Mutation-Driven S100A8 Overexpression Confers Aberrant Phenotypes in Type 1 CALR-Mutated MPN
Abstract
1. Introduction
2. Results
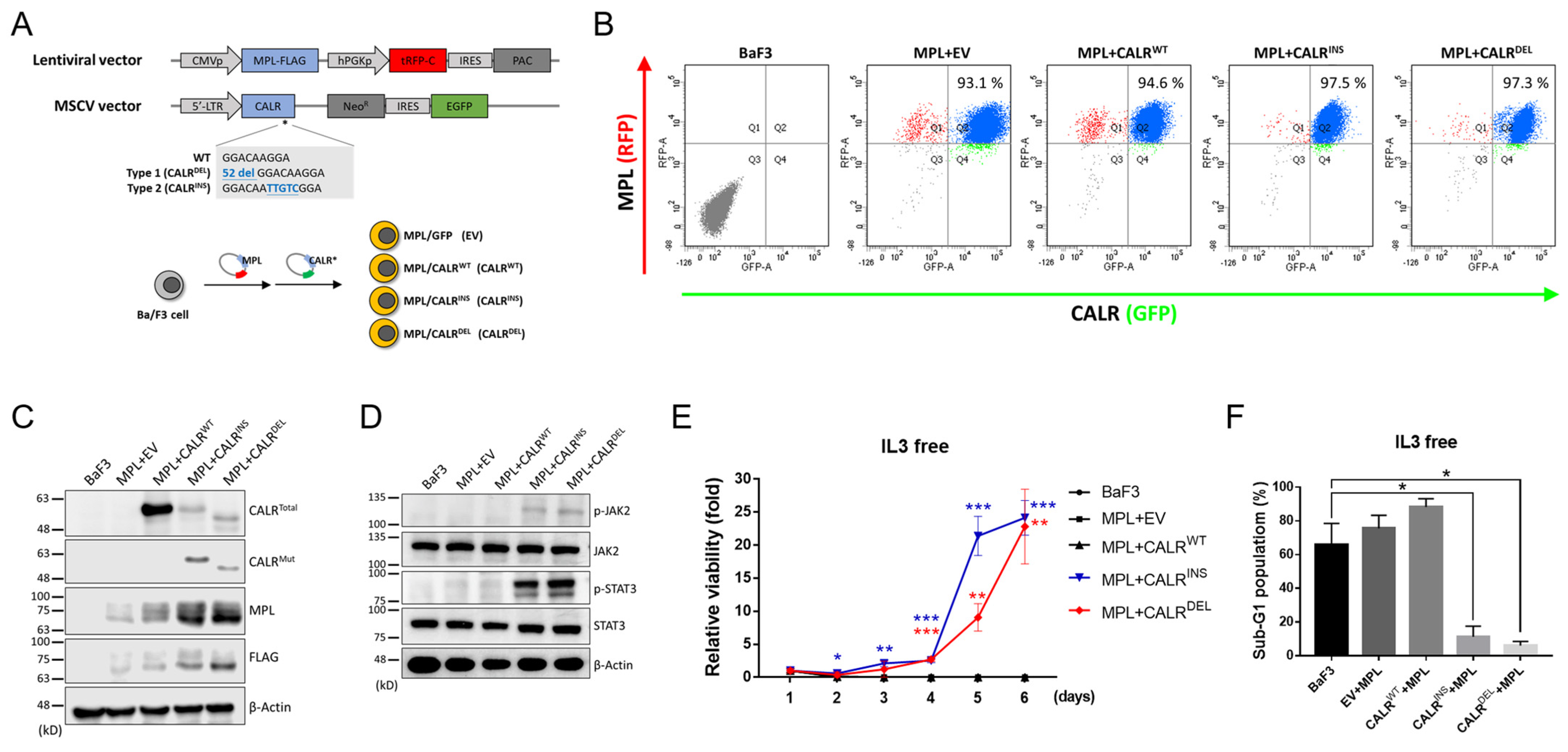
2.1. Establishment of CALR-Mutated MPN-Model Cells
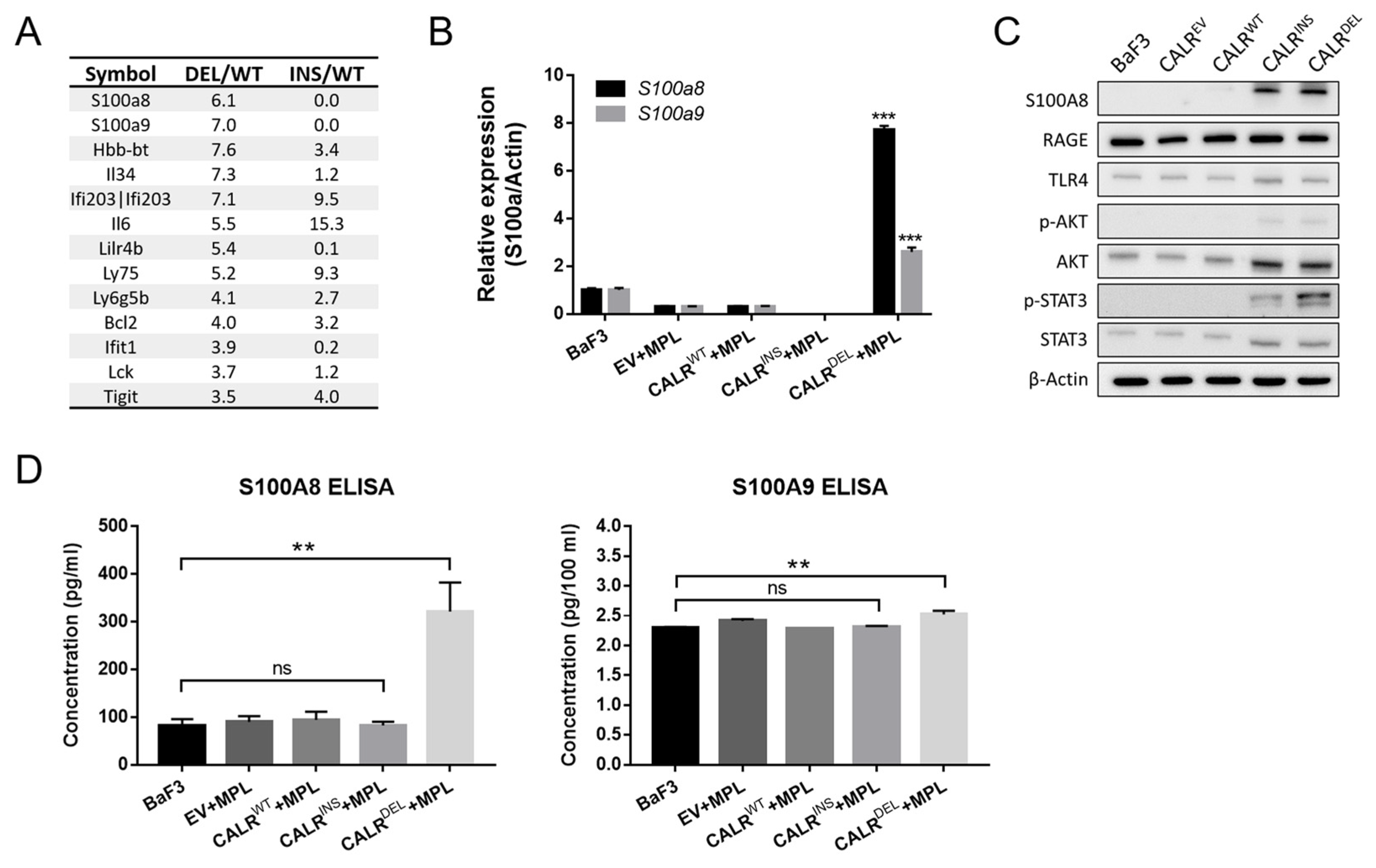
2.2. CALRDEL Cells but Not CALRINS Cells Exhibit Unequivocal Up-Regulation of S100a8
2.3. Inhibition of JAK/STAT3 Signaling Suppresses S100A8 Expression in CALRDEL MPN
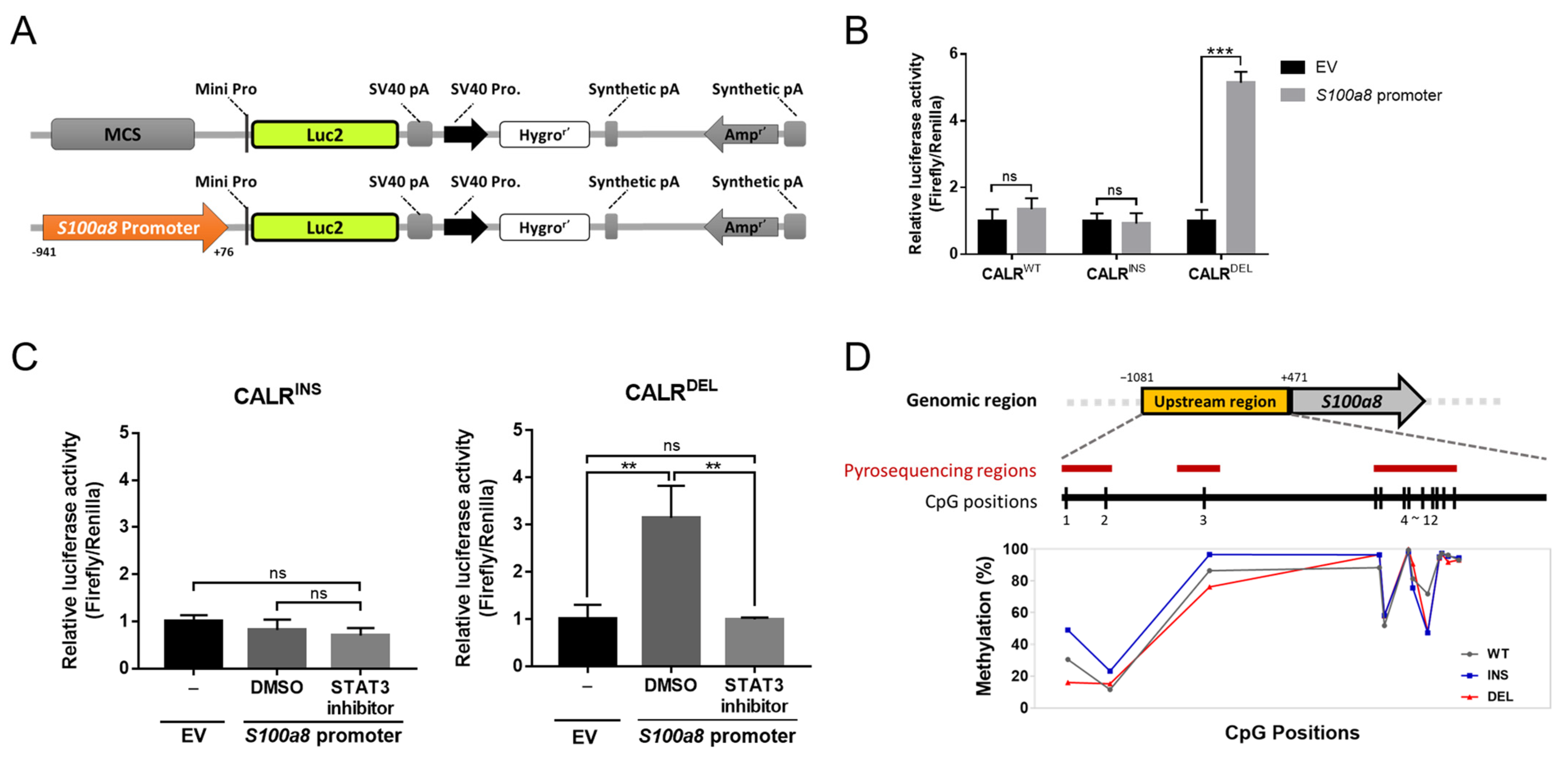
2.4. STAT3 Regulates S100a8 Expression in a Cell Type-Specific Manner, with Disparate Promoter Methylation Being One Potential Mechanism Involved in the Heterogeneous Regulation between CALRDEL and CALRINS Cells
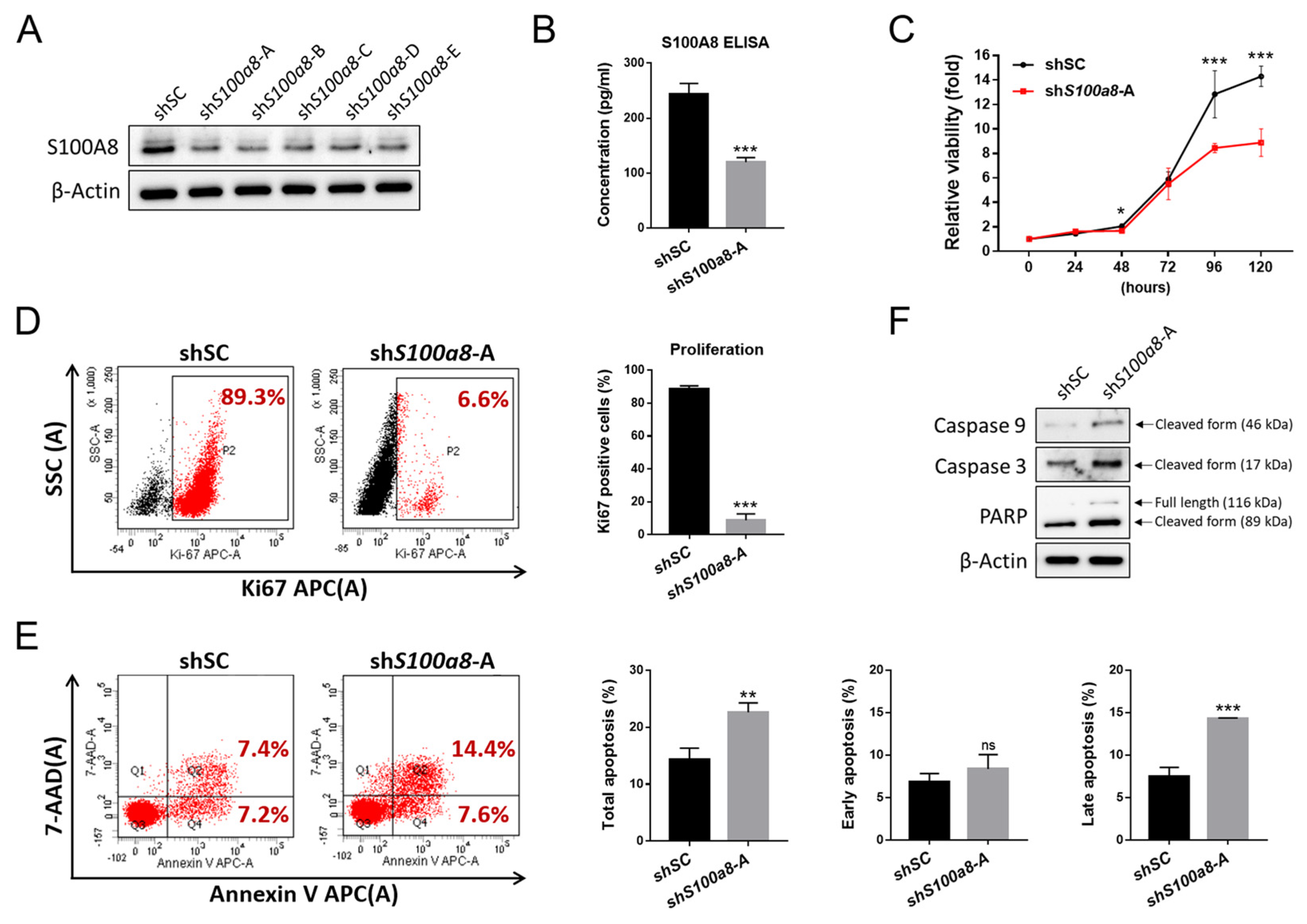
2.5. S100A8 Nonredundantly Contributes to Accelerated Cellular Proliferation and Reduced Apoptosis in CALRDEL Cells
2.6. S100A8-Overexpressing MPN Patients Exhibit Unique Clinical Characteristics
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Culture, and Viral Transduction
4.2. RNA-Seq, qRT-PCR, and ELISA
4.3. Cell Viability, Proliferation, and Apoptosis Assays
4.4. Western Blot
4.5. Luciferase Reporter Assay
4.6. Bisulfite Conversion and Pyrosequencing
4.7. Study Population and Mutational Analysis
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nangalia, J.; Green, T.R. The evolving genomic landscape of myeloproliferative neoplasms. Hematol. Am. Soc. Hematol. Educ. Program. 2014, 2014, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- How, J.; Hobbs, G.S.; Mullally, A. Mutant calreticulin in myeloproliferative neoplasms. Blood 2019, 134, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Finke, C.; Belachew, A.A.; Wassie, E.A.; Ketterling, R.P.; Hanson, C.A.; Pardanani, A. Type 1 vs. type 2 calreticulin mutations in primary myelofibrosis: Differences in phenotype and prognostic impact. Leukemia 2014, 28, 1568–1570. [Google Scholar] [CrossRef]
- Tefferi, A.; Wassie, E.A.; Guglielmelli, P.; Gangat, N.; Belachew, A.A.; Lasho, T.L.; Finke, C.; Ketterling, R.P.; Hanson, C.A.; Pardanani, A.; et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: A collaborative study of 1027 patients. Am. J. Hematol. 2014, 89, E121–E124. [Google Scholar] [CrossRef]
- Pietra, D.; Rumi, E.; Ferretti, V.V.; Di Buduo, C.A.; Milanesi, C.; Cavalloni, C.; Sant’Antonio, E.; Abbonante, V.; Moccia, F.; Casetti, I.C.; et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016, 30, 431–438. [Google Scholar] [CrossRef]
- Lasho, T.L.; Finke, C.M.; Tischer, A.; Pardanani, A.; Tefferi, A. Mayo CALR mutation type classification guide using alpha helix propensity. Am. J. Hematol. 2018, 93, E128–E129. [Google Scholar] [CrossRef]
- Ibarra, J.; Elbanna, Y.A.; Kurylowicz, K.; Ciboddo, M.; Greenbaum, H.S.; Arellano, N.S.; Rodriguez, D.; Evers, M.; Bock-Hughes, A.; Liu, C.; et al. Type I but Not Type II Calreticulin Mutations Activate the IRE1alpha/XBP1 Pathway of the Unfolded Protein Response to Drive Myeloproliferative Neoplasms. Blood Cancer Discov. 2022, 3, 298–315. [Google Scholar] [CrossRef]
- Arellano, N.S.; Kurylowicz, K.; Maxwell, L.; Greenbaum, H.S.; Ibarra, J.; Elf, S. Myeloproliferative Neoplasm-Associated Type 2 Calreticulin Mutations Differentially Activate and Depend on the ATF6 Pathway of the UPR. Blood 2021, 138, 3588. [Google Scholar] [CrossRef]
- Mondet, J.; Chevalier, S.; Mossuz, P. Pathogenic Roles of S100A8 and S100A9 Proteins in Acute Myeloid and Lymphoid Leukemia: Clinical and Therapeutic Impacts. Molecules 2021, 26, 1323. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Zenker, S.; Rossaint, J.; Holscher, A.; Pohlen, M.; Zarbock, A.; Roth, J.; Vogl, T. Alarmin S100A8 Activates Alveolar Epithelial Cells in the Context of Acute Lung Injury in a TLR4-Dependent Manner. Front. Immunol. 2017, 8, 1493. [Google Scholar] [CrossRef] [PubMed]
- Pruenster, M.; Vogl, T.; Roth, J.; Sperandio, M. S100A8/A9: From basic science to clinical application. Pharmacol. Ther. 2016, 167, 120–131. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Sunderkotter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: Is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 2012, 119, 3219–3225. [Google Scholar] [CrossRef]
- Strickland, M.; Quek, L.; Psaila, B. The immune landscape in BCR-ABL negative myeloproliferative neoplasms: Inflammation, infections and opportunities for immunotherapy. Br. J. Haematol. 2021, 196, 1149–1158. [Google Scholar] [CrossRef]
- Goyette, J.; Geczy, C.L. Inflammation-associated S100 proteins: New mechanisms that regulate function. Amino Acids 2011, 41, 821–842. [Google Scholar] [CrossRef]
- Yang, Y.; Akada, H.; Nath, D.; Hutchison, R.E.; Mohi, G. Loss of Ezh2 cooperates with Jak2V617F in the development of myelofibrosis in a mouse model of myeloproliferative neoplasm. Blood 2016, 127, 3410–3423. [Google Scholar] [CrossRef]
- Leimkuhler, N.B.; Gleitz, H.F.E.; Ronghui, L.; Snoeren, I.A.M.; Fuchs, S.N.R.; Nagai, J.S.; Banjanin, B.; Lam, K.H.; Vogl, T.; Kuppe, C.; et al. Heterogeneous bone-marrow stromal progenitors drive myelofibrosis via a druggable alarmin axis. Cell Stem Cell 2021, 28, 637–652. [Google Scholar] [CrossRef]
- Hsu, C.C.; Chen, Y.J.; Huang, C.E.; Wu, Y.Y.; Wang, M.C.; Pei, S.N.; Liao, C.K.; Lu, C.H.; Chen, P.T.; Tsou, H.Y.; et al. Molecular heterogeneity unravelled by single-cell transcriptomics in patients with essential thrombocythaemia. Br. J. Haematol. 2020, 188, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Marty, C.; Pecquet, C.; Nivarthi, H.; El-Khoury, M.; Chachoua, I.; Tulliez, M.; Villeval, J.-L.; Raslova, H.; Kralovics, R.; Constantinescu, S.N. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood 2016, 127, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Chachoua, I.; Pecquet, C.; El-Khoury, M.; Nivarthi, H.; Albu, R.-I.; Marty, C.; Gryshkova, V.; Defour, J.-P.; Vertenoeil, G.; Ngo, A. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 2016, 127, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Cabagnols, X.; Defour, J.P.; Ugo, V.; Ianotto, J.C.; Mossuz, P.; Mondet, J.; Girodon, F.; Alexandre, J.H.; Mansier, O.; Viallard, J.F.; et al. Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: Relevance for disease evolution. Leukemia 2015, 29, 249–252. [Google Scholar] [CrossRef]
- Prins, D.; Gonzalez Arias, C.; Klampfl, T.; Grinfeld, J.; Green, A.R. Mutant Calreticulin in the Myeloproliferative Neoplasms. Hemasphere 2020, 4, e333. [Google Scholar] [CrossRef]
- Sido, J.M.; Yang, X.; Nagarkatti, P.S.; Nagarkatti, M. Delta9-Tetrahydrocannabinol-mediated epigenetic modifications elicit myeloid-derived suppressor cell activation via STAT3/S100A8. J. Leukoc. Biol. 2015, 97, 677–688. [Google Scholar] [CrossRef]
- Bottcher, M.; Panagiotidis, K.; Bruns, H.; Stumpf, M.; Volkl, S.; Geyh, S.; Dietel, B.; Schroeder, T.; Mackensen, A.; Mougiakakos, D. Bone marrow stroma cells promote induction of a chemoresistant and prognostic unfavorable S100A8/A9high AML cell subset. Blood Adv. 2022, 6, 5685–5697. [Google Scholar] [CrossRef]
- Wang, Y.H.; Lin, C.C.; Yao, C.Y.; Amaral, F.; Yu, S.C.; Kao, C.J.; Shih, P.T.; Hou, H.A.; Chou, W.C.; Tien, H.F. High BM plasma S100A8/A9 is associated with a perturbed microenvironment and poor prognosis in myelodysplastic syndromes. Blood Adv. 2023, bloodadvances-2022008958. [Google Scholar] [CrossRef]
- Zambetti, N.A.; Ping, Z.; Chen, S.; Kenswil, K.J.G.; Mylona, M.A.; Sanders, M.A.; Hoogenboezem, R.M.; Bindels, E.M.J.; Adisty, M.N.; Van Strien, P.M.H.; et al. Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell 2016, 19, 613–627. [Google Scholar] [CrossRef]
- Tohumeken, S.; Baur, R.; Bottcher, M.; Stoll, A.; Loschinski, R.; Panagiotidis, K.; Braun, M.; Saul, D.; Volkl, S.; Baur, A.S.; et al. Palmitoylated Proteins on AML-Derived Extracellular Vesicles Promote Myeloid-Derived Suppressor Cell Differentiation via TLR2/Akt/mTOR Signaling. Cancer Res. 2020, 80, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, M.; Mitrović-Ajtić, O.; Beleslin-Čokić, B.; Djikić, D.; Subotički, T.; Diklić, M.; Leković, D.; Gotić, M.; Mossuz, P.; Čokić, V.P. TLR4 and RAGE conversely mediate pro-inflammatory S100A8/9-mediated inhibition of proliferation-linked signaling in myeloproliferative neoplasms. Cell. Oncol. 2018, 41, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Gleitz, H.F.E.; Benabid, A.; Schneider, R.K. Still a burning question: The interplay between inflammation and fibrosis in myeloproliferative neoplasms. Curr. Opin. Hematol. 2021, 28, 364–371. [Google Scholar] [CrossRef]
- Varricchio, L.; Hoffman, R. Megakaryocytes Are Regulators of the Tumor Microenvironment and Malignant Hematopoietic Progenitor Cells in Myelofibrosis. Front. Oncol. 2022, 12, 906698. [Google Scholar] [CrossRef]
- Chen, C.C.; Hsu, C.C.; Chen, S.L.; Lin, P.H.; Chen, J.P.; Pan, Y.R.; Huang, C.E.; Chen, Y.J.; Chen, Y.Y.; Wu, Y.Y.; et al. RAS Mediates BET Inhibitor-Endued Repression of Lymphoma Migration and Prognosticates a Novel Proteomics-Based Subgroup of DLBCL through Its Negative Regulator IQGAP3. Cancers 2021, 13, 5024. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Chen, C.C.; You, J.Y.; Lung, J.; Huang, C.E.; Chen, Y.Y.; Leu, Y.W.; Ho, H.Y.; Li, C.P.; Lu, C.H.; Lee, K.D.; et al. Aberrant let7a/HMGA2 signaling activity with unique clinical phenotype in JAK2-mutated myeloproliferative neoplasms. Haematologica 2017, 102, 509–518. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wang, Y.H.; Chen, Y.Y.; Chen, Y.J.; Lu, C.H.; Wu, Y.Y.; Yang, Y.R.; Tsou, H.Y.; Li, C.P.; Huang, C.E.; et al. The Genomic Landscape in Philadelphia-Negative Myeloproliferative Neoplasm Patients with Second Cancers. Cancers 2022, 14, 3435. [Google Scholar] [CrossRef]


| S100A8High N = 21 | S100A8Low N = 10 | p Value | |
|---|---|---|---|
| Age, years | 62.8 ± 16.5 | 56.6 ± 22.2 | 0.383 |
| Male, no. (%) | 12 (57.1%) | 7 (70.0%) | 0.492 |
| Subtype | 0.242 | ||
| ET | 16 | 10 | |
| PMF/PrePMF | 5 | 0 | |
| Splenomegaly | 0.673 | ||
| Yes | 5 | 4 | |
| No | 8 | 4 | |
| WBC count, ×109/L | 10.6 ± 6.5 | 9.3 ± 3.1 | 0.458 |
| Hemoglobin, g/L | 11.5 ± 2.3 | 12.5 ± 1.7 | 0.216 |
| Hematocrit, % | 35.0 ± 6.7 | 38.4 ± 4.7 | 0.163 |
| Platelet, ×109/L | 625 ± 318 | 975 ± 527 | 0.077 |
| LDH, U/L | 325 ± 130 | 257 ± 70 | 0.285 |
| Uric acid, mg/dL | 6.3 ± 1.5 | 7.0 ± 1.4 | 0.389 |
| Major thrombosis, no. (%) | 5 (23.8%) | 3 (30.0%) | 0.713 |
| Post-ET MF transformation, no. (%) | 2 (12.5%) | 0 (0%) | 0.260 |
| AML transformation, no. (%) | 1 (4.8%) | 0 (0%) | 0.483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-H.; Chen, Y.-J.; Lai, Y.-H.; Wang, M.-C.; Chen, Y.-Y.; Wu, Y.-Y.; Yang, Y.-R.; Tsou, H.-Y.; Li, C.-P.; Hsu, C.-C.; et al. Mutation-Driven S100A8 Overexpression Confers Aberrant Phenotypes in Type 1 CALR-Mutated MPN. Int. J. Mol. Sci. 2023, 24, 8747. https://doi.org/10.3390/ijms24108747
Wang Y-H, Chen Y-J, Lai Y-H, Wang M-C, Chen Y-Y, Wu Y-Y, Yang Y-R, Tsou H-Y, Li C-P, Hsu C-C, et al. Mutation-Driven S100A8 Overexpression Confers Aberrant Phenotypes in Type 1 CALR-Mutated MPN. International Journal of Molecular Sciences. 2023; 24(10):8747. https://doi.org/10.3390/ijms24108747
Chicago/Turabian StyleWang, Ying-Hsuan, Ying-Ju Chen, Yi-Hua Lai, Ming-Chung Wang, Yi-Yang Chen, Yu-Ying Wu, Yao-Ren Yang, Hsing-Yi Tsou, Chian-Pei Li, Chia-Chen Hsu, and et al. 2023. "Mutation-Driven S100A8 Overexpression Confers Aberrant Phenotypes in Type 1 CALR-Mutated MPN" International Journal of Molecular Sciences 24, no. 10: 8747. https://doi.org/10.3390/ijms24108747
APA StyleWang, Y.-H., Chen, Y.-J., Lai, Y.-H., Wang, M.-C., Chen, Y.-Y., Wu, Y.-Y., Yang, Y.-R., Tsou, H.-Y., Li, C.-P., Hsu, C.-C., Huang, C.-E., & Chen, C.-C. (2023). Mutation-Driven S100A8 Overexpression Confers Aberrant Phenotypes in Type 1 CALR-Mutated MPN. International Journal of Molecular Sciences, 24(10), 8747. https://doi.org/10.3390/ijms24108747






