Abstract
The fall armyworm (FAW), Spodoptera frugiperda, has become one of the most important pests on corn in China since it invaded in 2019. Although FAW has not been reported to cause widespread damage to rice plants in China, it has been sporadically found feeding in the field. If FAW infests rice in China, the fitness of other insect pests on rice may be influenced. However, how FAW and other insect pests on rice interact remains unknown. In this study, we found that the infestation of FAW larvae on rice plants prolonged the developmental duration of the brown planthopper (BPH, Nilaparvata lugens (Stål)) eggs and plants damaged by gravid BPH females did not induce defenses that influenced the growth of FAW larvae. Moreover, co-infestation by FAW larvae on rice plants did not influence the attractiveness of volatiles emitted from BPH-infested plants to Anagrus nilaparvatae, an egg parasitoid of rice planthoppers. FAW larvae were able to prey on BPH eggs laid on rice plants and grew faster compared to those larvae that lacked available eggs. Studies revealed that the delay in the development of BPH eggs on FAW-infested plants was probably related to the increase in levels of jasmonoyl-isoleucine, abscisic acid and the defensive compounds in the rice leaf sheaths on which BPH eggs were laid. These findings indicate that, if FAW invades rice plants in China, the population density of BPH may be decreased by intraguild predation and induced plant defenses, whereas the population density of FAW may be increased.
1. Introduction
Upon infestation by herbivores, plants recognize herbivore-associated molecular patterns and then initiate early signaling events; these include an increase in levels of cytosolic Ca2+ [1], a burst of reactive oxygen species (ROS) [2] and the activation of mitogen-activated protein kinases (MAPKs) [3]. Early signaling events thus activate signaling pathways mediated by phytohormones, such as jasmonic acid (JA), salicylic acid (SA), ethylene (ET) and abscisic acid (ABA); the activated phytohormone signaling pathways regulate the expression of defense-related genes and the biosynthesis of defense-related compounds, which in turn increase the resistance of plants to herbivores [4]. These herbivore-induced defense responses may affect the fitness of both conspecific and non-conspecific herbivores, and of other organisms sharing the same host plant [5,6,7].
Rice is one of the most important food crops in the world. It suffers from infestations by many insect pests, including the brown planthopper (BPH) Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). In China, BPH is a destructive insect pest on rice that generally results in yield losses of up to 30% if not controlled, and mainly immigrates from the north of Vietnam [8,9]. BPH damages rice plants by feeding on phloem sap, laying eggs in tissues and/or transmitting viruses [10,11,12,13]. It has been well documented that the infestation of BPH gravid females alters the biosynthesis of many defensive signals, such as JA, jasmonoyl-isoleucine (JA-Ile), SA, ET and H2O2; these signal-mediated pathways then enhance both the expression of defensive genes and the production of defensive compounds, such as volatiles, phenolamides and the activity of trypsin protease inhibitors (TrypPIs) [14,15,16]. Phenolamides and TrypPIs are reported to be defensive compounds that act directly against BPH [17]; moreover, volatiles emitted from rice plants infested by BPH are attractive to Anagrus nilarparvatae (Pang et Wang) (Hymenoptera: Mymaridae), the egg parasitoid of BPH [18]. Hence, BPH infestation enhances the direct and indirect resistance of rice to BPH.
The fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), is a destructive agricultural pest all over the world. In Brazil, for example, farmers need take about USD 600 million a year to control it [19]. FAW larvae feed on at least 353 plant species, including maize (Zea mays L.) and rice (Oryza sativa L.) [20]. Young FAW larvae generally aggregate to feed on leaves of host plants, whereas 3rd-instar and above FAW larvae disperse and consume leaves [21,22]. FAW originated in the tropical-subtropical part of the Western Hemisphere [23]. Since 2016, it has successfully invaded west and central Africa, damaging corn production [19,24]. In July 2018, FAW arrived in Yemen and India [25], and in January 2019, it was found in Yunnan province, China [26]. Nowadays, it has become one of the most important pests on corn in China [27]. FAW has two host plant-related strains, corn and rice [28]. The former strain primarily feeds on corn, cotton and sorghum, whereas the latter prefers rice and several pasture grasses. Despite their morphological similarity, these two strains exhibit distinct mating behavior, pheromone composition and developmental patterns [28]. Although the genetic background of its populations in China is corn [29], FAW has been sporadically found feeding on rice in the field in Guangxi, Fujian and Hubei provinces [27,30,31]. Moreover, because it can continue to reproduce on rice plants in the laboratory [30], FAW may well infest rice in China. FAW larvae infestation can induce defense responses, changing the physiological and biochemical status of plants [32,33,34], which may in turn influence the fitness of other insect pests on rice. In addition, besides cannibalizing its own species, FAW preys on other herbivores, such as Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) [35], Busseola fusca (Fuller) (Lepidoptera: Noctuidae) [36], Sesamia calamistis (Hampson) (Lepidoptera: Noctuidae) [37] and Chilo partellus (Swinhoe) (Lepidoptera: Crambidae) [36], a phenomenon known as intraguild predation [38,39]. However, whether and how current rice insect pests, such as BPH, and the potentially invasive leaf-chewer FAW interact, and how that interaction subsequently influences the interacting parties, remains unknown.
To address the above issues, in this study, we investigated the interaction between FAW and BPH. We found that FAW larvae not only prey on BPH eggs but also prolong the developmental duration of BPH eggs by triggering a systemic increase in levels of JA-Ile, ABA and defensive compounds in the rice leaf sheaths on which BPH eggs are laid. In addition, compared to those that do not prey on BPH eggs, FAW larvae preying on BPH eggs grow faster. The results demonstrate that, if FAW infests rice, FAW can benefit by preying on BPH eggs, whereas the BPH population might be inhibited because its eggs will be predated and its eggs’ development will be prolonged.
2. Results
2.1. Effects of FAW Larvae-Infested Rice Plants on BPH Performance
We investigated the influence of plants with different damage levels inflicted by FAW larvae on the feeding, development, survival and fecundity of BPH. No difference was observed in the amount of honeydew excreted by newly emerged BPH female adults for 1 day, an indicator of the amount of food intake, between plants that were pre-infested by a 3rd-instar FAW larva for 1–3 days and non-infested plants (Figure S1a; Table S1). The number of eggs laid by BPH female adults for 10 days on plants that were individually pre-infested by a 3rd-instar FAW larva for 1 or 4 days was similar to the number of eggs laid by BPH female adults for 10 days on non-infested plants (Figure S1b; Table S1). Pre-infestation of 3rd-instar FAW larvae (each plant had one larva) for 5 days, which resulted in the maximum damage to leaves (almost all of the leaves were eaten) (Figure S1c; Table S1), did not influence the developmental duration of BPH nymphs (Figure S1d; Table S1), the number of BPH eggs laid by BPH female adults for 24 h or the hatching rate and developmental duration of BPH eggs (Figure S2; Table S1).
We also measured the post-infestation of FAW 1st- and 2nd-instar larvae for 7 days on the survival and development of BPH eggs. Neither the number of BPH eggs laid by BPH female adults for 24 h, nor the hatching rate and developmental duration of BPH eggs were influenced by the post-infestation of 2 or 3 1st-instar FAW larvae per plant for 7 days (Figure 1a–f; Tables S2 and S3). However, when plants were individually post-infested by 3 2nd-instar FAW larvae for 7 days, the developmental duration of BPH eggs in these plants was longer by 1 day than that of BPH eggs on non-infested plants; however, the hatching rate of BPH eggs was not affected (Figure 1a–f; Tables S2 and S3).
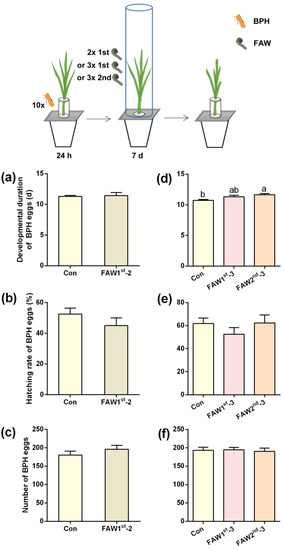
Figure 1.
FAW larvae infestation of plants prolongs the developmental duration of BPH eggs. Upper inset, schematic representation of experimental design. (a–f) Mean developmental duration of BPH eggs (a,d), hatching rate of BPH eggs (b,e) and number of eggs laid by 10 BPH gravid female adults for 24 h (c,f) (+SE, n = 9–10) on non-infested plants (Con) or rice plants that were post-infested by 2 1st-instar (FAW 1st-2), 3 1st-instar (FAW 1st-3) or 3 2nd-instar (FAW 2nd-3) FAW larvae for 7 days. Different letters indicate significant differences among treatments in (d) (p < 0.05, Tukey’s multiple comparison test).
2.2. Effects of BPH-Infested Rice Plants on Performance of FAW Larvae
When newly hatched FAW larvae had fed for 8 to 9 days on the non-BPH infested parts of plants (meaning FAW larvae could not reach BPH eggs) that were pre-infested by 15 gravid BPH females for 2 or 5 days (whether or not BPHs were later removed), their larval mass was similar to that of FAW fed on control plants (without BPH infestation) (Figure S3; Table S1). However, when newly hatched FAW larvae were allowed to feed on entire BPH-infested plants (meaning FAW could reach BPH eggs), they grew faster than those fed on control plants: by day 5.5, the mass of FAW larvae fed on BPH-infested plants was 1.57-fold higher than the mass of FAW larvae fed on control plants (Figure 2a; Table S4). To examine whether the fast growth of FAW larvae on BPH-infested plants was due to the presence of BPH eggs, we allowed 3rd-instar FAW larvae to feed on plants infested by gravid BPH females (containing BPH eggs). Our results showed that these larvae grew faster than those fed on non-infested plants; in addition, BPH eggs on BPH-infested plants were predated by FAW larvae (Figure 2b,c; Table S4). Moreover, by days 2 and 3, the mass of FAW larvae fed on rice leaf sheaths together with 400 BPH eggs per day was significantly higher than the mass of those fed on rice leaf sheaths alone (Figure 2d; Table S4). These data demonstrate that BPH infestation on rice can facilitate the growth of FAW larvae by providing eggs for FAW larvae, whereas the defense responses in rice induced by BPH infestation have no effect on the growth of FAW larvae.
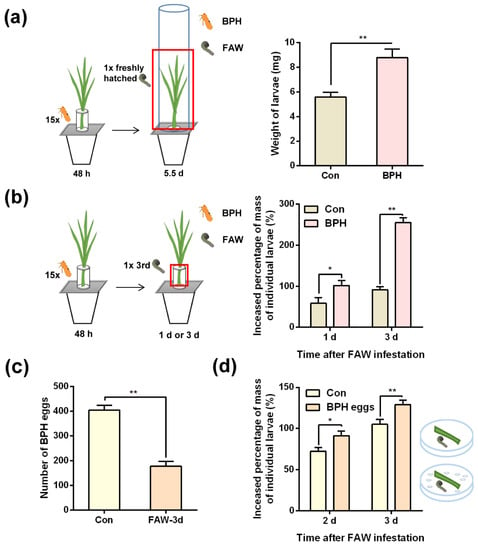
Figure 2.
BPH infestation-induced changes in rice do not influence the growth of FAW larvae, but the presence of BPH eggs does. (a) Mean mass of individual newly hatched FAW larvae (+SE, n = 23–30) 5.5 days after they fed on whole non-infested plants (Con) or BPH-infested plants (meaning FAW could reach BPH eggs). Inset, schematic representation of the experimental design; the red block indicates the location of FAW larvae placement. (b) Mean increased percentage of mass of individual 3rd-instar FAW larvae (+SE, n = 20–22) 1 or 3 days after they fed on lower parts of non-infested plants (Con) or plants that were pre-infested by 15 BPH gravid female adults for 48 h (afterwards, the BPHs were removed). Inset, schematic representation of the experimental design; the red block indicates the location of FAW larvae placement. (c) Mean number of remaining BPH eggs (+SE, n = 30) on BPH-infested plants as described (b) 3 days after the release of 1 3rd-instar FAW larva (FAW-3d) or not (Con). (d) Mean increased percentage of mass of 1 3rd-instar FAW larva (+SE, n = 28–30) 2 or 3 days after it fed on rice leaf sheaths containing BPH eggs or rice leaf sheaths alone (Con). Asterisks indicate significant differences between treatments (* p < 0.05; ** p < 0.01; Student’s t tests).
2.3. FAW Larvae Infestation Does Not Influence the Attractiveness of BPH-Infested Plants to the Parasitoid A. nilaparvatae
When given a choice between BPH eggs on BPH-infested plants and BPH eggs on BPH+FAW-infested plants, the parasitoid A. nilaparvatae showed no preference for BPH eggs: the parasitism rate by the parasitoid of BPH eggs on plants that were infested by 10 gravid BPH females for 24 h was similar to the parasitism rate of BPH eggs on plants that were infested by 10 gravid BPH females together with 1 4th-instar larva or 2 or 3 3rd-instar FAW larvae for 24 h (Figure S4a–c; Table S1). No difference in the number of BPH eggs was observed between pairs of plants (Figure S4d–f).
2.4. FAW Larvae Infestation Enhances BPH-Induced Levels of JA-Ile and ABA but Not Levels of JA, SA and H2O2 in Rice
We investigated the change in levels of BPH-induced JA, JA-Ile, SA, ABA and H2O2 in the leaf sheaths (the feeding and oviposition position of BPH) of rice plants after they were infested by FAW larvae. In summary, the levels of JA-Ile and ABA in the leaf sheaths of plants that were infested by 10 gravid BPH females for 24 h, followed by the infestation of 3 2nd-instar FAW larvae for 3 days, was 3-fold and 2.5-fold, respectively (higher than that in the leaf sheaths of plants that were infested by 10 gravid BPH females for 24 h alone), whereas no difference was observed in the levels of JA, SA and H2O2 between the 2 plant treatments (Figure 3a–e; Table S5).
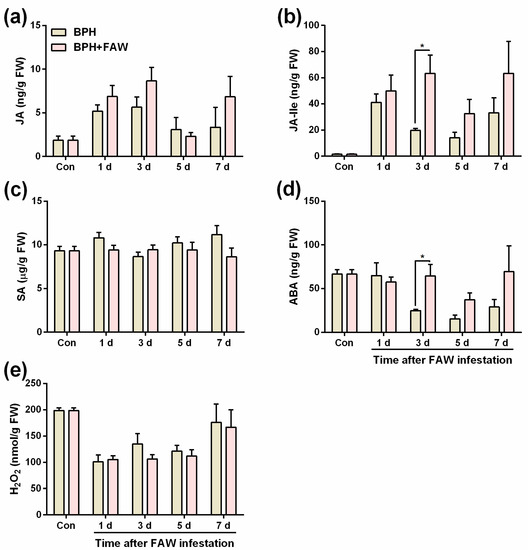
Figure 3.
Mean levels (+SE, n = 4~5) of JA (a), JA-Ile (b), SA (c), ABA (d) and H2O2 (e) in rice plants that were infested with 10 gravid BPH females for 24 h (and BPHs on them were removed), 1, 3, 5 and 7 days after plants were individually infested with 3 2nd-instar FAW larvae (BPH + FAW) or infested only by BPH. Plants confined in empty cages were used as controls (Con). Asterisks indicate significant difference between treatments (* p < 0.05; Student’s t tests).
2.5. FAW Infestation Influences BPH-Induced Levels of Phenolamides and Flavonoids in Rice
Levels of 3 flavonoids—astragalin (Figure 4c), luteolin 7-O-glucoside (Figure 4d) and schaftoside + isoschaftoside (Figure 4e)—in plants that were infested by gravid BPH females for 1 day, followed by infestation by FAW larvae for 1 day, was significantly higher than levels in plants that were infested only by gravid BPH females for 1 day (Table S6). Interestingly, FAW larvae infestation reduced levels of feruloylputrescine (Figure 4a; 1 day after FAW larvae infestation) and luteolin (Figure 4b; 1 and 7 days after FAW larvae infestation) in BPH-infested plants (Table S6). No difference was observed in levels of phenolamides and flavonoids at other time points between plants jointly infested by gravid BPH females and FAW larvae, and plants infested by gravid BPH females alone (Tables S7–S10).
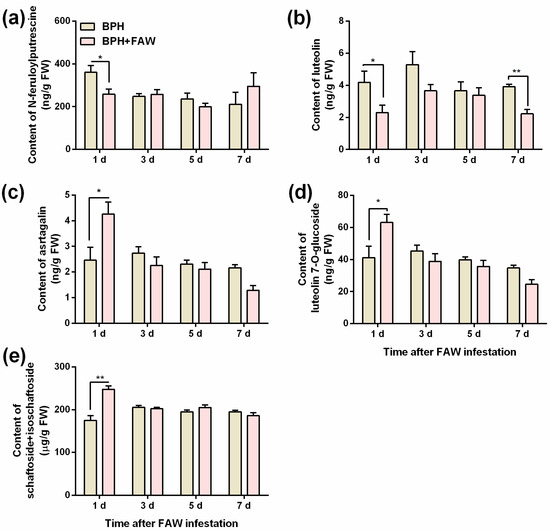
Figure 4.
Mean levels (+SE, n = 5~6) of N-feruloylputrescine (a), luteolin (b), astragalin (c), luteolin 7-O-glucoside (d) and schaftoside + isoschaftoside (e) in rice plants that were infested with 10 gravid BPH females for 24 h (and the BPHs on them were removed), 1, 3, 5 and 7 days after plants were individually infested with 3 2nd-instar FAW larvae (BPH + FAW) or controls (infested only by BPH) (BPH). Asterisks indicate significant differences between treatments (* p < 0.05, ** p < 0.01, Student’s t tests).
3. Discussion
In the study, we did not find that rice plants infested by BPH influenced the growth of FAW larvae (Figure S3). However, preying on BPH eggs on rice plants did accelerate the growth of FAW larvae (Figure 2). On the other hand, we found that post-infestation of FAW larvae (three 2nd-instar larvae) for seven days prolonged the developmental duration of BPH eggs, although rice plants with other damage levels inflicted by FAW larvae had no effect on the performance of BPH (Figure 1 and Figure S1). These findings demonstrate that the rice plant-mediated interaction between FAW and BPH was asymmetric: plants infested by FAW larvae influence the performance of BPH, but not vice versa. Moreover, FAW larvae could suppress the growth of the BPH population by preying on BPH eggs and prolonging the development of BPH eggs, and FAW could benefit from this interaction.
It has been well documented that chewing herbivores induce stronger defenses in plants than do sucking herbivores as the former generally cause more damage to plants than the latter do [39,40,41,42,43,44]. For instance, infestation by larvae of the rice striped stem borer (Chilo suppressalis (Walker) (Lepidoptera: Pyralidae), SSB), which bore into rice leaf sheaths or stems and feed inside these [40], induces a higher JA level than does BPH infestation [44]. Similarly, the expression level of LOX1, which encodes lipoxygenase (LOX) 1, a key enzyme in the biosynthesis of JA, in Arabidopsis infested by the green peach aphid (Myzus persicae (Sulzer) (Hemiptera: Aphidoidea)) is significantly lower than that in Arabidopsis infested by chewing herbivores [43,45]. In this study, BPH prefers to stay at the base of rice plants and damages plants by feeding on the phloem sap and laying eggs in tissues, whereas FAW is a chewing herbivore and usually feeds on the leaves of rice plants. Thus, the different feeding styles of the two herbivores probably underlie the asymmetric plant-mediated interaction between BPH and FAW. However, it should be noted that BPH infestation activates multiple metabolic pathways, and that these changes strongly depend on the genotype involved [46]. Therefore, it is also possible that BPH infestation may influence the performance of FAW with the change in rice genotype.
FAW larvae are omnivorous, and intraguild predation brings nutritional and energy benefits, increasing the size, growth and development of individuals [47,48,49,50]. Hence, the fast growth rate of FAW feeding on BPH eggs is probably related to the high nutritional and energy benefits of the eggs. In general, the larval mass of herbivores is correlated with potential fecundity [51,52,53]. In this study, we observed that the mass of 5.5-day-old FAW larvae fed on entire BPH-infested plants (meaning FAW could reach BPH eggs) increased by 57% compared to the mass of 5.5-day-old FAW larvae fed on control plants. Thus, it could be expected that co-existence between BPH and FAW promotes an increase in the population density of FAW.
The developmental duration and the number of eggs laid by female adults of BPH are two main factors that influence the population dynamics of BPH [54]. For developmental duration, it has been reported that a one-day delay in the development of immature-stage BPH at the fourth generation decreases the density by 11.4% of the peak of the total BPH population at the fifth generation. Moreover, this one-day delay also reduces the population density of the parasitoid A. nilaparvatae by 13.6% [54]. For the number of eggs laid by BPH female adults, a 10% decrease at the fourth generation results in reductions (by 23.2% and 18.8%, respectively) in the density of the peak of the total BPH population at the fifth generation and in the density of the A. nilaparvatae population. Therefore, a one-day delay in BPH egg development and the decrease in the number of BPH eggs caused by FAW larval infestation will probably have a relatively large effect on the population dynamics of BPH, especially in places such as Hainan Island, China, where 10–12 BPH generations occur per year [55].
JA, SA, H2O2 and ABA signaling pathways play an important role in the herbivore-induced defense responses of plants, including rice [14,15,16]. Therefore, to explain why the infestation of FAW larvae on rice plants prolonged the developmental duration of BPH eggs, we investigated the change in levels of signaling molecules JA, JA-Ile, ABA, SA and H2O2 in plants when they were pre-infested by BPH for 24 h, followed by the infestation of FAW larvae or infestation by BPH alone. We found that levels of JA-Ile and ABA in the leaf sheaths of plants infested by BPH gravid females are enhanced (the sheaths are the main location of BPH feeding and oviposition) 3 days after infestation (Figure 3b,d), suggesting that the co-infestation of both BPH and FAW larvae activated JA- and ABA-mediated signaling pathways in rice more strongly than did the infestation of BPH alone. Both JA and ABA signaling pathways have been reported to play important roles in regulating BPH resistance in rice [14,15,16]. For example, Xu et al. (2021) reported that the hatching rates of BPH eggs were significantly higher for those laid on JA-deficient lines than for those laid on wild-type plants [56]. Zhou et al. [57] found that significantly fewer eggs were laid by BPH females on the mutant osaba8ox3 (the knocked-down OsABA8ox3 gene; OsABA8ox3 is the key gene in ABA hydrolase genes) than on wild-type plants [57]. Moreover, the ABA signaling pathway has also been observed to reduce the hatching rate of BPH eggs [58]. Hence, the delay in the development of BPH eggs in rice plants that were infested by FAW larvae might be related to the activation of signaling pathways mediated by JA and ABA.
We also compared the difference in levels of phenolamides and flavonoids, two kinds of defensive compounds against herbivores in plants, including rice [59,60,61,62], using rice plants that were infested by BPH gravid females alone or co-infested by BPH gravid females and FAW larvae. Interestingly, FAW larvae infestation decreased levels of two compounds, feruloylputrescine and luteolin, in BPH-infested plants. Moreover, levels of three flavonoids—astragalin (Figure 4c), luteolin 7-O-glucoside (Figure 4d) and schaftoside + isoschaftoside (Figure 4e)—were higher in BPH-FAW-infested plants at one day after FAW infestation than in BPH-infested plants. Of these increased compounds, schaftoside and its isomers (isoschaftoside and neoschaftoside) have been reported to negatively influence BPH feeding [59,63]. Moreover, schaftoside can strongly bind with cyclin-dependent kinase 1 of BPH (NlCDK1) and inhibits the activation of NlCDK1 as a kinase by suppressing phosphorylation on its Thr-14 site [64]. Therefore, it is possible that increased levels of these compounds, such as schaftoside + isoschaftoside, in rice delay the development of BPH eggs in rice tissues by permeating these eggs. Future investigations should determine the compounds that influence the development of BPH eggs.
Thus far, several studies have reported that non-host herbivore infestation could influence the attractiveness of volatiles emitted from host-infested plants to parasitoids (predators). For instance, Cotesia marginiventris (Cresson) (Hymenoptera: Braconidae), a parasitoid of Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), was more attracted to tomato plants infested with both the aphid Macrosiphum euphorbiae (Thomas) (Hemiptera: Aphididae) and its host than were plants infested with S. exigua alone [65]. The infestation of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) decreases the attractiveness of spider mite-damaged plants to predatory mites [66]. More recently, Hu et al. (2020) reported that BPH females preferred to oviposit on plants that were infested by SSB larvae than on non-infested rice plants, as SSB larvae infestation results in an enemy-free space for BPH eggs: volatiles emitted from plants infested by SSB larvae and BPH gravid females were much less attractive to A. nilaparvatae, the egg parasitoid of BPH, than were volatiles emitted from plants infested by BPH gravid females alone [67]. However, here we did not find that FAW larvae infestation on plants influences the preference of the parasitoid A. nilaparvatae for BPH eggs (Figure S4), although there seem to be distinct differences in measurable volatiles between BPH-infested plants [18] and FAW-infested plants [68]. Similar results were also documented in other research systems. The infestation of Euscelidius variegatus (Kirschbaum) (Hemiptera: Cicadellidae), for example, did not alter the attractiveness of Spodoptera littoralis (Boisd) (Lepidoptera: Noctuidae)-infested maize plants to the parasitoid C. marginiventris [69]. These findings suggest that whether non-host herbivore infestation influences the attractiveness of host-damaged plants to parasitoids or predators depends on whether the volatile signals attractive to parasitoids or predators change.
In summary, we demonstrate that FAW larvae could facilitate their own growth by preying on BPH eggs on rice plants. Moreover, FAW larvae infestation could also prolong the development of BPH eggs by inducing the defense responses of rice. We propose that the delay in the development of BPH eggs in rice plants infested simultaneously by FAW larvae is probably due to the increase in signaling pathways mediated by JA and ABA and ensuing defensive compounds, such as flavonoids. These findings indicate that any infestation of rice plants in China by FAW—through intraguild predation and induced defensive responses—will probably decrease the population of BPH and change the composition of the arthropod community in rice. Moreover, because FAW benefits from its interaction with BPH, its population density and damage to plants may increase.
4. Materials and Methods
4.1. Plants and Insects
The seeds of the japonica rice variety, XiuShui 110 (XS110), were provided by China National Rice Research Institute, Hangzhou, China. Pre-germinated seeds of XS110 were cultured in a plastic bottle (diameter 8 cm, height 10 cm) in an illuminated incubator (28 ± 2 °C with a 14 h light period). Seven-day-old seedlings were grown in 25 L hydroponic boxes (length 51 cm, width 35 cm, height 17 cm) filled with a rice nutrient solution [70] and kept in a greenhouse (26 ± 2 °C 14 h light, 60% relative humidity). Twenty-five days later, seedlings were individually transferred to plastic pots (diameter 7 cm, height 9.5 cm) containing 350 mL of a nutrient solution. Plants were used for experiments 3–5 days after transplantation.
Colonies of BPH were originally obtained from rice fields in Hangzhou, China, and subsequently maintained on seedlings of the indica rice variety TN1 (susceptible to BPH) in a controlled climate room at 26 ± 2 °C, 12 h light phase and 80% relative humidity. Colonies of A. nilaparvatae, the egg parasitoid of BPH, were collected on rice plants containing BPH eggs from fields in Hangzhou, China, and propagated with BPH eggs on TN1 rice seedlings. FAW eggs were collected from maize fields in Hangzhou, China, and then larvae were individually reared on fresh maize leaves in Petri dishes (diameter 9 cm, height 1.5 cm).
4.2. Plant Treatment
For BPH infestation, plants were individually confined in glass cages (diameter 4 cm, height 8 cm, with 48 small holes, diameter 0.8 mm) into which 10 or 15 BPH gravid females were released (Figure S5a). Plants with empty glass cages were used as controls. For FAW infestation, one or more FAW larvae were placed on the upper part of the plants, each of which was individually confined in a plastic cylinder (diameter 4 cm, height 50 cm; with a side ventilation opening (length 15.5 cm, width 7 cm) and a top opening, all of which were covered with 140-mesh nylon nets) (Figure S5b). Plants confined in empty plastic cylinders were used as controls.
4.3. Bioassays
4.3.1. Effects of FAW Larvae-Infested Rice Plants on BPH Performance
Because 3rd-instar and above larvae of FAW disperse when they consume leaves, we investigated the effect of plants with different damage levels inflicted by FAW 3rd-instar larvae on the feeding, fecundity, survival and development of BPH. To assess the impact of FAW larvae-infested plants on BPH feeding, a newly emerged female adult was put into a parafilm bag (3 × 4 cm; weighed in advance) attached to the leaf sheath (Figure S5c) of a non-infested rice plant or a plant that was infested by a 3rd-instar FAW larva for 1, 2 or 3 days. Twenty-four hours later, the honeydew excreted by the BPH female adult was weighed by an analytical lab balance with a readability of 0.1 mg (to an accuracy of 0.1 mg). Each treatment was replicated 21~25 times.
To investigate the impact of FAW larvae-infested plants on the fecundity and oviposition behavior of BPH female adults, a non-infested plant or a plant that was infested by a 3rd-instar FAW larva for 1 or 4 days was confined in the glass cage into which a newly emerged BPH female and a male adult were introduced using the method described above. Ten days later, the number of eggs laid by BPH female adults on each plant was counted under a microscope. Each treatment was replicated 21~26 times.
To determine the impact of FAW larvae-infested plants on the survival of BPH nymphs, 15 newly emerged BPH nymphs were introduced on a non-infested plant or a plant that was infested by a 3rd-instar FAW larva for 5 days using the method described above (Figure S5a). The number of BPH nymphs surviving on each plant was recorded every day until all nymphs had become adults. Each treatment was replicated 8~9 times.
To assess the impact of FAW larvae-infested plants on the hatching rate and developmental duration of BPH eggs, 10 gravid BPH females were allowed to lay eggs for 24 h on the leaf sheaths of a non-infested plant or a plant that was infested by a 3rd-instar FAW larva for 5 days using the method described above. The newly hatched nymphs were counted every 24 h, until no new nymphs appeared. The number of unhatched eggs on each plant was counted under a microscope, and the hatching rate and developmental duration of BPH eggs for each treatment were calculated. Each treatment was replicated 9~10 times.
Considering that the developmental duration of BPH eggs in rice tissues laid by BPH female adults is 8–10 days at 25–28 °C [71] and that 1 FAW 3rd-instar larvae will eat all leaves within 5 days (Figure S1c), we also assess the impact that the post-infestation of FAW 1st- and 2nd-instar larvae had on the hatching rate and developmental duration of BPH eggs. A total of 10 gravid BPH females were allowed to lay eggs on the leaf sheath of a rice plant for 24 h using the method described above. BPHs were removed, and each plant was infested with two 1st- or three 1st- or 2nd-instar FAW larvae for seven days (controls were kept non-infested). The newly hatched nymphs were counted every 24 h, until no new nymphs appeared. The number of unhatched eggs on each plant was counted under a microscope, and the hatching rate and developmental duration of BPH eggs for each treatment were calculated. Each treatment set had 9~10 biological replicates.
4.3.2. Effects of BPH-Infested Rice Plants on the Performance of FAW Larvae
We first investigated the impact of plants with different damage levels inflicted by gravid BPH females on the growth of freshly hatched FAW larvae. The lower part of the rice plants was infested by 15 gravid BPH females using the method described above (Figure S5a). Two or five days later, BPHs were removed or not (glass cages covering plants (Figure S5a) were not removed; cages prevent FAW larvae from coming into contact with BPH eggs and eating them), and one newly hatched FAW larva was placed on the upper part of each plant. Plants with empty glass cages (without BPH infestation) were used as controls. FAW larvae were individually weighed 8 or 9 days later. Each treatment was replicated 12~28 times.
Considering that FAW larvae are omnivorous, we assess the impact of plants infested by gravid BPH females, when BPH eggs on these plants can be preyed upon by FAW larvae, on the growth of freshly hatched FAW larvae. The lower part of the rice plants was infested by 15 gravid BPH females using the method described above (Figure S5a). Two days later, BPHs, together with glass cages, were removed, and one newly hatched FAW larva was placed on the upper part of each plant. In this experiment, FAW larvae could consume whole plants and reach BPH eggs. Plants without BPH infestation (with empty glass cages) were used as controls. FAW larvae were individually weighed 5.5 days later. Each treatment was replicated 23~30 times. In another experiment, when plants were infested by 15 gravid BPH females for 2 days and BPHs were removed as stated above, these plants were divided into 2 groups. In one group, each plant received one pre-weighed 3rd-instar FAW larva introduced into the glass cage (meaning FAW larvae could reach BPH eggs); in the other group, plants did not receive any FAW larvae. FAW larvae reared on the lower parts of plants without BPH infestation (with empty glass cages) were used as controls. One or three days later, FAW larvae were individually weighed, and the number of eggs on each BPH-infested plant after the release of FAW larvae was counted under a microscope. Each treatment was replicated 20~30 times.
To decide whether the effect of BPH-infested plants on the growth of FAW larvae comes from BPH eggs, we performed the following experiment: pre-weighed 3rd-instar FAW larvae were individually fed on the leaf sheaths of rice plants plus 400 BPH eggs in Petri dishes (diameter 9 cm, height 1.5 cm); FAW larvae fed on rice leaf sheaths alone were used as controls. Rice leaf sheaths and BPH eggs were changed every day, and FAW larvae were weighed 2 and 3 days after the start of the experiment. Each treatment was replicated 28~30 times.
4.3.3. Effect of FAW Larvae Infestation on the Attractiveness of BPH-Infested Plants to the Parasitoid A. nilaparvatae
Rice plants were individually infested by 10 gravid BPH females that were confined in a glass cage as described above; meanwhile, one 4th-instar, two 3rd-instar or three 3rd-instar FAW larvae were placed on the rice leaves. Plants infested with BPH alone were used as controls. After 24 h, BPHs and FAW larvae were removed. A BPH-infested plant and a plant co-infested with BPH and FAW were transplanted into a plastic pot (inside diameter 12 cm, height 10 cm) containing 1 L nutrient solution and confined in the plastic cylinder, 4 cm apart (Figure S4, upper inset); 4 pairs of newly emerged parasitic wasps were released into the center of each cylinder. After 48 h, parasitic wasps were removed from the cylinder, and the plants were kept in the climate room. Five days after the release of the parasitoid, the parasitized BPH eggs have turned red. The parasitized and non-parasitized BPH eggs on each plant were counted under a microscope, and the parasitism rate of BPH eggs on plants subjected to different treatments was calculated. Each treatment was replicated 15~19 times.
4.4. Analysis of JA, JA-Ile, ABA and SA Levels
Plants were individually infested by 10 gravid BPH females using the method described above. Twenty-four hours later, BPHs were removed, and each plant was infested with three 2nd-instar FAW larvae (BPH + FAW) or kept non-infested (BPH). The outermost part of two leaf sheaths of each plant at one, three, five or seven days after FAW larvae infestation were harvested. Moreover, the same leaf sheaths of plants with empty cages (without BPH and FAW infestation) were also harvested. Samples were ground in liquid nitrogen (about 100 mg per sample for testing), and JA, JA-Ile, SA and ABA were extracted with ethyl acetate containing the labelled internal standards (D6-JA, D6-JA-Ile, D4-SA and D6-ABA) and then isolated and quantified by high-performance liquid chromatography-mass spectrometry-mass spectrometry (HPLC-MS-MS) (Agilent Technologies, Santa Clara, CA, USA) using the method described in [72]. Each treatment was replicated 4~5 times.
4.5. Hydrogen Peroxide Analysis
Samples were obtained using the method described above. H2O2 concentrations were determined using an Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen, Eugene, OR, USA) following the instructions. Each treatment was replicated 5 times.
4.6. Analysis of Phenolamides and Flavonoids
Samples were obtained using the method described above. Samples were ground in liquid nitrogen (about 100 mg per sample). Phenolamides and flavonoids were extracted with 800 μL of 70% methanol, and then were quantified by HPLC-MS-MS as previously described in [73] and [74], respectively. The contents of each compound were calculated using an external standard method. Each treatment was replicated 5~6 times.
4.7. Data Analysis
Two-treatment data were analyzed using Student’s t tests. Data from three or more treatments were compared using one-way ANOVA; if the ANOVA analysis was significant (p < 0.05), Tukey’s multiple comparison test was used to detect differences between groups. When necessary, data were log- or arc sine-transformed to meet requirements for the homogeneity of variance. All statistical analyses were conducted with SPSS software version 20 (SPSS).
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24108754/s1.
Author Contributions
Conceptualization, Y.L. and C.Q.; methodology, Y.L. and C.Q.; validation, Y.L. and C.Q.; formal analysis, Y.L. and C.Q.; investigation, C.Q., J.Z., Y.T., Q.G. and W.X.; resources, Y.L.; writing—original draft preparation, C.Q.; writing—review and editing, Y.L.; visualization, Y.L. and C.Q.; supervision, Y.L.; project administration, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly supported by the Key R&D Program of Zhejiang Province (2020C02003) and the earmarked fund for China Agriculture Research Systems (CARS-01-43).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Genshen Xie for assistance with BPH rearing and Emily Wheeler, Harwich, MA, USA, for editorial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, H.; Seo, P.J. Ca2+ talyzing initial responses to environmental stresses. Trends Plant Sci. 2021, 26, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, T.; Vandam, N. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 2005, 20, 617–624. [Google Scholar] [CrossRef]
- Soler, R.; Erb, M.; Kaplan, I. Long distance root–shoot signalling in plant–insect community interactions. Trends Plant Sci. 2013, 18, 149–156. [Google Scholar] [CrossRef]
- Papadopoulou, G.V.; van Dam, N.M. Mechanisms and ecological implications of plant-mediated interactions between belowground and aboveground insect herbivores. Ecol. Res. 2017, 32, 13–26. [Google Scholar] [CrossRef]
- Bottrell, D.G.; Schoenly, K.G. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia-Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Z.; Huang, C.; Lu, M.; Liu, J.; Yang, Q. Statistics and analysis of crop yield losses caused by main diseases and insect psets in recent 10 years. Plant Prot. 2016, 42, 1–9. [Google Scholar]
- Hogenhout, S.A.; Ammar, E.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Sogawa, K. Oviposition behavior of the rice brown planthopper, Nilaparvata lugens (Stål), and its electronic monitoring. J. Insect Behav. 2002, 15, 283–293. [Google Scholar] [CrossRef]
- Sogawa, K. The rice brown planthopper—Feeding physiology and host plant interactions. Annu. Rev. Entomol. 1982, 27, 49–73. [Google Scholar] [CrossRef]
- Muduli, L.; Pradhan, S.K.; Mishra, A.; Bastia, D.N.; Samal, K.C.; Agrawal, P.K.; Dash, M. Understanding brown planthopper resistance in rice: Genetics, biochemical and molecular breeding approaches. Rice Sci. 2021, 28, 532–546. [Google Scholar] [CrossRef]
- Mishra, A.; Barik, S.R.; Pandit, E.; Yadav, S.S.; Das, S.R.; Pradhan, S.K. Genetics, mechanisms and deployment of brown planthopper resistance genes in rice. Crit. Rev. Plant Sci. 2022, 41, 91–127. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Caarls, L.; Vos, I.A.; Pieterse, C.M.J.; Van Wees, S.C.M. Ethylene: Traffic controller on hormonal crossroads to defense. Plant Physiol. 2015, 169, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, Z.; Meng, J.; Zhou, P.; Luo, T.; Zhang, J.; Wu, J.; Lou, Y. Rice phenolamindes reduce the survival of female adults of the white-backed planthopper Sogatella furcifera. Sci. Rep. 2020, 10, 5778. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, B.; Cheng, J. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J. Chem. Ecol. 2005, 31, 2357–2372. [Google Scholar] [CrossRef]
- Erik, S. New crop pest takes Africa at lightning speed. Science 2017, 356, 473–474. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2022. [Google Scholar] [CrossRef]
- Kumar, R.M.; Gadratagi, B.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e165632. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chang, X. The occurrence, influence, prevention and control strategies of Spodoptera frugiperda in Aisa and Africa. China Plant Prot. 2019, 39, 88–90. [Google Scholar]
- Yang, X.; Liu, Y.; Luo, M.; Li, Y.; Wang, W.; Fei, W.; Hong, J. This is the first time that Spodoptera frugiperda has been found in Jiangcheng, Yunnan province. Yunnan Agric. 2019, 72. [Google Scholar]
- Jiang, Y.; Liu, J.; Xie, M.; Li, Y. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19. [Google Scholar] [CrossRef]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.; Henri, H.; Vavre, F.; et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.; Jiang, Y.; Liu, J.; Wu, K.; Xiao, Y. Molecular characterization analysis of fall armyworm populations in China. Plant Prot. 2019, 45, 20–27. [Google Scholar] [CrossRef]
- Zhang, H. A preliminary report on rice seedling damage caused by Spodoptera frugiperda in Yunxiao grassland of Fujian province and biotype identification of Spodoptera frugiperda. China Plant Prot. 2020, 20, 41–43. [Google Scholar]
- Yang, J.; Tao, Y.; Liu, Q.; Zheng, Z.; Zhou, H. In Wuxue, Hubei province, armyworm was found to harm rice seedlings. China Plant Prot. 2020, 40, 44–45. [Google Scholar]
- Pechan, T.; Ye, L.; Chang, Y.; Mitra, A.; Lin, L.; Davis, F.M.; Williams, W.P.; Luthe, D.S. A unique 33-KD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to fall armyworm and other lepidoptera. Plant Cell 2000, 12, 1031–1040. [Google Scholar] [CrossRef]
- Ingber, D.A.; Christensen, S.A.; Alborn, H.T.; Hiltpold, I. Detecting the conspecific: Herbivory-induced olfactory cues in the fall armyworm (Lepidoptera: Noctuidae). Metabolites 2021, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Glauser, G.; Marti, G.; Villard, N.; Doyen, G.A.; Wolfender, J.; Turlings, T.C.J.; Erb, M. Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. Plant J. 2011, 68, 901–911. [Google Scholar] [CrossRef]
- Bentivenha, J.P.F.; Baldin, E.L.L.; Montezano, D.G.; Hunt, T.E.; Paula-Moraes, S.V. Attack and defense movements involved in the interaction of Spodoptera frugiperda and Helicoverpa zea (Lepidoptera: Noctuidae). J. Pest Sci. 2017, 90, 433–445. [Google Scholar] [CrossRef]
- Mutua, J.M.; Mutyambai, D.M.; Asudi, G.O.; Khamis, F.; Niassy, S.; Jalloh, A.A.; Salifu, D.; Magara, H.J.O.; Calatayud, P.; Subramanian, S. Competitive plant-mediated and intraguild predation interactions of the invasive Spodoptera frugiperda and resident stemborers Busseola fusca and Chilo partellus in maize cropping systems in Kenya. Insects 2022, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Sokame, B.M.; Musyoka, B.; Mohammed, S.A.; Tamiru, A.; Bruce, A.; Anderson, P.; Karlsson Green, K.; Calatayud, P. Cannibalism and intraguild predation involved in the intra- and inter-specific interactions of the invasive fall armyworm, Spodoptera frugiperda, and lepidopteran maize stemborers. J. Pest Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Polis, G.A.; Holt, R.D. Intraguild predation: The dynamics of complex trophic interactions. Trends Ecol. Evol. 1992, 7, 151–154. [Google Scholar] [CrossRef]
- Polis, G.A.; Myers, C.A.; Holt, R.D. The ecology and evolution of intraguild predation—Potential competitors that eat each other. Annu. Rev. Ecol. Evol. Syst. 1989, 20, 297–330. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z.; Hua, H.; Ma, W. Comparative transcriptome analysis of defense response of rice to Nilaparvata lugens and Chilo suppressalis infestation. Int. J. Biol. Macromol. 2020, 163, 2270–2285. [Google Scholar] [CrossRef]
- Gosset, V.; Harmel, N.; Gobel, C.; Francis, F.; Haubruge, E.; Wathelet, J.P.; du Jardin, P.; Feussner, I.; Fauconnier, M.L. Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J. Exp. Bot. 2009, 60, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Leitner, M.; Boland, W.; Mithöfer, A. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol. 2005, 167, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J.P.J.; Thompson, G.A.G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001, 125, 1074–1085. [Google Scholar] [CrossRef]
- Wang, X. Influence of Infestation by Herbivores with Different Feeding Habits or Treatment by β-Glucosidase on Levels of Main Defense-Related Signals in Rice Plants. Master’s Thesis, Zhejiang University, Hangzhou, China, 2006; p. 31. [Google Scholar]
- Reymond, P.; Weber, H.; Damond, M.; Farmer, E.E. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 2000, 12, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhu, L.; He, G. Towards understanding of molecular interactions between rice and the brown planthopper. Mol. Plant 2013, 6, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.R. Cannibalism in natural populations. Annu. Rev. Ecol. Evol. Syst. 1975, 6, 87–106. [Google Scholar] [CrossRef]
- Polis, G.A. The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Evol. Syst. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Bose, A.P.H. Parent–offspring cannibalism throughout the animal kingdom: A review of adaptive hypotheses. Biol. Rev. 2022, 97, 1868–1885. [Google Scholar] [CrossRef]
- Wise, D.H. Cannibalism, food limitation, intraspecific competition, and the regulation of spider populations. Annu. Rev. Entomol. 2006, 51, 441–465. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Jimenez-Perez, A.; Wang, Q. Effect of body weight on reproductive performance in Cnephasia jactatana (Lepidoptera: Tortricidae). J. Insect Behav. 2004, 17, 511–522. [Google Scholar] [CrossRef]
- Rhainds, M. Size-dependent realized fecundity in two lepidopteran capital breeders. Environ. Entomol. 2015, 44, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Cheng, J. Simulation analysis on coordinated effects of rice varieties and Anagrus nilaparvatae Pang et Wang on brown planthopper. Nilaparvata lugens (Stål). J. Biomath. 1999, 14, 470–478. [Google Scholar]
- Wu, J. Agricultural Entomology (Northern Version); China Agriculture Press: Beijing, China, 2003; p. 146. [Google Scholar]
- Xu, J.; Wang, X.; Zu, H.; Zeng, X.; Baldwin, I.T.; Lou, Y.; Li, R. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol. 2021, 230, 1639–1652. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, L.; Wang, S.; Xie, P.; Liu, J. A key ABA hydrolase gene, OsABA8OX3 is involved in rice resistance to Nilaparvata lugens by affecting callose deposition. J. Asia-Pac. Entomol. 2019, 22, 625–631. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Zhou, S.; Lou, Y.; Lu, J. Silencing an E3 ubiquitin ligase gene OsJMJ715 enhances the resistance of rice to a piercing-sucking herbivore by activating ABA and JA signaling pathways. Int. J. Mol. Sci. 2021, 22, 13020. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Kimmins, F.M.; Grayer, R.J.; Raveendranath, S. Schaftosides from rice phloem as feeding inhibitors and resistance factors to brown planthoppers, Nilaparvata lugens. Entomol. Exp. Appl. 1996, 80, 246–249. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhou, P.Y.; Mo, X.C.; Hu, L.F.; Jin, N.; Chen, X.; Yu, Z.X.; Meng, J.P.; Erb, M.; Shang, Z.C.; et al. Induction of defense in cereals by 4-fluorophenoxyacetic acid suppresses insect pest populations and increases crop yields in the field. Proc. Natl. Acad. Sci. USA 2020, 117, 12017–12028. [Google Scholar] [CrossRef]
- Grayer, R.J.; Harborne, J.B.; Kimmins, F.M.; Stevenson, P.C.; Wijayagunasekera, H.N.P. Phenolics in rice phloem sap as sucking deterrents to the brown planthopper, Nilaparvata lugens. Acta Hortic. 1994, 381, 691–694. [Google Scholar] [CrossRef]
- Hao, P.; Feng, Y.; Zhou, Y.; Song, X.; Li, H.; Ma, Y.; Ye, C.; Yu, X. Schaftoside interacts with NlCDK1 protein: A mechanism of rice resistance to brown planthopper, Nilaparvata lugens. Front. Plant Sci. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Chalmers, J.A.; Raj, S.; Thaler, J.S. Induced plant responses to multiple damagers: Differential effects on an herbivore and its parasitoid. Oecologia 2005, 143, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.J.; Zheng, S.J.; van Loon, J.J.; Boland, W.; David, A.; Mumm, R.; Dicke, M. Whiteflies interfere with indirect plant defense against spider mites in lima bean. Proc. Natl. Acad. Sci. USA 2009, 106, 21202–21207. [Google Scholar] [CrossRef]
- Hu, X.; Su, S.; Liu, Q.; Jiao, Y.; Peng, Y.; Li, Y.; Turlings, T.C. Caterpillar-induced rice volatiles provide enemy-free space for the offspring of the brown planthopper. eLife 2020, 9, e55421. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Köllner, T.G.; Wiggins, G.; Grant, J.; Degenhardt, J.; Chen, F. Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J. 2008, 55, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Foresti, N.; Turlings, T.C. A tritrophic signal that attracts parasitoids to host-damaged plants withstands disruption by non-host herbivores. BMC Plant Biol. 2010, 10, 247. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Los Baños, PH, USA, 1976. [Google Scholar]
- Vattikuti, J.; Sailaja, V.; Prasad, Y.G.; Katti, G.R.; Chirutkar, P.M.; Rao, G.R.; Padmakumari, A.P.; Padmavathi, C.; Prabhakar, M. Temperature driven development of the rice brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). J. Agrometeorol. 2019, 21, 131–140. [Google Scholar] [CrossRef]
- Lu, J.; Robert, C.A.M.; Riemann, M.; Cosme, M.; Mène-Saffrané, L.; Massana, J.; Stout, M.J.; Lou, Y.; Gershenzon, J.; Erb, M. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015, 167, 1100–1116. [Google Scholar] [CrossRef]
- De Ascensao, A.R.F.D.; Dubery, I.A. Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f.sp. cubense. Phytochemistry 2003, 63, 679–686. [Google Scholar] [CrossRef]
- Caristi, C.; Bellocco, E.; Panzera, V.; Toscano, G.; Vadalà, R.; Leuzzi, U. Flavonoids detection by HPLC-DAD-MS-MS in lemon juices from Sicilian cultivars. J. Agric. Food Chem. 2003, 51, 3528–3534. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).