Genome-Wide Identification and Comparative Analysis of RALF Gene Family in Legume and Non-Legume Species
Abstract
1. Introduction
2. Results
2.1. Identification and Molecular Characterization of RALFs in Legume and Non-Legume Genomes
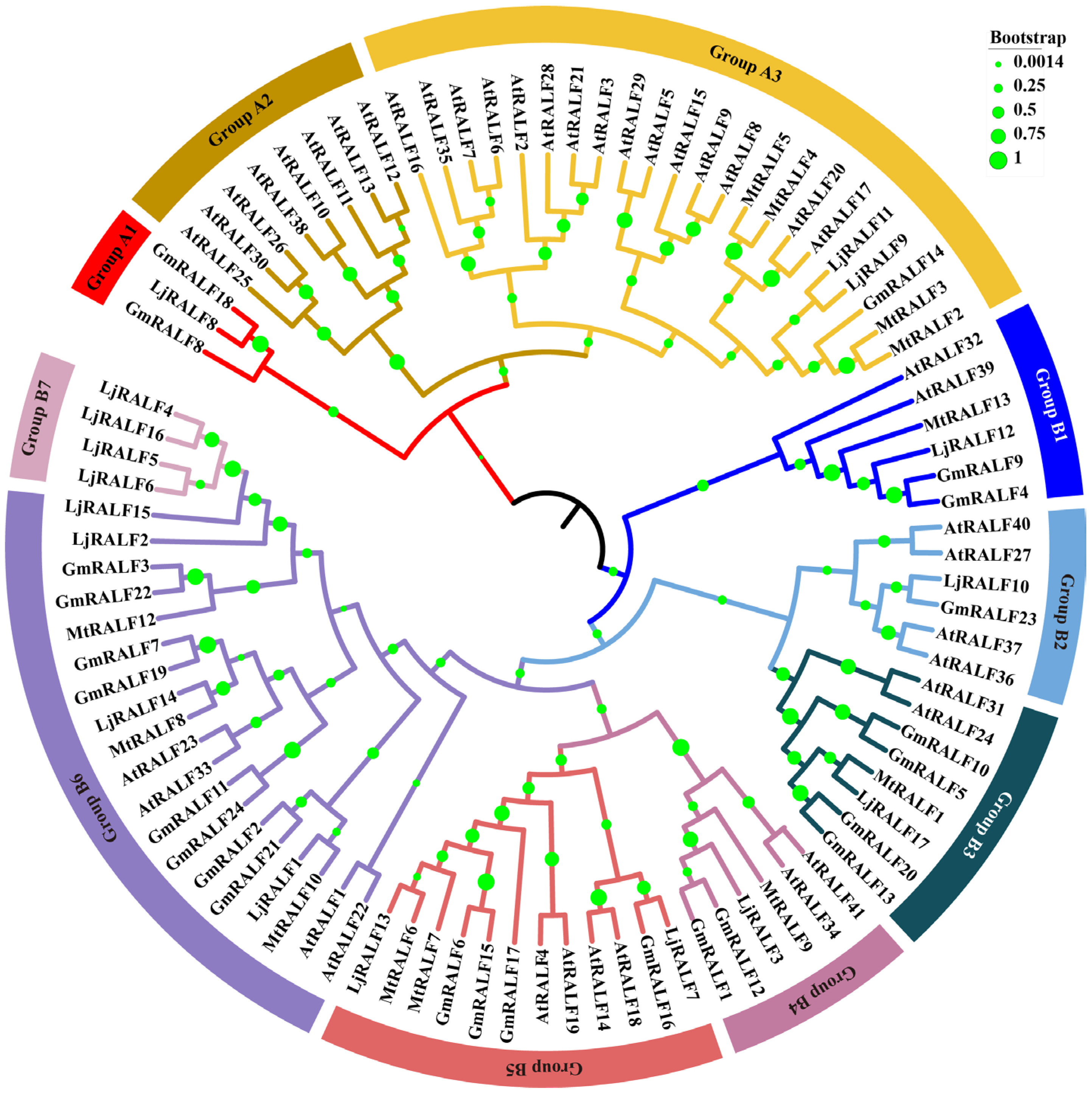
2.2. Phylogenetic Relationships and Classification of 94 RALF Family Genes
2.3. Conserved Motifs and Residues Analysis of 94 RALFs
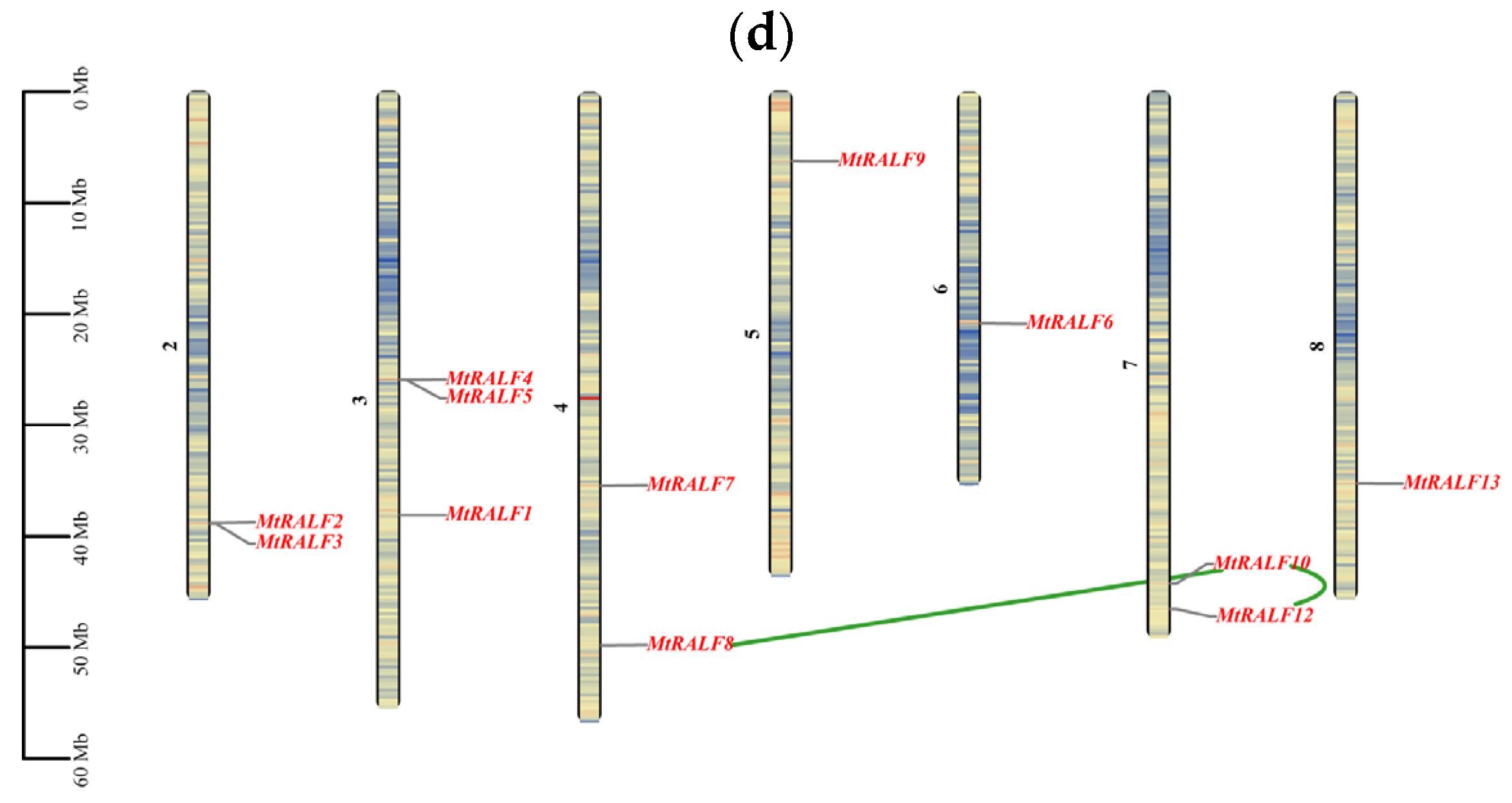
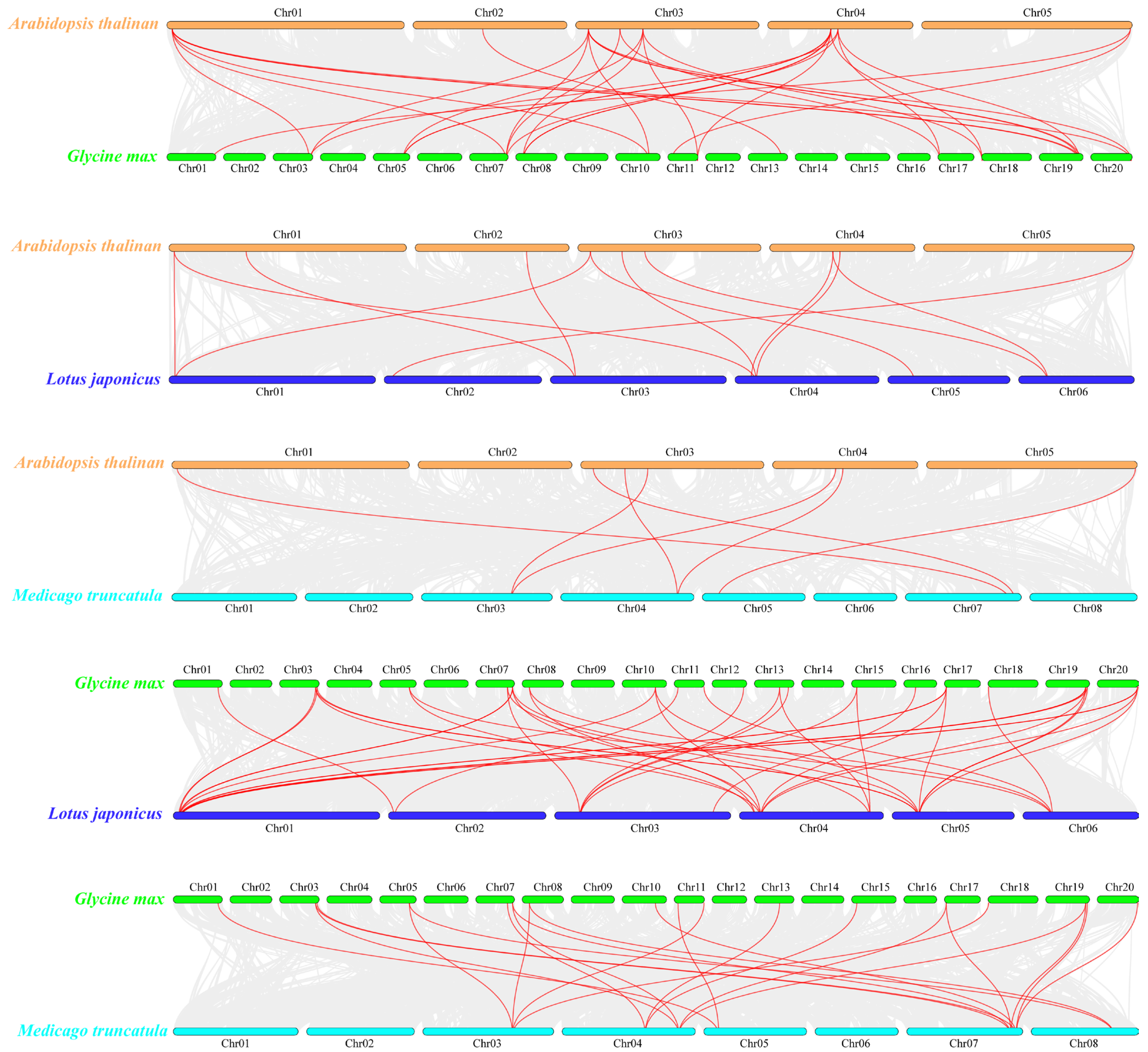
2.4. Chromosome Distribution and Synteny Analysis of RALF Family Genes
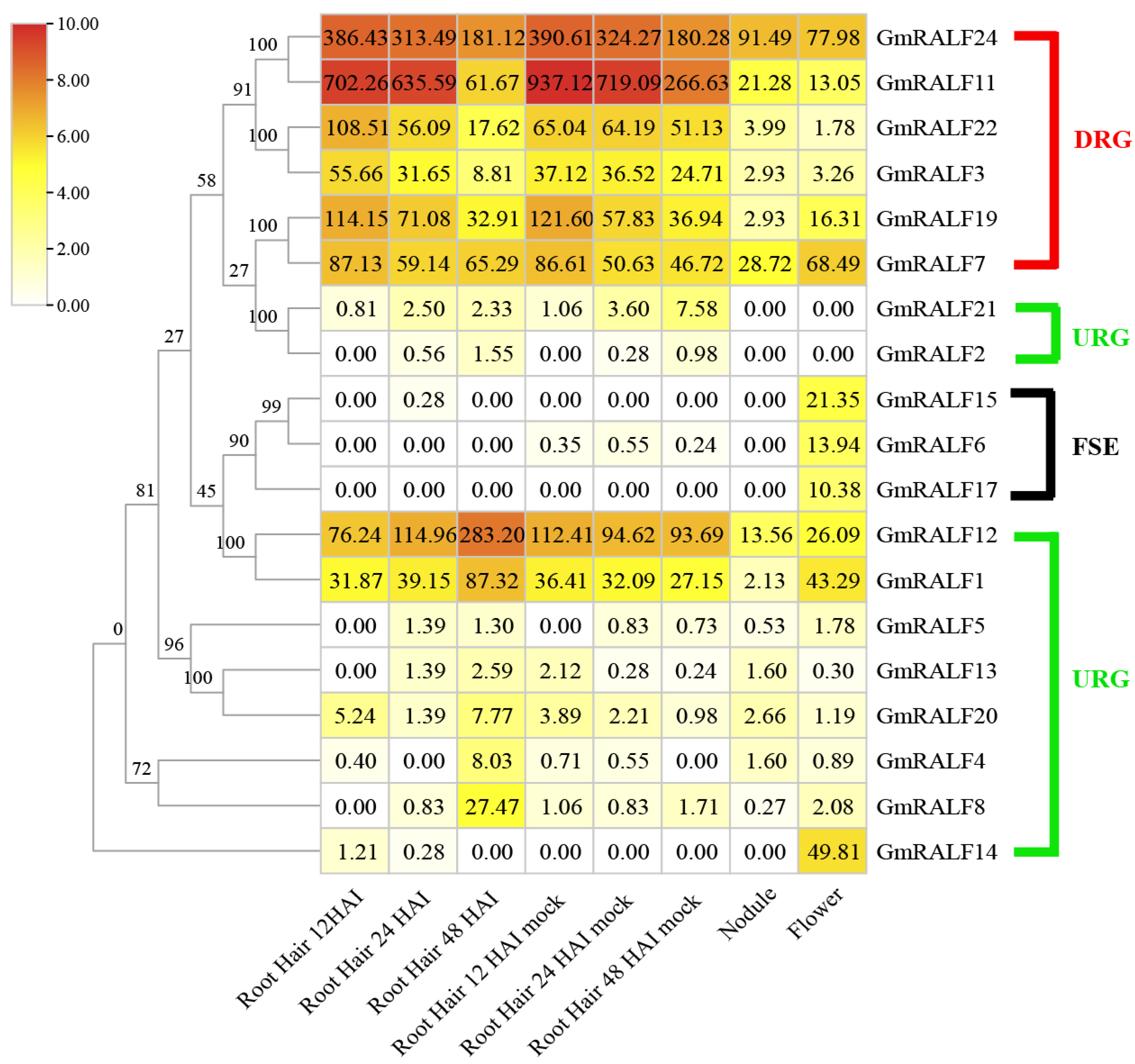
2.5. Expression Profile of GmRALFs in Soybean in Response to Bradyrhizobium Japonium Symbiosis
3. Discussion
4. Materials and Methods
4.1. Identification of RALF Family Members in Legume and Non-Legume Genomes
4.2. Sequence Alignments and Phylogenetic Tree Construction of RALF Members
4.3. Chromosome Locations and Synteny Analysis
4.4. Expression Analysis of Soybean RALF Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RALF | Rapid alkanization factor |
| At | Arabidopsis thaliana |
| Gm | Glycine max |
| Lj | Lotus japonicus |
| Mt | Medicago truncatula |
| MW | Molecular weight |
| Pi | Isoelectric point |
| Aa | Amino acid |
| CDS | Coding sequences |
| Ka/Ks | Non-synonymous/synonymous substitution |
| DRG | Down-regulated genes |
| URG | Up-regulated genes |
| FSE | Flower specific expression genes |
| Mb | Megabases |
| Kb | Kilobase |
References
- Sanders, D.; Bethke, P. Membrane transport. In Biochemistry and Molecular Biology of Plants; American Society of Plant Biologists: Rockville, MD, USA, 2000; pp. 110–158. [Google Scholar]
- Nurnberger, T.; Nennstiel, D.; Jabs, T.; Sacks, W.R.; Hahlbrock, K.; Scheel, D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 1994, 78, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999, 18, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; McEvoy, J.L.; Leverentz, B.; Conway, W.S. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol. Plant Microbe Interact. 2001, 14, 1105–1113. [Google Scholar] [CrossRef]
- Huffaker, A.; Pearce, G.; Ryan, C.A. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. USA 2006, 103, 10098–10103. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Regenass, M.; Boller, T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: Induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. Cell Mol. Biol. 2010, 4, 307–316. [Google Scholar] [CrossRef]
- Broughton, W.J.; Jabbouri, S.; Perret, X. Keys to symbiotic harmony. J. Bacteriol. 2000, 182, 5641–5652. [Google Scholar] [CrossRef]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sonksen, C.P.; Ludvigsen, S.; Raventos, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef]
- Phoenix, D.A.; Harris, F.; Dennison, S.R. Antimicrobial Peptides with pH-Dependent Activity and Alkaline Optima: Their Origins, Mechanisms of Action and Potential Applications. Curr. Protein Pept. Sci. 2021, 22, 775–799. [Google Scholar] [CrossRef]
- Ohkubo, T.; Matsumoto, Y.; Ogasawara, Y.; Sugita, T. Alkaline stress inhibits the growth of Staphylococcus epidermidis by inducing TCA cycle-triggered ROS production. Biochem. Biophys. Res. Commun. 2022, 588, 104–110. [Google Scholar] [CrossRef]
- Prusky, D.; Yakoby, N. Pathogenic fungi: Leading or led by ambient pH? Mol. Plant Pathol. 2003, 4, 509–516. [Google Scholar] [CrossRef]
- Motomitsu, A.; Sawa, S.; Ishida, T. Plant peptide hormone signalling. Essays Biochem. 2015, 58, 115–131. [Google Scholar] [PubMed]
- Kereszt, A.; Mergaert, P.; Montiel, J.; Endre, G.; Kondorosi, E. Impact of Plant Peptides on Symbiotic Nodule Development and Functioning. Front. Plant Sci. 2018, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, M.A.; Mohd-Radzman, N.A.; Imin, N. Small-peptide signals that control root nodule number, development, and symbiosis. J. Exp. Bot. 2015, 66, 5171–5181. [Google Scholar] [CrossRef]
- Imin, N.; Mohd-Radzman, N.A.; Ogilvie, H.A.; Djordjevic, M.A. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J. Exp. Bot. 2013, 64, 5395–5409. [Google Scholar] [CrossRef]
- Charon, C.; Johansson, C.; Kondorosi, E.; Kondorosi, A.; Crespi, M. enod40 induces dedifferentiation and division of root cortical cells in legumes. Proc. Natl. Acad. Sci. USA 1997, 94, 8901–8906. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, H.; Zhang, Z.; Yu, L.; Xu, X.; Hong, Z.; Luo, L. Phytosulfokine Is Involved in Positive Regulation of Lotus japonicus Nodulation. Mol. Plant Microbe Interact. 2015, 28, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Guefrachi, I.; Nagymihaly, M.; Pislariu, C.I.; Van de Velde, W.; Ratet, P.; Mars, M.; Udvardi, M.K.; Kondorosi, E.; Mergaert, P.; Alunni, B. Extreme specificity of NCR gene expression in Medicago truncatula. BMC Genom. 2014, 15, 712. [Google Scholar] [CrossRef]
- Kevei, Z.; Vinardell, J.M.; Kiss, G.B.; Kondorosi, A.; Kondorosi, E. Glycine-rich proteins encoded by a nodule-specific gene family are implicated in different stages of symbiotic nodule development in Medicago spp. Mol. Plant Microbe Interact. 2002, 15, 922–931. [Google Scholar] [CrossRef]
- Laporte, P.; Satiat-Jeunemaitre, B.; Velasco, I.; Csorba, T.; Van de Velde, W.; Campalans, A.; Burgyan, J.; Arevalo-Rodriguez, M.; Crespi, M. A novel RNA-binding peptide regulates the establishment of the Medicago truncatula-Sinorhizobium meliloti nitrogen-fixing symbiosis. Plant J. 2010, 62, 24–38. [Google Scholar] [CrossRef]
- Reid, D.E.; Ferguson, B.J.; Gresshoff, P.M. Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Mol. Plant Microbe Interact. 2011, 24, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Combier, J.P.; Kuster, H.; Journet, E.P.; Hohnjec, N.; Gamas, P.; Niebel, A. Evidence for the involvement in nodulation of the two small putative regulatory peptide-encoding genes MtRALFL1 and MtDVL1. Mol. Plant Microbe Interact. 2008, 21, 1118–1127. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Wu, J.; Wang, X.W. Cloning and expression analysis of a pollen preferential rapid alkalinization factor gene, BoRALF1, from broccoli flowers. Mol. Biol. Rep. 2010, 37, 3273–3281. [Google Scholar] [CrossRef] [PubMed]
- Covey, P.A.; Subbaiah, C.C.; Parsons, R.L.; Pearce, G.; Lay, F.T.; Anderson, M.A.; Ryan, C.A.; Bedinger, P.A. A pollen-specific RALF from tomato that regulates pollen tube elongation. Plant Physiol. 2010, 153, 703–715. [Google Scholar] [CrossRef]
- Mecchia, M.A.; Santos-Fernandez, G.; Duss, N.N.; Somoza, S.C.; Boisson-Dernier, A.; Gagliardini, V.; Martinez-Bernardini, A.; Fabrice, T.N.; Ringli, C.; Muschietti, J.P.; et al. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 2017, 358, 1600–1603. [Google Scholar] [CrossRef]
- Ge, Z.; Bergonci, T.; Zhao, Y.; Zou, Y.; Du, S.; Liu, M.C.; Luo, X.; Ruan, H.; Garcia-Valencia, L.E.; Zhong, S.; et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 2017, 358, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Gonneau, M.; Desprez, T.; Martin, M.; Doblas, V.G.; Bacete, L.; Miart, F.; Sormani, R.; Hematy, K.; Renou, J.; Landrein, B.; et al. Receptor Kinase THESEUS1 Is a Rapid Alkalinization Factor 34 Receptor in Arabidopsis. Curr. Biol. 2018, 28, 2452–2458.e4. [Google Scholar] [CrossRef]
- Zhu, S.; Estevez, J.M.; Liao, H.; Zhu, Y.; Yang, T.; Li, C.; Wang, Y.; Li, L.; Liu, X.; Pacheco, J.M.; et al. The RALF1-FERONIA Complex Phosphorylates eIF4E1 to Promote Protein Synthesis and Polar Root Hair Growth. Mol. Plant 2020, 13, 698–716. [Google Scholar] [CrossRef]
- Pearce, G.; Moura, D.S.; Stratmann, J.; Ryan, C.A., Jr. RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. USA 2001, 98, 12843–12847. [Google Scholar] [CrossRef]
- Masachis, S.; Segorbe, D.; Turra, D.; Leon-Ruiz, M.; Furst, U.; El Ghalid, M.; Leonard, G.; Lopez-Berges, M.S.; Richards, T.A.; Felix, G.; et al. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 2016, 1, 16043. [Google Scholar] [CrossRef] [PubMed]
- Thynne, E.; Saur, I.M.L.; Simbaqueba, J.; Ogilvie, H.A.; Gonzalez-Cendales, Y.; Mead, O.; Taranto, A.; Catanzariti, A.M.; McDonald, M.C.; Schwessinger, B.; et al. Fungal phytopathogens encode functional homologues of plant rapid alkalinization factor (RALF) peptides. Mol. Plant. Pathol. 2017, 18, 811–824. [Google Scholar] [CrossRef]
- Wood, A.K.M.; Walker, C.; Lee, W.S.; Urban, M.; Hammond-Kosack, K.E. Functional evaluation of a homologue of plant rapid alkalinisation factor (RALF) peptides in Fusarium graminearum. Fungal Biol. 2020, 124, 753–765. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Chen, J.; Wu, D.; Feng, X.; Yu, F. Nematode RALF-Like 1 Targets Soybean Malectin-Like Receptor Kinase to Facilitate Parasitism. Front. Plant Sci. 2021, 12, 775508. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kurten, E.L.; Monshausen, G.; Hummel, G.M.; Gilroy, S.; Baldwin, I.T. NaRALF, a peptide signal essential for the regulation of root hair tip apoplastic pH in Nicotiana attenuata, is required for root hair development and plant growth in native soils. Plant J. 2007, 52, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Haerizadeh, F.; Wong, C.E.; Bhalla, P.L.; Gresshoff, P.M.; Singh, M.B. Genomic expression profiling of mature soybean (Glycine max) pollen. BMC Plant Biol. 2009, 9, 25. [Google Scholar] [CrossRef]
- Stegmann, M.; Monaghan, J.; Smakowska-Luzan, E.; Rovenich, H.; Lehner, A.; Holton, N.; Belkhadir, Y.; Zipfel, C. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 2017, 355, 287–289. [Google Scholar] [CrossRef]
- Xiao, Y.; Stegmann, M.; Han, Z.; DeFalco, T.A.; Parys, K.; Xu, L.; Belkhadir, Y.; Zipfel, C.; Chai, J. Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature 2019, 572, 270–274. [Google Scholar] [CrossRef]
- Zhu, S.; Martinez Pacheco, J.; Estevez, J.M.; Yu, F. Autocrine regulation of root hair size by the RALF-FERONIA-RSL4 signaling pathway. New Phytol. 2020, 227, 45–49. [Google Scholar] [CrossRef]
- Tang, J.; Wu, D.; Li, X.; Wang, L.; Xu, L.; Zhang, Y.; Xu, F.; Liu, H.; Xie, Q.; Dai, S.; et al. Plant immunity suppression via PHR1-RALF-FERONIA shapes the root microbiome to alleviate phosphate starvation. EMBO J. 2022, 41, e109102. [Google Scholar] [CrossRef]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zayed, O.; Yu, Z.; Jiang, W.; Zhu, P.; Hsu, C.C.; Zhang, L.; Tao, W.A.; Lozano-Duran, R.; Zhu, J.K. Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 13123–13128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, H.; Zhu, S.; Xing, J.; Li, X.; Zhu, Z.; Zheng, J.; Wang, L.; Wang, B.; Chen, J.; et al. Nematode-Encoded RALF Peptide Mimics Facilitate Parasitism of Plants through the FERONIA Receptor Kinase. Mol. Plant 2020, 13, 1434–1454. [Google Scholar] [CrossRef] [PubMed]
- Solis-Miranda, J.; Juarez-Verdayes, M.A.; Nava, N.; Rosas, P.; Leija-Salas, A.; Cardenas, L.; Quinto, C. The Phaseolus vulgaris Receptor-Like Kinase PvFER1 and the Small Peptides PvRALF1 and PvRALF6 Regulate Nodule Number as a Function of Nitrate Availability. Int. J. Mol. Sci. 2023, 24, 5230. [Google Scholar] [CrossRef]
- Olsen, A.N.; Mundy, J.; Skriver, K. Peptomics, identification of novel cationic Arabidopsis peptides with conserved sequence motifs. Silico Biol. 2002, 2, 441–451. [Google Scholar]
- Matos, J.L.; Fiori, C.S.; Silva-Filho, M.C.; Moura, D.S. A conserved dibasic site is essential for correct processing of the peptide hormone AtRALF1 in Arabidopsis thaliana. FEBS Lett. 2008, 582, 3343–3347. [Google Scholar] [CrossRef]
- Pearce, G.; Yamaguchi, Y.; Munske, G.; Ryan, C.A. Structure-activity studies of RALF, Rapid Alkalinization Factor, reveal an essential--YISY--motif. Peptides 2010, 31, 1973–1977. [Google Scholar] [CrossRef]
- Frederick, R.O.; Haruta, M.; Tonelli, M.; Lee, W.; Cornilescu, G.; Cornilescu, C.C.; Sussman, M.R.; Markley, J.L. Function and solution structure of the Arabidopsis thaliana RALF8 peptide. Protein Sci. 2019, 28, 1115–1126. [Google Scholar] [CrossRef]
- Srivastava, R.; Liu, J.X.; Guo, H.; Yin, Y.; Howell, S.H. Regulation and processing of a plant peptide hormone, AtRALF23, in Arabidopsis. Plant J. 2009, 59, 930–939. [Google Scholar] [CrossRef]
- Sharma, A.; Hussain, A.; Mun, B.G.; Imran, Q.M.; Falak, N.; Lee, S.U.; Kim, J.Y.; Hong, J.K.; Loake, G.J.; Ali, A.; et al. Comprehensive analysis of plant rapid alkalization factor (RALF) genes. Plant Physiol. Biochem. 2016, 106, 82–90. [Google Scholar] [CrossRef]
- Campbell, L.; Turner, S.R. A Comprehensive Analysis of RALF Proteins in Green Plants Suggests There Are Two Distinct Functional Groups. Front. Plant Sci. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jing, X.; Chen, Y.; Liu, Z.; Xin, Y.; Qiao, Y. The Genome-Wide Analysis of RALF-Like Genes in Strawberry (Wild and Cultivated) and Five Other Plant Species (Rosaceae). Genes 2020, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Shi, F. Evolution of the RALF Gene Family in Plants: Gene Duplication and Selection Patterns. Evol. Bioinform. Online 2012, 8, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; De Smet, I. Understanding the RALF family: A tale of many species. Trends Plant Sci. 2014, 19, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, M.R.; Haruta, M.; Moura, D.S. Twenty Years of Progress in Physiological and Biochemical Investigation of RALF Peptides. Plant Physiol. 2020, 182, 1657–1666. [Google Scholar] [CrossRef]
- Abarca, A.; Franck, C.M.; Zipfel, C. Family-wide evaluation of RAPID ALKALINIZATION FACTOR peptides. Plant Physiol. 2021, 187, 996–1010. [Google Scholar] [CrossRef]
- Song, Q.; Jenkins, J.; Jia, G.; Hyten, D.L.; Pantalone, V.; Jackson, S.A.; Schmutz, J.; Cregan, P.B. Construction of high resolution genetic linkage maps to improve the soybean genome sequence assembly Glyma1.01. BMC Genom. 2016, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, F.; Wu, P.; Wang, K.; Cao, Y. A High-Quality Genome Sequence of Model Legume Lotus japonicus (MG-20) Provides Insights into the Evolution of Root Nodule Symbiosis. Genes 2020, 11, 483. [Google Scholar] [CrossRef]
- Tang, H.; Krishnakumar, V.; Bidwell, S.; Rosen, B.; Chan, A.; Zhou, S.; Gentzbittel, L.; Childs, K.L.; Yandell, M.; Gundlach, H.; et al. An improved genome release (version Mt4.0) for the model legume Medicago truncatula. BMC Genom. 2014, 15, 312. [Google Scholar] [CrossRef]
- Li, H.T.; Yi, T.S.; Gao, L.M.; Ma, P.F.; Zhang, T.; Yang, J.B.; Gitzendanner, M.A.; Fritsch, P.W.; Cai, J.; Luo, Y.; et al. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 2019, 5, 461–470. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, X.; Gao, Y.; Yang, S. Genome-Wide Identification and Characterization of TALE Superfamily Genes in Soybean (Glycine max L.). Int. J. Mol. Sci. 2021, 22, 4117. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Nolan, T.M.; Song, G.; Liu, S.; Xie, Z.; Chen, J.; Schnable, P.S.; Walley, J.W.; Yin, Y. FERONIA Receptor Kinase Contributes to Plant Immunity by Suppressing Jasmonic Acid Signaling in Arabidopsis thaliana. Curr. Biol. 2018, 28, 3316–3324.e6. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.; Downie, J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef]
- Andrio, E.; Marino, D.; Marmeys, A.; de Segonzac, M.D.; Damiani, I.; Genre, A.; Huguet, S.; Frendo, P.; Puppo, A.; Pauly, N. Hydrogen peroxide-regulated genes in the Medicago truncatula-Sinorhizobium meliloti symbiosis. New Phytol. 2013, 198, 179–189. [Google Scholar] [CrossRef]
- Holt, D.B.; Gupta, V.; Meyer, D.; Abel, N.B.; Andersen, S.U.; Stougaard, J.; Markmann, K. micro RNA 172 (miR172) signals epidermal infection and is expressed in cells primed for bacterial invasion in Lotus japonicus roots and nodules. New Phytol. 2015, 208, 241–256. [Google Scholar] [CrossRef]
- Liu, C.W.; Breakspear, A.; Stacey, N.; Findlay, K.; Nakashima, J.; Ramakrishnan, K.; Liu, M.; Xie, F.; Endre, G.; de Carvalho-Niebel, F.; et al. A protein complex required for polar growth of rhizobial infection threads. Nat. Commun. 2019, 10, 2848. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Jiang, H.; Shui, Z.; Zhong, Y.; Shang, J.; Yang, H.; Sun, X.; Du, J. Genome-wide characterization of soybean RALF genes and their expression responses to Fusarium oxysporum. Front. Plant Sci. 2022, 13, 1006028. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S. pI Determination of Native Proteins In Biological Samples. Curr. Protoc. Protein Sci. 2019, 96, e85. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Khan, A.; Hashem, A.; Abd Allah, E.F.; Al-Harrasi, A. The molecular mass and isoelectric point of plant proteomes. BMC Genom. 2019, 20, 631. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; Gao, Y.; Yang, S. Genome-wide identification and characterization of GRAS genes in soybean (Glycine max). BMC Plant Biol. 2020, 20, 415. [Google Scholar] [CrossRef]
- Haruta, M.; Monshausen, G.; Gilroy, S.; Sussman, M.R. A cytoplasmic Ca2+ functional assay for identifying and purifying endogenous cell signaling peptides in Arabidopsis seedlings: Identification of AtRALF1 peptide. Biochemistry 2008, 47, 6311–6321. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Lilley, C.J.; Urwin, P.E. Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiol. 2013, 162, 2028–2041. [Google Scholar] [CrossRef]
- Zhao, W.; Cheng, Y.; Zhang, C.; You, Q.; Shen, X.; Guo, W.; Jiao, Y. Genome-wide identification and characterization of circular RNAs by high throughput sequencing in soybean. Sci. Rep. 2017, 7, 5636. [Google Scholar] [CrossRef] [PubMed]
- Minami, E.; Kouchi, H.; Cohn, J.R.; Ogawa, T.; Stacey, G. Expression of the early nodulin, ENOD40, in soybean roots in response to various lipo-chitin signal molecules. Plant J. 1996, 10, 23–32. [Google Scholar] [CrossRef]
- Kumagai, H.; Kinoshita, E.; Ridge, R.W.; Kouchi, H. RNAi knock-down of ENOD40s leads to significant suppression of nodule formation in Lotus japonicus. Plant Cell Physiol. 2006, 47, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Libault, M.; Farmer, A.; Brechenmacher, L.; Drnevich, J.; Langley, R.J.; Bilgin, D.D.; Radwan, O.; Neece, D.J.; Clough, S.J.; May, G.D.; et al. Complete transcriptome of the soybean root hair cell, a single-cell model, and its alteration in response to Bradyrhizobium japonicum infection. Plant Physiol. 2010, 152, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Wang, L.; Hu, Y.; Yan, Q.; Lu, J.; Ren, Z.; Hong, Y.; Ji, H.; Wang, H.; et al. The B-type response regulator GmRR11d mediates systemic inhibition of symbiotic nodulation. Nat. Commun. 2022, 13, 7661. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Krishnakumar, V.; Chan, A.P.; Thibaud-Nissen, F.; Schobel, S.; Town, C.D. Araport11: A complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 2017, 89, 789–804. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.; Nelson, R.T.; Cannon, S.B.; Shoemaker, R.C. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res. 2010, 38, D843–D846. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Bannai, H.; Tamada, Y.; Maruyama, O.; Nakai, K.; Miyano, S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 2002, 18, 298–305. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Libault, M.; Farmer, A.; Joshi, T.; Takahashi, K.; Langley, R.J.; Franklin, L.D.; He, J.; Xu, D.; May, G.; Stacey, G. An integrated transcriptome atlas of the crop model Glycine max, and its use in comparative analyses in plants. Plant J. 2010, 63, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D.; Debellé, F.; Oldroyd, G.E.; Geurts, R.; Cannon, S.B.; Udvardi, M.K.; Benedito, V.A.; Mayer, K.F.; Gouzy, J.; Schoof, H.; et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 2011, 480, 520–524. [Google Scholar] [CrossRef] [PubMed]

| Species | Arabidopsis | Soybean | Lotus | Medicago |
|---|---|---|---|---|
| Gene number | 41 | 24 | 17 | 12 |
| Predicted S1P cleavage site (%) | 29.27 | 83.33 | 52.94 | 66.67 |
| YI/LXY motif (%) | 29.27 | 70.83 | 47.06 | 58.33 |
| Leu-11 | 26.83 | 83.33 | 52.94 | 66.67 |
| Conserved Cysteine 1 (%) | 26.83 | 79.17 | 52.94 | 66.67 |
| Conserved Cysteine 2 (%) | 26.83 | 66.67 | 41.18 | 50.00 |
| Conserved Cysteine 3 (%) | 29.27 | 83.33 | 52.94 | 66.67 |
| Conserved Cysteine 4 (%) | 29.27 | 79.17 | 52.94 | 66.67 |
| Mean Percentage (%) | 28.05 | 77.08 | 50.00 | 62.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Li, Y. Genome-Wide Identification and Comparative Analysis of RALF Gene Family in Legume and Non-Legume Species. Int. J. Mol. Sci. 2023, 24, 8842. https://doi.org/10.3390/ijms24108842
Jia Y, Li Y. Genome-Wide Identification and Comparative Analysis of RALF Gene Family in Legume and Non-Legume Species. International Journal of Molecular Sciences. 2023; 24(10):8842. https://doi.org/10.3390/ijms24108842
Chicago/Turabian StyleJia, Yancui, and Youguo Li. 2023. "Genome-Wide Identification and Comparative Analysis of RALF Gene Family in Legume and Non-Legume Species" International Journal of Molecular Sciences 24, no. 10: 8842. https://doi.org/10.3390/ijms24108842
APA StyleJia, Y., & Li, Y. (2023). Genome-Wide Identification and Comparative Analysis of RALF Gene Family in Legume and Non-Legume Species. International Journal of Molecular Sciences, 24(10), 8842. https://doi.org/10.3390/ijms24108842






