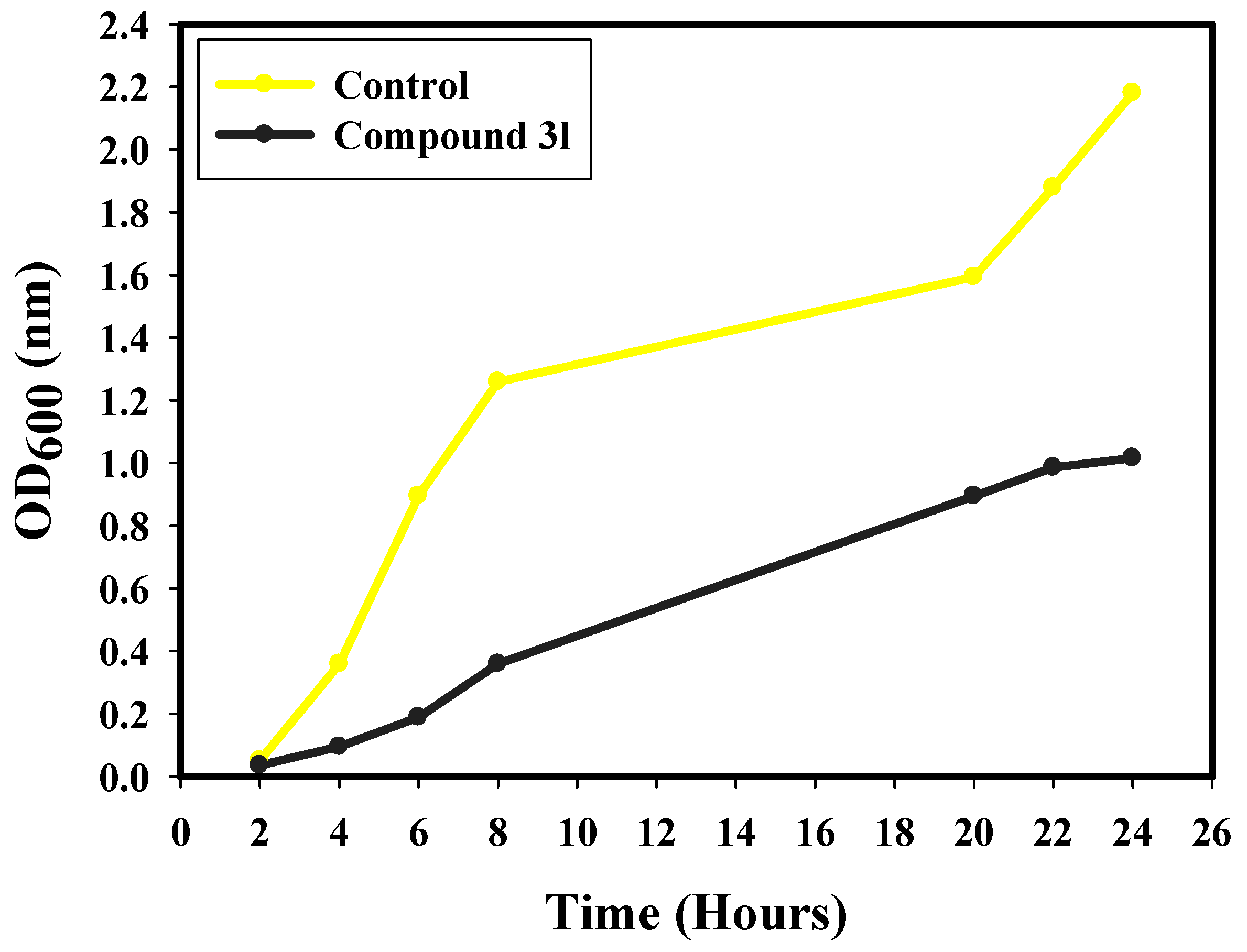
2.5. Kinetics of E. coli Growth (Growth Curve)
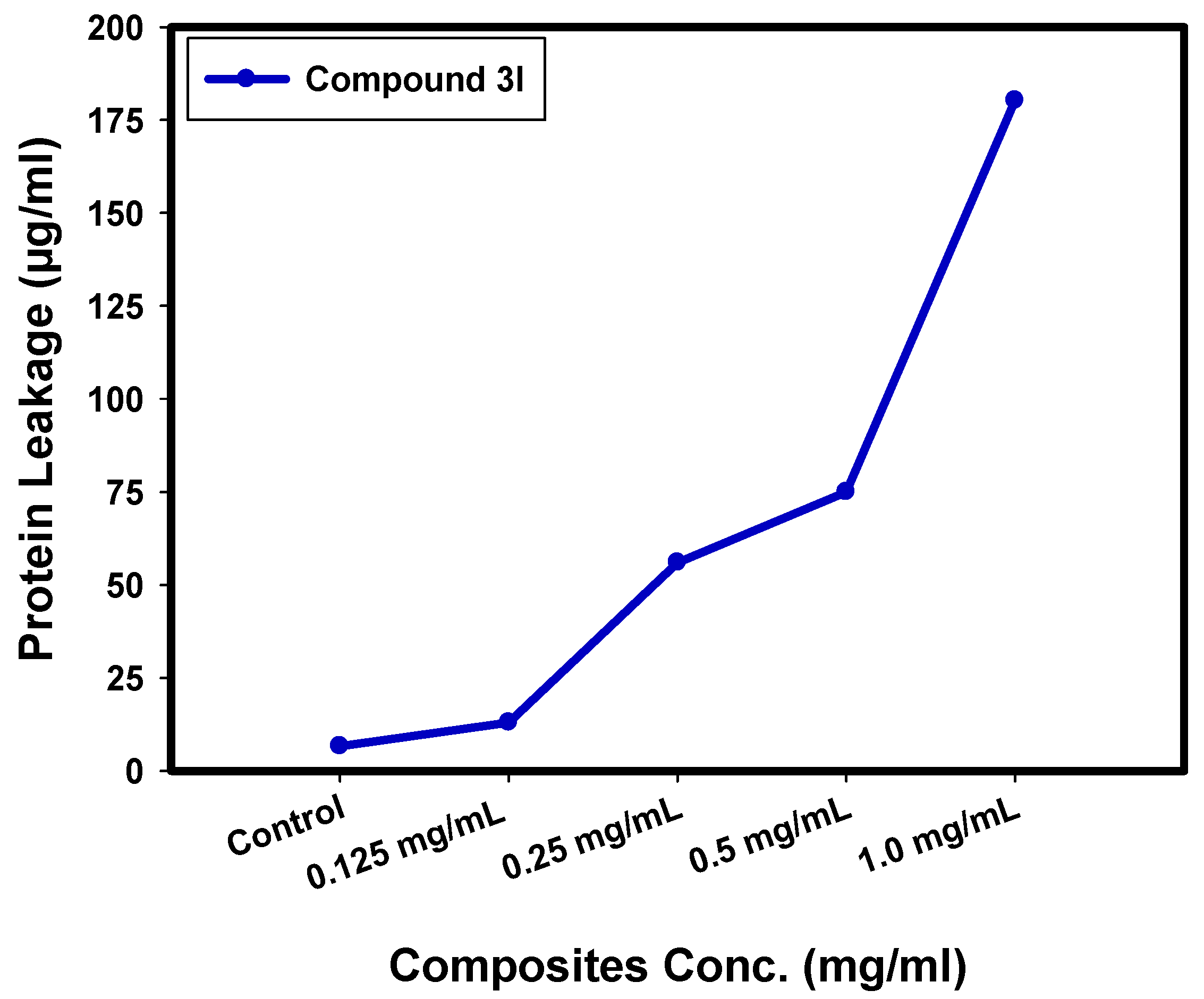
2.6. Determination of Protein Leakage from Bacterial Cell Membranes
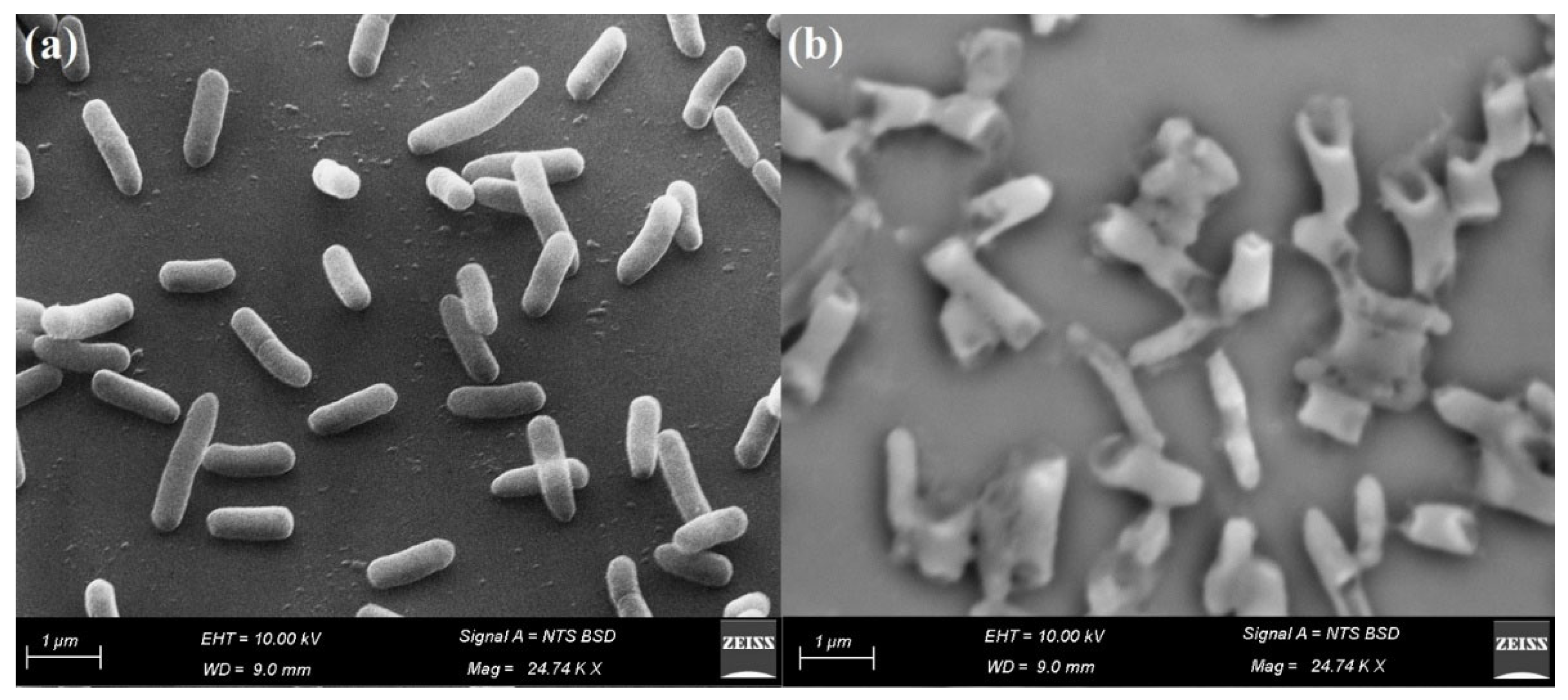
2.7. Reaction Mechanism Determination by SEM
2.8. In Silico ADME Study
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Synthesis of Compounds 3(a–s)
3.1.2. 4-((7-Methoxyquinolin-4-yl)amino)benzenesulfonamide (3a)
3.1.3. N-((4-((7-Methoxyquinolin-4-yl)amino)phenyl)sulfonyl)acetamide (3b)
3.1.4. N-(Diaminomethylene)-4-((7-methoxyquinolin-4-yl)amino)benzenesulfonamide (3c)
3.1.5. 4-((7-Methoxyquinolin-4-yl)amino)-N-(pyridin-2-yl)benzenesulfonamide (3d)
3.1.6. 4-((7-Methoxyquinolin-4-yl)amino)-N-(pyrimidin-2-yl)benzenesulfonamide (3e)
3.1.7. 4-((7-Methoxyquinolin-4-yl)amino)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (3f)
3.1.8. 4-((7-Methoxyquinolin-4-yl)amino)-N-(thiazol-2-yl)benzenesulfonamide (3g)
3.1.9. 4-((7-Methoxyquinolin-4-yl)amino)-N-(4-methylpyrimidin-2-yl)benzenesulfonamide (3h)
3.1.10. N-(4,5-Dimethyloxazol-2-yl)-4-((7-methoxyquinolin-4-yl)amino)benzenesulfonamide (3i)
3.1.11. 4-((7-Methoxyquinolin-4-yl)amino)-N-(5-methyl-1,3,4-thiadiazol-2-yl)benzenesulfonamide (3j)
3.1.12. N-(2,6-Dimethylpyrimidin-4-yl)-4-((7-methoxyquinolin-4-yl)amino)benzenesulfonamide (3k)
3.1.13. N-(4,6-Dimethylpyrimidin-2-yl)-4-((7-methoxyquinolin-4-yl)amino)benzenesulfonamide (3l)
3.1.14. N-(5-Methoxypyrimidin-2-yl)-4-((7-methoxyquinolin-4-yl)amino)benzenesulfonamide (3m)
3.1.15. N-(6-Methoxypyrimidin-4-yl)-4-((7-methoxyquinolin-4-yl)amino)benzenesulfonamide (3n)
3.1.16. N-(1H-Indazol-6-yl)-4-((7-methoxyquinolin-4-yl)amino)benzenesulfonamide (3o)
3.1.17. 4-((7-Methoxyquinolin-4-yl)amino)-N-(quinoxalin-2-yl)benzenesulfonamide (3p)
3.1.18. N-(2,6-Dimethoxypyrimidin-4-yl)-4-((7-methoxyquinolin-4-yl)amino) benzenesulfonamide (3q)
3.1.19. N-(5,6-Dimethoxypyrimidin-4-yl)-4-((7-methoxyquinolin-4-yl)amino) benzenesulfonamide (3r)
3.1.20. 4-((7-Methoxyquinolin-4-yl)amino)-N-(1-phenyl-1H-pyrazol-5-yl)benzenesulfonamide (3s)
3.2. Antimicrobial Activity
3.3. Antibiofilm Potential
3.4. Growth Curve Assay
3.5. Effect of Compound 3l on Protein Leakage from Bacterial Cell Membranes
3.6. Reaction Mechanism Using SEM Analysis
3.7. Physicochemical and Pharmacokinetic Parameters
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, R.A.; M’ikanatha, N.M.; Read, A.F. Antibiotic resistance: A primer and call to action. Health Commun. 2015, 30, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Badawy, M.S.E.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green biosynthesis of selenium nanoparticles using orange peel waste: Characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Klahn, P.; Brönstrup, M. New structural templates for clinically validated and novel targets in antimicrobial drug research and development. In How to Overcome the Antibiotic Crisis; Springer: Cham, Switzerland, 2016; Volume 398, pp. 365–417. [Google Scholar]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M.; Group, E.P. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Bush, K.; Courvalin, P.; Dantas, G.; Davies, J.; Eisenstein, B.; Huovinen, P.; Jacoby, G.A.; Kishony, R.; Kreiswirth, B.N.; Kutter, E. Tackling antibiotic resistance. Nat. Rev. Microbiol. 2011, 9, 894–896. [Google Scholar] [CrossRef]
- Blair, J.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef]
- Peters, L.; Olson, L.; Khu, D.T.; Linnros, S.; Le, N.K.; Hanberger, H.; Hoang, N.T.; Tran, D.M.; Larsson, M. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: A cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS ONE 2019, 14, e0215666. [Google Scholar] [CrossRef]
- Verma, S.K.; Verma, R.; Kumar, K.S.S.; Banjare, L.; Shaik, A.B.; Bhandare, R.R.; Rakesh, K.P.; Rangappa, K.S. A key review on oxadiazole analogs as potential methicillin-resistant Staphylococcus aureus (MRSA) activity: Structure-activity relationship studies. Eur. J. Med. Chem. 2021, 219, 113442. [Google Scholar] [CrossRef]
- Mühldorfer, I.; Ziebuhr, W.; Hacker, J. Escherichia coli in urinary tract infections. In Molecular medical microbiology; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1515–1540. [Google Scholar]
- Yamamoto, S. Molecular epidemiology of uropathogenic Escherichia coli. J. Infect. Chemother. 2007, 13, 68–73. [Google Scholar] [CrossRef]
- Johnson, J.R. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 1991, 4, 80–128. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.; Mobley, H.L. Virulence and fitness determinants of uropathogenic Escherichia coli. Urin. Tract Infect. Mol. Pathog. Clin. Manag. 2017, 235–261. [Google Scholar]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic discovery: Combatting bacterial resistance in cells and in biofilm communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Reisner, A.; Maierl, M.; Jörger, M.; Krause, R.; Berger, D.; Haid, A.; Tesic, D.; Zechner, E.L. Type 1 fimbriae contribute to catheter-associated urinary tract infections caused by Escherichia coli. J. Bacteriol. 2014, 196, 931–939. [Google Scholar] [CrossRef]
- Mobley, H.; Chippendale, G.; Tenney, J.; Hull, R.; Warren, J. Expression of type 1 fimbriae may be required for persistence of Escherichia coli in the catheterized urinary tract. J. Clin. Microbiol. 1987, 25, 2253–2257. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef]
- Mittal, S.; Sharma, M.; Chaudhary, U. Biofilm and multidrug resistance in uropathogenic Escherichia coli. Pathog. Glob. Health 2015, 109, 26–29. [Google Scholar] [CrossRef]
- Vila, J.; Simon, K.; Ruiz, J.; Horcajada, J.P.; Velasco, M.; Barranco, M.; Moreno, A.; Mensa, J. Are quinolone-resistant uropathogenic Escherichia coli less virulent? J. Infect. Dis. 2002, 186, 1039–1042. [Google Scholar] [CrossRef]
- Webber, M.; Piddock, L.J. Quinolone resistance in Escherichia coli. Vet. Res. 2001, 32, 275–284. [Google Scholar] [CrossRef]
- Neupane, S.; Pant, N.D.; Khatiwada, S.; Chaudhary, R.; Banjara, M.R. Correlation between biofilm formation and resistance toward different commonly used antibiotics along with extended spectrum beta lactamase production in uropathogenic Escherichia coli isolated from the patients suspected of urinary tract infections visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob. Resist. Infect. Control. 2016, 5, 5. [Google Scholar] [PubMed]
- Dabholkar, V.V.; Ansari, F.Y. Synthesis and characterization of selected fused isoxazole and pyrazole derivatives and their antimicrobial activity. J. Serb. Chem. Soc. 2009, 74, 1219–1228. [Google Scholar] [CrossRef]
- Yadav, D.K.; Rai, R.; Kumar, N.; Singh, S.; Misra, S.; Sharma, P.; Shaw, P.; Pérez-Sánchez, H.; Mancera, R.L.; Choi, E.H. New arylated benzo [h] quinolines induce anti-cancer activity by oxidative stress-mediated DNA damage. Sci. Rep. 2016, 6, 38128. [Google Scholar] [CrossRef] [PubMed]
- Senerovic, L.; Opsenica, D.; Moric, I.; Aleksic, I.; Spasić, M.; Vasiljevic, B. Quinolines and quinolones as antibacterial, antifungal, anti-virulence, antiviral and anti-parasitic agents. Adv. Microbiol. Infect. Dis. Public Health 2019, 1282, 37–69. [Google Scholar]
- Borsoi, A.F.; Paz, J.D.; Abbadi, B.L.; Macchi, F.S.; Sperotto, N.; Pissinate, K.; Rambo, R.S.; Ramos, A.S.; Machado, D.; Viveiros, M. Design, synthesis, and evaluation of new 2-(quinoline-4-yloxy) acetamide-based antituberculosis agents. Eur. J. Med. Chem. 2020, 192, 112179. [Google Scholar] [CrossRef]
- Olateju, O.A.; Babalola, C.P.; Olubiyi, O.O.; Kotila, O.A.; Kwasi, D.A.; Oaikhena, A.O.; Okeke, I.N. Quinoline Antimalarials Increase the Antibacterial Activity of Ampicillin. Front. Microbiol. 2021, 12, 556550. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Colson, P.; Rolain, J.-M.; Lagier, J.-C.; Brouqui, P.; Raoult, D. Chloroquine and Hydroxychloroquine as Available Weapons to Fight COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105932. [Google Scholar] [CrossRef]
- O’donnell, F.; Smyth, T.; Ramachandran, V.; Smyth, W. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef]
- Celik, I.; Erol, M.; Puskullu, M.O.; Uzunhisarcikli, E.; Ince, U.; Kuyucuklu, G.; Suzen, S. In vitro and in silico studies of quinoline-2-carbaldehyde hydrazone derivatives as potent antimicrobial agents. Polycycl. Aromat. Compd. 2022, 42, 1942–1958. [Google Scholar] [CrossRef]
- Ribeiro, A.G.; de Almeida, S.M.V.; de Oliveira, J.F.; de Lima Souza, T.R.C.; Dos Santos, K.L.; de Barros Albuquerque, A.P.; Nogueira, M.C.d.B.L.; de Carvalho Junior, L.B.; de Moura, R.O.; da Silva, A.C. Novel 4-quinoline-thiosemicarbazone derivatives: Synthesis, antiproliferative activity, in vitro and in silico biomacromolecule interaction studies and topoisomerase inhibition. Eur. J. Med. Chem. 2019, 182, 111592. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Joshi, G.; Kler, H.; Kashyap, A.; Amrutkar, S.M.; Sharma, P.; Bhilare, K.D.; Banerjee, U.C.; Singh, S.; Kumar, R. Dual inhibitors of epidermal growth factor receptor and topoisomerase IIα derived from a quinoline scaffold. RSC Adv. 2016, 6, 77717–77734. [Google Scholar] [CrossRef]
- Bhatnagar, K.; Wong, A. The mutational landscape of quinolone resistance in Escherichia coli. PLoS ONE 2019, 14, e0224650. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Groundwater, P.W.; Todd, A.; Worsley, A. Antibacterial Agents: Chemistry, Mode of Action, Mechanisms of Resistance and Clinical Applications; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Bisacchi, G.S. Origins of the quinolone class of antibacterials: An expanded “discovery story” miniperspective. J. Med. Chem. 2015, 58, 4874–4882. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.T.; Barker, R.; Rehal, R.; Vandera, K.-K.A.; Harvey, R.D.; Coates, A.R. Mechanism of action of a membrane-active quinoline-based antimicrobial on natural and model bacterial membranes. Biochemistry 2017, 56, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mitscher, L.A.; Shen, L.L. The 2-pyridone antibacterial agents: Bacterial topoisomerase inhibitors. Med. Res. Rev. 2000, 20, 231–293. [Google Scholar] [CrossRef]
- Finklestone-Sayliss, H.; Paine, C.; Patrick, L. The Bacteriostatie Action of p-Aminobenzenesulphonamide upon Haemolytic Streptococci. Lancet 1937, 230, 792–795. [Google Scholar] [CrossRef]
- El Ella, D.A.A.; Ghorab, M.M.; Heiba, H.I.; Soliman, A.M. Synthesis of some new thiazolopyrane and thiazolopyranopyrimidine derivatives bearing a sulfonamide moiety for evaluation as anticancer and radiosensitizing agents. Med. Chem. Res. 2012, 21, 2395–2407. [Google Scholar] [CrossRef]
- Alsaid, M.S.; Al-Mishari, A.A.; Soliman, A.M.; Ragab, F.A.; Ghorab, M.M. Discovery of Benzo [g] quinazolin benzenesulfonamide derivatives as dual EGFR/HER2 inhibitors. Eur. J. Med. Chem. 2017, 141, 84–91. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Alsaid, M.S.; El-Gaby, M.S.; Safwat, N.A.; Elaasser, M.M.; Soliman, A.M. Biological evaluation of some new N-(2,6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2016, 124, 299–310. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Alsaid, M.S.; Soliman, A.M.; Ragab, F.A. VEGFR-2 inhibitors and apoptosis inducers: Synthesis and molecular design of new benzo [g] quinazolin bearing benzenesulfonamide moiety. J. Enzyme Inhib. Med. Chem. 2017, 32, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, W.-K.; Cao, Q.; Chu, W.-J.; Lan, L.-F.; Hu, W.-H.; Yang, Y.-S. Design, synthesis and biological evaluation of LpxC inhibitors with novel hydrophilic terminus. Chin. Chem. Lett. 2015, 26, 763–767. [Google Scholar] [CrossRef]
- Supuran, C.T. Bacterial carbonic anhydrases as drug targets: Toward novel antibiotics? Front. Pharmacol. 2011, 2, 34. [Google Scholar] [CrossRef]
- Mockenhaupt, M.; Viboud, C.; Dunant, A.; Naldi, L.; Halevy, S.; Bavinck, J.N.B.; Sidoroff, A.; Schneck, J.; Roujeau, J.C.; Flahault, A. Stevens–Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J. Invest. Dermatol. 2008, 128, 35–44. [Google Scholar] [CrossRef]
- Letko, E.; Papaliodis, D.N.; Papaliodis, G.N.; Daoud, Y.J.; Ahmed, A.R.; Foster, C.S. Stevens-Johnson syndrome and toxic epidermal necrolysis: A review of the literature. Ann. Allergy Asthma Immunol. 2005, 94, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alqahtani, A.S.; Soliman, A.M.; Askar, A.A. Novel N-(Substituted) Thioacetamide Quinazolinone Benzenesulfonamides as Antimicrobial Agents. Int. J. Nanomed. 2020, 15, 3161. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Alqahtani, A.S.; Soliman, A.M.; Askar, A.A. Antimicrobial, anticancer and immunomodulatory potential of new quinazolines bearing benzenesulfonamide moiety. Future Med. Chem. 2023, 3, 17. [Google Scholar] [CrossRef]
- Bazine, I.; Bendjedid, S.; Boukhari, A. Potential antibacterial and antifungal activities of novel sulfamidophosphonate derivatives bearing the quinoline or quinolone moiety. Arch. Pharm. 2021, 354, 2000291. [Google Scholar] [CrossRef]
- Jin, G.; Li, Z.; Xiao, F.; Qi, X.; Sun, X. Optimization of activity localization of quinoline derivatives: Design, synthesis, and dual evaluation of biological activity for potential antitumor and antibacterial agents. Bioorg. Chem. 2020, 99, 103837. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M.; Basisi, H.M.; Asad, M.; Zayed, M.E.; Sharma, K.; Wani, M.Y. Synthesis and evaluation of Quinoline-3-carbonitrile derivatives as potential antibacterial agents. Bioorg. Chem. 2019, 88, 102968. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Soliman, A.M.; Alsaid, M.S.; Askar, A.A. Synthesis, antimicrobial activity and docking study of some novel 4-(4, 4-dimethyl-2, 6-dioxocyclohexylidene) methylamino derivatives carrying biologically active sulfonamide moiety. Arab. J. Chem. 2020, 13, 545–556. [Google Scholar] [CrossRef]
- Abu-Elghait, M.; Hasanin, M.; Hashem, A.H.; Salem, S.S. Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles: Characterization, antibiofilm and biocompatibility. Int. J. Biol. Macromol. 2021, 175, 294–303. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, G.S.; Abd Elkodous, M.; El-Khawaga, A.M.; Elsayed, M.A.; El-Batal, A.I.; Gobara, M. Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: Implication for wastewater treatment. RSC Adv. 2020, 10, 5241–5259. [Google Scholar] [CrossRef] [PubMed]
- Tailor, S.M.; Patel, U.H. Synthesis, spectroscopic characterization, antimicrobial activity and crystal structure of silver and copper complexes of sulfamethazine. J. Coord. Chem. 2015, 68, 2192–2207. [Google Scholar] [CrossRef]
- Ragab, A.; Fouad, S.A.; Ali, O.A.A.; Ahmed, E.M.; Ali, A.M.; Askar, A.A.; Ammar, Y.A. Sulfaguanidine Hybrid with Some New Pyridine-2-One Derivatives: Design, Synthesis, and Antimicrobial Activity against Multidrug-Resistant Bacteria as Dual DNA Gyrase and DHFR Inhibitors. Antibiotics 2021, 10, 162. [Google Scholar] [CrossRef]
- Chen, L.; Yang, D.; Pan, Z.; Lai, L.; Liu, J.; Fang, B.; Shi, S. Synthesis and antimicrobial activity of the hybrid molecules between sulfonamides and active antimicrobial pleuromutilin derivative. Chem. Biol. Drug. Des. 2015, 86, 239–245. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Al-Hazmi, N.E.; Gobara, M. Antibiofilm and antimicrobial activities of silver boron nanoparticles synthesized by PVP polymer and gamma rays against urinary tract pathogens. J. Clust. Sci. 2019, 30, 947–964. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Nada, H.G.; El-Behery, R.R.; Gobara, M.; El-Sayyad, G.S. Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Adv. 2020, 10, 9274–9289. [Google Scholar] [CrossRef]
- de Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Souza Filho, A.G.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Boegli, L.; Agostinho, A.; Sánchez, E.M.; Bach, H.; Ruiz, F.; James, G. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 2013, 29, 651–660. [Google Scholar] [CrossRef]
- Mahamuni, P.P.; Patil, P.M.; Dhanavade, M.J.; Badiger, M.V.; Shadija, P.G.; Lokhande, A.C.; Bohara, R.A. Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem. Biophys. Rep. 2019, 17, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Bradford, N. A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal. Biochem. 1976, 72, e254. [Google Scholar] [CrossRef]
- Rajesh, S.; Dharanishanthi, V.; Kanna, A.V. Antibacterial mechanism of biogenic silver nanoparticles of Lactobacillus acidophilus. J. Exp. Nanosci. 2015, 10, 1143–1152. [Google Scholar] [CrossRef]
- Azam, Z.; Ayaz, A.; Younas, M.; Qureshi, Z.; Arshad, B.; Zaman, W.; Ullah, F.; Nasar, M.Q.; Bahadur, S.; Irfan, M.M. Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 2020, 144, 104188. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Maiti, S.; Sethi, D.P.; Neogi, S. Bi-functional NiO-ZnO nanocomposite: Synthesis, characterization, antibacterial and photo assisted degradation study. Adv. Powder Technol. 2021, 32, 131–143. [Google Scholar] [CrossRef]
- El-Sayyad, G.S.; El-Bastawisy, H.S.; Gobara, M.; El-Batal, A.I. Gentamicin-Assisted Mycogenic Selenium Nanoparticles Synthesized Under Gamma Irradiation for Robust Reluctance of Resistant Urinary Tract Infection-Causing Pathogens. Biol. Trace Elem. Res. 2020, 195, 323–342. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Refsgaard, H.H.; Jensen, B.F.; Brockhoff, P.B.; Padkjær, S.B.; Guldbrandt, M.; Christensen, M.S. In silico prediction of membrane permeability from calculated molecular parameters. J. Med. Chem. 2005, 48, 805–811. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Bisno, A.L.; Beachey, E.H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 1982, 37, 318–326. [Google Scholar] [CrossRef]
- Narisawa, N.; Furukawa, S.; Ogihara, H.; Yamasaki, M. Estimation of the biofilm formation of Escherichia coli K-12 by the cell number. J. Biosci. Bioeng. 2005, 99, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Pal, R. Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Appl. Nanosci. 2014, 4, 859–868. [Google Scholar] [CrossRef]
- Huang, W.; Wang, J.-Q.; Song, H.-Y.; Zhang, Q.; Liu, G.-F. Chemical analysis and in vitro antimicrobial effects and mechanism of action of Trachyspermum copticum essential oil against Escherichia coli. Asian Pac. J. Trop. Med. 2017, 10, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, M.; El-Sabbagh, S.H.; Mohamed, R.M.; El-Sayyad, G.S.; Sokary, R. Mechanical, Thermal and Antimicrobial Properties of LLDPE/EVA/MMT/Ag Nanocomposites Films Synthesized by Gamma Irradiation. J. Inorg. Organomet. Polym. Mater. 2021, 32, 631–645. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Menon, S.; Shanmugam, V. Eco-friendly synthesis of zinc oxide nanoparticles using Cinnamomum Tamala leaf extract and its promising effect towards the antibacterial activity. J. Drug Deliv. Sci. Technol. 2019, 53, 101212. [Google Scholar] [CrossRef]
- El-Shazly, A.N.; El-Sayyad, G.S.; Hegazy, A.H.; Hamza, M.A.; Fathy, R.M.; El Shenawy, E.; Allam, N.K. Superior visible light antimicrobial performance of facet engineered cobalt doped TiO2 mesocrystals in pathogenic bacterium and fungi. Sci. Rep. 2021, 11, 5609. [Google Scholar] [CrossRef] [PubMed]
- El-Khawaga, A.M.; Farrag, A.A.; Elsayed, M.A.; El-Sayyad, G.S.; El-Batal, A.I. Antimicrobial and Photocatalytic Degradation Activities of Chitosan-coated Magnetite Nanocomposite. J. Clust. Sci. 2021, 32, 1–13. [Google Scholar] [CrossRef]
- Brown, A.M. A new software for carrying out one-way ANOVA post hoc tests. Comput. Methods Programs Biomed. 2005, 79, 89–95. [Google Scholar] [CrossRef]


| Test Organism | Compound 3c | Compound 3d | Compound 3l | AMC/Nystatin | ||||
|---|---|---|---|---|---|---|---|---|
| IZ (mm) | MIC µg/mL | IZ (mm) | MIC µg/mL | IZ (mm) | MIC µg/mL | IZ (mm) | MIC µg/mL | |
| E. coli | 15.0 ± 0.55 e | 62.50 | 18.8 ± 0.76 e | 31.25 | 21.0 ± 0.58 f | 7.812 | 15.3 ± 0.46 d | 250 |
| P. aeruginosa | 10.1 ± 0.40 b | 250 | 12.7 ± 0.58 d | 125 | 16.2 ± 1.53 bcd | 125 | 21.0 ± 1.00 c | 500 |
| S. aureus | 14.2 ± 0.85 d | 125 | 10.4 ± 0.53 d | 250 | 18.0 ± 0.76 de | 31.25 | 29.5 ± 0.50 b | 250 |
| B. subtilis | 9.0 ± 0.98 bc | 500 | 9.0 ± 0.87 bc | 250 | 16.0 ± 0.50 a | 125 | 26.0 ± 1.00 a | 31.25 |
| C. albicans | 11.2 ± 0.72 f | 125 | 12.0 ± 0.45 f | 125 | 18.0 ± 0.58 f | 31.25 | 11.3 ± 0.58 f | 125 |
| C. neoformans | 9.0 ± 0.68 bcd | 500 | 8.0 ± 0.69 c | 500 | 10.0 ± 1.15 cde | 500 | 19.8 ± 0.68 c | 250 |
| Test Organism | O.D. of Crystal Violet Stain at 570.0 nm | Inhibition % | |
|---|---|---|---|
| Control | Treated | Compound 3l | |
| B. subtilis | 0.808 d ± 0.0080 | 0.399 c ± 0.0021 | 62.64 |
| P. aeruginosa | 0.950 a ± 0.0062 | 0.122 e ± 0.0047 | 91.74 |
| S. aureus | 0.945 b ± 0.0070 | 0.445 b ± 0.0053 | 55.98 |
| E. coli | 0.454 f ± 0.0025 | 0.259 d ± 0.0062 | 94.60 |
| C. albicans | 0.789 e ± 0.0046 | 0.478 a ± 0.0036 | 49.95 |
| C. neoformans | 0.845 cd ± 0.0046 | 0.145 e ± 0.0036 | 98.03 |
| Parameters | Compound 3c | Compound 3d | Compound 3l |
|---|---|---|---|
| TSPA (A2) | 141.07 | 101.59 | 114.48 |
| n-ROTB | 5 | 6 | 6 |
| Mol. Wt. | 371.41 | 406.46 | 435.50 |
| Molar Vol. | 99.97 | 112.69 | 120.41 |
| Log P | 1.76 | 2.12 | 3.08 |
| n-HB donor | 3 | 2 | 2 |
| n-HB acceptor | 5 | 5 | 6 |
| Lipinski’s violation | 0 | 0 | 0 |
| GI absorption | Low | High | Low |
| CYP1A2 inhibitor | Yes | Yes | Yes |
| CYP3A4 inhibitor | No | Yes | Yes |
| Log Kp (skin permeation) | −6.89 cm/s | −5.87 cm/s | −5.94 cm/s |
| Bioavailability score | 0.55 | 0.55 | 0.55 |
| PAINS | 0 alert | 0 alert | 0 alert |
| Synthetic accessibility | 2.91 | 3.17 | 3.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorab, M.M.; M. Soliman, A.; El-Sayyad, G.S.; Abdel-Kader, M.S.; El-Batal, A.I. Synthesis, Antimicrobial, and Antibiofilm Activities of Some Novel 7-Methoxyquinoline Derivatives Bearing Sulfonamide Moiety against Urinary Tract Infection-Causing Pathogenic Microbes. Int. J. Mol. Sci. 2023, 24, 8933. https://doi.org/10.3390/ijms24108933
Ghorab MM, M. Soliman A, El-Sayyad GS, Abdel-Kader MS, El-Batal AI. Synthesis, Antimicrobial, and Antibiofilm Activities of Some Novel 7-Methoxyquinoline Derivatives Bearing Sulfonamide Moiety against Urinary Tract Infection-Causing Pathogenic Microbes. International Journal of Molecular Sciences. 2023; 24(10):8933. https://doi.org/10.3390/ijms24108933
Chicago/Turabian StyleGhorab, Mostafa M., Aiten M. Soliman, Gharieb S. El-Sayyad, Maged S. Abdel-Kader, and Ahmed I. El-Batal. 2023. "Synthesis, Antimicrobial, and Antibiofilm Activities of Some Novel 7-Methoxyquinoline Derivatives Bearing Sulfonamide Moiety against Urinary Tract Infection-Causing Pathogenic Microbes" International Journal of Molecular Sciences 24, no. 10: 8933. https://doi.org/10.3390/ijms24108933
APA StyleGhorab, M. M., M. Soliman, A., El-Sayyad, G. S., Abdel-Kader, M. S., & El-Batal, A. I. (2023). Synthesis, Antimicrobial, and Antibiofilm Activities of Some Novel 7-Methoxyquinoline Derivatives Bearing Sulfonamide Moiety against Urinary Tract Infection-Causing Pathogenic Microbes. International Journal of Molecular Sciences, 24(10), 8933. https://doi.org/10.3390/ijms24108933








