Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment
Abstract
1. Introduction
2. Overview of Mitochondrial Transfer and Its Mechanisms
3. Methodology
4. Methods for Measuring Mitochondrial Transfer In Vitro and In Vivo
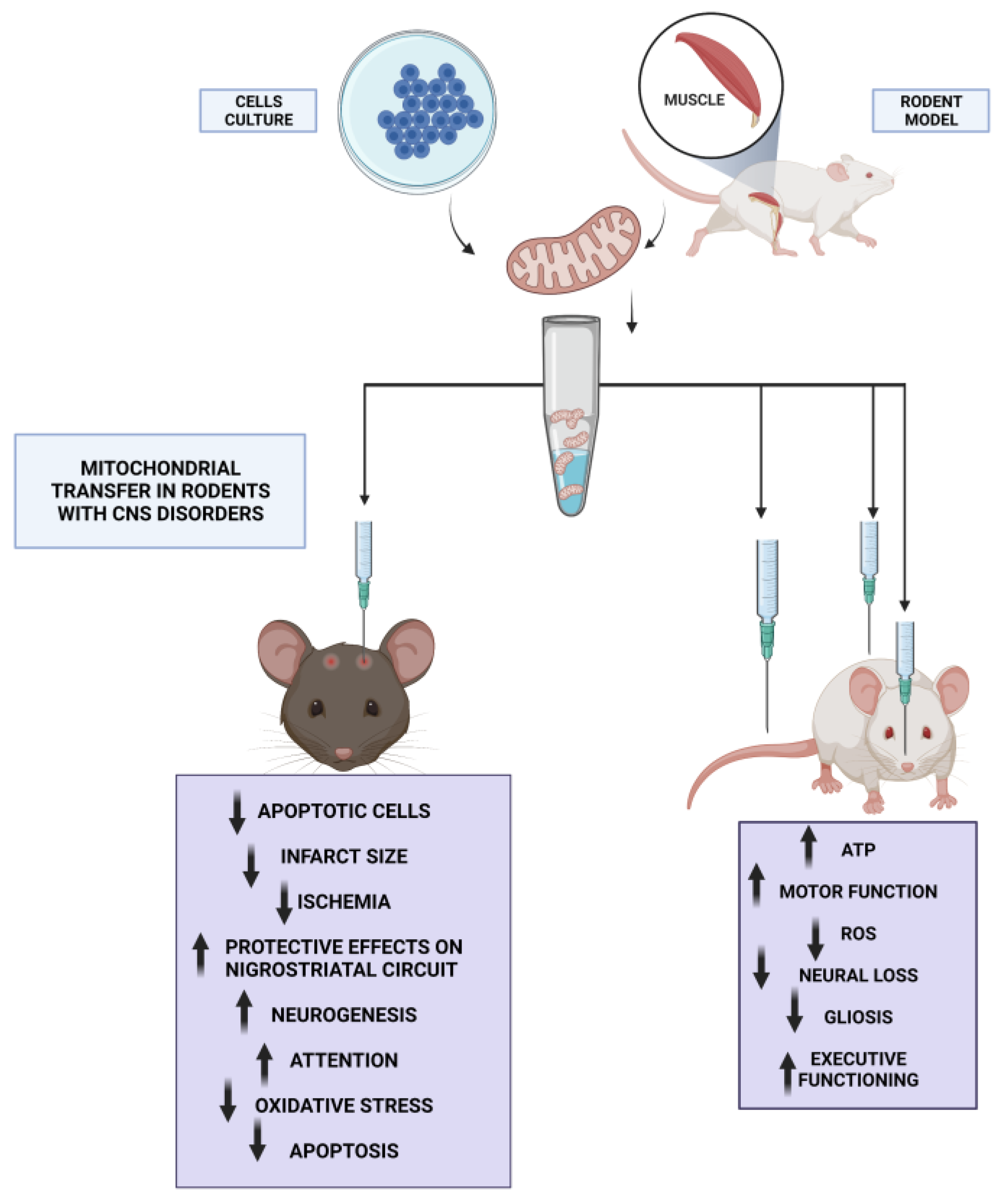
5. Therapeutic Potential of Mitochondrial Transfer in Neurodegenerative Diseases
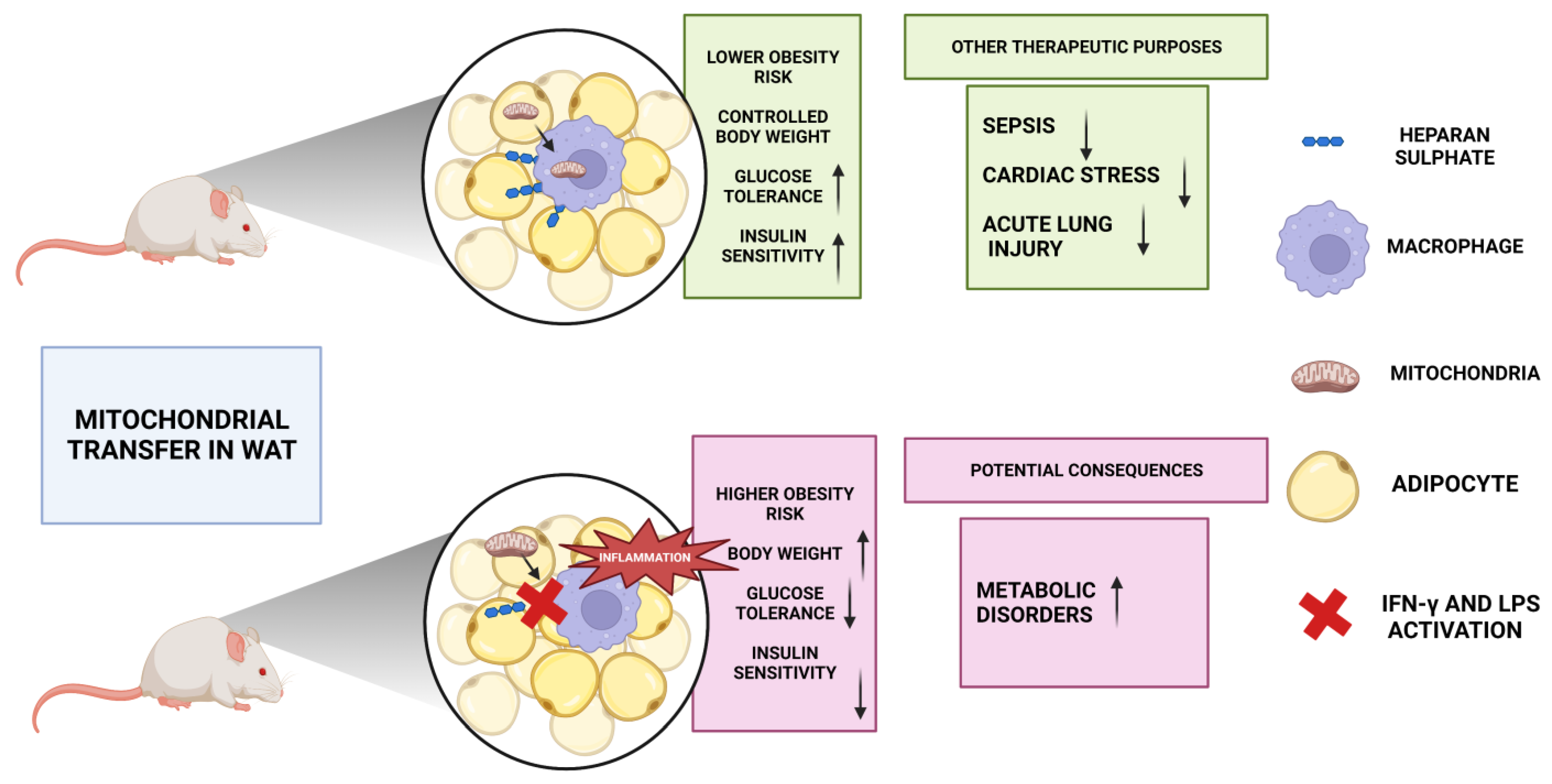
6. Therapeutic Potential of Mitochondrial Transfer in Metabolic Disorders
7. Therapeutic Potential of Mitochondrial Transfer in Cancer
7.1. Mechanisms of Mitochondrial Transfer
7.2. Implications of Mitochondrial Transfer in Cancer Cells
7.3. Potential Therapeutic Applications
8. Impact of Mitochondrial Transfer on Cell Death Pathways
9. Mitochondrial Transfer and Drug Resistance in Cancer: A Possible Approach to New Therapies
10. Mitochondrial Transfer as a Tool for Tissue Engineering and Regenerative Medicine
11. Limitations and Challenges in the Field of Mitochondrial Transfer
11.1. Availability of Donor Mitochondria
11.2. Technical Challenges
11.3. Ethical Concerns
11.4. Safety Concerns
11.5. Regulatory Challenges
11.6. Cost
12. Future Directions for the Development of Mitochondrial-Transfer-Based Therapies
13. Conclusions and Implications for the Use of Mitochondrial Transfer in Disease Diagnosis
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.T. Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Area-Gomez, E.; Groof, A.J.C.; Boldogh, I.; Bird, T.D.; Gibson, G.E.; Koehler, C.M.; Yu, W.H.; Duff, K.E.; Yaffe, M.P.; Pon, L.A.; et al. Presenilins Are Enriched in Endoplasmic Reticulum Membranes Associated with Mito-chondria. Am. J. Pathol. 2009, 175, 1810–1816. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and Reactive Oxygen Species. Free. Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S.M. More than Just a Powerhouse. Curr. Biol. 2006, 16, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Sirtori, C.; Peracino, A.; Gheorghiade, M.; Borum, P.; Remuzzi, G.; Ardehali, H. Translating the Basic Knowledge of Mitochondrial Functions to Metabolic Therapy: Role of L-Carnitine. Transl. Res. 2013, 161, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular Highways for Intercellular Organelle Transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Koyanagi, M.; Brandes, R.P.; Haendeler, J.; Zeiher, A.M.; Dimmeler, S. Cell-to-Cell Connection of Endothelial Progenitor Cells with Cardiac Myocytes by Nanotubes: A Novel Mechanism for Cell Fate Changes? Circ. Res. 2005, 96, 1039–1041. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Qi, Z.; Cao, L.; Ding, S. Mitochondrial Transfer/Transplantation: An Emerging Therapeutic Approach for Multiple Diseases. Cell Biosci. 2022, 12, 66. [Google Scholar] [CrossRef]
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial Transfer between Cells Can Rescue Aerobic Respiration. Proc. Natl. Acad. Sci. USA 2006, 103, 1283–1288. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial Transfer from Bone-Marrow-Derived Stromal Cells to Pulmonary Alveoli Protects against Acute Lung Injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Chan, S.J.; Mandeville, E.T.; Park, J.H.; Bruzzese, M.; Montaner, J.; Arai, K.; Rosell, A.; Lo, E.H. Protective Effects of Endothelial Progenitor Cell-Derived Extracellular Mitochondria in Brain Endothelium. Stem Cells 2018, 36, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Vignais, P.M.; Billoud, B.O. Classification, and Biological Function of Hydrogenases: An Overview. Chem. Rev. 2007, 107, 4206–4272. [Google Scholar] [CrossRef]
- Zhang, T.-G.; Miao, C.-Y. Mitochondrial Transplantation as a Promising Therapy for Mitochondrial Diseases. Acta Pharm. Sin. B. 2023, 13, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.; Zhu, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Wang, X.; Su, B.; Lee, H.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer’s Disease. J. Neurosci. J. Soc. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired Mitochondrial Activity in the Insulin-Resistant Offspring of Patients with Type 2 Diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef]
- Bullon, P.; Newman, H.N.; Battino, M.O. Diabetes Mellitus, Atherosclerosis and Chronic Periodontitis: A Shared Pathology via Oxidative Stress and Mitochondrial Dysfunction? Periodontol 2000, 64, 139–153. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders-A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A Key Regulator of Energy Metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Vernucci, E.; Tomino, C.; Molinari, F.; Limongi, D.; Aventaggiato, M.; Sansone, L.; Tafani, M.; Russo, M.A. Mitophagy and Oxidative Stress in Cancer and Aging: Focus on Sirtuins and Nanomaterials. Oxid. Med. Cell. Longev. 2019, 2019, 6387357. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Kras-nodembskaya, A.D. Mitochondrial Transfer via Tunneling Nanotubes Is an Important Mechanism by Which Mesen-Chymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Inter-Cellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.M.A.; Krasnodembskaya, A.D. Concise Review: Intercellular Communication Via Organelle Transfer in the Biology and Therapeutic Applications of Stem Cells. Stem Cells Dayt. Ohio 2019, 37, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Khryapenkova, T.G.; Vasileva, A.K.; Marey, M.V.; Galkina, S.I.; Isaev, N.K.; Sheval, E.V.; Polyakov, V.Y.; Sukhikh, G.T.; Zorov, D.B. Cell-to-Cell Cross-Talk between Mesenchymal Stem Cells and Cardiomyocytes in Co-Culture. J. Cell Mol. Med. 2008, 12, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Rafelski, S.M. Mitochondrial Network Morphology: Building an Integrative, Geometrical View. BMC Biol. 2013, 11, 71. [Google Scholar] [CrossRef]
- Morris, R.L.; Hollenbeck, P.J. The Regulation of Bidirectional Mitochondrial Transport Is Coordinated with Axonal Outgrowth. J. Cell Sci. 1993, 104 Pt 3, 917–927. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular Mitochondrial Transfer as a Means of Tissue Revitalization. Signal. Transduct. Target. Ther. 2021, 6, 65. [Google Scholar] [CrossRef]
- Tan, Y.L.; Eng, S.P.; Hafez, P.; Abdul Karim, N.; Law, J.X.; Ng, M.H. Mesenchymal Stromal Cell Mitochondrial Transfer as a Cell Rescue Strategy in Regenerative Medicine: A Review of Evidence in Preclinical Models. Stem Cells Transl. Med. 2022, 11, 814–827. [Google Scholar] [CrossRef]
- Zhou, F.; Gao, S.; Han, D.; Han, W.; Chen, S.; Patalano, S.; Macoska, J.A.; He, H.H.; Cai, C. TMPRSS2-ERG Activates NO-CGMP Signaling in Prostate Cancer Cells. Oncogene 2019, 38, 4397–4411. [Google Scholar] [CrossRef]
- Hough, K.P.; Trevor, J.L.; Strenkowski, J.G.; Wang, Y.; Chacko, B.K.; Tousif, S.; Chanda, D.; Steele, C.; Antony, V.B.; Dokland, T.; et al. Exosomal Transfer of Mitochondria from Airway Myeloid-Derived Regulatory Cells to T Cells. Redox Biol. 2018, 18, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, 6478. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, J.; Guerrouahen, B.S.; Al Thawadi, H.; Ghiabi, P.; Maleki, M.; Abu-Kaoud, N.; Jacob, A.; Mirshahi, M.; Galas, L.; Rafii, S.; et al. Preferential Transfer of Mitochondria from Endothelial to Cancer Cells through Tunneling Nanotubes Modulates Chemoresistance. J. Transl. Med. 2013, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mamma-Lian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Gorman, G.S.; Schaefer, A.M.; Ng, Y.; Gomez, N.; Blakely, E.L.; Alston, C.L.; Feeney, C.; Horvath, R.; Yu-Wai-Man, P.; Chinnery, P.F.; et al. Prevalence of Nuclear and Mitochondrial DNA Mutations Related to Adult Mitochondrial Disease. Ann. Neurol. 2015, 77, 753–759. [Google Scholar] [CrossRef]
- Schatten, H.; Sun, Q.-Y.; Prather, R. The Impact of Mitochondrial Function/Dysfunction on IVF and New Treatment Possibilities for Infertility. Reprod. Biol. Endocrinol. 2014, 12, 111. [Google Scholar] [CrossRef]
- Babajani, A.; Hosseini-Monfared, P.; Abbaspour, S.; Jamshidi, E.; Niknejad, H. Targeted Mitochondrial Therapy With Over-Expressed MAVS Protein From Mesenchymal Stem Cells: A New Therapeutic Approach for COVID-19. Front. Cell Dev. Biol. 2021, 9, 695362. [Google Scholar] [CrossRef]
- Smeets, H.J.M. Preventing the Transmission of Mitochondrial DNA Disorders: Selecting the Good Guys or Kicking out the Bad Guys. Reprod. Biomed. Online 2013, 27, 599–610. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria Dysfunction in the Pathogenesis of Alzheimer’s Disease: Recent Advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in Skeletal Muscle Mitochondrial Function with Aging in Humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Greenlund, L.J.; Asmann, Y.W.; Short, K.R.; McCrady, S.K.; Levine, J.A.; Nair, K.S. Impact of Endurance Training on Murine Spontaneous Activity, Muscle Mitochondrial DNA Abundance, Gene Transcripts, and Function. J. Appl. Physiol. Bethesda Md. 1985 2007, 102, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, A.; Halvatsiotis, P.; Williamson, B.; Juhasz, P.; Martin, S.; Nair, K.S. Identification of Amadori-Modified Plasma Proteins in Type 2 Diabetes and the Effect of Short-Term Intensive Insulin Treatment. Diabetes Care 2005, 28, 645–652. [Google Scholar] [CrossRef]
- Cogswell, A.M.; Stevens, R.J.; Hood, D.A. Properties of Skeletal Muscle Mitochondria Isolated from Subsarcolemmal and Intermyofibrillar Regions. Am. J. Physiol. 1993, 264, C383–C389. [Google Scholar] [CrossRef] [PubMed]
- Elander, A.; Sjöström, M.; Lundgren, F.; Scherstén, T.; Bylund-Fellenius, A.C. Biochemical and Morphometric Properties of Mitochondrial Populations in Human Muscle Fibres. Clin. Sci. 1985, 69, 153–164. [Google Scholar] [CrossRef]
- Lanza, I.R.; Nair, K.S. Functional Assessment of Isolated Mitochondria In Vitro. Methods Enzymol. 2009, 457, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Asmann, Y.W.; Stump, C.S.; Short, K.R.; Coenen-Schimke, J.M.; Guo, Z.; Bigelow, M.L.; Nair, K.S. Skeletal Muscle Mitochondrial Functions, Mitochondrial DNA Copy Numbers, and Gene Transcript Profiles in Type 2 Diabetic and Nondiabetic Subjects at Equal Levels of Low or High Insulin and Euglycemia. Diabetes 2006, 55, 3309–3319. [Google Scholar] [CrossRef]
- Grassi, D.; Howard, S.; Zhou, M.; Diaz-Perez, N.; Urban, N.T.; Guerrero-Given, D.; Kamasawa, N.; Volpicelli-Daley, L.A.; LoGrasso, P.; Lasmézas, C.I. Identification of a Highly Neurotoxic α-Synuclein Species Inducing Mitochondrial Damage and Mitophagy in Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2018, 115, E2634–E2643. [Google Scholar] [CrossRef]
- Hall, A.M.; Rhodes, G.J.; Sandoval, R.M.; Corridon, P.R.; Molitoris, B.A. In Vivo Multiphoton Imaging of Mitochondrial Structure and Function during Acute Kidney Injury. Kidney Int. 2013, 83, 72–83. [Google Scholar] [CrossRef]
- Marti Gutierrez, N.; Mikhalchenko, A.; Ma, H.; Koski, A.; Li, Y.; Dyken, C.; Tippner-Hedges, R.; Yoon, D.; Liang, D.; Hayama, T.; et al. Horizontal MtDNA Transfer between Cells Is Common during Mouse Development. iScience 2022, 25, 103901. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing Mitochondrial Dysfunction in Cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Diminished Mitochondrial DNA Integrity and Repair Capacity in OA Chondrocytes. Osteoarthr. Cartil. 2009, 17, 107–113. [CrossRef] [PubMed]
- Lee, W.T.; Sun, X.; Tsai, T.-S.; Johnson, J.L.; Gould, J.A.; Garama, D.J.; Gough, D.J.; McKenzie, M.; Trounce, I.A.; St. John, J.C. Mitochondrial DNA Haplotypes Induce Differential Patterns of DNA Methylation that Result in Differential Chromosomal Gene Expression Patterns. Cell Death Discov. 2017, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.; Yeung, K.Y.; Donoghue, J.; Baker, M.J.; Kelly, R.D.; McKenzie, M.; Johns, T.G.; St John, J.C. The Regulation of Mitochondrial DNA Copy Number in Glioblastoma Cells. Cell Death Differ. 2013, 20, 1644–1653. [Google Scholar] [CrossRef]
- Kelly, R.D.W.; Rodda, A.E.; Dickinson, A.; Mahmud, A.; Nefzger, C.M.; Lee, W.; Forsythe, J.S.; Polo, J.M.; Trounce, I.A.; McKenzie, M.; et al. Mitochondrial DNA Haplotypes Define Gene Expression Patterns in Pluripotent and Differentiating Embryonic Stem Cells. Stem Cells 2013, 31, 703–716. [Google Scholar] [CrossRef]
- Lee, W.T.Y.; Cain, J.E.; Cuddihy, A.; Johnson, J.; Dickinson, A.; Yeung, K.-Y.; Kumar, B.; Johns, T.G.; Watkins, D.N.; Spencer, A.; et al. Mitochondrial DNA Plasticity Is an Essential Inducer of Tumorigenesis. Cell Death Discov. 2016, 2, 16016. [Google Scholar] [CrossRef]
- Wallace, D.C. Bioenergetics in Human Evolution and Disease: Implications for the Origins of Biological Complexity and the Missing Genetic Variation of Common Diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120267. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Lapeña, A.C.; Díez-Sánchez, C.; Pérez-Martos, A.; Montoya, J.; Alvarez, E.; Díaz, M.; Urriés, A.; Montoro, L.; López-Pérez, M.J.; et al. Human MtDNA Haplogroups Associated with High or Reduced Spermatozoa Motility. Am. J. Hum. Genet. 2000, 67, 682–696. [Google Scholar] [CrossRef]
- Pan, J.; Wang, L.; Lu, C.; Zhu, Y.; Min, Z.; Dong, X.; Sha, H. Matching Mitochondrial DNA Haplotypes for Circumventing Tissue-Specific Segregation Bias. iScience 2019, 13, 371–379. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Sun, J.; Lo, H.T.J.; Fan, L.; Yiu, T.L.; Shakoor, A.; Li, G.; Lee, W.Y.W.; Sun, D. High-Efficiency Quantitative Control of Mitochondrial Transfer Based on Droplet Microfluidics and Its Application on Muscle Regeneration. Sci. Adv. 2022, 8, eabp9245. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, S.; Kassir, N.; Keshavarz Moraveji, M. Correction: Droplet Microfluidics: Fundamentals and Its Advanced Applications. RSC Adv. 2020, 10, 32843–32844. [Google Scholar] [CrossRef]
- Gollihue, J.L.; Patel, S.P.; Eldahan, K.C.; Cox, D.H.; Donahue, R.R.; Taylor, B.K.; Sullivan, P.G.; Rabchevsky, A.G. Effects of Mitochondrial Transplantation on Bioenergetics, Cellular Incorporation, and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 2018, 35, 1800–1818. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Heo, C.H.; Choi, D.J.; Sen, D.; Joe, E.-H.; Cho, B.R.; Kim, H.M. A Ratiometric Two-Photon Fluorescent Probe Reveals Reduction in Mitochondrial H2S Production in Parkinson’s Disease Gene Knockout Astrocytes. J. Am. Chem. Soc. 2013, 135, 9915–9923. [Google Scholar] [CrossRef]
- McCully, J.D.; Cowan, D.B.; Pacak, C.A.; Toumpoulis, I.K.; Dayalan, H.; Levitsky, S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H94–H105. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zeng, J.; Zheng, Y.; Li, T.; Ren, J.; Chen, K.; Zhang, Q.; Xie, R.; Xu, F.; Zhu, J. Mitochondrial transplantation attenuates cerebral ischemia-reperfusion injury: Possible involvement of mitochondrial component separation. Oxidative Med. Cell. Longev. 2021, 2021, 1006636. [Google Scholar] [CrossRef]
- Blitzer, D.; Guariento, A.; Doulamis, I.P.; Shin, B.; Moskowitzova, K.; Barbieri, G.R.; Orfany, A.; del Nido, P.J.; McCully, J.D. Delayed transplantation of autologous mitochondria for cardioprotection in a porcine model. Annals Thorac. Surg. 2020, 109, 711–719. [Google Scholar] [CrossRef]
- Chang, J.-C.; Wu, S.-L.; Liu, K.-H.; Chen, Y.-H.; Chuang, C.-S.; Cheng, F.-C.; Su, H.-L.; Wei, Y.-H.; Kuo, S.-J.; Liu, C.-S. Allogeneic/Xenogeneic Transplantation of Peptide-Labeled Mitochondria in Parkinson’s Disease: Restoration of Mitochondria Functions and Attenuation of 6-Hydroxydopamine-Induced Neurotoxicity. Transl. Res. J. Lab. Clin. Med. 2016, 170, 40–56.e3. [Google Scholar] [CrossRef]
- Onyango, I.G. Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer’s disease. Neural Regen. Res. 2018, 13, 19. [Google Scholar] [CrossRef]
- Nitzan, K.; Benhamron, S.; Valitsky, M.; Kesner, E.E.; Lichtenstein, M.; Ben-Zvi, A.; Ella, E.; Segalstein, Y.; Saada, A.; Lorberboum-Galski, H.; et al. Mitochondrial Transfer Ameliorates Cognitive Deficits, Neuronal Loss, and Gliosis in Alzheimer’s Disease Mice. J. Alzheimers Dis. 2019, 72, 587–604. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, L.; Li, L.; Liu, F.; Liu, J.; Chen, Y.; Cheng, J.; Lu, Y. Mitochondrial Transfer from Mesenchymal Stem Cells to Macrophages Restricts Inflammation and Alleviates Kidney Injury in Diabetic Nephropathy Mice via PGC-1α Activation. Stem Cells 2021, 39, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Konari, N.; Nagaishi, K.; Kikuchi, S.; Fujimiya, M. Mitochondria Transfer from Mesenchymal Stem Cells Structurally and Functionally Repairs Renal Proximal Tubular Epithelial Cells in Diabetic Nephropathy in Vivo. Sci. Rep. 2019, 9, 5184. [Google Scholar] [CrossRef] [PubMed]
- Doulamis, I.P.; Guariento, A.; Duignan, T.; Kido, T.; Orfany, A.; Saeed, M.Y.; Weixler, V.H.; Blitzer, D.; Shin, B.; Snay, E.R.; et al. Mitochondrial Transplantation by Intra-Arterial Injection for Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2020, 319, F403–F413. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; McCully, J.D. Mitochondrial transplantation: Applications for pediatric patients with congenital heart disease. Translational pediatrics 2018, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Guariento, A.; Piekarski, B.L.; Doulamis, I.P.; Blitzer, D.; Ferraro, A.M.; Harrild, D.M.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D.; Emani, S.M. Autologous Mitochondrial Transplantation for Cardiogenic Shock in Pediatric Patients Following Ischemia-Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2021, 162, 992–1001. [Google Scholar] [CrossRef]
- Mobarak, H.; Heidarpour, M.; Tsai, P.-S.J.; Rezabakhsh, A.; Rahbarghazi, R.; Nouri, M.; Mahdipour, M. Autologous Mitochondrial Microinjection; a Strategy to Improve the Oocyte Quality and Subsequent Reproductive Outcome during Aging. Cell Biosci. 2019, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Gamage, U.S.K.; Yamochi, T.; Saeki, N.; Morimoto, N.; Yamanaka, M.; Koike, A.; Miyamoto, Y.; Tanaka, K.; Fukuda, A.; et al. Mitochondrial Transfer into Human Oocytes Improved Embryo Quality and Clinical Outcomes in Recurrent Pregnancy Failure Cases. Int. J. Mol. Sci. 2023, 24, 2738. [Google Scholar] [CrossRef]
- Espino De la Fuente-Muñoz, C.; Arias, C. The Therapeutic Potential of Mitochondrial Transplantation for the Treatment of Neurodegenerative Disorders. Rev. Neurosci. 2021, 32, 203–217. [Google Scholar] [CrossRef]
- Davis, C.O.; Kim, K.-Y.; Bushong, E.A.; Mills, E.A.; Boassa, D.; Shih, T.; Kinebuchi, M.; Phan, S.; Zhou, Y.; Bihlmeyer, N.A.; et al. Transcellular Degradation of Axonal Mitochondria. Proc. Natl. Acad. Sci. USA 2014, 111, 9633–9638. [Google Scholar] [CrossRef]
- Park, J.-H.; Hayakawa, K. Extracellular Mitochondria Signals in CNS Disorders. Front. Cell Dev. Biol. 2021, 9, 642853. [Google Scholar] [CrossRef]
- Sweetat, S.; Nitzan, K.; Suissa, N.; Haimovich, Y.; Lichtenstein, M.; Zabit, S.; Benhamron, S.; Akarieh, K.; Mishra, K.; Barasch, D.; et al. The Beneficial Effect of Mitochondrial Transfer Therapy in 5XFAD Mice via Liver–Serum–Brain Response. Cells 2023, 12, 1006. [Google Scholar] [CrossRef]
- Atlante, A.; Amadoro, G.; Latina, V.; Valenti, D. Therapeutic Potential of Targeting Mitochondria for Alzheimer’s Disease Treatment. J. Clin. Med. 2022, 11, 6742. [Google Scholar] [CrossRef]
- Wang, W.-W.; Han, R.; He, H.-J.; Wang, Z.; Luan, X.-Q.; Li, J.; Feng, L.; Chen, S.-Y.; Aman, Y.; Xie, C.-L. Delineating the Role of Mitophagy Inducers for Alzheimer Disease Patients. Aging Dis. 2021, 12, 852–867. [Google Scholar] [CrossRef]
- Mukherjee, A.; Becerra Calixto, A.D.; Chavez, M.; Delgado, J.P.; Soto, C. Mitochondrial Transplant to Replenish Damaged Mitochondria: A Novel Therapeutic Strategy for Neurodegenerative Diseases? Prog. Mol. Biol. Transl. Sci. 2021, 177, 49–63. [Google Scholar] [CrossRef]
- Anderson, R.M.; Hadjichrysanthou, C.; Evans, S.; Wong, M.M. Why Do so Many Clinical Trials of Therapies for Alzheimer’s Disease Fail? Lancet 2017, 390, 2327–2329. [Google Scholar] [CrossRef]
- Mckean, N.E.; Handley, R.R.; Snell, R.G. A Review of the Current Mammalian Models of Alzheimer’s Disease and Challenges That Need to Be Overcome. Int. J. Mol. Sci. 2021, 22, 13168. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Vacca, R.A.; Moro, L.; Atlante, A. Mitochondria Can Cross Cell Boundaries: An Overview of the Biological Relevance, Pathophysiological Implications and Therapeutic Perspectives of Intercellular Mitochondrial Transfer. Int. J. Mol. Sci. 2021, 22, 8312. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Mahad, D. Neurodegeneration in Progressive Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028985. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Licht-Mayer, S.; Mahad, D. Targeting Mitochondria to Protect Axons in Progressive MS. Neurosci. Lett. 2019, 710, 134258. [Google Scholar] [CrossRef]
- Campbell, G.; Mahad, D.J. Mitochondrial Dysfunction and Axon Degeneration in Progressive Multiple Sclerosis. FEBS Lett. 2018, 592, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, J.; Koper, O.M.; Piechal, K.; Kemona, H. Multiple Sclerosis-Etiology and Diagnostic Potential. Postepy Hig. Med. Dosw. Online 2017, 71, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. Axonal Conduction and Injury in Multiple Sclerosis: The Role of Sodium Channels. Nat. Rev. Neurosci. 2006, 7, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Nuzzo, D. Promising Treatment for Multiple Sclerosis: Mitochondrial Transplantation. Int. J. Mol. Sci. 2022, 23, 2245. [Google Scholar] [CrossRef]
- Park, A.; Oh, M.; Lee, S.J.; Oh, K.-J.; Lee, E.-W.; Lee, S.C.; Bae, K.-H.; Han, B.S.; Kim, W.K. Mitochondrial Transplantation as a Novel Therapeutic Strategy for Mitochondrial Diseases. Int. J. Mol. Sci. 2021, 22, 4793. [Google Scholar] [CrossRef]
- Gollihue, J.L.; Patel, S.P.; Rabchevsky, A.G. Mitochondrial Transplantation Strategies as Potential Therapeutics for Central Nervous System Trauma. Neural Regen. Res. 2018, 13, 194–197. [Google Scholar] [CrossRef]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; Del Nido, P.J.; McCully, J.D. Autologous Mitochondrial Transplantation for Dysfunction after Ischemia-Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Sokolowski, J.D.; Gorick, C.M.; Kumar, J.S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M.R.; et al. Mitochondrial Dysfunction in Neurological Disorders: Exploring Mitochondrial Transplantation. NPJ Regen. Med. 2020, 5, 22. [Google Scholar] [CrossRef]
- Cai, Q.; Jeong, Y.Y. Mitophagy in Alzheimer’s Disease and Other Age-Related Neurodegenerative Diseases. Cells 2020, 9, 150. [Google Scholar] [CrossRef]
- Kubat, G.B.; Ulger, O.; Akin, S. Requirements for Successful Mitochondrial Transplantation. J. Biochem. Mol. Toxicol. 2021, 35, e22898. [Google Scholar] [CrossRef]
- Alastrue-Agudo, A.; Rodriguez-Jimenez, F.J.; Mocholi, E.L.; De Giorgio, F.; Erceg, S.; Moreno-Manzano, V. FM19G11 and Ependymal Progenitor/Stem Cell Combinatory Treatment Enhances Neuronal Preservation and Oligodendrogenesis after Severe Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 200. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B.; et al. Transplantation of Autologously Derived Mitochondria Protects the Heart from Ischemia-Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H966–H982. [Google Scholar] [CrossRef]
- Huang, P.-J.; Kuo, C.-C.; Lee, H.-C.; Shen, C.-I.; Cheng, F.-C.; Wu, S.-F.; Chang, J.-C.; Pan, H.-C.; Lin, S.-Z.; Liu, C.-S.; et al. Transferring Xenogenic Mitochondria Provides Neural Protection Against Ischemic Stress in Ischemic Rat Brains. Cell Transpl. 2016, 25, 913–927. [Google Scholar] [CrossRef]
- Roushandeh, A.M.; Kuwahara, Y.; Roudkenar, M.H. Mitochondrial Transplantation as a Potential and Novel Master Key for Treatment of Various Incurable Diseases. Cytotechnology 2019, 71, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhao, M.; Fu, C.; Fu, A. Intravenous Administration of Mitochondria for Treating Experimental Parkinson’s Disease. Mitochondrion 2017, 34, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gollihue, J.L.; Patel, S.P.; Mashburn, C.; Eldahan, K.C.; Sullivan, P.G.; Rabchevsky, A.G. Optimization of Mitochondrial Isolation Techniques for Intraspinal Transplantation Procedures. J. Neurosci. Methods 2017, 287, 1–12. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Su, H.-L.; Chang, T.-L.; Chiang, C.-Y.; Sheu, M.-L.; Cheng, F.-C.; Chen, C.-J.; Sheehan, J.; Pan, H.-C. Preven-Tion of Axonal Degeneration by Perineurium Injection of Mitochondria in a Sciatic Nerve Crush Injury Model. Neuro-Surg. 2017, 80, 475–488. [Google Scholar] [CrossRef]
- Liu, K.; Guo, L.; Zhou, Z.; Pan, M.; Yan, C. Mesenchymal Stem Cells Transfer Mitochondria into Cerebral Microvasculature and Promote Recovery from Ischemic Stroke. Microvasc. Res. 2019, 123, 74–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Yan, C.; Pu, K.; Wu, M.; Bai, J.; Li, Y.; Wang, Q. Muscle-Derived Autologous Mitochondrial Transplantation: A Novel Strategy for Treating Cerebral Ischemic Injury. Behav. Brain Res. 2019, 356, 322–331. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Li, Q.; Sun, D.; Dong, X.; Li, X.; Xin, W.; Zhang, J. Effects of Brain-Derived Mitochondria on the Function of Neuron and Vascular Endothelial Cell After Traumatic Brain Injury. World Neurosurg. 2020, 138, e1–e9. [Google Scholar] [CrossRef]
- Robicsek, O.; Ene, H.M.; Karry, R.; Ytzhaki, O.; Asor, E.; McPhie, D.; Cohen, B.M.; Ben-Yehuda, R.; Weiner, I.; Ben-Shachar, D. Isolated Mitochondria Transfer Improves Neuronal Differentiation of Schizophrenia-Derived Induced Pluripotent Stem Cells and Rescues Deficits in a Rat Model of the Disorder. Schizophr. Bull. 2018, 44, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.-Y.; Lan, J.; Esposito, E.; Ning, M.; Balaj, L.; Ji, X.; Lo, E.H.; Hayakawa, K. Extracellular Mitochondria in Cerebrospinal Fluid and Neurological Recovery After Subarachnoid Hemorrhage. Stroke 2017, 48, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C.; O’Rourke, B. Mitochondrial Transplantation in Humans: “Magical” Cure or Cause for Concern? J. Clin. Investig. 2018, 128, 5191–5194. [Google Scholar] [CrossRef]
- Alexander, J.F.; Seua, A.V.; Arroyo, L.D.; Ray, P.R.; Wangzhou, A.; Heiβ-Lückemann, L.; Schedlowski, M.; Price, T.J.; Kavelaars, A.; Heijnen, C.J. Nasal Administration of Mitochondria Reverses Chemotherapy-Induced Cognitive Deficits. Theranostics 2021, 11, 3109–3130. [Google Scholar] [CrossRef] [PubMed]
- Ulger, O.; Kubat, G.B. Therapeutic Applications of Mitochondrial Transplantation. Biochimie 2022, 195, 1–15. [Google Scholar] [CrossRef]
- Chang, J.-C.; Hoel, F.; Liu, K.-H.; Wei, Y.-H.; Cheng, F.-C.; Kuo, S.-J.; Tronstad, K.J.; Liu, C.-S. Peptide-Mediated Delivery of Donor Mitochondria Improves Mitochondrial Function and Cell Viability in Human Cybrid Cells with the MELAS A3243G Mutation. Sci. Rep. 2017, 7, 10710. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Hanipah, Z.N.; Schauer, P.R. Bariatric Surgery as a Long-Term Treatment for Type 2 Diabetes/Metabolic Syndrome. Annu. Rev. Med. 2020, 71, 1–15. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Yuan, R.; Jia, B.; Zhang, Y.; Wang, S.; Zhang, Y.; Liu, M.; Wang, T. Mitochondrial Targeting Therapeutics: Promising Role of Natural Products in Non-Alcoholic Fatty Liver Disease. Front. Pharmacol. 2021, 12, 796207. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Chu, Y.; Yun, Z.; Yan, Y.; Jin, J. Mitochondrial Transplantation: Opportunities and Challenges in the Treatment of Obesity, Diabetes, and Nonalcoholic Fatty Liver Disease. J. Transl. Med. 2022, 20, 483. [Google Scholar] [CrossRef] [PubMed]
- Babenko, V.A.; Silachev, D.N.; Popkov, V.A.; Zorova, L.D.; Pevzner, I.B.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules 2018, 23, 687. [Google Scholar] [CrossRef]
- Zappulli, V.; Friis, K.P.; Fitzpatrick, Z.; Maguire, C.A.; Breakefield, X.O. Extracellular Vesicles and Intercellular Communication within the Nervous System. J. Clin. Investig. 2016, 126, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Bátiz, L.F.; Contreras, R.A.; Ortúzar, M.I.; Kurte, M.; Elizondo-Vega, R.; et al. Mitochondrial Transfer from MSCs to T Cells Induces Treg Differentiation and Restricts Inflammatory Response. EMBO Rep. 2020, 21, 48052. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.; Lumeng, C.N. The Initiation of Metabolic Inflammation in Childhood Obesity. J. Clin. Investig. 2017, 127, 65–73. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Wollam, J.; Olefsky, J.M. An Integrated View of Immunometabolism. Cell 2018, 172, 22–40. [Google Scholar] [CrossRef]
- Maeda, H.; Kami, D.; Maeda, R.; Murata, Y.; Jo, J.-I.; Kitani, T.; Tabata, Y.; Matoba, S.; Gojo, S. TAT-Dextran-Mediated Mitochondrial Transfer Enhances Recovery from Models of Reperfusion Injury in Cultured Cardiomyocytes. J. Cell. Mol. Med. 2020, 24, 5007–5020. [Google Scholar] [CrossRef]
- Rackham, C.L.; Hubber, E.L.; Czajka, A.; Malik, A.N.; King, A.J.F.; Jones, P.M. Optimizing Beta Cell Function through Mesenchymal Stromal Cell-Mediated Mitochondria Transfer. Stem Cells 2020, 38, 574–584. [Google Scholar] [CrossRef]
- Nicolás-Ávila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martínez, L.; Sánchez-Díaz, M.; Díaz-García, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Wilen, C.B.; Moley, J.R.; Li, Y.; Zou, W.; Malvin, N.P.; Rowen, M.N.; Saunders, B.T.; Ma, H.; Mack, M.R.; et al. Intercellular Mitochondria Transfer to Macrophages Regulates White Adipose Tissue Homeostasis and Is Impaired in Obesity. Cell Metab. 2021, 33, 270–282.e8. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Microbiota and Metabolites in Metabolic Diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhang, C.; Gao, J. Macrophages as Emerging Key Players in Mitochondrial Transfers. Front. Cell Dev. Biol. 2021, 9, 747377. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; DelProposto, J.B.; Westcott, D.J.; Saltiel, A.R. Phenotypic Switching of Adipose Tissue Macrophages with Obesity Is Generated by Spatiotemporal Differences in Macrophage Subtypes. Diabetes 2008, 57, 3239–3246. [Google Scholar] [CrossRef]
- Patoli, D.; Mignotte, F.; Deckert, V.; Dusuel, A.; Dumont, A.; Rieu, A.; Jalil, A.; Van Dongen, K.; Bourgeois, T.; Gautier, T.; et al. Inhibition of Mitophagy Drives Macrophage Activation and Antibacterial Defense during Sepsis. J. Clin. Investig. 2020, 130, 5858–5874. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Chen, L.; Li, W.; Chen, L.; Wang, Y.-P. Mitochondria Transfer and Transplantation in Human Health and Diseases. Mitochondrion 2022, 65, 80–87. [Google Scholar] [CrossRef]
- McCully, J.D.; Del Nido, P.J.; Pacak, C.A.; Emani, S.M. Mitochondrial transplantation for organ recue. Am. J. Physiol. Heart Circ. Mitochondrion 2022, 64, 27–33. [Google Scholar] [CrossRef]
- Kim, M.J.; Hwang, J.W.; Yun, C.-K.; Lee, Y.; Choi, Y.-S. Delivery of Exogenous Mitochondria via Centrifugation Enhances Cellular Metabolic Function. Sci. Rep. 2018, 8, 3330. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Yeung, S.C.; Liang, Y.; Liang, X.; Ding, Y.; Ip, M.S.M.; Tse, H.-F.; Mak, J.C.W.; Lian, Q. Mitochondrial Transfer of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells to Airway Epithelial Cells Attenuates Cigarette Smoke-Induced Damage. Am. J. Respir. Cell Mol. Biol. 2014, 51, 455–465. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Lee, K.S.; Choi, K.H.; Kim, K.H.; Yang, S.C.; Han, W.K. Preconditioning Strategies for Kidney Ischemia Reperfusion Injury: Implications of the “Time-Window” in Remote Ischemic Preconditioning. PLoS ONE 2015, 10, 0124130. [Google Scholar] [CrossRef]
- Steichen, C.; Giraud, S.; Bon, D.; Barrou, B.; Badet, L.; Salamé, E.; Kerforne, T.; Allain, G.; Roumy, J.; Jayle, C.; et al. Barriers and Advances in Kidney Preservation. BioMed. Res. Int. 2018, 2018, 9206257. [Google Scholar] [CrossRef] [PubMed]
- Guariento, A.; Blitzer, D.; Doulamis, I.; Shin, B.; Moskowitzova, K.; Orfany, A.; Ramirez-Barbieri, G.; Staffa, S.J.; Zurakowski, D.; Del Nido, P.J.; et al. Preischemic Autologous Mitochondrial Transplantation by Intracoronary Injection for Myocardial Protection. J. Thorac. Cardiovasc. Surg. 2020, 160, e15–e29. [Google Scholar] [CrossRef]
- Louwagie, E.J.; Larsen, T.D.; Wachal, A.L.; Gandy, T.C.T.; Baack, M.L. Mitochondrial Transfer Improves Cardiomyocyte Bioenergetics and Viability in Male Rats Exposed to Pregestational Diabetes. Int. J. Mol. Sci. 2021, 22, 2382. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Shi, X.; Zhang, H.; Fu, B. Mitotherapy for Fatty Liver by Intravenous Administration of Exogenous Mitochondria in Male Mice. Front. Pharmacol. 2017, 8, 241. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer Metabolism: A Therapeutic Perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Solaini, G.; Sgarbi, G.; Baracca, A. Oxidative Phosphorylation in Cancer Cells. Biochim. Biophys. Acta 2011, 1807, 534–542. [Google Scholar] [CrossRef]
- Kreuzaler, P.; Panina, Y.; Segal, J.; Yuneva, M. Adapt and Conquer: Metabolic Flexibility in Cancer Growth, Invasion and Evasion. Mol. Metab. 2020, 33, 83–101. [Google Scholar] [CrossRef]
- Hekmatshoar, Y.; Nakhle, J.; Galloni, M.; Vignais, M.-L. The Role of Metabolism and Tunneling Nanotube-Mediated Intercellular Mitochondria Exchange in Cancer Drug Resistance. Biochem. J. 2018, 475, 2305–2328. [Google Scholar] [CrossRef]
- Guha, M.; Avadhani, N.G. Mitochondrial Retrograde Signaling at the Crossroads of Tumor Bioenergetics, Genetics and Epigenetics. Mitochondrion 2013, 13, 577–591. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial Mutations and Mitoepigenetics: Focus on Regulation of Oxidative Stress-Induced Responses in Breast Cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef]
- Zampieri, L.X.; Silva-Almeida, C.; Rondeau, J.D.; Sonveaux, P. Mitochondrial Transfer in Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 3245. [Google Scholar] [CrossRef]
- Yamada, M.; Emmanuele, V.; Sanchez-Quintero, M.J.; Sun, B.; Lallos, G.; Paull, D.; Zimmer, M.; Pagett, S.; Prosser, R.W.; Sauer, M.V.; et al. Genetic Drift Can Compromise Mitochondrial Replacement by Nuclear Transfer in Human Oocytes. Cell Stem Cell 2016, 18, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Karhu, E.; Wang, J.-S.; Delgado, S.; Zukerman, R.; Mittal, J.; Jhaveri, V.M. Cell Communication by Tunneling Nanotubes: Implications in Disease and Therapeutic Applications. J. Cell Physiol. 2019, 234, 1130–1146. [Google Scholar] [CrossRef] [PubMed]
- Elfawy, H.A.; Das, B. Crosstalk between Mitochondrial Dysfunction, Oxidative Stress, and Age Related Neurodegen-Erative Disease: Etiologies and Therapeutic Strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug. Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sancheti, H.; Cadenas, E. Mitochondrial Thiols in the Regulation of Cell Death Pathways. Antioxid. Redox Signal. 2012, 17, 1714–1727. [Google Scholar] [CrossRef]
- Hoffmann, L.; Rust, M.B.; Culmsee, C. Actin(g) on Mitochondria-a Role for Cofilin1 in Neuronal Cell Death Pathways. Biol. Chem. 2019, 400, 1089–1097. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The Role of Mitochondria in Metabolism and Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Newell, C.; Sabouny, R.; Hittel, D.S.; Shutt, T.E.; Khan, A.; Klein, M.S.; Shearer, J. Mesenchymal Stem Cells Shift Mi-Tochondrial Dynamics and Enhance Oxidative Phosphorylation in Recipient Cells. Front. Physiol. 2018, 9, 1572. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; Robson, C.A.; Yip, J.Y.H. Induction of Mitochondrial Alternative Oxidase in Response to a Cell Signal Pathway Down-Regulating the Cytochrome Pathway Prevents Programmed Cell Death. Plant. Physiol. 2002, 129, 1829–1842. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, X.; Yang, Q.; Zhao, J.; Zhou, Q.; Zhou, Y. The Functions, Methods, and Mobility of Mitochondrial Transfer between Cells. Front. Oncol. 2021, 11, 672781. [Google Scholar] [CrossRef] [PubMed]
- Golstein, P.; Kroemer, G. Cell Death by Necrosis: Towards a Molecular Definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Re-Sistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Moschoi, R.; Imbert, V.; Nebout, M.; Chiche, J.; Mary, D.; Prebet, T.; Saland, E.; Castellano, R.; Pouyet, L.; Collette, Y.; et al. Protective Mitochondrial Transfer from Bone Marrow Stromal Cells to Acute Myeloid Leukemic Cells during Chemotherapy. Blood 2016, 128, 253–264. [Google Scholar] [CrossRef]
- Suzuki, R.; Ogiya, D.; Ogawa, Y.; Kawada, H.; Ando, K. Targeting CAM-DR and Mitochondrial Transfer for the Treatment of Multiple Myeloma. Curr. Oncol. 2022, 29, 8529–8539. [Google Scholar] [CrossRef]
- Zampieri, L.X.; Sboarina, M.; Cacace, A.; Grasso, D.; Thabault, L.; Hamelin, L.; Vazeille, T.; Dumon, E.; Rossignol, R.; Frédérick, R.; et al. Olaparib Is a Mitochondrial Complex I Inhibitor That Kills Temozolomide-Resistant Human Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 11938. [Google Scholar] [CrossRef]
- Kollet, O.; Dar, A.; Shivtiel, S.; Kalinkovich, A.; Lapid, K.; Sztainberg, Y.; Tesio, M.; Samstein, R.M.; Goichberg, P.; Spiegel, A.; et al. Osteoclasts Degrade Endosteal Components and Promote Mobilization of Hematopoietic Progenitor Cells. Nat. Med. 2006, 12, 657–664. [Google Scholar] [CrossRef]
- Rueff, J.; Rodrigues, A.S. Cancer Drug Resistance: A Brief Overview from a Genetic Viewpoint. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–18. [Google Scholar] [CrossRef]
- Vinogradov, S.; Wei, X. Cancer Stem Cells and Drug Resistance: The Potential of Nanomedicine. Nanomedicine 2012, 7, 597–615. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]
- Tyagi, K.; Mandal, S.; Roy, A. Recent Advancements in Therapeutic Targeting of the Warburg Effect in Refractory Ovarian Cancer: A Promise towards Disease Remission. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188563. [Google Scholar] [CrossRef]
- Zong, W.-X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Zheng, L.; Tennant, D.A.; Papkovsky, D.B.; Hedley, B.A.; Kalna, G.; Watson, D.G.; Gottlieb, E. Metabolic Profiling of Hypoxic Cells Revealed a Catabolic Signature Required for Cell Survival. PLoS ONE 2011, 6, 24411. [Google Scholar] [CrossRef]
- Jin, P.; Jiang, J.; Zhou, L.; Huang, Z.; Nice, E.C.; Huang, C.; Fu, L. Mitochondrial Adaptation in Cancer Drug Resistance: Prevalence. Mech. Manag. J. Hematol. Oncol. 2022, 15, 97. [Google Scholar] [CrossRef]
- Genovese, I.; Carinci, M.; Modesti, L.; Aguiari, G.; Pinton, P.; Giorgi, C.M. Insights into Crucial Features to Overcome Cancer Chemoresistance. Int. J. Mol. Sci. 2021, 22, 4770. [Google Scholar] [CrossRef]
- Brummer, C.; Faerber, S.; Bruss, C.; Blank, C.; Lacroix, R.; Haferkamp, S.; Herr, W.; Kreutz, M.; Renner, K. Metabolic Targeting Synergizes with MAPK Inhibition and Delays Drug Resistance in Melanoma. Cancer Lett. 2019, 442, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dang, C.V. Time to Hit Pause on Mitochondria-Targeting Cancer Therapies. Nat. Med. 2023, 29, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Matula, Z.; Mikala, G.; Lukácsi, S.; Matkó, J.; Kovács, T.; Monostori, É.; Uher, F.; Vályi-Nagy, I. Stromal Cells Serve Drug Resistance for Multiple Myeloma via Mitochondrial Transfer: A Study on Primary Myeloma and Stromal Cells. Cancers 2021, 13, 3461. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Y.; Zhang, Z. Crosstalk between Oxidative Phosphorylation and Immune Escape in Cancer: A New Concept of Therapeutic Targets Selection. Cell Oncol. Dordr. 2023. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Gaskins, K.; Vasko, V.V.; Boufraqech, M.; Patel, D.; Sourbier, C.; Reece, J.; Cheng, S.-Y.; Kebebew, E.; et al. Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 2018, 24, 4030–4043. [Google Scholar] [CrossRef]
- Jin, J.; Gu, H.; Anders, N.M.; Ren, T.; Jiang, M.; Tao, M.; Peng, Q.; Rudek, M.A.; Duan, W. Metformin Protects Cells from Mutant Huntingtin Toxicity through Activation of AMPK and Modulation of Mitochondrial Dynamics. Neuromol. Med. 2016, 18, 581–592. [Google Scholar] [CrossRef]
- De, A.; Kuppusamy, G. Metformin in Breast Cancer: Preclinical and Clinical Evidence. Curr. Probl. Cancer 2020, 44, 100488. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Khawaja, K.I.; Masud, F.; Saif, M.W. Metformin and Pancreatic Cancer: Is There a Role? Cancer Chemother. Pharmacol. 2016, 77, 235–242. [Google Scholar] [CrossRef]
- Ahn, H.K.; Lee, Y.H.; Koo, K.C. Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 8540. [Google Scholar] [CrossRef]
- Alistar, A.; Morris, B.B.; Desnoyer, R.; Klepin, H.D.; Hosseinzadeh, K.; Clark, C.; Cameron, A.; Leyendecker, J.; D’Agostino, R.J.; Topaloglu, U.; et al. Safety and Tolerability of the First-in-Class Agent CPI-613 in Combination with Modified FOLFIRINOX in Patients with Metastatic Pancreatic Cancer: A Single-Centre, Open-Label, Dose-Escalation, Phase 1 Trial. Lancet Oncol. 2017, 18, 770–778. [Google Scholar] [CrossRef]
- Pardee, T.S.; Anderson, R.G.; Pladna, K.M.; Isom, S.; Ghiraldeli, L.P.; Miller, L.D.; Chou, J.W.; Jin, G.; Zhang, W.; Ellis, L.R.; et al. A Phase I Study of CPI-613 in Combination with High-Dose Cytarabine and Mitoxantrone for Relapsed or Refractory Acute Myeloid Leukemia. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 2018, 24, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, Z.; Huang, Z.; Tang, Y.; Yang, D.; Huang, J.; He, L.; Liu, M.; Chen, Z.; Teng, Y. CPI-613 Rewires Lipid Me-Tabolism to Enhance Pancreatic Cancer Apoptosis via the AMPK-ACC Signaling. J. Exp. Clin. Cancer Res. 2020, 39, 73. [Google Scholar] [CrossRef] [PubMed]
- Dijk, S.N.; Protasoni, M.; Elpidorou, M.; Kroon, A.M.; Taanman, J.-W. Mitochondria as Target to Inhibit Proliferation and Induce Apoptosis of Cancer Cells: The Effects of Doxycycline and Gemcitabine. Sci. Rep. 2020, 10, 4363. [Google Scholar] [CrossRef]
- Sahinbegovic, H.; Jelinek, T.; Hrdinka, M.; Bago, J.R.; Turi, M.; Sevcikova, T.; Kurtovic-Kozaric, A.; Hajek, R.; Simicek, M. Intercellular Mitochondrial Transfer in the Tumor Microenvironment. Cancers 2020, 12, 1787. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, A.; Dumbali, S.P.; Wenzel, P.L. Mitochondrial Transfer and Regulators of Mesenchymal Stromal Cell Function and Therapeutic Efficacy. Front. Cell Dev. Biol. 2020, 8, 603292. [Google Scholar] [CrossRef]
- Tello, J.A.; Williams, H.E.; Eppler, R.M.; Steinhilb, M.L.; Khanna, M. Animal Models of Neurodegenerative Disease: Recent Advances in Fly Highlight Innovative Approaches to Drug Discovery. Front. Mol. Neurosci. 2022, 15, 883358. [Google Scholar] [CrossRef]
- Akbar, M.; Essa, M.M.; Daradkeh, G.; Abdelmegeed, M.A.; Choi, Y.; Mahmood, L.; Song, B.-J. Mitochondrial Dysfunction and Cell Death in Neurodegenerative Diseases through Nitroxidative Stress. Brain Res. 2016, 1637, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Sendra, L.; García-Mares, A.; Herrero, M.J.; Aliño, S.F. Mitochondrial DNA Replacement Techniques to Prevent Human Mitochondrial Diseases. Int. J. Mol. Sci. 2021, 22, 551. [Google Scholar] [CrossRef] [PubMed]
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Mitochondria Donation by Mesenchymal Stem Cells: Current Under-Standing and Mitochondria Transplantation Strategies. Front. Cell Dev. Biol. 2021, 9, 653322. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Varela, C.; Labarta, E. Role of Mitochondria Transfer in Infertility: A Commentary. Cells 2022, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 626755. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef]
- Heyck, M.; Bonsack, B.; Zhang, H.; Sadanandan, N.; Cozene, B.; Kingsbury, C.; Lee, J.-Y.; Borlongan, C.V. The Brain and Eye. Treat. Cereb. Retin. Ischemia Mitochondrial Transf. Exp. Biol. Med. 2019, 244, 1485–1492. [Google Scholar]
- Saxena, N.; Taneja, N.; Shome, P.; Mani, S. Mitochondrial Donation: A Boon or Curse for the Treatment of Incurable Mitochondrial Diseases. J. Hum. Reprod. Sci. 2018, 11, 3–9. [Google Scholar] [CrossRef]
- Caicedo, A.; Aponte, P.M.; Cabrera, F.; Hidalgo, C.; Khoury, M. Artificial Mitochondria Transfer: Current Challenges, Advances, and Future Applications. Stem Cells Int. 2017, 2017, e7610414. [Google Scholar] [CrossRef]
- Committee on the Ethical and Social Policy Considerations of Novel Techniques for Prevention of Maternal Transmission of Mitochondrial DNA Diseases; Board on Health Sciences Policy; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine; Claiborne, A.; English, R.; Kahn, J.; Diseases, C. (Eds.) Introduction. In Mitochondrial Replacement Techniques: Ethical, Social, and Policy Considerations; National Academies Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Newson, A.J.; Wilkinson, S.; Wrigley, A. Ethical and Legal Issues in Mitochondrial Transfer. EMBO Mol. Med. 2016, 8, 589–591. [Google Scholar] [CrossRef]
- Craven, L.; Murphy, J.; Turnbull, D.M.; Taylor, R.W.; Gorman, G.S.; McFarland, R. Scientific and Ethical Issues in Mi-Tochondrial Donation. New. Bioeth. Multidiscip. J. Biotechnol. Body 2018, 24, 57–73. [Google Scholar] [CrossRef]
- Castro, R.J. Mitochondrial Replacement Therapy: The UK and US Regulatory Landscapes. J. Law. Biosci. 2016, 3, 726–735. [Google Scholar] [CrossRef]
- McCully, J.D.; Levitsky, S.; Del Nido, P.J.; Cowan, D.B. Mitochondrial Transplantation for Therapeutic Use. Clin. Transl. Med. 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, P.F.; Craven, L.; Mitalipov, S.; Stewart, J.B.; Herbert, M.; Turnbull, D.M. The Challenges of Mitochondrial Replacement. PLoS Genet. 2014, 10, 1004315. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J. The Mesenchymal Stromal Cells Dilemma–Does a Negative Phase III Trial of Random Donor Mesenchymal Stromal Cells in Steroid-Resistant Graft-versus-Host Disease Represent a Death Knell or a Bump in the Road? Cytotherapy 2013, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Folmes, C.D.; Ma, H.; Mitalipov, S.; Terzic, A. Mitochondria in Pluripotent Stem Cells: Stemness Regulators and Disease Targets. Curr. Opin. Genet. Dev. 2016, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Morris, J.K. New Therapeutics to Modulate Mitochondrial Function in Neurodegenerative Disorders. Curr. Pharm. Des. 2017, 23, 731–752. [Google Scholar] [CrossRef]
- Gammage, P.A.; Moraes, C.T.; Minczuk, M. Mitochondrial Genome Engineering: The Revolution May Not Be CRISPR-Ized. Trends Genet. 2018, 34, 101–110. [Google Scholar] [CrossRef]
| Preclinical Trials | |||
|---|---|---|---|
| Disease or Injury Model Animal | Outcomes | Title | Author |
| Cardiac ischemia (White Rabbits) | The transplanted mitochondria enhanced oxygen consumption, high-energy phosphate synthesis, and the induction of cytokine mediators and proteomic pathways that are important in preserving myocardial energetics. | Transplantation of Autologously Derived Mitochondria Protects the Heart from Ischemia-Reperfusion Injury | Masuzawa et al. (2013) [65] |
| Middle cerebral artery occlusion (Rats) | The transplantation of mitochondria decreased brain infarct volume and reversed neurological deficits. | Muscle-Derived Autologous Mitochondrial Transplantation: A Novel Strategy for Treating Cerebral Ischemic Injury | Zhang et al. (2019) [66] |
| Ischemia–reperfusion injury (IRI) (Pigs) | Preischemic MT by single or serial intracoronary injections provides prophylactic myocardial protection from IRI. | Preischemic Autologous Mitochondrial Transplantation by Intracoronary Injection for Myocardial Protection | Guariento et al. (2018) [67] |
| Parkinson’s disease (Rats) | Allogeneic and xenogeneic transplantation of peptide-labeled mitochondria after 3 months improved the locomotive activity in the PD rats. | Allogeneic/Xenogeneic Transplantation of Peptide-Labeled Mitochondria in Parkinson’s Disease: Restoration of Mitochondria Functions and Attenuation of 6-Hydroxydopamine-Induced Neurotoxicity | Chang et al. (2016) [68] |
| Brain ischemic (Rats) | Significantly restored the motor performance. | Transferring Xenogenic Mitochondria Provides Neural Protection Against Ischemic Stress in Ischemic Rat Brains | Huang et al. (2016) [69] |
| Alzheimer’s disease (Mice) | A significantly better cognitive performance was noticed in the mitochondria-treated AD mice. | Mitochondrial Transfer Ameliorates Cognitive Deficits, Neuronal Loss, and Gliosis in Alzheimer’s Disease Mice | Nitzan et al. (2019) [70] |
| Diabetic nephropathy (Mice) | MSCs elicited macrophages into anti-inflammatory phenotype and ameliorated kidney injury through mitochondrial transfer. | Mitochondrial Transfer from Mesenchymal Stem Cells to Macrophages Restricts Inflammation and Alleviates Kidney Injury in Diabetic Nephropathy Mice via PGC-1α Activation. | Yuan et al. (2021) [71] |
| Diabetes (Rats) | Isolated Mt also inhibited nuclear translocation of PGC-1α and restored the expression of megalin and SGLT2 under high glucose condition (HG) in PTECs. | Mitochondria Transfer from Mesenchymal Stem Cells Structurally and Functionally Repairs Renal Proximal Tubular Epithelial Cells in Diabetic Nephropathy in Vivo | Konari et al. (2019) [72] |
| Acute renal injury (Pigs) | Mitochondrial transplantation by intra-arterial injection provides renal protection from ischemia–reperfusion injury. | Mitochondrial Transplantation by Intra-Arterial Injection for Acute Kidney Injury | Doulamis et al. (2020) [73] |
| Clinical Trials | |||
|---|---|---|---|
| Disease | Outcomes | Title | Author |
| Acute respiratory distress syndrome | MSCs promote an anti-inflammatory and highly phagocytic macrophage phenotype through EV-mediated mitochondrial transfer. | Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle 1281 Mitochondrial Transfer | Morrison et al. (2017) [74] |
| Myocardial ischemia | All five patients showed qualitative improvement in left ventricular function within days, without short-term complications. | Autologous Mitochondrial Transplantation for Dysfunction after Ischemia-Reperfusion Injury | Emani et al. (2017) [75] |
| Ischemia–reperfusion injury | MT was associated with successful separation from ECMO and enhanced ventricular strain in patients requiring postcardiotomy ECMO for severe refractory cardiogenic shock after IRI. | Autologous Mitochondrial Transplantation for Cardiogenic Shock in Pediatric Patients Following Ischemia-Reperfusion Injury | Gauriento et al. (2020) [76] |
| Infertility | Mitochondrial transfer from ovarian cells and healthy oocytes could lead to improved fertility outcome in low-quality oocytes. | Autologous Mitochondrial Microinjection: A Strategy to Improve the Oocyte Quality and Subsequent Reproductive Outcome During Aging | Mobarak et al. (2019) [77] |
| Infertility | Mitochondrial transfer technology is a beneficial clinical option to improve oocyte quality and the subsequent clinical results for patients with recurrent failures. | Mitochondrial Transfer into Human Oocytes Improved Embryo Quality and Clinical Outcomes in Recurrent Pregnancy Failure Cases | Morimoto et al. (2023) [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 8848. https://doi.org/10.3390/ijms24108848
Clemente-Suárez VJ, Martín-Rodríguez A, Yáñez-Sepúlveda R, Tornero-Aguilera JF. Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment. International Journal of Molecular Sciences. 2023; 24(10):8848. https://doi.org/10.3390/ijms24108848
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Alexandra Martín-Rodríguez, Rodrigo Yáñez-Sepúlveda, and José Francisco Tornero-Aguilera. 2023. "Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment" International Journal of Molecular Sciences 24, no. 10: 8848. https://doi.org/10.3390/ijms24108848
APA StyleClemente-Suárez, V. J., Martín-Rodríguez, A., Yáñez-Sepúlveda, R., & Tornero-Aguilera, J. F. (2023). Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment. International Journal of Molecular Sciences, 24(10), 8848. https://doi.org/10.3390/ijms24108848








