Identification of Torquetenovirus Species in Patients with Kawasaki Disease Using a Newly Developed Species-Specific PCR Method
Abstract
1. Introduction
2. Results
2.1. Quantification of Total TTV DNA in Individual Samples
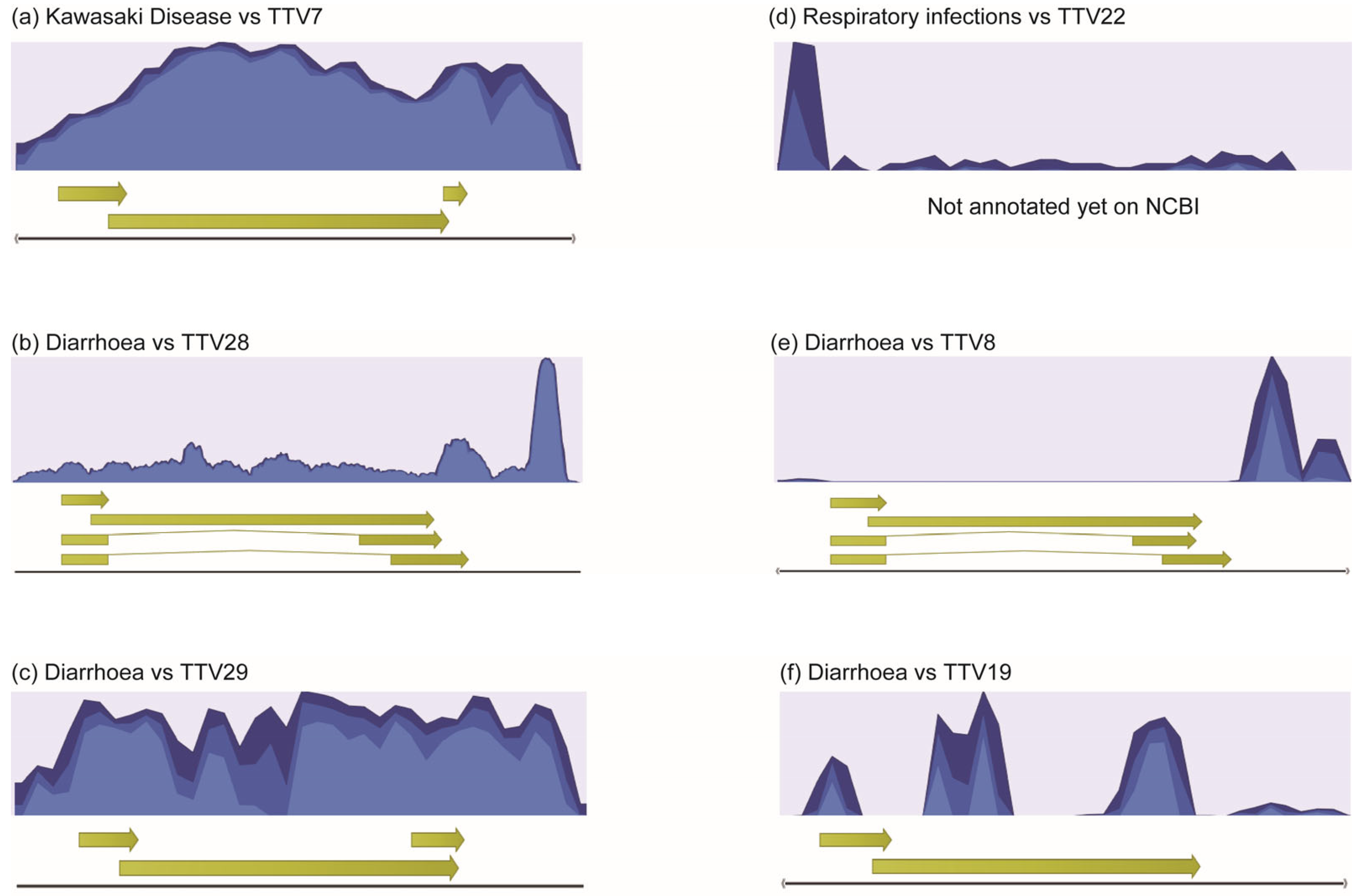
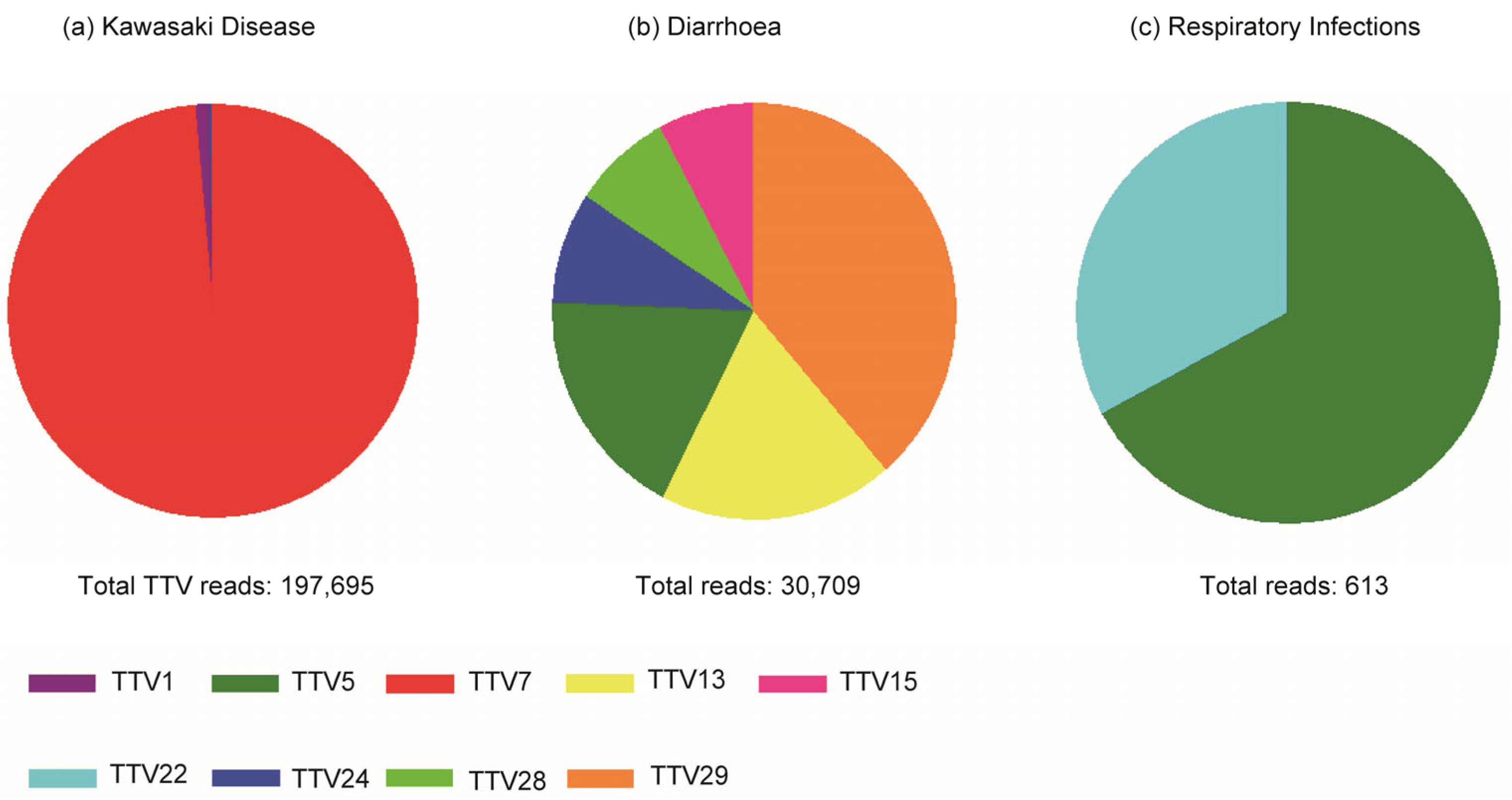
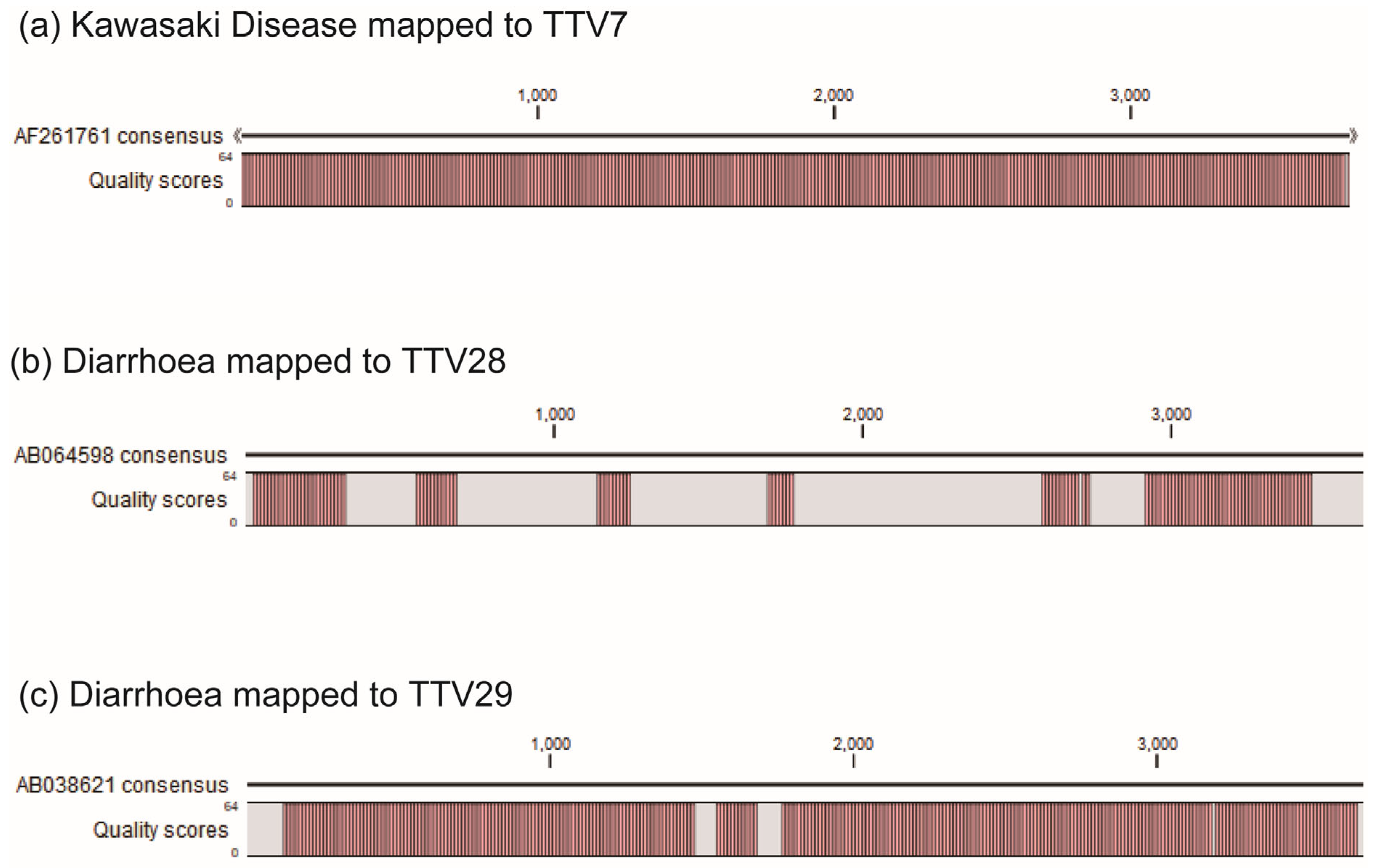
2.2. High-Throughput NGS Sequencing and Bioinformatical Analysis
2.3. Selection of TTV Species Based on High-Throughput NGA Sequencing
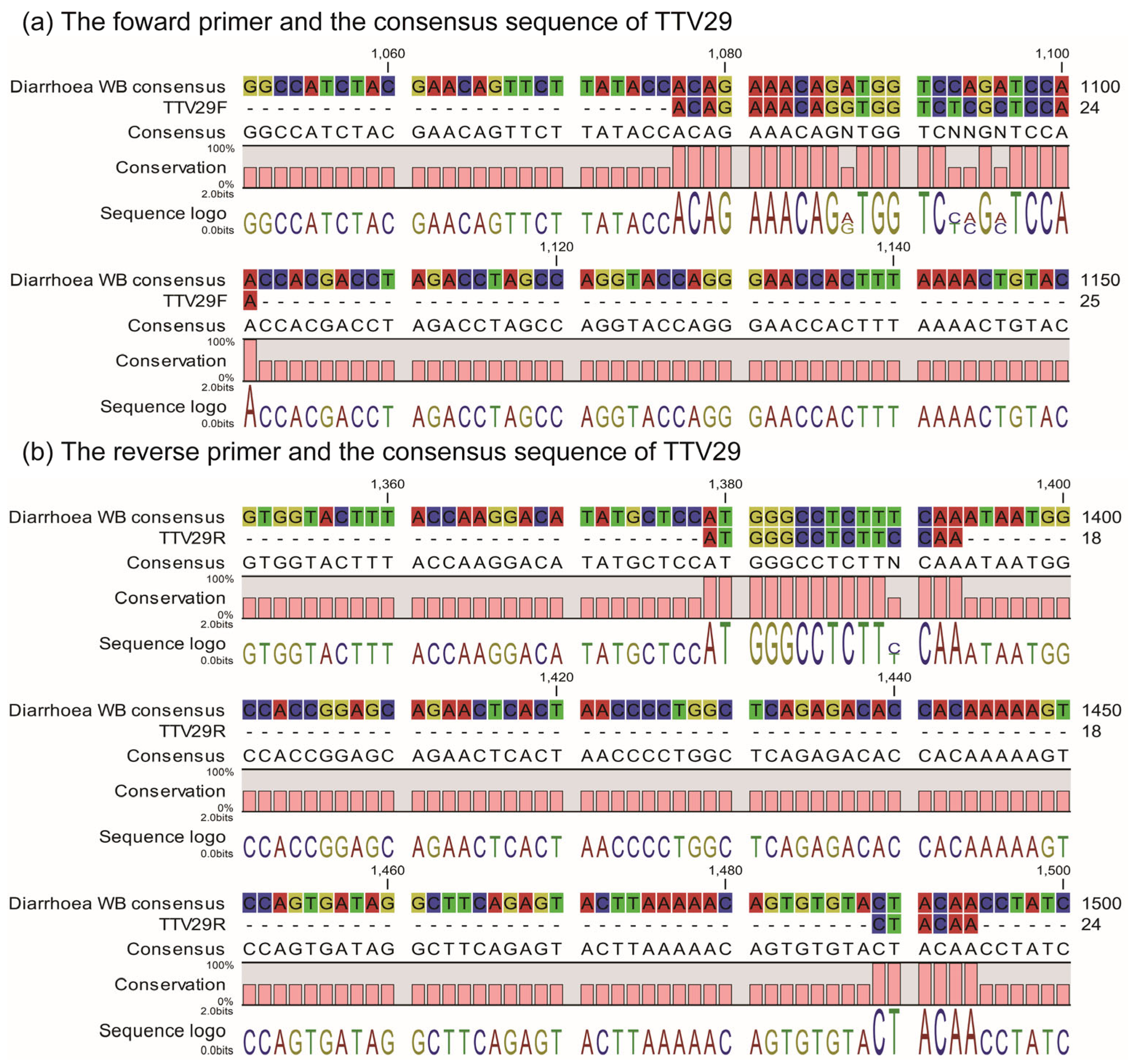
2.4. ssTTV-PCR
2.5. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Participants and Samples
4.2. Quantification of Total TTV DNA in Individual Samples
4.3. NGS Sequencing of Pooled Samples
4.4. Identification of TTV Species in Individual WB Samples by ssTTV-PCR
4.5. Statistical Analysis of the Association between Each TTV and KD
4.6. Bioinformatical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawasaki, T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967, 16, 178–222. (In Japanese) [Google Scholar] [PubMed]
- Ogata, S.; Tremoulet, A.H.; Sato, Y.; Ueda, K.; Shimizu, C.; Sun, X.; Jain, S.; Silverstein, L.; Baker, A.L.; Tanaka, N.; et al. Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int. J. Cardiol. 2013, 168, 3825–3828. [Google Scholar] [CrossRef] [PubMed]
- Makino, N.; Nakamura, Y.; Yashiro, M.; Kosami, K.; Matsubara, Y.; Ae, R.; Aoyama, Y.; Yanagawa, H. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr. Int. 2019, 61, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Lin, K.M.; Ho, S.C.; Yan, J.H.; Lo, M.H.; Kuo, H.C. Increased Incidence of Kawasaki Disease in Taiwan in Recent Years: A 15 Years Nationwide Population-Based Cohort Study. Front Pediatr. 2019, 7, 121. [Google Scholar] [CrossRef]
- Kim, G.B.; Eun, L.Y.; Han, J.W.; Kim, S.H.; Yoon, K.L.; Han, M.Y.; Yu, J.J.; Choi, J.W.; Rhim, J.W. Epidemiology of Kawasaki Disease in South Korea: A Nationwide Survey 2015–2017. Pediatr. Infect. Dis. J. 2020, 39, 1012–1016. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chang, L.Y.; Lu, C.Y.; Shao, P.L.; Fan, T.Y.; Cheng, A.L.; Hu, J.J.; Yeh, S.J.; Chang, C.C.; Huang, L.M. Transmission of acute infectious illness among cases of Kawasaki disease and their household members. J. Formos. Med. Assoc. 2014, 114, 72–76. [Google Scholar] [CrossRef]
- Nagao, Y.; Urabe, C.; Nakamura, H.; Hatano, N. Predicting the characteristics of the aetiologic agent for Kawasaki disease from other paediatric infectious diseases in Japan. Epidemiol. Infect. 2016, 144, 478–492. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, Y.K.; Min, S.H.; Kim, S.W.; Lee, Y.H.; Lee, J.M. Seasonal Trends of Viral Prevalence and Incidence of Kawasaki Disease: A Korea Public Health Data Analysis. J. Clin. Med. 2021, 10, 3301. [Google Scholar] [CrossRef]
- Onouchi, Y.; Ozaki, K.; Burns, J.C.; Shimizu, C.; Terai, M.; Hamada, H.; Honda, T.; Suzuki, H.; Suenaga, T.; Takeuchi, T.; et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat. Genet. 2012, 44, 517–521. [Google Scholar] [CrossRef]
- Onouchi, Y.; Gunji, T.; Burns, J.C.; Shimizu, C.; Newburger, J.W.; Yashiro, M.; Nakamura, Y.; Yanagawa, H.; Wakui, K.; Fukushima, Y.; et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat. Genet. 2008, 40, 35–42. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kuo, H.C.; Chang, J.S.; Chang, L.Y.; Huang, L.M.; Chen, M.R.; Liang, C.D.; Chi, H.; Huang, F.Y.; Lee, M.L.; et al. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat. Genet. 2012, 44, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.C.; Davila, S.; Breunis, W.B.; Lee, Y.C.; Shimizu, C.; Wright, V.J.; Yeung, R.S.; Tan, D.E.; Sim, K.S.; Wang, J.J.; et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat. Genet. 2011, 43, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Wu, Y.T.; Liu, C.A.; Kuo, H.C.; Yang, K.D. Kawasaki disease: Infection, immunity and genetics. Pediatr. Infect. Dis. J. 2005, 24, 998–1004. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, J.J.; Lee, J.; Kim, M.N.; Ko, H.K.; Choi, H.S.; Kim, Y.H.; Ko, J.K. Detection rate and clinical impact of respiratory viruses in children with Kawasaki disease. Korean J. Pediatr. 2012, 55, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Lu, C.Y.; Shao, P.L.; Lee, P.I.; Lin, M.T.; Fan, T.Y.; Cheng, A.L.; Lee, W.L.; Hu, J.J.; Yeh, S.J.; et al. Viral infections associated with Kawasaki disease. J. Formos. Med. Assoc. 2014, 113, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Thissen, J.B.; Isshiki, M.; Jaing, C.; Nagao, Y.; Lebron Aldea, D.; Allen, J.E.; Izui, M.; Slezak, T.R.; Ishida, T.; Sano, T. A novel variant of torque teno virus 7 identified in patients with Kawasaki Disease. PLoS ONE 2018, 13, e0209683. [Google Scholar] [CrossRef]
- Strassl, R.; Doberer, K.; Rasoul-Rockenschaub, S.; Herkner, H.; Görzer, I.; Kläger, J.P.; Schmidt, R.; Haslacher, H.; Schiemann, M.; Eskandary, F.A.; et al. Torque Teno Virus for Risk Stratification of Acute Biopsy-Proven Alloreactivity in Kidney Transplant Recipients. J. Infect. Dis. 2019, 219, 1934–1939. [Google Scholar] [CrossRef]
- Doberer, K.; Haupenthal, F.; Nackenhorst, M.; Bauernfeind, F.; Dermuth, F.; Eigenschink, M.; Schiemann, M.; Kläger, J.; Görzer, I.; Eskandary, F.; et al. Torque Teno Virus Load Is Associated with Subclinical Alloreactivity in Kidney Transplant Recipients: A Prospective Observational Trial. Transplantation 2021, 105, 2112–2118. [Google Scholar] [CrossRef]
- Albert, E.; Torres, I.; Talaya, A.; Giménez, E.; Piñana, J.L.; Hernández-Boluda, J.C.; Focosi, D.; Macera, L.; Maggi, F.; Solano, C.; et al. Kinetics of torque teno virus DNA load in saliva and plasma following allogeneic hematopoietic stem cell transplantation. J. Med. Virol. 2018, 90, 1438–1443. [Google Scholar] [CrossRef]
- Biagini, P.; Bendinelli, M.; Hino, S.; Kakkola, L.; Mankertz, A.; Niel, C.; Okamoto, H.; Raidal, S.; Teo, C.G.; Todd, D. Family Anelloviridae. In IXth Report of the International Committee for the Taxonomy of Viruses; King, A.M.Q., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2012; pp. 331–341. [Google Scholar]
- Varsani, A.; Opriessnig, T.; Celer, V.; Maggi, F.; Okamoto, H.; Blomström, A.L.; Cadar, D.; Harrach, B.; Biagini, P.; Kraberger, S. Taxonomic update for mammalian anelloviruses (family Anelloviridae). Arch. Virol. 2021, 166, 2943–2953. [Google Scholar] [CrossRef]
- Stadhouders, R.; Pas, S.D.; Anber, J.; Voermans, J.; Mes, T.H.; Schutten, M. The effect of primer-template mismatches on the detection and quantification of nucleic acids using the 5′ nuclease assay. J. Mol. Diagn. 2010, 12, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Riollano-Cruz, M.; Akkoyun, E.; Briceno-Brito, E.; Kowalsky, S.; Reed, J.; Posada, R.; Sordillo, E.M.; Tosi, M.; Trachtman, R.; Paniz-Mondolfi, A. Multisystem inflammatory syndrome in children related to COVID-19: A New York City experience. J. Med. Virol. 2021, 93, 424–433. [Google Scholar] [CrossRef]
- Keshavarz, P.; Yazdanpanah, F.; Azhdari, S.; Kavandi, H.; Nikeghbal, P.; Bazyar, A.; Rafiee, F.; Nejati, S.F.; Sadabad, F.E.; Rezaei, N. Coronavirus disease 2019 (COVID-19): A systematic review of 133 Children that presented with Kawasaki-like multisystem inflammatory syndrome. J. Med. Virol. 2021, 93, 5458–5473. [Google Scholar] [CrossRef]
- Pitzer, V.E.; Burgner, D.; Viboud, C.; Simonsen, L.; Andreasen, V.; Steiner, C.A.; Lipsitch, M. Modelling seasonal variations in the age and incidence of Kawasaki disease to explore possible infectious aetiologies. Proc. R. Soc. B Biol. Sci. 2012, 279, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Huang, L.M.; Chang, I.S.; Chang, L.Y.; Chiang, B.L.; Chen, P.J.; Wu, M.H.; Lue, H.C.; Lee, C.Y. Epidemiologic features of Kawasaki disease in Taiwan, 2003–2006. Pediatrics 2009, 123, e401–e405. [Google Scholar] [CrossRef] [PubMed]
- Melonari, P.; Abate, H.; Llano López, L.H.; Cutlca, R.J.; Apaz, M.T.; Battagliotti, C.; Vilca, I.; Cancellara, A.; Calvari, M.; Ellis, A.; et al. Clinical-epidemiological characteristics and predictors of coronary complications in children of Argentina with Kawasaki disease. Rev. Chil. Infectol. 2019, 36, 636–641. (In Spanish) [Google Scholar] [CrossRef]
- Jakob, A.; Whelan, J.; Kordecki, M.; Berner, R.; Stiller, B.; Arnold, R.; von Kries, R.; Neumann, E.; Roubinis, N.; Robert, M.; et al. Kawasaki Disease in Germany: A Prospective, Population-based Study Adjusted for Underreporting. Pediatr. Infect. Dis. J. 2016, 35, 129–134. [Google Scholar] [CrossRef]
- Fernandez-Cooke, E.; Barrios Tascón, A.; Sánchez-Manubens, J.; Antón, J.; Grasa Lozano, C.D.; Aracil Santos, J.; Villalobos Pinto, E.; Clemente Garulo, D.; Mercader Rodríguez, B.; Bustillo Alonso, M.; et al. Epidemiological and clinical features of Kawasaki disease in Spain over 5 years and risk factors for aneurysm development. (2011–2016): KAWA-RACE study group. PLoS ONE 2019, 14, e0215665. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford University Press: New York, NY, USA, 1991. [Google Scholar]
- Nishizawa, T.; Okamoto, H.; Konishi, K.; Yoshizawa, H.; Miyakawa, Y.; Mayumi, M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem. Biophys. Res. Commun. 1997, 241, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Maggi, F.; Diciommo, V.; Caione, D.; Marcellini, M.; Bendinelli, M. Is torquetenovirus a potential cause of liver damage in children? J. Infect. 2005, 50, 368–369. [Google Scholar] [CrossRef] [PubMed]
- McElvania TeKippe, E.; Wylie, K.M.; Deych, E.; Sodergren, E.; Weinstock, G.; Storch, G.A. Increased prevalence of anellovirus in pediatric patients with fever. PLoS ONE 2012, 7, e50937. [Google Scholar] [CrossRef] [PubMed]
- Del Rosal, T.; García-García, M.L.; Casas, I.; Iglesias-Caballero, M.; Pozo, F.; Alcolea, S.; Bravo, B.; Rodrigo-Muñoz, J.M.; Del Pozo, V.; Calvo, C. Torque Teno Virus in Nasopharyngeal Aspirate of Children with Viral Respiratory Infections. Pediatr. Infect. Dis. J. 2023, 42, 184–188. [Google Scholar] [CrossRef]
- Zehender, G.; Manzin, A.; De Maddalena, C.; Colasante, C.; Solforosi, L.; Corsi, F.; Bianchi-Bosisio, A.; Girotto, M.; Schirru, I.; Russo, U.; et al. Molecular epidemiology of TT virus in Italy and phylogenesis of viral isolates from subjects at different risk for parenteral exposure. J. Med. Virol. 2001, 63, 76–84. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Leijonhufvud, G.; Bajalan, A.; Teixeira Soratto, T.A.; Gustafsson, B.; Bogdanovic, G.; Bjerkner, A.; Allander, T.; Ljungman, G.; Andersson, B. Better detection of Torque teno virus in children with leukemia by metagenomic sequencing than by quantitative PCR. J. Med. Virol. 2022, 94, 634–641. [Google Scholar] [CrossRef]
- Touinssi, M.; Gallian, P.; Biagini, P.; Attoui, H.; Vialettes, B.; Berland, Y.; Tamalet, C.; Dhiver, C.; Ravaux, I.; De Micco, P.; et al. TT virus infection: Prevalence of elevated viraemia and arguments for the immune control of viral load. J. Clin. Virol. 2001, 21, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, M.; Puchhammer-Stöckl, E.; Eskandary, F.; Kohlbeck, P.; Rasoul-Rockenschaub, S.; Heilos, A.; Kozakowski, N.; Görzer, I.; Kikić, Ž.; Herkner, H.; et al. Torque Teno Virus Load-Inverse Association with Antibody-Mediated Rejection After Kidney Transplantation. Transplantation 2017, 101, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Maggi, F.; Albani, M.; Macera, L.; Ricci, V.; Gragnani, S.; Di Beo, S.; Ghimenti, M.; Antonelli, G.; Bendinelli, M.; et al. Torquetenovirus viremia kinetics after autologous stem cell transplantation are predictable and may serve as a surrogate marker of functional immune reconstitution. J. Clin. Virol. 2010, 47, 189–192. [Google Scholar] [CrossRef]
- Studenic, P.; Bond, G.; Kerschbaumer, A.; Bécède, M.; Pavelka, K.; Karateev, D.; Stieger, J.; Puchner, R.; Mueller, R.B.; Puchhammer-Stöckl, E.; et al. Torque Teno Virus Quantification for Monitoring of Immunomodulation with Biological Compounds in the Treatment of Rheumatoid Arthritis. Rheumatology 2021, 61, 2815–2825. [Google Scholar] [CrossRef]
- Giménez, E.; Monzó, C.; Albert, E.; Fuentes-Trillo, A.; Seda, E.; Piñana, J.L.; Hernández Boluda, J.C.; Solano, C.; Chaves, J.; Navarro, D. Diversity and dynamic changes of anelloviruses in plasma following allogeneic hematopoietic stem cell transplantation. J. Med. Virol. 2021, 93, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Uhl, P.; Heilos, A.; Bond, G.; Meyer, E.; Böhm, M.; Puchhammer-Stöckl, E.; Arbeiter, K.; Müller-Sacherer, T.; Csaicsich, D.; Aufricht, C.; et al. Torque teno viral load reflects immunosuppression in paediatric kidney-transplanted patients-a pilot study. Pediatr. Nephrol. 2021, 36, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Bueno, F.; Albert, E.; Piñana, J.L.; Pérez, A.; Úbeda, C.; Gómez, M.D.; Hernández-Boluda, J.C.; Gonzalez-Barberá, E.M.; Montoro, J.; Giménez, E.; et al. Kinetics of Torque Teno virus DNA in stools may predict occurrence of acute intestinal graft versus host disease early after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2021, 23, e13507. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; Albert, E.; Giménez, E.; Ruiz-Merlo, T.; Parra, P.; López-Medrano, F.; San Juan, R.; Polanco, N.; Andrés, A.; Navarro, D.; et al. Monitoring of alphatorquevirus DNA levels for the prediction of immunosuppression-related complications after kidney transplantation. Am. J. Transplant. 2019, 19, 1139–1149. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Albert, E.; Giménez, E.; Rodríguez-Goncer, I.; Andrés, A.; Navarro, D.; Aguado, J.M. Early kinetics of Torque Teno virus DNA load and BK polyomavirus viremia after kidney transplantation. Transpl. Infect. Dis. 2020, 22, e13240. [Google Scholar] [CrossRef]
- Albert, E.; Solano, C.; Giménez, E.; Focosi, D.; Pérez, A.; Macera, L.; Piñana, J.L.; Mateo, E.M.; Boluda, J.C.H.; Maggi, F.; et al. Kinetics of Alphatorquevirus plasma DNAemia at late times after allogeneic hematopoietic stem cell transplantation. Med. Microbiol. Immunol. 2019, 208, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Solano, C.; Giménez, E.; Focosi, D.; Pérez, A.; Macera, L.; Piñana, J.L.; Boluda, J.C.H.; Maggi, F.; Navarro, D. The kinetics of torque teno virus plasma DNA load shortly after engraftment predicts the risk of high-level CMV DNAemia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2018, 53, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, S.; Cabezas, S.; Perez, A.B.; Pupo, M.; Ruiz, D.; Calzada, N.; Bernardo, L.; Castro, O.; Gonzalez, D.; Serrano, T.; et al. Kinetics of antibodies in sera, saliva, and urine samples from adult patients with primary or secondary dengue 3 virus infections. Int. J. Infect. Dis. 2006, 11, 256–262. [Google Scholar] [CrossRef]
- Rigante, D.; Andreozzi, L.; Fastiggi, M.; Bracci, B.; Natale, M.F.; Esposito, S. Critical Overview of the Risk Scoring Systems to Predict Non-Responsiveness to Intravenous Immunoglobulin in Kawasaki Syndrome. Int. J. Mol. Sci. 2016, 17, 278. [Google Scholar] [CrossRef]
- Japan’s Ministry of Education and Ministry of Health Labour and Welfare Ethical Guideline for the Medical Researches Involving Human Individuals. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000153339.pdf (accessed on 23 August 2022). (In Japanese)
- Maggi, F.; Fornai, C.; Vatteroni, M.L.; Siciliano, G.; Menichetti, F.; Tascini, C.; Specter, S.; Pistello, M.; Bendinelli, M. Low prevalence of TT virus in the cerebrospinal fluid of viremic patients with central nervous system disorders. J. Med. Virol. 2001, 65, 418–422. [Google Scholar] [CrossRef]
- Maggi, F.; Pifferi, M.; Fornai, C.; Andreoli, E.; Tempestini, E.; Vatteroni, M.; Presciuttini, S.; Marchi, S.; Pietrobelli, A.; Boner, A.; et al. TT virus in the nasal secretions of children with acute respiratory diseases: Relations to viremia and disease severity. J. Virol. 2003, 77, 2418–2425. [Google Scholar] [CrossRef] [PubMed]

| Individual | Sex | Kawasaki Disease | Diarrhea | Respiratory Infections |
|---|---|---|---|---|
| Age (years) | ||||
| 1 | M | 1.1 | 1.1 | 1.5 |
| 2 | F | 3.3 | 3.3 | 3.7 |
| 3 | F | 8.0 | 4.7 | 5.3 |
| 4 | M | 2.8 | 3.0 | 2.0 |
| 5 | F | 2.3 | 1.9 | 2.6 |
| 6 | F | 0.83 | 1.34 | 0.96 |
| 7 | M | 4.2 | 3.8 | 3.7 |
| 8 | M | 0.27 | 0.87 | 0.88 |
| 9 | M | 0.75 | 1.03 | 0.92 |
| 10 | M | 3.5 | 2.7 | 3.1 |
| 11 | F | 0.45 | 1.1 | 0.85 |
| Primer | Sequence (5′-> 3′) | Position (nt) | Amplicon Size (bp) |
|---|---|---|---|
| TTV-1F | ACAGATCTTTGTGACATGGTGC | 1285–1306 | 103 |
| TTV-1R | GGAAGTTCACAACCACAGAGTC | 1387–1366 | |
| TTV-5F | TTGTGGTTATAGAGGCAACGGT | 1195–1216 | 89 |
| TTV-5R | AAGAACCTGGAAGTTGCAACAG | 1283–1262 | |
| TTV-7F | TCCCCCTAGTAACTATCAGTGCCT | 1323–1346 | 113 |
| TTV-7R | GTTATACCAGTAGGGATCTAAAATCTG | 1435–1409 | |
| TTV-13F | TCCAACTGAACTTAACCGCAGC | 1224–1245 | 131 |
| TTV-13R | AAATTGCGGAAGGTCTATATTGAGAC | 1354–1329 | |
| TTV-14F | TGCGACGTTAACCTTGTGCAA | 1343–1363 | 98 |
| TTV-14R | ACTTGGAAGGTCACACAAGGAGT | 1440–1418 | |
| TTV-15F | TTGTAACTTTGCGGTCAACTGC | 1327–1348 | 88 |
| TTV-15R | AACACCTGGAAGGTGGTGCAA | 1414–1394 | |
| TTV-22F | TGGTTACTGCGGCTGACTTTC | 1563–1583 | 81 |
| TTV-22R | CTTTCAACACTTGGAACGTTGTG | 1643–1621 | |
| TTV-24F | TTTCTGCGGCTAGCTTCATGC | 1247–1267 | 81 |
| TTV-24R | TGTCTTTCAACACCTGGAAGG | 1327–1307 | |
| TTV probe * | FAM/TCCGTTCKGCTCACCACWAAC/BHQ1 | ||
| TTV-28F | ACAGCCCATACTTTTTAACACCGCGA | 1655–1680 | 722 |
| TTV-28R | TGACACTCTTTTAAGACTTGCCGAGCT | 2376–2350 | |
| TTV-29F | ACAGAAACAGGTGGTCTCGCTCCAA | 1078–1102 | 324 |
| TTV-29R | CTTGTAGTTGGAAGAGGCCCATGCT | 1401–1377 |
| Group † | NGS ‡ | ssTTV-PCR ¶ | Comparison | |
|---|---|---|---|---|
| TTV1 | KD | + | + | Consistent |
| Diarrhea | - | - | Consistent | |
| Respiratory | - | - | Consistent | |
| TTV5 | KD | + | + | Consistent |
| Diarrhea | + | + | Consistent | |
| Respiratory | + | + | Consistent | |
| TTV7 | KD | + | + | Consistent |
| Diarrhea | - | - | Consistent | |
| Respiratory | - | - | Consistent | |
| TTV13 | KD | - | - | Consistent |
| Diarrhea | + | + | Consistent | |
| Respiratory | - | - | Consistent | |
| TTV15 | KD | + | + | Consistent |
| Diarrhea | + | + | Consistent | |
| Respiratory | - | + | Inconsistent | |
| TTV22 | KD | - | - | Consistent |
| Diarrhea | - | + | Inconsistent | |
| Respiratory | + | + | Consistent | |
| TTV24 | KD | + | + | Consistent |
| Diarrhea | + | + | Consistent | |
| Respiratory | - | - | Consistent | |
| TTV28 | KD | - | - | Consistent |
| Diarrhea | + | - | Inconsistent | |
| Respiratory | - | - | Consistent | |
| TTV29 | KD | - | + | Inconsistent |
| Diarrhea | + | - | Inconsistent | |
| Respiratory | - | - | Consistent |
| TTV | KD† | Diarrhea † | Respiratory † | Odds Ratio ‡ | p-Value ‡ |
|---|---|---|---|---|---|
| Any TTV | 9 | 8 | 9 | 1.69 | 1.0000 |
| TTV1 | 1 | 0 | 0 | 2.00 | 0.3333 |
| TTV5 | 1 | 2 | 3 | 0.295 | 0.5926 |
| TTV7 | 2 | 0 | 0 | 4.83 | 0.1111 |
| TTV13 | 0 | 2 | 0 | 0.828 | 0.5556 |
| TTV15 | 3 | 3 | 1 | 1.82 | 0.6461 |
| TTV22 | 0 | 1 | 2 | 0.520 | 0.5556 |
| TTV24 | 2 | 4 | 0 | 1.00 | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spezia, P.G.; Filippini, F.; Nagao, Y.; Sano, T.; Ishida, T.; Maggi, F. Identification of Torquetenovirus Species in Patients with Kawasaki Disease Using a Newly Developed Species-Specific PCR Method. Int. J. Mol. Sci. 2023, 24, 8674. https://doi.org/10.3390/ijms24108674
Spezia PG, Filippini F, Nagao Y, Sano T, Ishida T, Maggi F. Identification of Torquetenovirus Species in Patients with Kawasaki Disease Using a Newly Developed Species-Specific PCR Method. International Journal of Molecular Sciences. 2023; 24(10):8674. https://doi.org/10.3390/ijms24108674
Chicago/Turabian StyleSpezia, Pietro Giorgio, Fabio Filippini, Yoshiro Nagao, Tetsuya Sano, Takafumi Ishida, and Fabrizio Maggi. 2023. "Identification of Torquetenovirus Species in Patients with Kawasaki Disease Using a Newly Developed Species-Specific PCR Method" International Journal of Molecular Sciences 24, no. 10: 8674. https://doi.org/10.3390/ijms24108674
APA StyleSpezia, P. G., Filippini, F., Nagao, Y., Sano, T., Ishida, T., & Maggi, F. (2023). Identification of Torquetenovirus Species in Patients with Kawasaki Disease Using a Newly Developed Species-Specific PCR Method. International Journal of Molecular Sciences, 24(10), 8674. https://doi.org/10.3390/ijms24108674






