Low Magnesium Concentration Enforces Bone Calcium Deposition Irrespective of 1,25-Dihydroxyvitamin D3 Concentration
Abstract
1. Introduction
2. Results
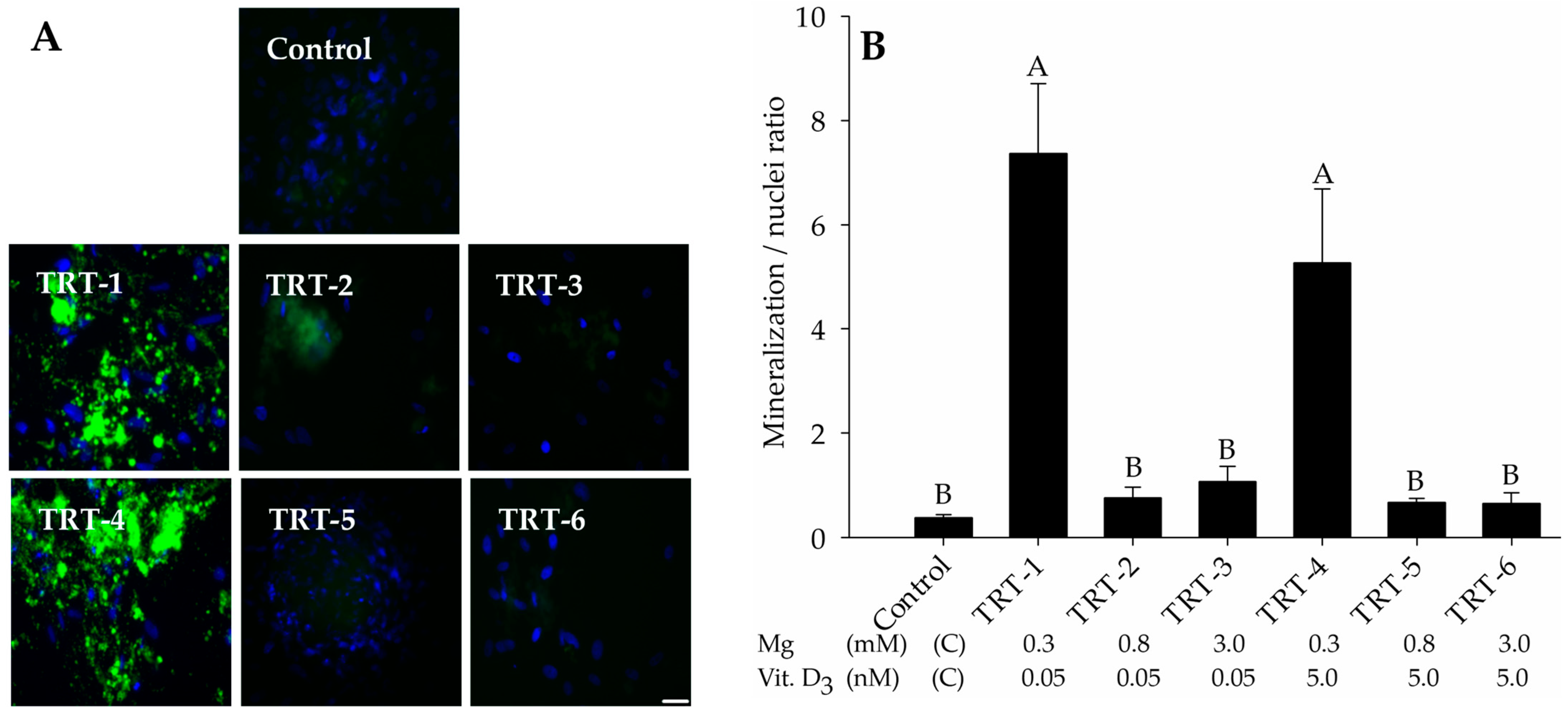
2.1. Role of 1,25D and Magnesium in Hydroxyapatite Deposition
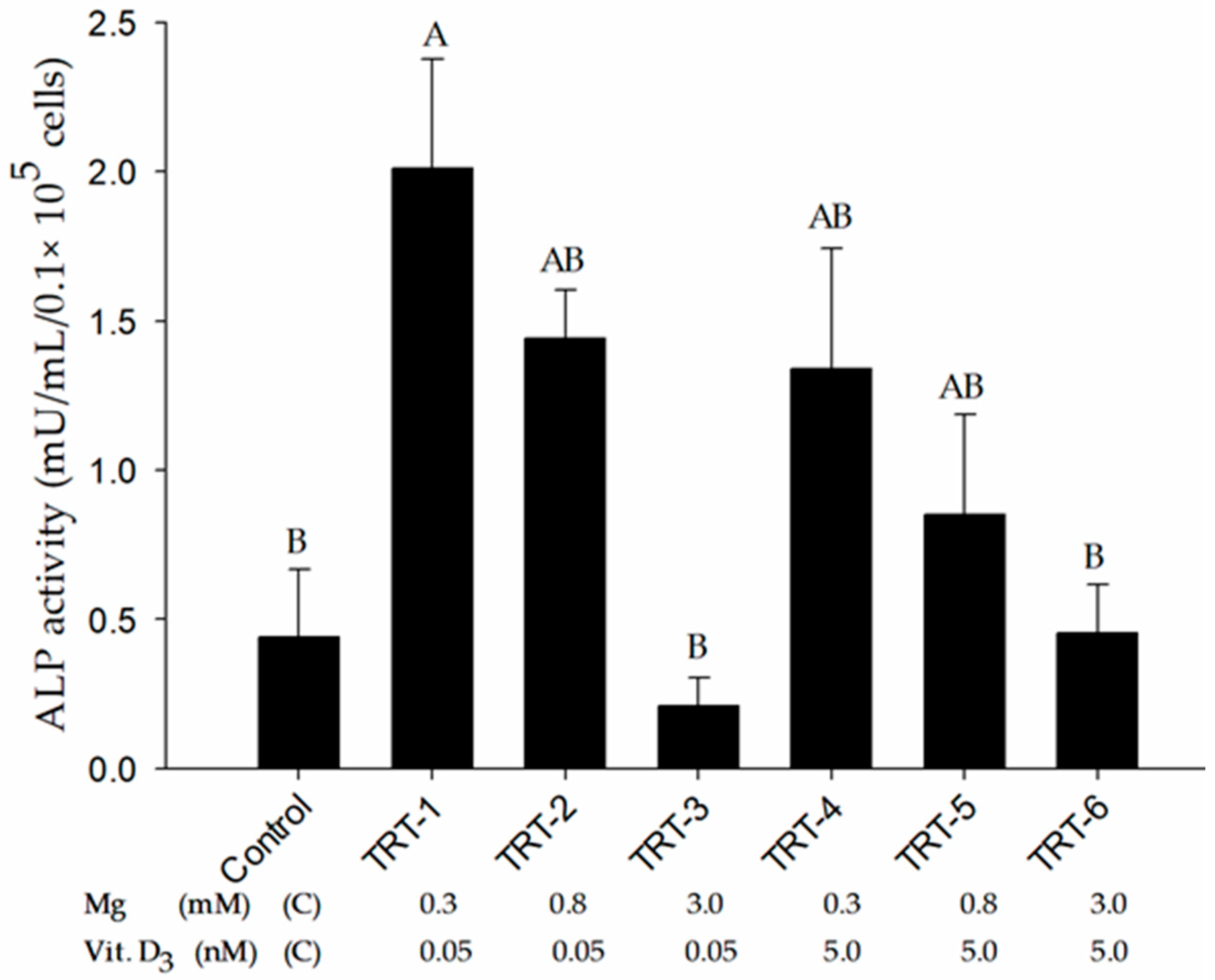
2.2. Role of 1,25D and Magnesium in Alkaline Phosphatase Activity
2.3. Immunocytochemical Analysis
2.4. PCR Analysis
3. Discussion
4. Materials and Methods
4.1. Preadipocytes Isolation and Culture
4.2. Osteogenic Differentiation
4.3. Alkaline Phosphatase Activity
4.4. Microscopic Validation of Preadipocyte Differentiation and Hydroxyapatite Formation
4.5. RNA Isolation and RT-qPCR Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvao, K.; Santos, J.E. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P. Calcium and magnesium disorders. Vet. Clin. N. Am. Food Anim. Pract. 2014, 30, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P. Treatment of calcium, phosphorus, and magnesium balance disorders. Vet. Clin. N. Am. Food Anim. Pract. 1999, 15, 619–639. [Google Scholar] [CrossRef]
- Wilms, J.; Wang, G.; Doelman, J.; Jacobs, M.; Martin-Tereso, J. Intravenous calcium infusion in a calving protocol disrupts calcium homeostasis compared with an oral calcium supplement. J. Dairy Sci. 2019, 102, 6056–6064. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ahdoot, R.S.; Kalantar-Zadeh, K.; Ghobry, L.; Kurtz, I. Calcium Transport in the Kidney and Disease Processes. Front. Endocrinol. 2021, 12, 762130. [Google Scholar] [CrossRef]
- Wysolmerski, J.J. Osteocytic osteolysis: Time for a second look? Bonekey Rep. 2012, 1, 229. [Google Scholar] [CrossRef]
- Goltzman, D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef]
- Lou, Y.R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D(3) induces osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 2017, 7, 42816. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stenslokken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Mann, S.; McArt, J.; Abuelo, A.J.I.P. Production-related metabolic disorders of cattle: Ketosis, milk fever and grass staggers. Practice 2019, 41, 205–219. [Google Scholar] [CrossRef]
- Jurek, S.; Sandhu, M.A.; Trappe, S.; Bermudez-Pena, M.C.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Optimizing adipogenic transdifferentiation of bovine mesenchymal stem cells: A prominent role of ascorbic acid in FABP4 induction. Adipocyte 2020, 9, 35–50. [Google Scholar] [CrossRef]
- Rashid, U.; Yousaf, A.; Yaqoob, M.; Saba, E.; Moaeen-Ud-Din, M.; Waseem, S.; Becker, S.K.; Sponder, G.; Aschenbach, J.R.; Sandhu, M.A. Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet. Res. 2021, 17, 388. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef]
- Horst, R.L.; Jorgensen, N.A.; DeLuca, H.F. Plasma 1,25-dihydroxyvitamin D and parathyroid hormone levels in paretic dairy cows. Am. J. Physiol. 1978, 235, E634–E637. [Google Scholar] [CrossRef]
- Barton, B.A.; Horst, R.L.; Jorgensen, N.A.; DeLuca, H.F. Concentration of calcium, phosphorus, and 1,25-dihydroxyvitamin D in plasma of dairy cows during the lactation cycle. J. Dairy Sci. 1981, 64, 850–852. [Google Scholar] [CrossRef]
- Martin-Tereso, J.; Martens, H. Calcium and magnesium physiology and nutrition in relation to the prevention of milk fever and tetany (dietary management of macrominerals in preventing disease). Vet. Clin. N. Am. Food Anim. Pract. 2014, 30, 643–670. [Google Scholar] [CrossRef]
- Halse, K. Individual variation in blood magnesium and susceptibility to hypomagnesaemia in cows. Acta Vet. Scand. 1970, 11, 394–414. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin D activation and function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Long, S.; Romani, A.M.P. Role of cellular magnesium in human diseases. Austin J. Nutr. Food Sci. 2014, 2, 10. [Google Scholar]
- Deng, X.; Song, Y.; Manson, J.E.; Signorello, L.B.; Zhang, S.M.; Shrubsole, M.J.; Ness, R.M.; Seidner, D.L.; Dai, Q. Magnesium, vitamin D status and mortality: Results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Reddi, S.; Shanmugam, V.P.; Tanedjeu, K.S.; Kapila, S.; Kapila, R. Effect of buffalo casein-derived novel bioactive peptides on osteoblast differentiation. Eur. J. Nutr. 2018, 57, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A. High magnesium inhibits human osteoblast differentiation in vitro. Magnes Res. 2011, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, I.M.; Landis, W.J.; Risbud, M.V. Matrix vesicles: Are they anchored exosomes? Bone 2015, 79, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in bone mineralization and potential use as therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.C.; Kuzynski, M.; Bottini, M.; Beniash, E.; Dokland, T.; Mobley, C.G.; Yadav, M.C.; Poliard, A.; Kellermann, O.; Millan, J.L.; et al. Phosphate induces formation of matrix vesicles during odontoblast-initiated mineralization in vitro. Matrix Biol. 2016, 52–54, 284–300. [Google Scholar] [CrossRef]
- Michigami, T.; Ozono, K. Roles of phosphate in skeleton. Front. Endocrinol. 2019, 10, 180. [Google Scholar] [CrossRef]
- Chu, W.; Li, T.; Jia, G.; Chang, Y.; Liu, Z.; Pei, J.; Yu, D.; Zhai, Z. Exposure to high levels of magnesium disrupts bone mineralization in vitro and in vivo. Ann. Transl. Med. 2020, 8, 1419. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Li, J.; Zhu, Y.; Zhang, X. High extracellular magnesium inhibits mineralized matrix deposition and modulates intracellular calcium signaling in human bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 1390–1395. [Google Scholar] [CrossRef]
- Lee, H.J.; Song, Y.M.; Baek, S.; Park, Y.H.; Park, J.B. Vitamin D enhanced the osteogenic differentiation of cell spheroids composed of bone marrow stem cells. Medicina 2021, 57, 1271. [Google Scholar] [CrossRef]
- Chang, C.B.; Han, S.A.; Kim, E.M.; Lee, S.; Seong, S.C.; Lee, M.C. Chondrogenic potentials of human synovium-derived cells sorted by specific surface markers. Osteoarthr. Cartil. 2013, 21, 190–199. [Google Scholar] [CrossRef]
- Sandhu, M.A.; Jurek, S.; Trappe, S.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Influence of bovine serum lipids and fetal bovine serum on the expression of cell surface markers in cultured bovine preadipocytes. Cells Tissues Organs 2017, 204, 13–24. [Google Scholar] [CrossRef]
- McClellan, P.; Jacquet, R.; Yu, Q.; Landis, W.J. A method for the immunohistochemical identification and localization of osterix in periosteum-wrapped constructs for tissue engineering of bone. J. Histochem. Cytochem. 2017, 65, 407–420. [Google Scholar] [CrossRef]
- Peng, Y.; Shi, K.; Wang, L.; Lu, J.; Li, H.; Pan, S.; Ma, C. Characterization of osterix protein stability and physiological role in osteoblast differentiation. PLoS ONE 2013, 8, e56451. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef]
- Louvet, L.; Metzinger, L.; Buchel, J.; Steppan, S.; Massy, Z.A. Magnesium attenuates phosphate-induced deregulation of a microRNA signature and prevents modulation of smad1 and osterix during the course of vascular calcification. BioMed Res. Int. 2016, 2016, 7419524. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Huang, Y.J.; Wu, H.H.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef]
- Guney, G.; Sener-Simsek, B.; Tokmak, A.; Yucel, A.; Buyukkagnici, U.; Yilmaz, N.; Engin-Ustun, Y.; Ozgu-Erdinc, A.S. Assessment of the relationship between serum vitamin D and osteocalcin levels with metabolic syndrome in non-osteoporotic postmenopausal women. Geburtshilfe Frauenheilkd 2019, 79, 293–299. [Google Scholar] [CrossRef]
- Nourisa, J.; Zeller-Plumhoff, B.; Helmholz, H.; Luthringer-Feyerabend, B.; Ivannikov, V.; Willumeit-Romer, R. Magnesium ions regulate mesenchymal stem cells population and osteogenic differentiation: A fuzzy agent-based modeling approach. Comput. Struct. Biotechnol. J. 2021, 19, 4110–4122. [Google Scholar] [CrossRef]
- Shahsavani, Z.; Asadi, A.; Shamshirgardi, E.; Akbarzadeh, M. Vitamin D, magnesium and their interactions: A review. Int. J. Nutr. Sci. 2021, 6, 113–118. [Google Scholar]
- Tashiro, K.; Abe, T.; Oue, N.; Yasui, W.; Ryoji, M. Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol. Cell Endocrinol. 2004, 226, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tsang, H.T.; Edwards, T.L.; Wang, X.; Connell, J.W.; Davies, R.J.; Durrington, H.J.; O’Kane, C.J.; Luzio, J.P.; Reid, E. The hereditary spastic paraplegia proteins NIPA1, spastin and spartin are inhibitors of mammalian BMP signalling. Hum. Mol. Genet. 2009, 18, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Sahni, J.; Nelson, B.; Scharenberg, A.M. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem. J. 2007, 401, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Kolisek, M.; Nestler, A.; Vormann, J.; Schweigel-Rontgen, M. Human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. Am. J. Physiol. Cell Physiol. 2012, 302, C318–C326. [Google Scholar] [CrossRef]
- Mastrototaro, L.; Smorodchenko, A.; Aschenbach, J.R.; Kolisek, M.; Sponder, G. Solute carrier 41A3 encodes for a mitochondrial Mg2+ efflux system. Sci. Rep. 2016, 6, 27999. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef]
- Fong, J.; Khan, A. Hypocalcemia: Updates in diagnosis and management for primary care. Can. Fam. Physician 2012, 58, 158–162. [Google Scholar]
- Liu, R.H.; Razavi, C.R.; Chang, H.Y.; Tufano, R.P.; Eisele, D.W.; Gourin, C.G.; Russell, J.O. Association of hypocalcemia and magnesium disorders with thyroidectomy in commercially insured patients. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 237–246. [Google Scholar] [CrossRef]
- Becker, S.K.; Sponder, G.; Sandhu, M.A.; Trappe, S.; Kolisek, M.; Aschenbach, J.R. The combined influence of magnesium and insulin on central metabolic functions and expression of genes involved in magnesium homeostasis of cultured bovine adipocytes. Int. J. Mol. Sci. 2021, 22, 5897. [Google Scholar] [CrossRef]


| Treatments | Mg (mM) | 1,25D 1 (nM) |
|---|---|---|
| Control | 0.80 | 0 |
| TRT-1 | 0.30 | 0.05 |
| TRT-2 | 0.80 | 0.05 |
| TRT-3 | 3.00 | 0.05 |
| TRT-4 | 0.30 | 5.00 |
| TRT-5 | 0.80 | 5.00 |
| TRT-6 | 3.00 | 5.00 |
| Gene | Sense Primer (5′-3′) | Anti-Sense Primer (3′-5′) | Amplicon Size, bp | |
|---|---|---|---|---|
| Stem cell markers | NT5E (CD73) | TTTGGAGGCACCTTTGACC | AGAGGCTCATAACTGGGCAC | 212 |
| THY1 (CD90) | CAACTTCACCACCAAGGATG | TCTGGATCAGCAGGCTTATG | 140 | |
| ENG (endoglin, CD105) | CCTCAGCGTGAACAAATCC | CGTGAAAGACCAGTTTGGAG | 89 | |
| Osteogenic genes | SP7 (osterix) | TGCTTGAGGAGGAAGCTCAC | TTTGGAGGCCGAAAGGTCAC | 160 |
| BGLAP (osteocalcin) | GCAAAGGCGCAGCCTTCGTG | AAGCCGATGTGGTCAGCTAG | 174 | |
| Magnesium-responsive genes | SLC41A1 | TGGTGTTCCTCTATACCATCAG | TCAAGTACGGGATGGAGAAG | 186 |
| SLC41A2 | CTGCTTTTAGTGATACCTGGAC | TTCCTTTCCTCCAGAAATGATG | 178 | |
| SLC41A3 | CTTCTGCACTATTTCCAGCAC | TCATCTCCAGGTTGCCCTTC | 100 | |
| TRPM6 | ACAAACCATTCCCTACACTCC | CGTTGTTGTTGTTGTACTTCC | 125 | |
| TRPM7 | ATACAAGAGGGGAGTTACTGG | GGGCCAAAAACCATATCACAG | 112 | |
| NIPA1 | TCCCCGAAATCTGAGAGTGTG | AGAAGATGAGCAGCAGCAGC | 115 | |
| Vitamin D-responsive genes | VDR | TTCGCTCCAACCAGTCCTTC | CTCTTCGTGCAAATTCAGCTTC | 166 |
| CYP24A1 | CAATTTATCCCGTAATCCCCAC | AGCATATTCCCCCAGAACC | 204 | |
| Housekeeping gene | GAPDH | AAGAAGGTGGTGAAGCAGG | GCATCGAAGGTAGAAGAGTGAG | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, U.; Becker, S.K.; Sponder, G.; Trappe, S.; Sandhu, M.A.; Aschenbach, J.R. Low Magnesium Concentration Enforces Bone Calcium Deposition Irrespective of 1,25-Dihydroxyvitamin D3 Concentration. Int. J. Mol. Sci. 2023, 24, 8679. https://doi.org/10.3390/ijms24108679
Rashid U, Becker SK, Sponder G, Trappe S, Sandhu MA, Aschenbach JR. Low Magnesium Concentration Enforces Bone Calcium Deposition Irrespective of 1,25-Dihydroxyvitamin D3 Concentration. International Journal of Molecular Sciences. 2023; 24(10):8679. https://doi.org/10.3390/ijms24108679
Chicago/Turabian StyleRashid, Usman, Sandra K. Becker, Gerhard Sponder, Susanne Trappe, Mansur A. Sandhu, and Jörg R. Aschenbach. 2023. "Low Magnesium Concentration Enforces Bone Calcium Deposition Irrespective of 1,25-Dihydroxyvitamin D3 Concentration" International Journal of Molecular Sciences 24, no. 10: 8679. https://doi.org/10.3390/ijms24108679
APA StyleRashid, U., Becker, S. K., Sponder, G., Trappe, S., Sandhu, M. A., & Aschenbach, J. R. (2023). Low Magnesium Concentration Enforces Bone Calcium Deposition Irrespective of 1,25-Dihydroxyvitamin D3 Concentration. International Journal of Molecular Sciences, 24(10), 8679. https://doi.org/10.3390/ijms24108679







