The High Level of RANKL Improves IκB/p65/Cyclin D1 Expression and Decreases p-Stat5 Expression in Firm Udder of Dairy Goats
Abstract
1. Introduction
2. Results
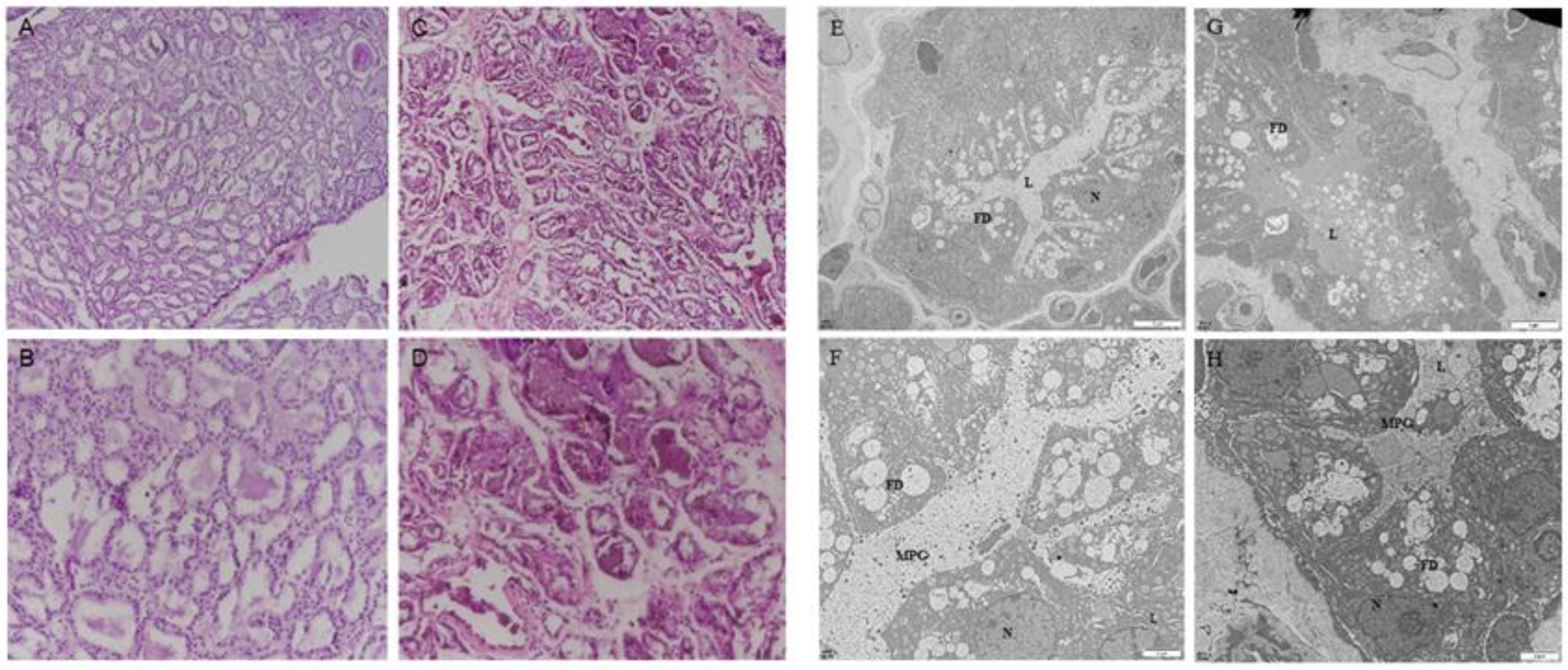
2.1. The Firm Udder Structure of Guanzhong Dairy Goats
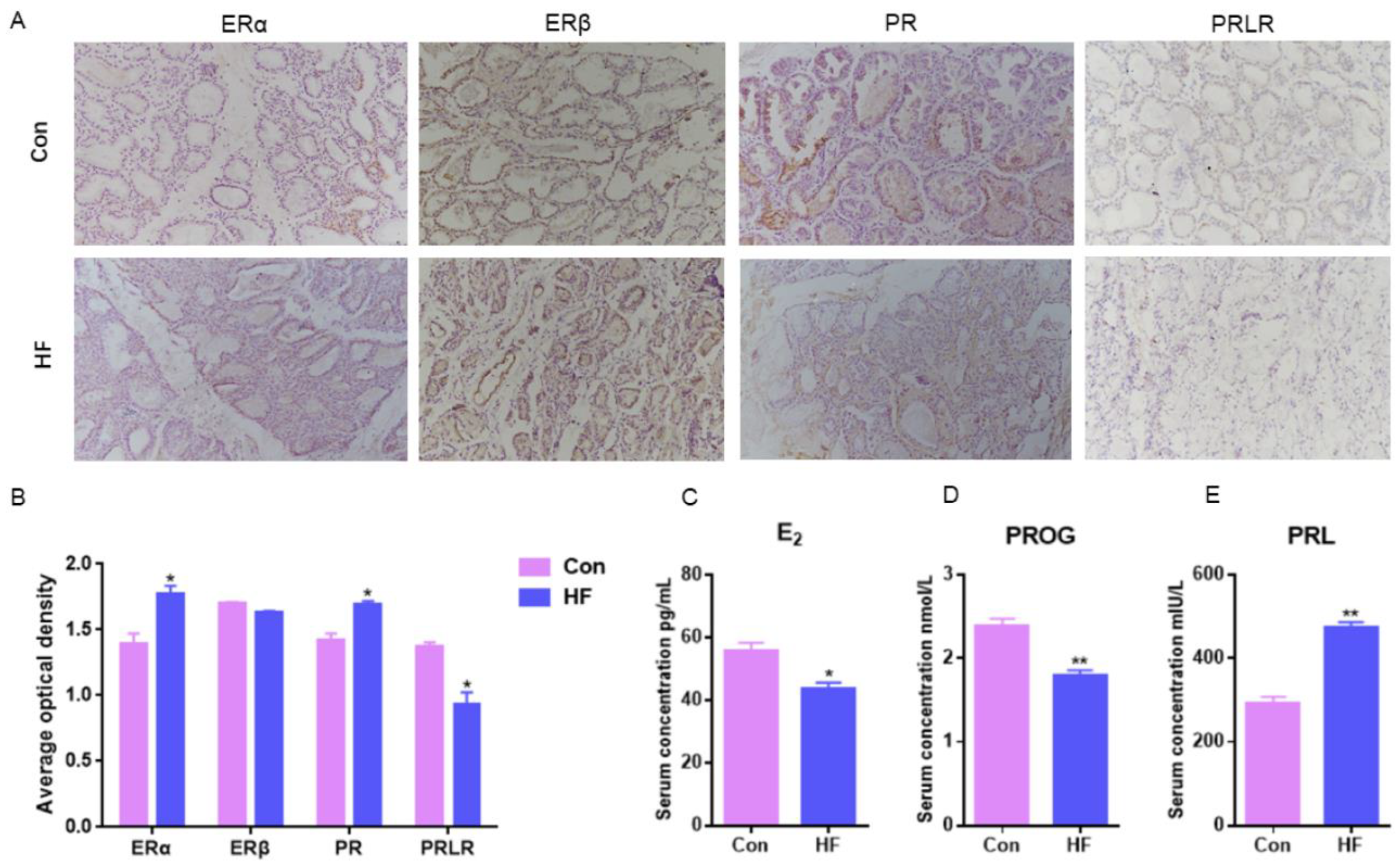
2.2. Hormones Serum Levels and Receptor Mammary Expression in Goats with Firm Udder
2.3. Transcriptome Sequencing and Validation of Mammary Gland in Guanzhong Dairy Goats with Firm Udder
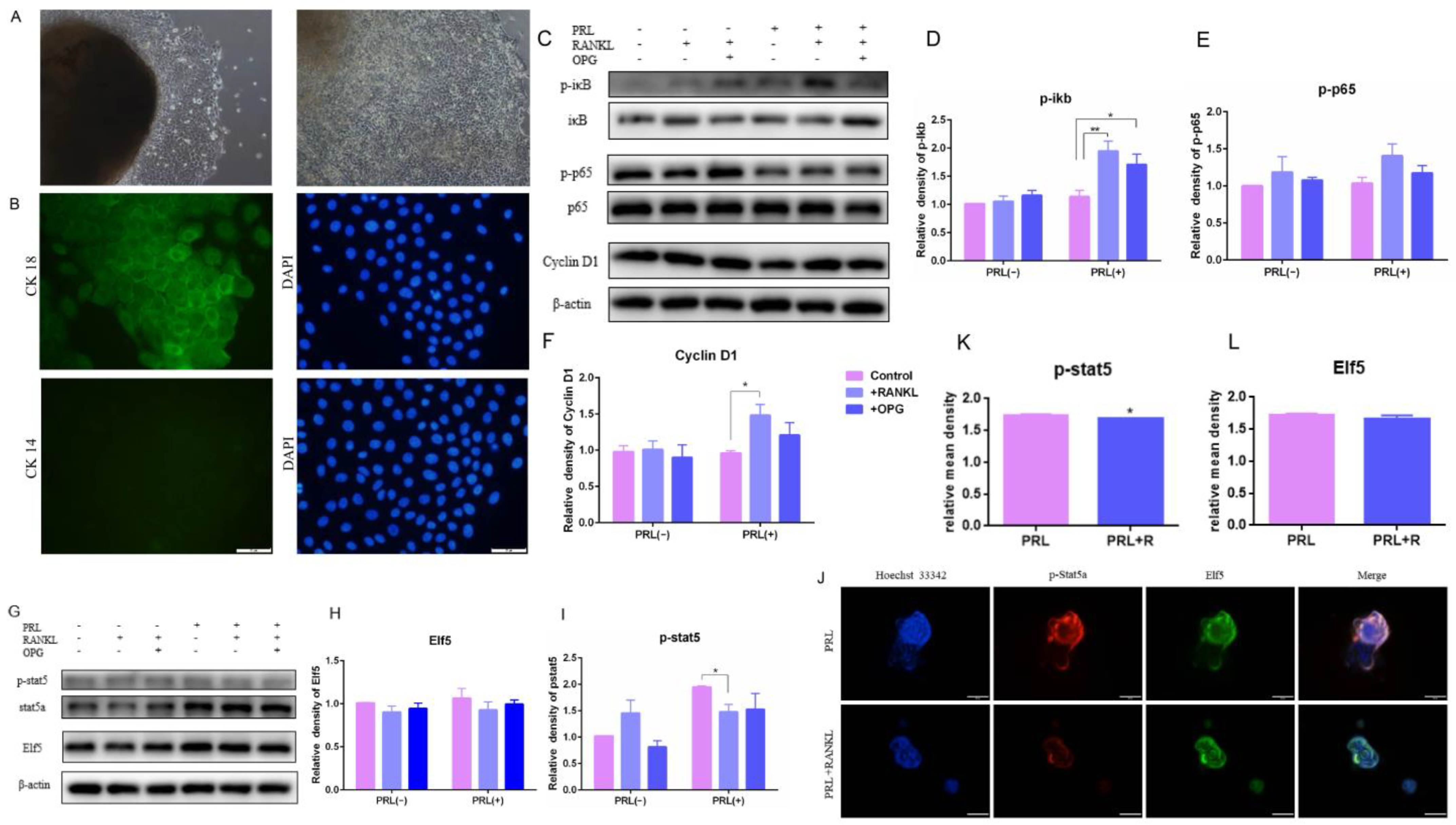
2.4. The Effects of High RANKL Level on Primary GMECs Proliferation, Function and Formatting Acini
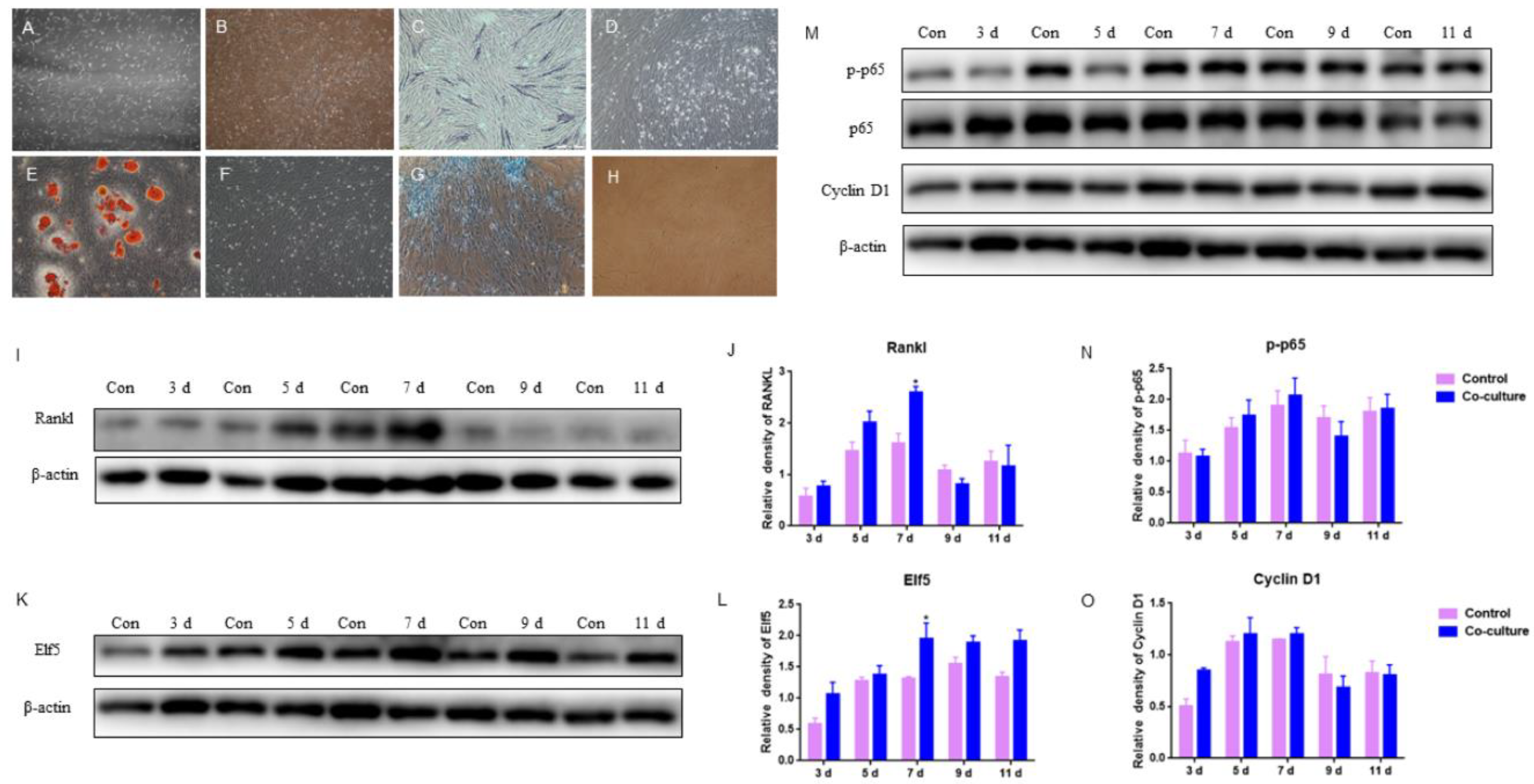
2.5. The Effects of GMECs and Adipocyte-like Cells Co-Culturing on IκB/p65/Cyclin D1 and Elf5 in GMECs
3. Discussion
4. Materials and Methods
4.1. Chemicals and Guanzhong Dairy Goat
4.2. Paraffin Embedment, Hematoxylin-Eosin (HE) and IHC Staining of Goat Mammary Gland
4.3. Transmission Electron Microscope Sample Preparation and Dyeing
4.4. RNA Extraction SOP for Mammary Gland
4.5. RNA-Seq Library Preparation Protocol (DNBSEQ)
4.6. Determination of Serum E2, PROG, PRL, RANKL and OPG Levels in Dairy Goats with Firm Udder
4.7. Primary Culture and RANKL/OPG Treatment of Goat Mammary Epithelial Cells (GMECs)
4.8. Three-Dimensional Culture of GMECs
4.9. Isolation and Culture of Adipose Derived Stem Cells (ADSCs) from Goat Mammary Gland
4.10. Induction of Adipogenic Differentiation of ADSCs from Goat Mammary Gland
4.11. Induction of Osteogenesis Differentiation of ADSCs from Goat Mammary Gland
4.12. Induction of Chondrogenesis Differentiation of ADSCs from Goat Mammary Gland
4.13. Co-Culture of GMECs and Adipocyte-like Cells Induced by ADSCs
4.14. Total Protein Extraction and Western Blotting
4.15. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.16. Immunofluorescence Staining
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazar, M.; Abdalla, I.M.; Chen, Z.; Ullah, N.; Liang, Y.; Chu, S.; Xu, T.; Mao, Y.; Yang, Z.; Lu, X. Genome-Wide Association Study for Udder Conformation Traits in Chinese Holstein Cattle. Animals 2022, 12, 2542. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, M.S. Genetic parameters of udder traits and their relationship with milk yield in Sahiwal cows of Pakistan. J. Anim. Plant Sci. 2016, 26, 880–886. [Google Scholar]
- Rosenberger, G. Clinical Examination of Cattle; Paul Parey: Berlin/Hamburg, Germany, 1979. [Google Scholar]
- Houe, H.; Vaarst, M.; Enevoldsen, C. Clinical parameters for assessment of udder health in Danish dairy herds. Acta Vet. Scand. 2002, 43, 173–184. [Google Scholar] [PubMed]
- Kern, E.L.; Cobuci, J.A.; Costa, C.N.; Pimentel, C.M. Factor analysis of linear type traits and their relation with longevity in brazilian holstein cattle. Asian-Australas. J. Anim. Sci. 2014, 27, 784–790. [Google Scholar] [CrossRef]
- Xue, X.; Hu, H.; Zhang, J.; Ma, Y.; Han, L.; Hao, F.; Jiang, Y.; Ma, Y. Estimation of Genetic Parameters for Conformation Traits and Milk Production Traits in Chinese Holsteins. Animals 2022, 13, 100. [Google Scholar] [CrossRef]
- Bohlouli, M.; Alijani, S.; Varposhti, M.R. Genetic relationships among linear type traits and milk production traits of Holstein dairy cattle. Ann. Anim. Sci. 2015, 15, 903–917. [Google Scholar] [CrossRef]
- Devani, K.; Valente, T.S.; Crowley, J.J.; Orsel, K. Development of optimal genetic evaluations for teat and udder structure in Canadian Angus cattle. J. Anim. Sci. 2019, 97, 4445–4452. [Google Scholar] [CrossRef]
- Margatho, G.; Quintas, H.; Rodríguez-Estévez, V.; Simões, J. Udder Morphometry and Its Relationship with Intramammary Infections and Somatic Cell Count in Serrana Goats. Animals 2020, 10, 1534. [Google Scholar] [CrossRef]
- Suntinger, M.; Fuerst-Waltl, B.; Obritzhauser, W.; Firth, C.L.; Köck, A.; Egger-Danner, C. Usability of bacteriological milk analyses for genetic improvement of udder health in Austrian Fleckvieh cows. J. Dairy Sci. 2022, 105, 5167–5177. [Google Scholar] [CrossRef]
- Rees, A.; Fischer-Tenhagen, C.; Heuwieser, W. Udder firmness as a possible indicator for clinical mastitis. J. Dairy Sci. 2017, 100, 2170–2183. [Google Scholar] [CrossRef]
- van Hoeij, R.J.; Lam, T.J.G.M.; Bruckmaier, R.M.; Dijkstra, J.; Remmelink, G.J.; Kemp, B.; van Knegsel, A.T.M. Udder health of dairy cows fed different dietary energy levels after a short or no dry period without use of dry cow antibiotics. J. Dairy Sci. 2018, 101, 4570–4585. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Sinha, B.; Kumari, R.; Vineeth, M.R.; Shrivastava, K.; Verma, A.; Gupta, I.D. Udder and teat morphometry in relation to clinical mastitis in dairy cows. Trop. Anim. Health Prod. 2022, 54, 99. [Google Scholar] [CrossRef]
- Hovey, R.C.; Aimo, L. Diverse and active roles for adipocytes during mammary gland growth and function. J. Mammary Gland. Biol. Neoplasia 2010, 15, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Scherer, P.E. Remodeling of Murine Mammary Adipose Tissue during Pregnancy, Lactation, and Involution. J. Mammary Gland. Biol. Neoplasia 2019, 24, 207–212. [Google Scholar] [CrossRef]
- Zwick, R.K.; Rudolph, M.C.; Shook, B.A.; Holtrup, B.; Roth, E.; Lei, V.; Van Keymeulen, A.; Seewaldt, V.; Kwei, S.; Wysolmerski, J.; et al. Adipocyte hypertrophy and lipid dynamics underlie mammary gland remodeling after lactation. Nat. Commun. 2018, 9, 3592. [Google Scholar] [CrossRef]
- Wang, X.; Reagan, M.R.; Kaplan, D.L. Synthetic adipose tissue models for studying mammary gland development and breast tissue engineering. J. Mammary Gland. Biol. Neoplasia 2010, 15, 365–376. [Google Scholar] [CrossRef]
- Lee, S.H.; Yap, Y.H.Y.; Lim, C.L.; Woo, A.R.E.; Lin, V.C.L. Activation function 1 of progesterone receptor is required for mammary development and regulation of RANKL during pregnancy. Sci. Rep. 2022, 12, 12286. [Google Scholar] [CrossRef] [PubMed]
- Aupperlee, M.D.; Smith, K.T.; Kariagina, A.; Haslam, S.Z. Progesterone receptor isoforms A and B: Temporal and spatial differences in expression during murine mammary gland development. Endocrinology 2005, 146, 3577–3588. [Google Scholar] [CrossRef]
- Beleut, M.; Rajaram, R.D.; Caikovski, M.; Ayyanan, A.; Germano, D.; Choi, Y.; Schneider, P.; Brisken, C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl. Acad. Sci. USA 2010, 107, 2989–2994. [Google Scholar] [CrossRef]
- Tanos, T.; Sflomos, G.; Echeverria, P.C.; Ayyanan, A.; Gutierrez, M.; Delaloye, J.F.; Raffoul, W.; Fiche, M.; Dougall, W.; Schneider, P. Progesterone/RANKL is a major regulatory axis in the human breast. Sci. Transl. Med. 2013, 5, 182ra55. [Google Scholar] [CrossRef]
- Joshi, P.A.; Jackson, H.W.; Beristain, A.G.; Di Grappa, M.A.; Mote, P.A.; Clarke, C.L.; Stingl, J.; Waterhouse, P.D.; Khokha, R. Progesterone induces adult mammary stem cell expansion. Nature 2010, 465, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Asselin-Labat, M.L.; Vaillant, F.; Sheridan, J.M.; Pal, B.; Wu, D.; Simpson, E.R.; Yasuda, H.; Smyth, G.K.; Martin, T.J.; Lindeman, G.J. Control of mammary stem cell function by steroid hormone signaling. Nature 2010, 465, 798–802. [Google Scholar] [CrossRef]
- De Matteis, R.; Zingaretti, M.C.; Murano, I.; Vitali, A.; Frontini, A.; Giannulis, I.; Barbatelli, G.; Marcucci, F.; Bordicchia, M.; Sarzani, R. In Vivo Physiological Transdifferentiation of Adult Adipose Cells. Stem Cells 2009, 27, 2761–2768. [Google Scholar] [CrossRef]
- Morroni, M.; Giordano, A.; Zingaretti, M.C.; Boiani, R.; De Matteis, R.; Kahn, B.B.; Nisoli, E.; Tonello, C.; Pisoschi, C.; Luchetti, M.M. Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proc. Natl. Acad. Sci. USA 2004, 101, 16801–16806. [Google Scholar] [CrossRef] [PubMed]
- Prokesch, A.; Smorlesi, A.; Perugini, J.; Manieri, M.; Ciarmela, P.; Mondini, E.; Trajanoski, Z.; Kristiansen, K.; Giordano, A.; Bogner-Strauss, J.G. Molecular Aspects of Adipoepithelial Transdifferentiation in Mouse Mammary Gland. Stem Cells 2014, 32, 2756–2766. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell. Metab. 2018, 27, 68–83. [Google Scholar] [CrossRef]
- Brisken, C.; Ataca, D. Endocrine hormones and local signals during the development of the mouse mammary gland. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 181–195. [Google Scholar] [CrossRef]
- Brenot, A.; Hutson, I.; Harris, C. Epithelial-adipocyte interactions are required for mammary gland development, but not for milk production or fertility. Dev. Biol. 2020, 458, 153–163. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hugo, E. Prolactin (PRL) in Adipose Tissue: Regulation and Functions; Springer: Cham, Switzerland, 2015; pp. 1–35. [Google Scholar]
- Hugo, E.R.; Borcherding, D.C.; Gersin, K.S.; Loftus, J.; Ben-Jonathan, N. Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. J. Clin. Endocrinol. Metab. 2008, 93, 4006–4012. [Google Scholar] [CrossRef]
- Goffin, V. Prolactin receptor targeting in breast and prostate cancers: New insights into an old challenge. Pharmacol. Ther. 2017, 179, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, M.; Albarella, S.; Dario, C.; Peretti, V.; Ciotola, F. Association of STAT5A Gene Variants with Milk Production Traits in Agerolese Cattle. Biochem. Genet. 2017, 55, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Luo, J.; Huang, L.; Zang, S.; He, Q.; Wu, J.; Huang, J. Mutation of signal transducer and activator of transcription 5 (STAT5) binding sites decreases milk allergen αS1-Casein content in goat mammary epithelial cells. Foods 2022, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wang, H.R.; Wang, M.Z.; Wang, C.; Liu, S.M. Lactogenic hormones regulate mammary protein synthesis in bovine mammary epithelial cells via the mTOR and JAK-STAT signal pathways. Anim. Prod. Sci. 2016, 56, 1803–1809. [Google Scholar] [CrossRef]
- Lacasse, P.; Ollier, S.; Lollivier, V.; Boutinaud, M. New insights into the importance of prolactin in dairy ruminants. J. Dairy Sci. 2016, 99, 864–874. [Google Scholar] [CrossRef]
- Klaas, I.C.; Enevoldsen, C.; Vaarst, M.; Houe, H. Systematic clinical examinations for identification of latent udder health types in Danish dairy herds. J. Dairy Sci. 2004, 87, 1217–1228. [Google Scholar] [CrossRef]
- Mao, Y.; Yang, H.; Ma, X.; Wang, C.; Zhang, L.; Cui, Y. Prolactin regulates RANKL expression via signal transducer and activator of transcription 5a signaling in mammary epithelial cells of dairy cows. Cell. Biol. Int. 2023, 47, 920–928. [Google Scholar] [CrossRef]
- González-Suárez, E.; Sanz-Moreno, A. RANK as a therapeutic target in cancer. FEBS J. 2016, 283, 2018–2033. [Google Scholar] [CrossRef]
- Geoghegan, I.P.; McNamara, L.M.; Hoey, D.A. Estrogen withdrawal alters cytoskeletal and primary ciliary dynamics resulting in increased Hedgehog and osteoclastogenic paracrine signalling in osteocytes. Sci. Rep. 2021, 11, 9272. [Google Scholar] [CrossRef]
- Hanada, R.; Hanada, T.; Sigl, V.; Schramek, D.; Penninger, J.M. RANKL/RANK-beyond bones. J. Mol. Med. 2011, 89, 647–656. [Google Scholar] [CrossRef]
- Rao, S.; Cronin, S.J.F.; Sigl, V.; Penninger, J.M. RANKL and RANK: From mammalian physiology to Cancer treatment. Trends Cell. Biol. 2018, 28, 213–223. [Google Scholar] [CrossRef]
- Mukherjee, A.; Soyal, S.M.; Li, J.; Ying, Y.; He, B.; DeMayo, F.J.; Lydon, J.P. Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEB J. 2010, 24, 4408–4419. [Google Scholar] [CrossRef] [PubMed]
- Obr, A.E.; Grimm, S.L.; Bishop, K.A.; Wesley, P.J.; Lydon, J.P.; Edwards, D.P. Progesterone receptor and stat5 signaling cross talk through RANKL in mammary epithelial cells. Mol. Endocrinol. 2013, 27, 1808–1824. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Fabi, A.; Cognetti, F.; Gorini, S.; Caprio, M.; Fabbri, A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 12. [Google Scholar] [CrossRef]
- Ledesma-Colunga, M.G.; Adán, N.; Ortiz, G.; Solís-Gutiérrez, M.; López-Barrera, F.; Martínez de la Escalera, G.; Clapp, C. Prolactin blocks the expression of receptor activator of nuclear factor κB ligand and reduces osteoclastogenesis and bone loss in murine inflammatory arthritis. Arthritis Res. Ther. 2017, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Gallego-Ortega, D.; Ledger, A.; Schramek, D.; Joshi, P.; Szwarc, M.M. Progesterone drives mammary secretory differentiation via RANKL-mediated induction of Elf5 in luminal progenitor cells. Development 2013, 140, 1397–1401. [Google Scholar] [CrossRef]
- Fernandez-Valdivia, R.; Mukherjee, A.; Yan, Y.; Jie, L.; Paquet, M.; Demayo, F.J. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev. Biol. 2009, 328, 127–139. [Google Scholar] [CrossRef]
- Gonzalez-Suarez, E.; Branstetter, D.; Armstrong, A.; Dinh, H.; Blumberg, H.; Dougall, W.C. RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol. Cell. Biol. 2007, 27, 1442–1454. [Google Scholar] [CrossRef]
- Arun, S.J.; Thomson, P.C.; Sheehy, P.A.; Khatkar, M.S.; Raadsma, H.W.; Williamson, P. Targeted Analysis Reveals an Important Role of JAK-STAT-SOCS Genes for Milk Production Traits in Australian Dairy Cattle. Front. Genet. 2015, 6, 342. [Google Scholar] [CrossRef]
- Choi, Y.S.; Chakrabarti, R.; Escamilla-Hernandez, R.; Sinha, S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: Failure of stat5 activation and functional differentiation in the absence of elf5. Dev. Biol. 2009, 329, 227–241. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Wei, Y.; Romano, R.A. Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem Cells 2012, 30, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Stanford, P.M.; Sutherland, K.; Oakes, S.R.; Naylor, M.J.; Robertson, F.G. Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol. Endocrinol. 2006, 20, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Boopalan, T.; Arumugam, A.; Parada, J.; Saltzstein, E.; Lakshmanaswamy, R. Receptor activator for nuclear factor-κb ligand signaling promotes progesterone-mediated estrogen-induced mammary carcinogenesis. Cancer Sci. 2015, 106, 25–33. [Google Scholar] [CrossRef]
- Cordero, A.; Pellegrini, P.; Sanz-Moreno, A.; Trinidad, E.M.; Serra-Musach, J.; Deshpande, C.; Dougall, W.C.; Pujana, M.A.; González-Suárez, E. RANKL Impairs Lactogenic Differentiation Through Inhibition of the Prolactin/Stat5 Pathway at Midgestation. Stem Cells 2016, 34, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Marzan, C.V.; Kupumbati, T.S.; Bertran, S.P.; Samuels, T.; Leibovitch, B.; Mira-y-Lopez, R.; Ossowski, L.; Farias, E.F. Adipocyte derived paracrine mediators of mammary ductal morphogenesis controlled by retinoic acid receptors. Dev. Biol. 2011, 349, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Darcy, K.M.; Zangani, D.; Shea-Eaton, W.; Shoemaker, S.F.; Lee, P.P.; Mead, L.H.; Mudipalli, A.; Megan, R.; Ip, M.M. Mammary fibroblasts stimulate growth, alveolar morphogenesis, and functional differentiation of normal rat mammary epithelial cells. Vitr. Cell. Dev. Biol. 2000, 36, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Zangani, D.; Darcy, K.M.; Shoemaker, S.; Ip, M.M. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp. Cell Res. 1999, 247, 399–409. [Google Scholar] [CrossRef]
- Yao, D.; Yang, C.; Ma, J.; Chen, L.; Luo, J.; Ma, Y.; Loor, J.J. cAMP Response Element Binding Protein 1 (CREB1) Promotes Monounsaturated Fatty Acid Synthesis and Triacylglycerol Accumulation in Goat Mammary Epithelial Cells. Animals 2020, 10, 1871. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, X.; Han, T.; Li, P.; Wang, J.; Liu, G.; Wang, Z.; Ge, C.; Gao, S. Dietary pseudopurpurin improves bone geometry architecture and metabolism in red-bone Guishan goats. PLoS ONE 2012, 7, e37469. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Shao, D.; Zhao, C.; Liu, H.; Zhao, X.; Wei, Q.; Ma, B. The High Level of RANKL Improves IκB/p65/Cyclin D1 Expression and Decreases p-Stat5 Expression in Firm Udder of Dairy Goats. Int. J. Mol. Sci. 2023, 24, 8841. https://doi.org/10.3390/ijms24108841
Gao Z, Shao D, Zhao C, Liu H, Zhao X, Wei Q, Ma B. The High Level of RANKL Improves IκB/p65/Cyclin D1 Expression and Decreases p-Stat5 Expression in Firm Udder of Dairy Goats. International Journal of Molecular Sciences. 2023; 24(10):8841. https://doi.org/10.3390/ijms24108841
Chicago/Turabian StyleGao, Zhen, Dan Shao, Chunrui Zhao, Haokun Liu, Xiaoe Zhao, Qiang Wei, and Baohua Ma. 2023. "The High Level of RANKL Improves IκB/p65/Cyclin D1 Expression and Decreases p-Stat5 Expression in Firm Udder of Dairy Goats" International Journal of Molecular Sciences 24, no. 10: 8841. https://doi.org/10.3390/ijms24108841
APA StyleGao, Z., Shao, D., Zhao, C., Liu, H., Zhao, X., Wei, Q., & Ma, B. (2023). The High Level of RANKL Improves IκB/p65/Cyclin D1 Expression and Decreases p-Stat5 Expression in Firm Udder of Dairy Goats. International Journal of Molecular Sciences, 24(10), 8841. https://doi.org/10.3390/ijms24108841





