Gene Expression Changes in Cytokine and Chemokine Receptors in Association with Melanoma Liver Metastasis
Abstract
1. Introduction
2. Results
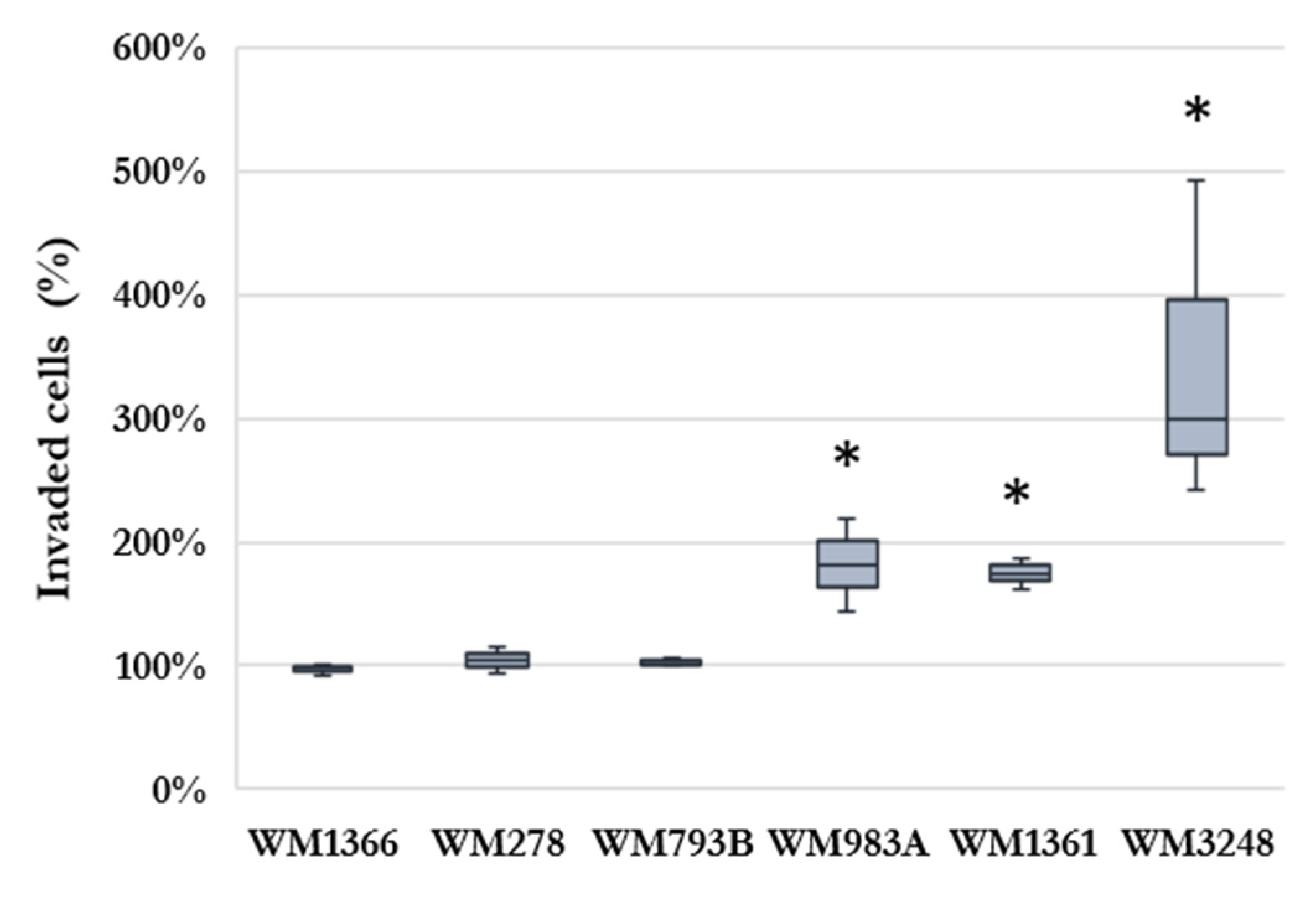
2.1. Effect of HHSEC on Melanoma Cell Invasion
2.2. Chemokine and Cytokine Receptor Expression of Melanoma Cells
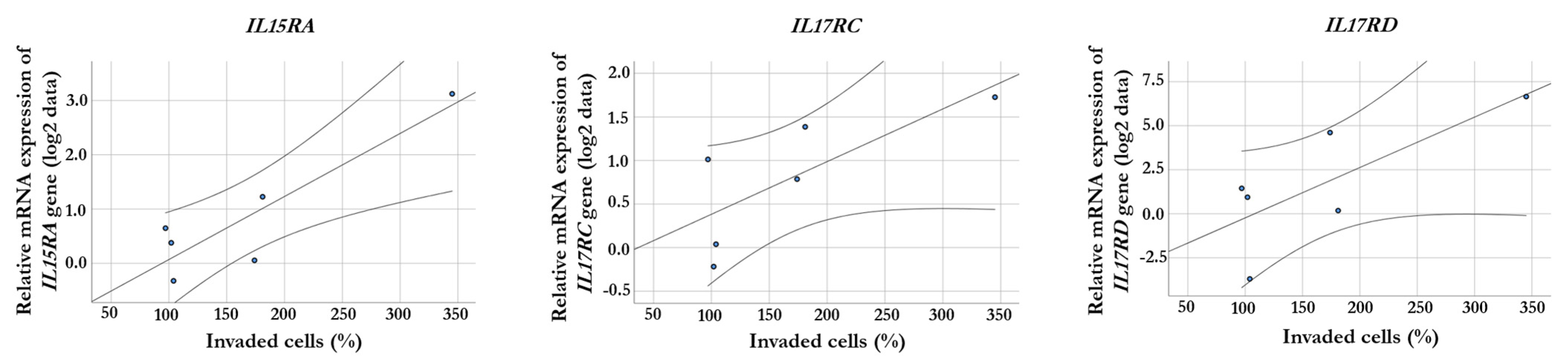
2.3. Correlation between Gene Expression and Invasiveness
2.4. Proteome Profile of HHSECs
2.5. Gene Expression of Chemokine and Cytokine Receptors in Melanoma Tissue Samples
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culturing
4.2. Melanoma Tissue Samples Used for qRT-PCR
4.3. In Vitro Invasion Assay
4.4. Co-Culturing of Melanoma Cell Lines and Endothelial Cells
4.5. Real-Time Quantitative PCR Analysis
4.6. Protein Expression Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Lupardus, P.; Laporte, S.L.; Garcia, K.C. Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 2009, 27, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef] [PubMed]
- Cucanic, O.; Farnsworth, R.H.; Stacker, S.A. The cellular and molecular mediators of metastasis to the lung. Growth Factors 2022, 40, 119–152. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Gerashchenko, T.S.; Schegoleva, A.A.; Khozyainova, A.A.; Choinzonov, E.L.; Denisov, E.V. Metastasis prevention: How to catch metastatic seeds. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188867. [Google Scholar] [CrossRef]
- Obenauf, A.C.; Massague, J. Surviving at a distance: Organ specific metastasis. Trends Cancer 2015, 1, 76–91. [Google Scholar] [CrossRef]
- Nascentes Melo, L.M.; Kumar, S.; Riess, V.; Szylo, K.J.; Eisenburger, R.; Schadendorf, D.; Ubellacker, J.M.; Tasdogan, A. Advancements in melanoma cancer metastasis models. Pigment Cell Melanoma Res. 2023, 36, 206–223. [Google Scholar] [CrossRef]
- Signore, A.; Chianelli, M.; Bei, R.; Oyen, W.; Modesti, A. Targeting cytokine/chemokine receptors: A challenge for molecular nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 149–156. [Google Scholar] [CrossRef]
- Muller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Bos, P.D.; Massague, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Amos, S.E.; Choi, Y.S. The Cancer Microenvironment: Mechanical Challenges of the Metastatic Cascade. Front. Bioeng. Biotechnol. 2021, 9, 625859. [Google Scholar] [CrossRef] [PubMed]
- Floros, T.; Tarhini, A.A. Anticancer Cytokines: Biology and Clinical Effects of Interferon-alpha2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin. Oncol. 2015, 42, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Majidpoor, J. Checkpoint inhibitor/interleukin-based combination therapy of cancer. Cancer Med. 2022, 11, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.E.; Rosenbaum, L.E.; Bosenberg, M. Decoding melanoma metastasis. Cancers 2010, 3, 126–163. [Google Scholar] [CrossRef]
- Kastelan, S.; Mrazovac Zimak, D.; Ivankovic, M.; Markovic, I.; Gverovic Antunica, A. Liver metastasis in uveal melanoma—Treatment options and clinical outcome. Front. Biosci. 2022, 27, 72. [Google Scholar] [CrossRef]
- Burns, D.; George, J.; Aucoin, D.; Bower, J.; Burrell, S.; Gilbert, R.; Bower, N. The Pathogenesis and Clinical Management of Cutaneous Melanoma: An Evidence-Based Review. J. Med. Imaging Radiat. Sci. 2019, 50, 460–469.e1. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibanes, E.; Pawlik, T.M. Liver metastases. Nat. Rev. Dis. Prim. 2021, 7, 27. [Google Scholar] [CrossRef]
- DeLeve, L.D.; Maretti-Mira, A.C. Liver Sinusoidal Endothelial Cell: An Update. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2017; Volume 37, pp. 377–387. [Google Scholar] [CrossRef]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; Qurashi, M.; Shetty, S. The Role of Sinusoidal Endothelial Cells in the Axis of Inflammation and Cancer within the Liver. Front. Physiol. 2020, 11, 990. [Google Scholar] [CrossRef]
- Clark, A.M.; Ma, B.; Taylor, D.L.; Griffith, L.; Wells, A. Liver metastases: Microenvironments and ex-vivo models. Exp. Biol. Med. 2016, 241, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Cardier, J.E. Activation of the CXCR4 chemokine receptor enhances biological functions associated with B16 melanoma liver metastasis. Melanoma Res. 2017, 27, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.; Erben, U.; Kruse, N.; Wechsung, K.; Schumann, M.; Klugewitz, K.; Scheffold, A.; Kuhl, A.A. Chemokine Transfer by Liver Sinusoidal Endothelial Cells Contributes to the Recruitment of CD4+ T Cells into the Murine Liver. PLoS ONE 2015, 10, e0123867. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Pavan, W.J.; Bastian, B.C. The melanomas: A synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res. 2011, 24, 879–897. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.E.; Theodosakis, N.; Bosenberg, M. Melanoma metastasis: New concepts and evolving paradigms. Oncogene 2014, 33, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Gupta, B.; Wong, G.Y.M. Prognostic circulating proteomic biomarkers in colorectal liver metastases. Comput. Struct. Biotechnol. J. 2023, 21, 2129–2136. [Google Scholar] [CrossRef]
- Kitsel, Y.; Cooke, T.; Sotirchos, V.; Sofocleous, C.T. Colorectal Cancer Liver Metastases: Genomics and Biomarkers with Focus on Local Therapies. Cancers 2023, 15, 1679. [Google Scholar] [CrossRef]
- Rossi, E.; Croce, M.; Reggiani, F.; Schinzari, G.; Ambrosio, M.; Gangemi, R.; Tortora, G.; Pfeffer, U.; Amaro, A. Uveal Melanoma Metastasis. Cancers 2021, 13, 5684. [Google Scholar] [CrossRef]
- Karami Fath, M.; Azargoonjahromi, A.; Jafari, N.; Mehdi, M.; Alavi, F.; Daraei, M.; Mohammadkhani, N.; Mueller, A.L.; Brockmueller, A.; Shakibaei, M.; et al. Exosome application in tumorigenesis: Diagnosis and treatment of melanoma. Med. Oncol. 2022, 39, 19. [Google Scholar] [CrossRef]
- Bendas, G.; Borsig, L. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef] [PubMed]
- Reymond, N.; d’Agua, B.B.; Ridley, A.J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 2013, 13, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Strilic, B.; Offermanns, S. Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, P.; Haier, J.; Schluter, K.; Domikowsky, B.; Wendel, C.; Wiesner, U.; Kubitza, R.; Engers, R.; Schneider, S.W.; Homey, B.; et al. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia 2009, 11, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Ceausu, R.A.; Cimpean, A.M.; Gaje, P.; Gurzu, S.; Jung, I.; Raica, M. CD105/Ki67 double immunostaining expression in liver metastasis from colon carcinoma. Rom. J. Morphol. Embryol. 2011, 52, 613–616. [Google Scholar]
- Li, Y.; Zhai, Z.; Liu, D.; Zhong, X.; Meng, X.; Yang, Q.; Liu, J.; Li, H. CD105 promotes hepatocarcinoma cell invasion and metastasis through VEGF. Tumour Biol. 2015, 36, 737–745. [Google Scholar] [CrossRef]
- Vidal-Vanaclocha, F.; Fantuzzi, G.; Mendoza, L.; Fuentes, A.M.; Anasagasti, M.J.; Martin, J.; Carrascal, T.; Walsh, P.; Reznikov, L.L.; Kim, S.H.; et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc. Natl. Acad. Sci. USA 2000, 97, 734–739. [Google Scholar] [CrossRef]
- Carrascal, M.T.; Mendoza, L.; Valcarcel, M.; Salado, C.; Egilegor, E.; Telleria, N.; Vidal-Vanaclocha, F.; Dinarello, C.A. Interleukin-18 binding protein reduces b16 melanoma hepatic metastasis by neutralizing adhesiveness and growth factors of sinusoidal endothelium. Cancer Res. 2003, 63, 491–497. [Google Scholar]
- Helfrich, I.; Edler, L.; Sucker, A.; Thomas, M.; Christian, S.; Schadendorf, D.; Augustin, H.G. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin. Cancer Res. 2009, 15, 1384–1392. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef]
- Abdul Pari, A.A.; Singhal, M.; Hubers, C.; Mogler, C.; Schieb, B.; Gampp, A.; Gengenbacher, N.; Reynolds, L.E.; Terhardt, D.; Geraud, C.; et al. Tumor Cell-Derived Angiopoietin-2 Promotes Metastasis in Melanoma. Cancer Res. 2020, 80, 2586–2598. [Google Scholar] [CrossRef] [PubMed]
- Urosevic, J.; Blasco, M.T.; Llorente, A.; Bellmunt, A.; Berenguer-Llergo, A.; Guiu, M.; Canellas, A.; Fernandez, E.; Burkov, I.; Clapes, M.; et al. ERK1/2 Signaling Induces Upregulation of ANGPT2 and CXCR4 to Mediate Liver Metastasis in Colon Cancer. Cancer Res. 2020, 80, 4668–4680. [Google Scholar] [CrossRef]
- Bentebibel, S.E.; Diab, A. Cytokines in the Treatment of Melanoma. Curr. Oncol. Rep. 2021, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef]
- Ardolino, M.; Hsu, J.; Raulet, D.H. Cytokine treatment in cancer immunotherapy. Oncotarget 2015, 6, 19346–19347. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Perez-Gracia, J.L.; Rodriguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castanon, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71. [Google Scholar] [CrossRef]
- Matsuo, Y.; Raimondo, M.; Woodward, T.A.; Wallace, M.B.; Gill, K.R.; Tong, Z.; Burdick, M.D.; Yang, Z.; Strieter, R.M.; Hoffman, R.M.; et al. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int. J. Cancer 2009, 125, 1027–1037. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nannuru, K.C.; Sadanandam, A.; Varney, M.L.; Singh, R.K. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br. J. Cancer 2009, 100, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.M.; Li, J. A small-molecule antagonist of CXCR1 and CXCR2 inhibits cell proliferation, migration and invasion in melanoma via PI3K/AKT pathway. Med. Clín. 2019, 152, 425–430. [Google Scholar] [CrossRef]
- Bie, Y.; Ge, W.; Yang, Z.; Cheng, X.; Zhao, Z.; Li, S.; Wang, W.; Wang, Y.; Zhao, X.; Yin, Z.; et al. The Crucial Role of CXCL8 and Its Receptors in Colorectal Liver Metastasis. Dis. Markers 2019, 2019, 8023460. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wen, M.; Pan, D.; Lin, X.; Mo, J.; Dong, X.; Liao, S.; Ma, Y. IL-33/ST2 Axis Regulates Vasculogenic Mimicry via ERK1/2-MMP-2/9 Pathway in Melanoma. Dermatology 2019, 235, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Deng, S.; Ye, H.; Yu, X.; Deng, Q.; Zhang, Y.; Jiang, L.; Li, J.; Yu, Y.; Han, W. The IL-33/ST2 pathway suppresses murine colon cancer growth and metastasis by upregulating CD40 L signaling. Biomed. Pharm. 2020, 127, 110232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Davis, C.; Shah, S.; Hughes, D.; Ryan, J.C.; Altomare, D.; Pena, M.M. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol. Carcinog. 2017, 56, 272–287. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X.; Yang, Q.; Zhao, X.; Wen, W.; Li, G.; Lu, J.; Qin, W.; Qi, Y.; Xie, F.; et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J. Immunol. 2015, 194, 438–445. [Google Scholar] [CrossRef]
- Sotiriou, C.; Lacroix, M.; Lespagnard, L.; Larsimont, D.; Paesmans, M.; Body, J.J. Interleukins-6 and -11 expression in primary breast cancer and subsequent development of bone metastases. Cancer Lett. 2001, 169, 87–95. [Google Scholar] [CrossRef]
- Wu, C.Y.; Liu, J.F.; Tsai, H.C.; Tzeng, H.E.; Hsieh, T.H.; Wang, M.; Lin, Y.F.; Lu, C.C.; Lien, M.Y.; Tang, C.H. Interleukin-11/gp130 upregulates MMP-13 expression and cell migration in OSCC by activating PI3K/Akt and AP-1 signaling. J. Cell. Physiol. 2022, 237, 4551–4562. [Google Scholar] [CrossRef]
- Putoczki, T.L.; Thiem, S.; Loving, A.; Busuttil, R.A.; Wilson, N.J.; Ziegler, P.K.; Nguyen, P.M.; Preaudet, A.; Farid, R.; Edwards, K.M.; et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 2013, 24, 257–271. [Google Scholar] [CrossRef]
- To, S.Q.; Dmello, R.S.; Richards, A.K.; Ernst, M.; Chand, A.L. STAT3 Signaling in Breast Cancer: Multicellular Actions and Therapeutic Potential. Cancers 2022, 14, 429. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Xu, L.; Liu, H.; Zhang, W.; Liu, W.; Liu, Y.; Fu, Q.; Xu, J. High expression of interleukin-11 is an independent indicator of poor prognosis in clear-cell renal cell carcinoma. Cancer Sci. 2015, 106, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Yamazumi, K.; Nakayama, T.; Kusaba, T.; Wen, C.Y.; Yoshizaki, A.; Yakata, Y.; Nagayasu, T.; Sekine, I. Expression of interleukin-11 and interleukin-11 receptor alpha in human colorectal adenocarcinoma; immunohistochemical analyses and correlation with clinicopathological factors. World J. Gastroenterol. 2006, 12, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Koroknai, V.; Szasz, I.; Jambor, K.; Balazs, M. Cytokine and Chemokine Receptor Patterns of Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 2644. [Google Scholar] [CrossRef] [PubMed]
- Szasz, I.; Koroknai, V.; Kiss, T.; Vizkeleti, L.; Adany, R.; Balazs, M. Molecular alterations associated with acquired resistance to BRAFV600E targeted therapy in melanoma cells. Melanoma Res. 2019, 29, 390–400. [Google Scholar] [CrossRef]



| Cell Line | Growth Phase 1 | Histologic Subtype 2 | BRAF Mutation Status 3 | NRAS Mutation Status 4 |
|---|---|---|---|---|
| WM793B | RGP/VGP | SSM | V600E | wt |
| WM1361 | VGP | SSM | wt | Q61L |
| WM278 | VGP | NM | V600E | wt |
| WM983A | VGP | n.a. | V600E | wt |
| WM1366 | VGP | n.a. | wt | Q61L |
| WM3248 | VGP | n.a. | V600E | wt |
| Sample Number | Gender 1 | Age at Initial Diagnosis (Years) | Location | Histological Subtype 2 | Breslow Thickness (mm) | Ulceration |
|---|---|---|---|---|---|---|
| Primary melanoma with no metastasis 3 | ||||||
| 1 | F | 64 | Extremities | SSM | 0.4 | No |
| 2 | M | 67 | Head | NM | 0.1 | No |
| 3 | M | 72 | Trunk | NM | 4.5 | No |
| 4 | M | 59 | Trunk | SSM | 0.7 | No |
| Primary melanoma with liver metastasis 3 | ||||||
| 5 | M | 71 | Trunk | SSM | 2.3 | No |
| 6 | M | 40 | Extremities | NM | 3.0 | No |
| 7 | M | 69 | Trunk | SSM/NM | 8.0 | Yes |
| 8 | M | 63 | Trunk | SSM | 2.2 | No |
| 9 | F | 71 | Trunk | NM | 7.0 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koroknai, V.; Szász, I.; Balázs, M. Gene Expression Changes in Cytokine and Chemokine Receptors in Association with Melanoma Liver Metastasis. Int. J. Mol. Sci. 2023, 24, 8901. https://doi.org/10.3390/ijms24108901
Koroknai V, Szász I, Balázs M. Gene Expression Changes in Cytokine and Chemokine Receptors in Association with Melanoma Liver Metastasis. International Journal of Molecular Sciences. 2023; 24(10):8901. https://doi.org/10.3390/ijms24108901
Chicago/Turabian StyleKoroknai, Viktória, István Szász, and Margit Balázs. 2023. "Gene Expression Changes in Cytokine and Chemokine Receptors in Association with Melanoma Liver Metastasis" International Journal of Molecular Sciences 24, no. 10: 8901. https://doi.org/10.3390/ijms24108901
APA StyleKoroknai, V., Szász, I., & Balázs, M. (2023). Gene Expression Changes in Cytokine and Chemokine Receptors in Association with Melanoma Liver Metastasis. International Journal of Molecular Sciences, 24(10), 8901. https://doi.org/10.3390/ijms24108901








