Enantiomeric Separation and Degradation of Benoxacor Enantiomers in Horticultural Soil by Normal-Phase and Reversed-Phase High Performance Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
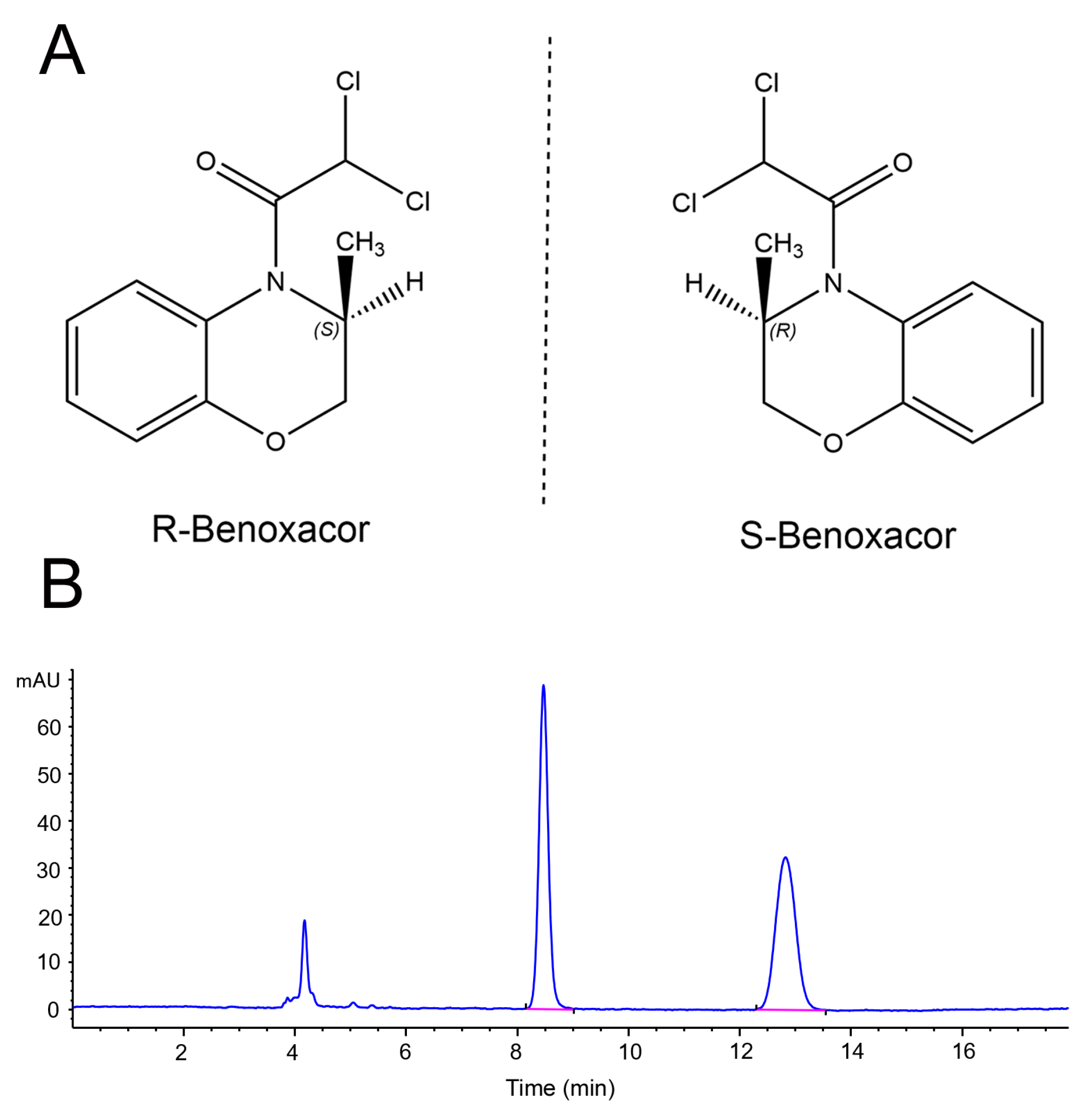
2.1. Benoxacor Enantiomer Separation with Normal-Phase HPLC
2.2. Benoxacor Enantiomer Separation with Reversed-Phase HPLC
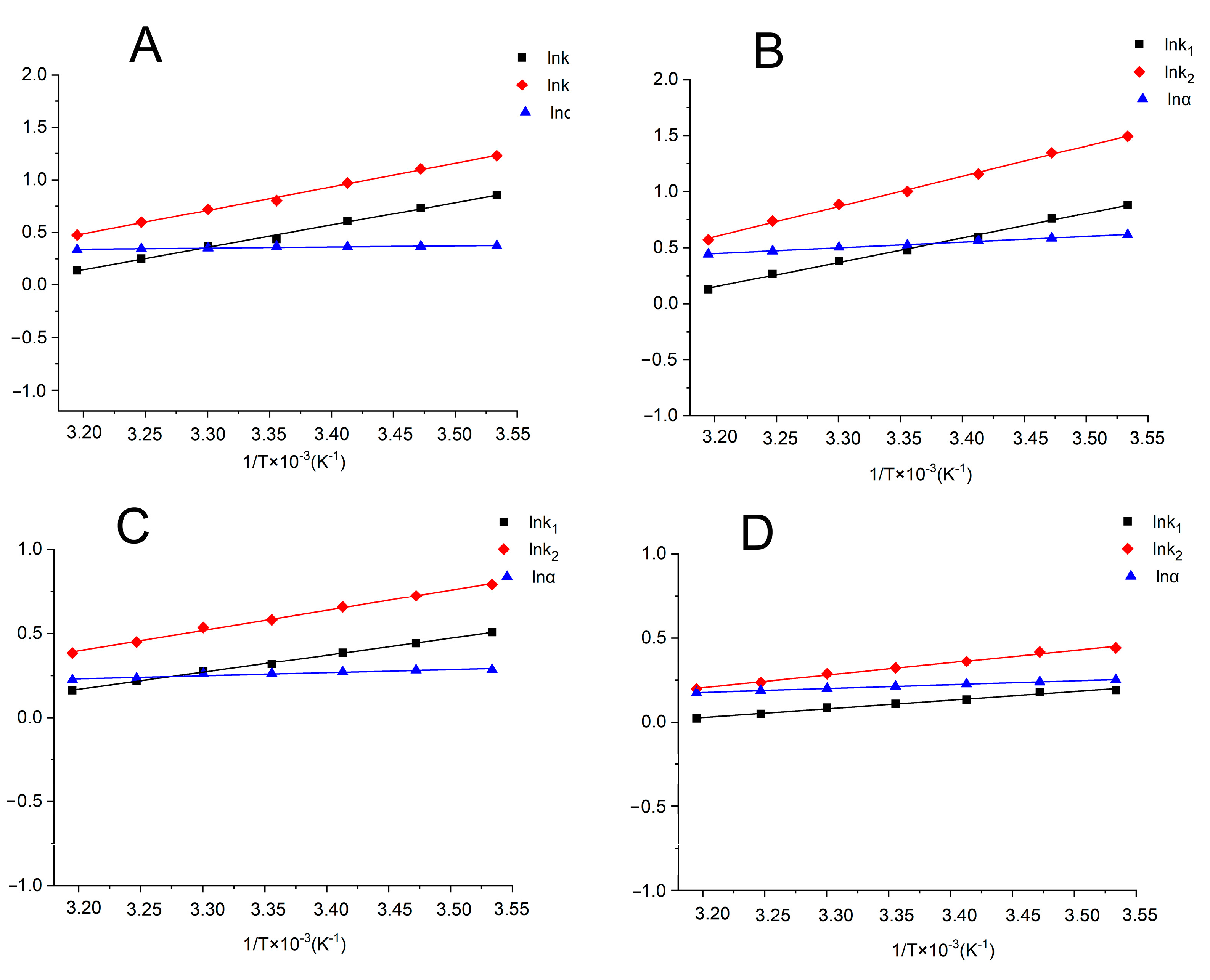
2.3. Influence of Temperature on Separation
2.4. Thermodynamic Parameters
2.5. Enantiomers Stability and Racemization in Solvents
2.6. Detection of Benoxacor Enantiomer in Horticultural Soils
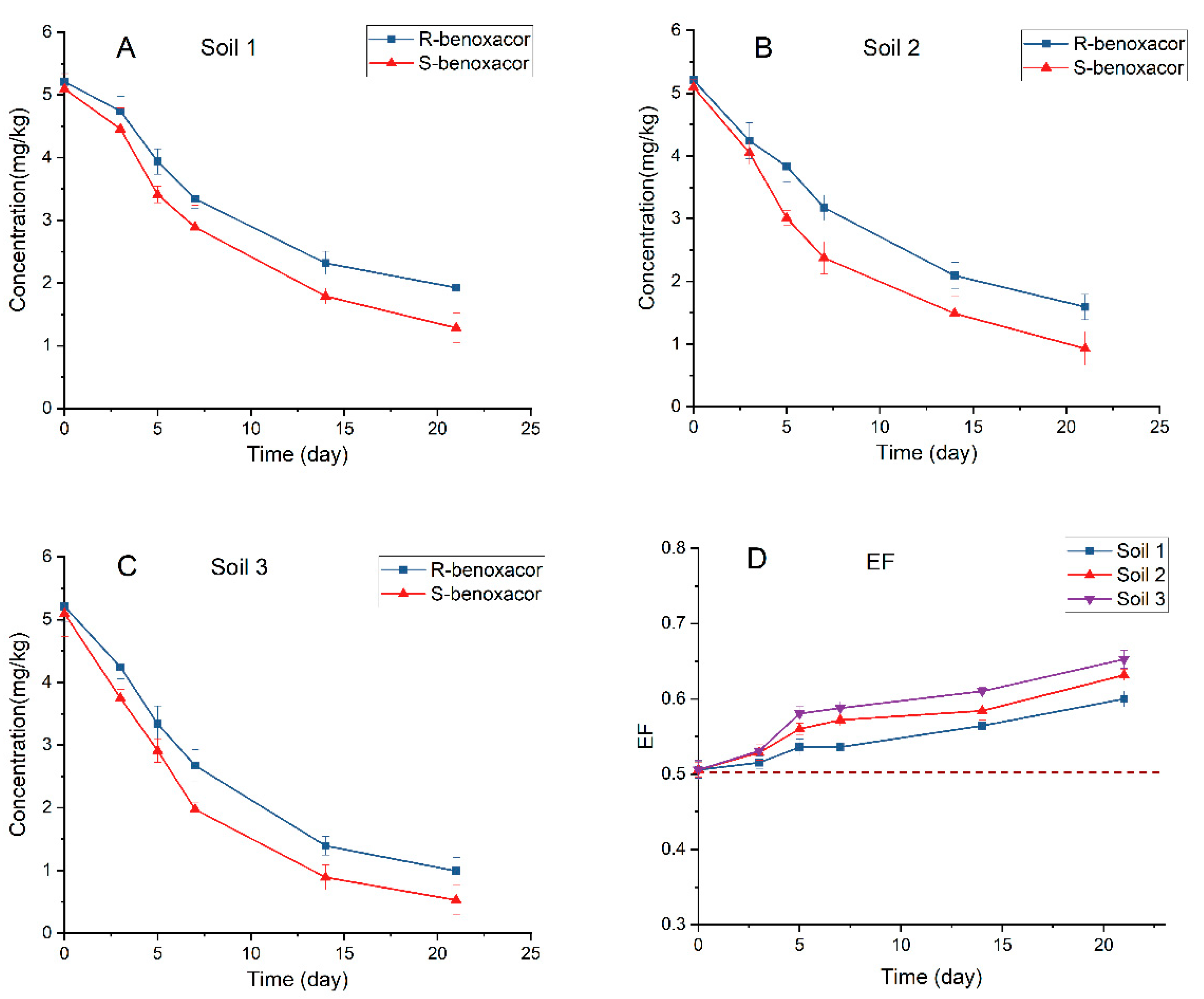
2.7. Degradation of Benoxacor Enantiomers in Horticultural Soils
3. Materials and Methods
3.1. Chemicals and Regents
3.2. Apparatus
3.3. Chiral Columns and Chromatographic Conditions
3.4. Enantiomer Stability and Racemization in Solvents
3.5. Data Analysis
3.6. Degradation of Benoxacor Enantiomers in Horticultural Soils
3.7. Sample Preparation
3.8. Benoxacor Enantiomer Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, L.; Caywood, L.M.; Sivey, J.D.; Dai, N. Sunlight Photolysis of Safener Benoxacor and Herbicide Metolachlor as Mixtures on Simulated Soil Surfaces. Environ. Sci. Technol. 2019, 53, 6784–6793. [Google Scholar] [CrossRef]
- Liu, S.; Deng, X.; Bai, L. Developmental toxicity and transcriptome analysis of zebrafish (Danio rerio) embryos following exposure to chiral herbicide safener benoxacor. Sci. Total Environ. 2021, 761, 143273. [Google Scholar] [CrossRef] [PubMed]
- Sivey, J.D.; Roberts, A.L. Abiotic Reduction Reactions of Dichloroacetamide Safeners: Transformations of “Inert” Agrochemical Constituents. Environ. Sci. Technol. 2012, 46, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Fan, J.; Chen, R.; Guo, D.; Zhao, M.; Zhang, Z.; Liang, C.; Chen, M.; Song, H.; Zhang, W. Stereoselective in vitro metabolism of cyproconazole in rat liver microsomes and identification of major metabolites. Chemosphere 2021, 264, 128495. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhao, S.; Shi, H.; Zhang, Z.; Li, L.; He, Z.; Wen, Y.; Covaci, A.; Wang, M. Enantioselective disposition and metabolic products of isofenphos-methyl in rats and the hepatotoxic effects. Environ. Int. 2020, 143, 105940. [Google Scholar] [CrossRef]
- Chai, T.; Cui, F.; Di, S.; Wu, S.; Zhang, Y.; Wang, X. New insights into cardiotoxicity induced by chiral fluoxetine at environmental-level: Enantioselective arrhythmia in developmental zebrafish (Danio rerio). Environ. Pollut. 2021, 270, 116182. [Google Scholar] [CrossRef]
- Jiang, X.; Song, B.; Wang, S.; Ran, L.; Lu, P.; Hu, D. Oxidative Stress and Enantioselective Degradation of Dufulin on Tubifex. Environ. Toxicol. Chem. 2020, 39, 2136–2146. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Tang, S.; Meng, X.; Wang, F.; Hu, D.; Zhang, Y. Enantioselective Analysis and Degradation Studies of Four Stereoisomers of Difenoconazole in Citrus by Chiral Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 501–510. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, X.; Sun, X.; Wang, Z.; Zhang, J.; Li, L.; Shi, H.; Wang, M. Enantioselective Detection, Bioactivity, and Degradation of the Novel Chiral Fungicide Oxathiapiprolin. J. Agric. Food Chem. 2021, 69, 3289–3297. [Google Scholar] [CrossRef]
- He, Z.; Li, C.; Xia, W.; Wang, Z.; Li, R.; Zhang, Y.; Wang, M. Comprehensive Enantioselectivity Evaluation of Insecticidal Activity and Mammalian Toxicity of Fenobucarb. J. Agric. Food Chem. 2022, 70, 5330–5338. [Google Scholar] [CrossRef]
- Liu, R.; Deng, Y.; Zhang, W.; He, R.; Fan, J.; Zhu, W.; Zhou, Z.; Diao, J. Risk Assessment of the Chiral Fungicide Triticonazole: Enantioselective Effects, Toxicity, and Fate. J. Agric. Food Chem. 2022, 70, 2712–2721. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jia, X.; Wu, C.; Liu, X.; Dong, F. Chiral Fungicide Famoxadone: Stereoselective Bioactivity, Aquatic Toxicity, and Environmental Behavior in Soils. J. Agric. Food Chem. 2021, 69, 8530–8535. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Magnuson, J.T.; Zhang, W.; Zhao, M. New insight into the enantioselective cytotoxicity of cypermethrin: Imbalance between cell cycle and apoptosis. J. Hazard. Mater. 2021, 403, 123893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Zhao, X.; Gao, B.; He, Z.; Li, L.; Shi, H.; Wang, M. Stereoselective uptake and metabolism of prothioconazole caused oxidative stress in zebrafish (Danio rerio). J. Hazard. Mater. 2020, 396, 122756. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, K.; Zhang, X.; Liu, T.; Wang, X. Enantioselective toxicity and oxidative stress effects of acetochlor on earthworms (Eisenia fetida) by mediating the signaling pathway. Sci. Total Environ. 2021, 766, 142630. [Google Scholar] [CrossRef]
- Li, R.; Pan, X.; Wang, Q.; Tao, Y.; Chen, Z.; Jiang, D.; Wu, C.; Dong, F.; Xu, J.; Liu, X.; et al. Development of S-Fluxametamide for Bioactivity Improvement and Risk Reduction: Systemic Evaluation of the Novel Insecticide Fluxametamide at the Enantiomeric Level. Environ. Sci. Technol. 2019, 53, 13657–13665. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, X.; Dong, F.; Duan, H.; Shao, X.; Chen, X.; Yang, T.; Wang, G.; Zheng, Y. Ecological toxicity reduction of dinotefuran to honeybee: New perspective from an enantiomeric level. Environ. Int. 2019, 130, 104854. [Google Scholar] [CrossRef]
- Fox, S.; Strasdeit, H.; Haasmann, S.; Bruckner, H. Gas chromatographic separation of stereoisomers of non-protein amino acids on modified gamma-cyclodextrin stationary phase. J. Chromatogr. A 2015, 1411, 101–109. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, S.; Liu, D.; Zhang, H.; Zhou, Z. Enantiomeric resolution of chiral pesticides by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 1577–1583. [Google Scholar] [CrossRef]
- Tian, Q.; Zhou, Z.Q.; Lv, C.G.; Yang, J.J. Direct enantiomeric separation of chiral pesticides by liquid chromatography on polysaccharide-based chiral stationary phases under reversed phase conditions. Anal. Methods 2012, 4, 2307–2317. [Google Scholar] [CrossRef]
- Perez-Fernandez, V.; Dominguez-Vega, E.; Chankvetadze, B.; Crego, A.L.; Garcia, M.A.; Marina, M.L. Evaluation of new cellulose-based chiral stationary phases Sepapak-2 and Sepapak-4 for the enantiomeric separation of pesticides by nano liquid chromatography and capillary electrochromatography. J. Chromatogr. A 2012, 1234, 22–31. [Google Scholar] [CrossRef]
- Shea, D.; Penmetsa, K.V.; Leidy, R.B. Enantiomeric and isomeric separation of pesticides by cyclodextrin-modified micellar electrokinetic chromatography. J. AOAC Int. 1999, 82, 1550–1561. [Google Scholar] [CrossRef]
- Tan, Q.; Fan, J.; Gao, R.; He, R.; Wang, T.; Zhang, Y.; Zhang, W. Stereoselective quantification of triticonazole in vegetables by supercritical fluid chromatography. Talanta 2017, 164, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dong, F.; Xu, J.; Liu, X.; Chen, Z.; Pan, X.; Chen, X.; Zheng, Y. Enantioselective separation and pharmacokinetic dissipation of cyflumetofen in field soil by ultra-performance convergence chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Baker, B.A. Chiral separations. Anal. Chem. 2008, 80, 4363–4372. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhao, M.; Liu, J.; Liu, W. Enantioselectivity in environmental risk assessment of modern chiral pesticides. Environ. Pollut. 2010, 158, 2371–2383. [Google Scholar] [CrossRef]
- Jeschke, P. Current status of chirality in agrochemicals. Pest Manag. Sci. 2018, 74, 2389–2404. [Google Scholar] [CrossRef]
- Liu, W.; Gan, J.; Schlenk, D.; Jury, W.A. Enantioselectivity in environmental safety of current chiral insecticides. Proc. Natl. Acad. Sci. USA 2005, 102, 701–706. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, Q.; He, Y.; Zhu, W.; Zhou, Z.; He, L. Chiral pyrethroid insecticide fenpropathrin and its metabolite: Enantiomeric separation and pharmacokinetic degradation in soils by reverse-phase high-performance liquid chromatography. Anal. Methods 2017, 9, 4439–4446. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.; Jiang, S.; Xu, Y.; Gu, X.; Zhou, Z. The chiral resolution of pesticides on amylose-tris(3,5-dimethylphenylcarbamate) CSP by HPLC and the enantiomeric identification by circular dichroism. Chirality 2008, 20, 40–46. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, Q.; He, X.; Qian, K.; Xiao, W.; Xu, Z.; Li, T.; He, L. Enantiomeric separation of type I and type II pyrethroid insecticides with different chiral stationary phases by reversed-phase high-performance liquid chromatography. Chirality 2018, 30, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, R.; Li, K.; Cheng, Z.; Zhong, G.; Zhang, G.; Li, J. Experimental Study on the Role of Sedimentation and Degradation Processes on Atmospheric Deposition of Persistent Organic Pollutants in a Subtropical Water Column. Environ. Sci. Technol. 2017, 51, 4424–4433. [Google Scholar] [CrossRef] [PubMed]

| Column | Mobile Phase | Ratio (v/v) | α | Rs | Mobile Phase | Ratio (v/v) | α | Rs |
|---|---|---|---|---|---|---|---|---|
| Lux Cellulose-1 | HEX/IPA | 95/5 | 1.02 | 0.38 | MEOH/H2O | 100/0 | 1.13 | 0.54 |
| 90/10 | 1.04 | 0.50 | 95/5 | 1.09 | 0.53 | |||
| 85/15 | 1.04 | 0.63 | 90/10 | 1.07 | 0.52 | |||
| 80/20 | 1.05 | 0.70 | 85/15 | 1.06 | 0.47 | |||
| 75/25 | 1.06 | 0.71 | 80/20 | 1.04 | 0.43 | |||
| HEX/ETOH | 98/2 | 1.21 | 2.57 | ACN/H2O | / | / | / | |
| 95/5 | 1.17 | 2.22 | / | / | / | |||
| 90/10 | 1.13 | 1.48 | / | / | / | |||
| 85/15 | 1.11 | 1.00 | / | / | / | |||
| 80/20 | 1.09 | 0.72 | / | / | / | |||
| 75/25 | 1.08 | 0.72 | / | / | / | |||
| Lux Cellulose-2 | HEX/IPA | / | / | / | MEOH/H2O | / | / | / |
| HEX/ETOH | 98/2 | 1.04 | 0.68 | ACN/H2O | / | / | / | |
| 95/5 | 1.06 | 0.71 | / | / | / | |||
| 90/10 | 1.06 | 0.66 | / | / | / | |||
| Lux Cellulose-3 | HEX/IPA | 95/5 | 1.49 | 4.15 | MEOH/H2O | 95/5 | 1.96 | 2.92 |
| 90/10 | 1.47 | 3.30 | 90/10 | 2.08 | 3.91 | |||
| 85/15 | 1.46 | 3.38 | 85/15 | 2.18 | 5.13 | |||
| 80/20 | 1.45 | 2.74 | 80/20 | 2.18 | 6.08 | |||
| 75/25 | 1.43 | 2.53 | / | / | / | |||
| HEX/ETOH | 98/2 | 1.52 | 4.69 | ACN/H2O | 90/10 | 2.40 | 0.95 | |
| 95/5 | 1.50 | 3.42 | 80/20 | 2.29 | 1.46 | |||
| 90/10 | 1.69 | 5.81 | 70/30 | 2.19 | 2.19 | |||
| 85/15 | 1.69 | 6.16 | 60/40 | 2.12 | 3.26 | |||
| 80/20 | 1.70 | 5.64 | 50/50 | 2.51 | 5.17 | |||
| 75/25 | 1.68 | 4.86 | / | / | / | |||
| Lux Cellulose-4 | HEX/IPA | / | / | / | MEOH/H2O | / | / | / |
| HEX/ETOH | / | / | / | ACN/H2O | / | / | / | |
| Chiralpak AD | HEX/IPA | 98/2 | 1.09 | 1.14 | MEOH/H2O | / | / | / |
| 95/5 | 1.09 | 0.98 | / | / | / | |||
| 90/10 | 1.09 | 0.88 | / | / | / | |||
| 85/15 | 1.09 | 0.84 | / | / | / | |||
| 80/20 | 1.09 | 0.81 | / | / | / | |||
| 75/25 | 1.09 | 0.79 | / | / | / | |||
| HEX/ETOH | 98/2 | 1.35 | 3.52 | ACN/H2O | / | / | / | |
| 95/5 | 1.18 | 1.79 | / | / | / | |||
| 90/10 | 1.11 | 1.23 | / | / | / | |||
| 85/15 | 1.07 | 0.81 | / | / | / | |||
| 80/20 | 1.04 | 0.53 | / | / | / | |||
| 75/25 | 1.03 | 0.34 | / | / | / | |||
| Chiralpak IC | HEX/IPA | 98/2 | 1.28 | 3.45 | MEOH/H2O | 90/10 | 1.15 | 0.57 |
| 95/5 | 1.28 | 2.38 | 85/15 | 1.14 | 0.69 | |||
| 90/10 | 1.30 | 2.66 | 80/20 | 1.13 | 0.74 | |||
| 85/15 | 1.29 | 2.17 | 75/25 | 1.12 | 0.84 | |||
| 80/20 | 1.29 | 1.83 | 70/30 | 1.11 | 0.96 | |||
| 75/25 | 1.29 | 1.79 | / | / | / | |||
| HEX/ETOH | 98/2 | 1.24 | 2.50 | ACN/H2O | / | / | / | |
| 95/5 | 1.27 | 2.97 | / | / | / | |||
| 90/10 | 1.24 | 1.50 | / | / | / | |||
| 85/15 | 1.21 | 1.28 | / | / | / | |||
| 80/20 | 1.21 | 1.37 | / | / | / | |||
| 75/25 | 1.19 | 1.04 | / | / | / |
| Column | Mobile Phase | Lnk = −△H/RT + △S/R + lnφ | R2 | △H (KJ mol−1) | △S/R + lnφ | lnα = −∆∆H/RT + ∆∆S/R | R2 | △△H (KJ mol−1) | △△S (J mol−1) |
|---|---|---|---|---|---|---|---|---|---|
| Lux Cellulose-1 | MEOH/H2O (95/5) | y = 750.98x − 3.5623 | 0.982 | −6.24 | −3.56 | y = 218.66x − 0.6079 | 0.961 | −1.82 | −5.05 |
| y = 969.63x − 4.1702 | 0.980 | −8.06 | −4.17 | ||||||
| Lux Cellulose-1 | HEX/IPA (75/25) | y = 1347x − 4.4026 | 0.994 | −11.20 | −4.40 | y = 124.7x − 0.358 | 0.959 | −1.04 | −2.98 |
| y = 1471.7x − 4.7606 | 0.995 | −12.24 | −4.76 | ||||||
| Lux Cellulose11 | HEX/ETOH (95/5) | y = 1088.3x − 3.0047 | 0.993 | −9.05 | −3.00 | y = 312.42x − 0.8844 | 0.994 | −2.60 | −7.35 |
| y = 1400.8x − 3.8891 | 0.993 | −11.65 | −3.89 | ||||||
| Lux Cellulose-2 | HEX/ETOH (95/5) | y = 849.05x − 2.4806 | 0.985 | −7.06 | −2.48 | y = 69.467x − 0.1735 | 0.958 | −0.58 | −1.44 |
| y = 918.52x − 2.654 | 0.984 | −7.64 | −2.65 | ||||||
| Lux Cellulose-3 | MEOH/H2O (90/10) | y = 1456x − 5.0499 | 0.982 | −12.11 | −5.05 | y = 268.1x − 0.2293 | 0.987 | −2.23 | −1.91 |
| y = 1724.1x − 5.2792 | 0.985 | −14.33 | −5.28 | ||||||
| Lux Cellulose-3 | ACN/H2O (60/40) | y = 532.77x − 3.5984 | 0.968 | −4.43 | −3.60 | y = 412.04x − 0.5817 | 0.952 | −3.43 | −4.84 |
| y = 944.82x − 4.1801 | 0.976 | −7.86 | −4.18 | ||||||
| Lux Cellulose-3 | HEX/IPA (80/20) | y = 2126.3x − 6.6577 | 0.995 | −17.68 | −6.66 | y = 108.67x − 0.007 | 0.866 | −0.90 | −0.06 |
| y = 2235x − 6.6647 | 0.997 | −18.58 | −6.66 | ||||||
| Lux Cellulose-3 | HEX/ETOH (85/15) | y = 2188x − 6.852 | 0.996 | −18.19 | −6.85 | y = 507.3x − 1.1748 | 0.995 | −4.22 | −9.77 |
| y = 2695.3x − 8.0267 | 0.998 | −22.41 | −8.03 | ||||||
| Lux Amylose-1 | HEX/IPA (95/5) | y = 1210x − 2.9493 | 0.997 | −10.06 | −2.95 | y = −87.024x + 0.3683 | 0.667 | 0.72 | 3.06 |
| y = 1122.9x − 2.581 | 0.990 | −9.34 | −2.58 | ||||||
| Lux Amylose-1 | HEX/ETOH (95/5) | y = 1316.1x − 3.538 | 0.977 | −10.94 | −3.54 | y = −27.113x + 0.3046 | 0.339 | 0.23 | 2.53 |
| y = 1289x − 3.2334 | 0.984 | −10.72 | −3.23 | ||||||
| Chirapak IC | MEOH/H2O (90/10) | y = 1110.5x − 3.5073 | 0.985 | −9.23 | −3.51 | y = 96.821x − 0.2132 | 0.993 | −0.80 | −1.77 |
| y = 1207.4x − 3.7205 | 0.987 | −10.04 | −3.72 | ||||||
| Chirapak IC | HEX/IPA (90/10) | y = 1015x − 3.0798 | 0.999 | −8.44 | −3.08 | y = 182.81x − 0.3551 | 0.916 | −1.52 | −2.95 |
| y = 1197.8x − 3.4348 | 0.997 | −9.96 | −3.43 | ||||||
| Chirapak IC | HEX/ETOH (95/5) | y = 511.9x − 1.6097 | 0.988 | −4.26 | −1.61 | y = 228.8x − 0.5555 | 0.999 | −1.90 | −4.62 |
| y = 740.7x − 2.1653 | 0.994 | −6.16 | −2.17 |
| Compound | Spiked Level (μg/g) | Intraday | Interday | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||||||
| Recovery (%) | CV a (%) | Recovery (%) | CV a (%) | Recovery (%) | CV a (%) | CV b (%) | ||
| R-benoxacor | 0.05 | 91.78 | 1.47 | 93.66 | 3.78 | 94.78 | 1.63 | 2.86 |
| 0.5 | 91.48 | 5.03 | 92.58 | 4.70 | 92.31 | 4.25 | 4.70 | |
| 5 | 96.57 | 2.85 | 94.46 | 1.89 | 96.16 | 5.05 | 3.66 | |
| S-benoxacor | 0.05 | 91.15 | 3.71 | 91.99 | 4.80 | 90.12 | 1.95 | 3.79 |
| 0.5 | 93.50 | 3.41 | 89.05 | 2.03 | 92.25 | 5.24 | 4.34 | |
| 5 | 92.18 | 5.61 | 93.01 | 5.77 | 93.88 | 4.00 | 5.23 | |
| Soil No. | Site | BENO Enantiomer | k (day−1) | t1/2 (day) | R2 |
|---|---|---|---|---|---|
| Soil 1 | Guangxi | R-benoxacor | 0.0500 | 13.9 | 0.8766 |
| S-benoxacor | 0.0678 | 10.2 | 0.9321 | ||
| Soil 2 | Chongqing | R-benoxacor | 0.0574 | 12.1 | 0.9114 |
| S-benoxacor | 0.0809 | 8.6 | 0.9200 | ||
| Soil 3 | Gansu | R-benoxacor | 0.0825 | 8.4 | 0.9715 |
| S-benoxacor | 0.1115 | 6.2 | 0.9783 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Qin, K.; Zhang, P.; Wang, H. Enantiomeric Separation and Degradation of Benoxacor Enantiomers in Horticultural Soil by Normal-Phase and Reversed-Phase High Performance Liquid Chromatography. Int. J. Mol. Sci. 2023, 24, 8887. https://doi.org/10.3390/ijms24108887
Zhu H, Qin K, Zhang P, Wang H. Enantiomeric Separation and Degradation of Benoxacor Enantiomers in Horticultural Soil by Normal-Phase and Reversed-Phase High Performance Liquid Chromatography. International Journal of Molecular Sciences. 2023; 24(10):8887. https://doi.org/10.3390/ijms24108887
Chicago/Turabian StyleZhu, Haoxiang, Kunrong Qin, Ping Zhang, and Haiyang Wang. 2023. "Enantiomeric Separation and Degradation of Benoxacor Enantiomers in Horticultural Soil by Normal-Phase and Reversed-Phase High Performance Liquid Chromatography" International Journal of Molecular Sciences 24, no. 10: 8887. https://doi.org/10.3390/ijms24108887
APA StyleZhu, H., Qin, K., Zhang, P., & Wang, H. (2023). Enantiomeric Separation and Degradation of Benoxacor Enantiomers in Horticultural Soil by Normal-Phase and Reversed-Phase High Performance Liquid Chromatography. International Journal of Molecular Sciences, 24(10), 8887. https://doi.org/10.3390/ijms24108887





