Stromal Lineage Precursors from Rodent Femur and Tibia Bone Marrows after Hindlimb Unloading: Functional Ex Vivo Analysis
Abstract
1. Introduction
2. Results
2.1. Bone Marrow (BM) Cellularity
2.2. The Ex Vivo Analysis of Stromal Progenitors from Femur and Tibia BM
2.2.1. Fibroblast Colony-Forming Units (CFU-f)
2.2.2. Multipotent Mesenchymal Stromal Cells (MMSCs)
Cell Growth
MSC Osteogenic Potential
Transcriptomic Activity
Paracrine Activity
3. Discussion
4. Materials and Methods
4.1. Animals and Procedures
4.2. Bone Marrow Cells Isolation and Enumeration
4.3. Cell Cultures
4.4. Fibroblast Colony-Forming Units (CFU-f)
4.5. Proliferative Activity of MMSCs
4.6. Osteogenic Differentiation
4.7. Paracrine Activity
4.8. Quantitative Real-Time PCR
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oganov, V.S. Modern Analysis of Bone Loss Mechanisms in Microgravity. J. Gravit. Physiol. 2004, 11, P143–P146. [Google Scholar] [PubMed]
- Vico, L.; Collet, P.; Guignandon, A.; Lafage-Proust, M.-H.; Thomas, T.; Rehailia, M.; Alexandre, C. Effects of Long-Term Microgravity Exposure on Cancellous and Cortical Weight-Bearing Bones of Cosmonauts. Lancet 2000, 355, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human Pathophysiological Adaptations to the Space Environment. Front. Physiol. 2017, 8, 547. [Google Scholar] [CrossRef]
- Vailas, A.C.; Zernicke, R.F.; Grindeland, R.E.; Kaplansky, A.; Durnova, G.N.; Li, K.C.; Martinez, D.A. Effects of Spaceflight on Rat Humerus Geometry, Biomechanics, and Biochemistry. FASEB J. 1990, 4, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. The Mesengenic Process. Clin. Plast. Surg. 1994, 21, 429–435. [Google Scholar] [CrossRef]
- Bigildeev, A.E.; Zhironkina, O.A.; Shipounova, I.N.; Sats, N.V.; Kotyashova, S.Y.; Drize, N.I. Clonal Composition of Human Multipotent Mesenchymal Stromal Cells. Exp. Hematol. 2012, 40, 847–856.e4. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional Heterogeneity of Mesenchymal Stem Cells from Natural Niches to Culture Conditions: Implications for Further Clinical Uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef]
- Domaratskaya, E.I.; Michurina, T.V.; Bueverova, E.I.; Bragina, E.V.; Nikonova, T.A.; Starostin, V.I.; Khrushchov, N.G. Studies on Clonogenic Hemopoietic Cells of Vertebrate in Space: Problems and Perspectives. Adv. Space Res. 2002, 30, 771–776. [Google Scholar] [CrossRef]
- Markina, E.; Andreeva, E.; Andrianova, I.; Sotnezova, E.; Buravkova, L. Stromal and Hematopoietic Progenitors from C57/BI/6N Murine Bone Marrow after 30-Day “BION-M1” Spaceflight. Stem Cells Dev. 2018, 27, 1268–1277. [Google Scholar] [CrossRef]
- Rogacheva, I.V.; Stupakov, G.P.; Volozhin, A.I.; Pavlova, M.N.; Poliakov, A.N. Characteristics of bone tissue of rats after flight aboard biosputnik Kosmos-1129. Kosm. Biol. Aviakosm Med. 1984, 18, 39–44. [Google Scholar] [PubMed]
- Durnova, G.; Kaplansky, A.; Morey-Holton, E. Histomorphometric Study of Tibia of Rats Exposed Aboard American Spacelab Life Sciences 2 Shuttle Mission. J. Gravit. Physiol. 1996, 3, 80–81. [Google Scholar]
- Evans, G.L.; Morey-Holton, E.; Turner, R.T. Spaceflight Has Compartment- and Gene-Specific Effects on MRNA Levels for Bone Matrix Proteins in Rat Femur. J. Appl. Physiol. 1998, 84, 2132–2137. [Google Scholar] [CrossRef] [PubMed]
- Globus, R.K.; Morey-Holton, E. Hindlimb Unloading: Rodent Analog for Microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef]
- Smith, S.M.; Heer, M.; Shackelford, L.C.; Sibonga, J.D.; Spatz, J.; Pietrzyk, R.A.; Hudson, E.K.; Zwart, S.R. Bone Metabolism and Renal Stone Risk during International Space Station Missions. Bone 2015, 81, 712–720. [Google Scholar] [CrossRef]
- Basso, N.; Jia, Y.; Bellows, C.G.; Heersche, J.N.M. The Effect of Reloading on Bone Volume, Osteoblast Number, and Osteoprogenitor Characteristics: Studies in Hind Limb Unloaded Rats. Bone 2005, 37, 370–378. [Google Scholar] [CrossRef]
- Sanesi, L.; Storlino, G.; Dicarlo, M.; Oranger, A.; Zerlotin, R.; Pignataro, P.; Suriano, C.; Guida, G.; Grano, M.; Colaianni, G.; et al. Time-Dependent Unloading Effects on Muscle and Bone and Involvement of FNDC5/Irisin Axis. NPJ Microgravity 2023, 9, 4. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Rinaldi, A.M.; Marini, M.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Recombinant Irisin Prevents Cell Death and Mineralization Defects Induced by Random Positioning Machine Exposure in Primary Cultures of Human Osteoblasts: A Promising Strategy for the Osteoporosis Treatment. Front. Physiol. 2023, 14, 1107933. [Google Scholar] [CrossRef]
- Morabito, C.; Guarnieri, S.; Cucina, A.; Bizzarri, M.; Mariggiò, M.A. Antioxidant Strategy to Prevent Simulated Microgravity-Induced Effects on Bone Osteoblasts. Int. J. Mol. Sci. 2020, 21, 3638. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.d.S.; Nardi, N.B. Murine Marrow-Derived Mesenchymal Stem Cell: Isolation, in Vitro Expansion, and Characterization: Characterization of Murine Mesenchymal Stem Cells. Br. J. Haematol. 2003, 123, 702–711. [Google Scholar] [CrossRef]
- Paiushina, O.V.; Domaratskaia, E.I. Heterogeneity and possible structure of mesenchymal stromal cell population. Tsitologiia 2015, 57, 31–38. [Google Scholar] [PubMed]
- Murray, I.R.; Péault, B. Q&A: Mesenchymal Stem Cells—Where Do They Come from and Is It Important? BMC Biol. 2015, 13, 99. [Google Scholar] [CrossRef]
- Markina, E.A.; Alekseeva, O.Y.; Andreeva, E.R.; Buravkova, L.B. Short-Term Reloading after Prolonged Unloading Ensures Restoration of Stromal but Not Hematopoietic Precursor Activity in Tibia Bone Marrow of C57Bl/6N Mice. Stem Cells Dev. 2021, 30, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Visigalli, D.; Strangio, A.; Palmieri, D.; Manduca, P. Hind Limb Unloading of Mice Modulates Gene Expression at the Protein and MRNA Level in Mesenchymal Bone Cells. BMC Musculoskelet. Disord. 2010, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Grigor’ev, A.I.; Il’in, E.A. Animals in Space: On the 50th Anniversary of Space Biology. Her. Russ. Acad. Sci. 2007, 77, 541–549. [Google Scholar] [CrossRef]
- Basso, N.; Bellows, C.G.; Heersche, J.N.M. Effect of Simulated Weightlessness on Osteoprogenitor Cell Number and Proliferation in Young and Adult Rats. Bone 2005, 36, 173–183. [Google Scholar] [CrossRef]
- Sakai, A.; Sakata, T.; Tanaka, S.; Okazaki, R.; Kunugita, N.; Norimura, T.; Nakamura, T. Disruption of the P53 Gene Results in Preserved Trabecular Bone Mass and Bone Formation After Mechanical Unloading. J. Bone Miner. Res. 2002, 17, 119–127. [Google Scholar] [CrossRef]
- Sakai, A.; Nakamura, T. Changes in Trabecular Bone Turnover and Bone Marrow Cell Development in Tail-Suspended Mice. J. Musculoskelet. Neuronal Interact. 2001, 1, 387–392. [Google Scholar]
- Shahnazari, M.; Kurimoto, P.; Boudignon, B.M.; Orwoll, B.E.; Bikle, D.D.; Halloran, B.P. Simulated Spaceflight Produces a Rapid and Sustained Loss of Osteoprogenitors and an Acute but Transitory Rise of Osteoclast Precursors in Two Genetic Strains of Mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1354–E1362. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W.; Shi, X.; Zhang, W.; Pennington, C.; Thakore, H.; Haque, M.; Kang, B.; Isales, C.M.; Fulzele, S.; Wenger, K.H. Loss of Myostatin (GDF8) Function Increases Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells but the Osteogenic Effect Is Ablated with Unloading. Bone 2007, 40, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Colleran, P.N.; Wilkerson, M.K.; Bloomfield, S.A.; Suva, L.J.; Turner, R.T.; Delp, M.D. Alterations in Skeletal Perfusion with Simulated Microgravity: A Possible Mechanism for Bone Remodeling. J. Appl. Physiol. 2000, 89, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Stabley, J.N.; Prisby, R.D.; Behnke, B.J.; Delp, M.D. Chronic Skeletal Unloading of the Rat Femur: Mechanisms and Functional Consequences of Vascular Remodeling. Bone 2013, 57, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Prisby, R.D.; Behnke, B.J.; Allen, M.R.; Delp, M.D. Effects of Skeletal Unloading on the Vasomotor Properties of the Rat Femur Principal Nutrient Artery. J. Appl. Physiol. 2015, 118, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Markina, E.A.; Kokhan, V.S.; Roe, M.P.; Andrianova, I.V.; Shtemberg, A.S.; Buravkova, L.B. The Effects of Radiation and Hindlimb Unloading on Rat Bone Marrow Progenitor Cells. Cell Tiss. Biol. 2018, 12, 183–196. [Google Scholar] [CrossRef]
- Ignatenko, G.A.; Nemsadze, I.G.; Mirovich, E.D.; Churilov, A.V.; Maylyan, E.A.; Glazkov, I.S.; Rumyantceva, Z.S. The Role of Cytokines in Bone Remodeling and the Pathogenesis of Postmenopausal Osteoporosis. Med. Vestn. Ûga Ross. 2020, 11, 6–18. [Google Scholar] [CrossRef]
- Barantseva, M.Y.; Ozerov, D.S.; Dadasheva, O.A. Effect of tail-suspension on levels of pro- and anti-inflammatory cytokins and morphological changes in rat’s pulmonary tissues. AEM 2021, 55, 56–60. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb Unloading Rodent Model: Technical Aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Morey-Holton, E.; Globus, R.K.; Kaplansky, A.; Durnova, G. The Hindlimb Unloading Rat Model: Literature Overview, Technique Update and Comparison with Space Flight Data. In Advances in Space Biology and Medicine; Elsevier: Amsterdam, The Netherlands, 2005; Volume 10, pp. 7–40. ISBN 978-0-444-51907-8. [Google Scholar]
- Wolfrom, C.; Raynaud, N.; Maigne, J.; Papathanassiou, S.; Conti, M.; Kadhom, N.; Hecquet, B.; Levi, F.; Gautier, M.; Deschatrette, J. Periodic Fluctuations in Proliferation of SV-40 Transformed Human Skin Fibroblast Lines with Prolonged Lifespan. Cell Biol. Toxicol. 1994, 10, 247–254. [Google Scholar] [CrossRef]
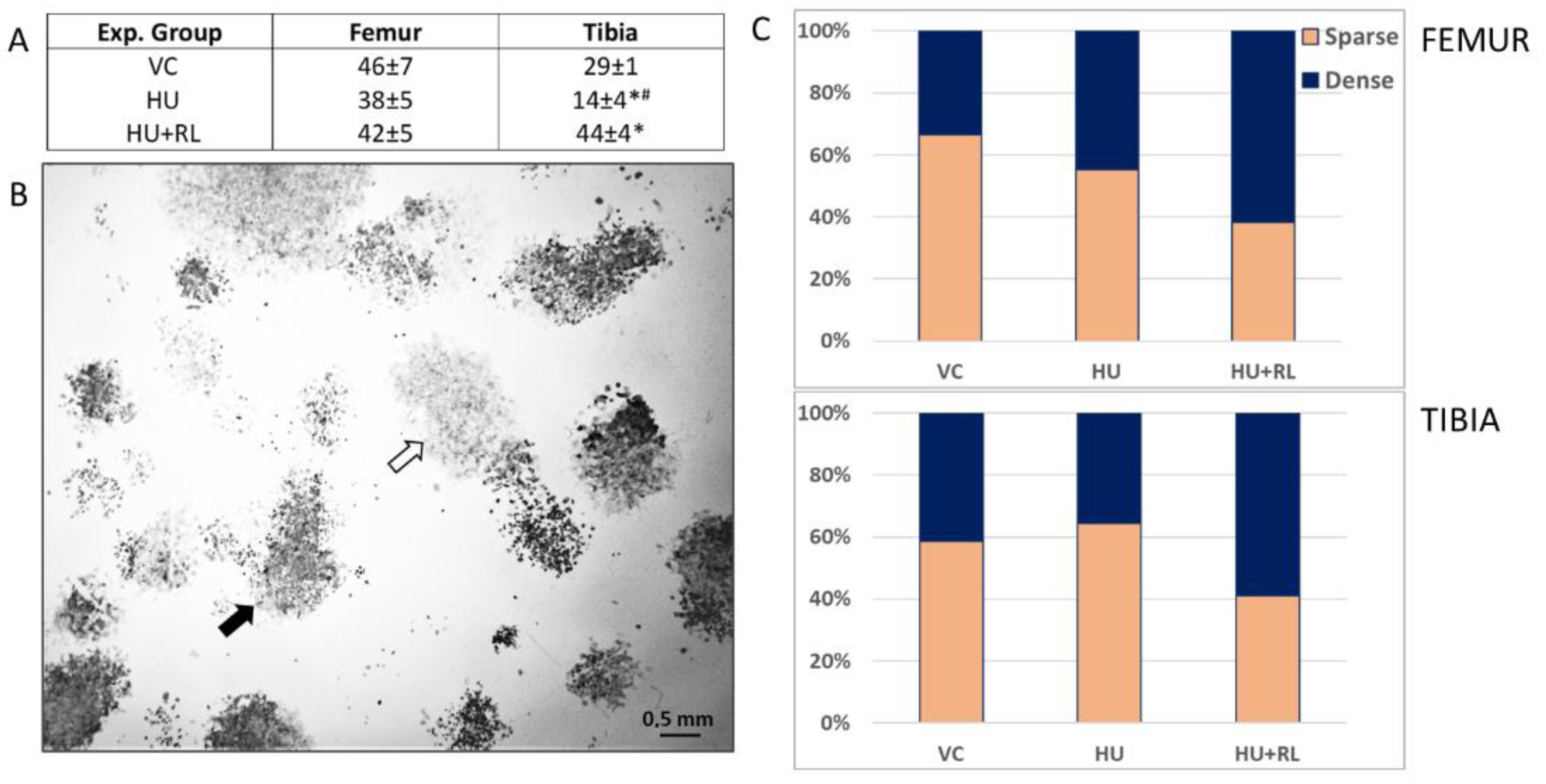
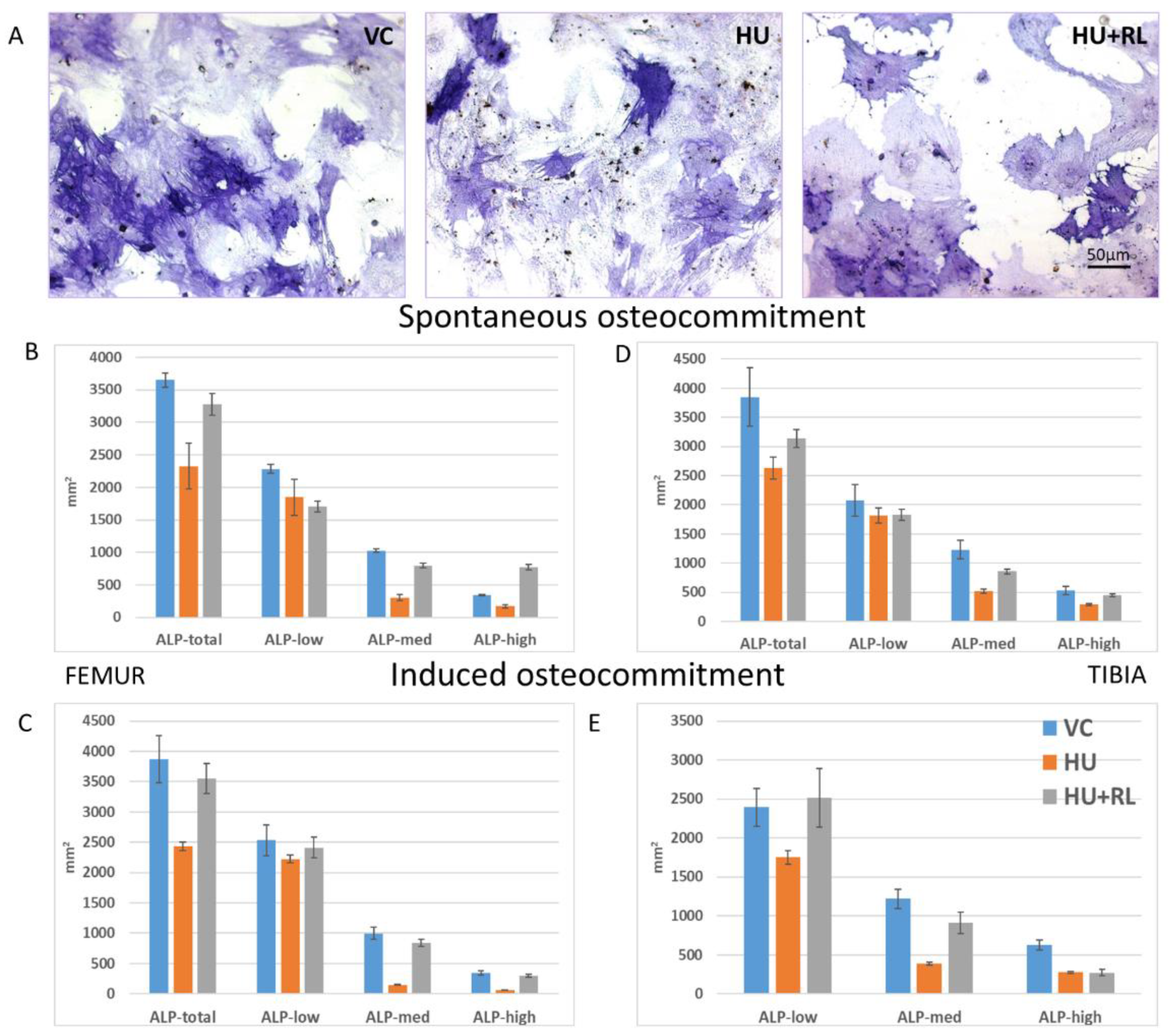
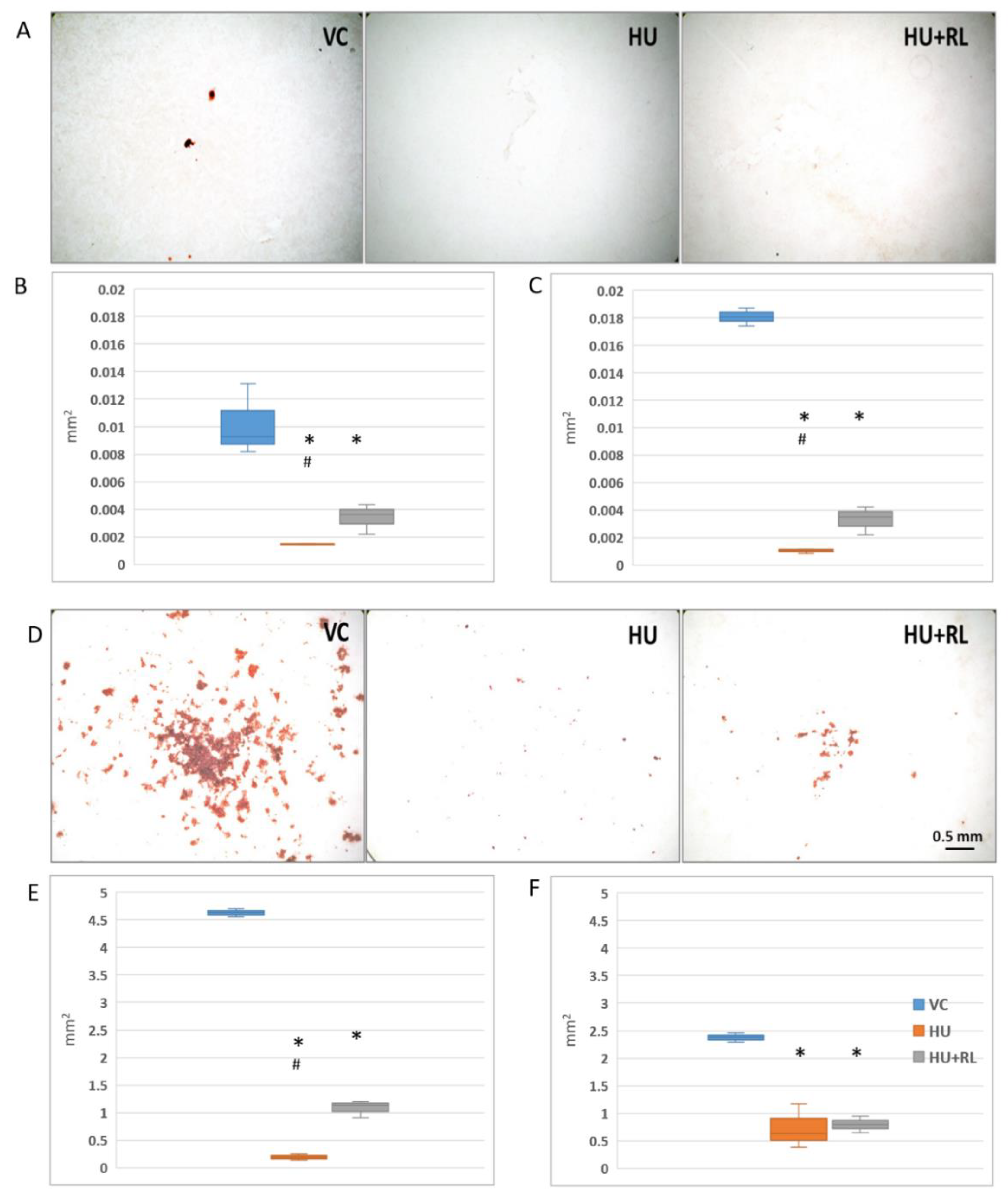

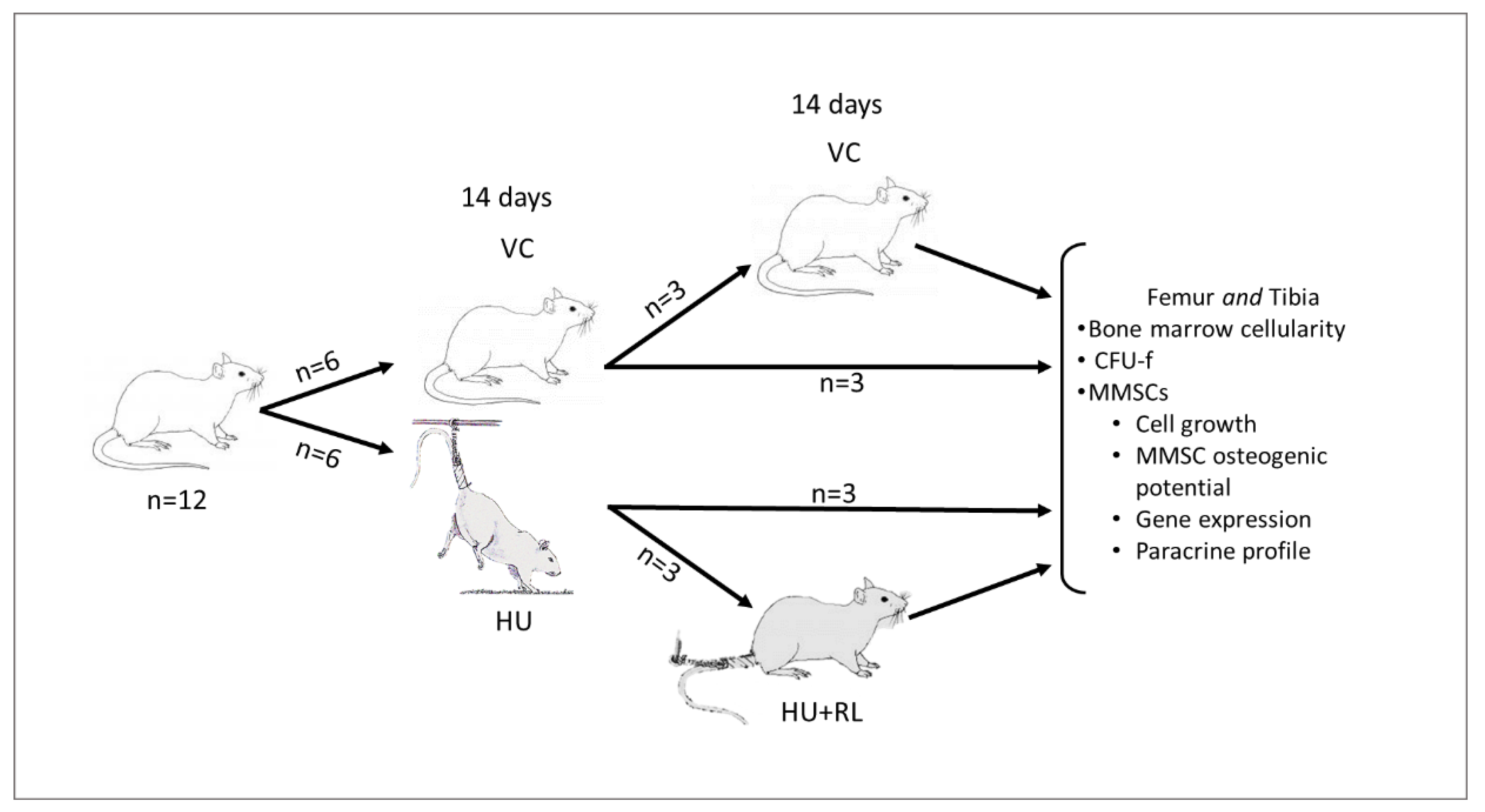

| Experimental Group | Femur | Tibia |
|---|---|---|
| VC | 326 ± 65 | 193 ± 25 |
| HU | 149 ± 23 * | 112 ± 3 *# |
| HU + RL | 173 ± 17 * | 150 ± 12 * |
| Exp. Group | Femur | Tibia |
|---|---|---|
| VC | 3.9 ± 0.4 | 3.6 ± 0.4 |
| HU | 1.4 ± 0.5 *# | 1.2 ±0.3 *# |
| HU + RL | 3.6 ± 0.2 | 3.6 ± 0.2 |
| BM Source | Femur | Tibia | ||||
|---|---|---|---|---|---|---|
| Experimental Groups | ||||||
| Level, pg/mL | VC | HU | HU + RL | VC | HU | HU + RL |
| IL-5 | 15.3 ± 1.4 | 17.5 ± 3.9 | 14.0 ± 5.8 | 15.3 ± 1.2 | 20.0 ± 5.5 | 14.0 ± 6.1 |
| IL-6 | 155.2 ± 46.7 | 2971.0 ± 186.4 *# | 79.2 ± 24.4 * | 141.6 ± 35.5 | 1314.5 ± 142.8 *# | 353.7 ± 59.7 * |
| IL-10 | 47.8 ± 3.6 | 124.1 ± 11.1 *# | 14.0 ± 2.7 * | 69.8 ± 15.6 | 99.6 ± 7.5 *# | 41.8 ± 8.3 |
| IL-13 | 15.4 ± 1.9 | 17.3 ± 1.4 | 13.6 ± 5.1 | 14.8 ± 1.4 | 13.6 ± 1.1 | 0 |
| IP-10 | 213.1 ± 28.6 | 301.8 ± 24.7 * | 360.2 ± 36.6 * | 238.0 ± 25.7 | 360.3 ± 30.2 * | 360.0 ± 20.1 * |
| TNF-a | 0 | 5.6 ± 1.1 *# | 0 | 0 | 3.5 ± 1.2 *# | 0 |
| OPG | 113.0 ± 28.3 | 57.3 ± 1.6 *# | 188.1 ± 1.2 * | 88.8 ± 9.4 | 59.1 ± 7.0 *# | 113.7 ± 12.1 * |
| SOST | 172.5 ± 13.0 | 50.4 ± 8.6 * | 67.7 ± 6.83 * | 383.7 ± 74.8 | 94.6 ± 23.1 *# | 322.7 ± 55.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markina, E.; Andreeva, E.; Buravkova, L. Stromal Lineage Precursors from Rodent Femur and Tibia Bone Marrows after Hindlimb Unloading: Functional Ex Vivo Analysis. Int. J. Mol. Sci. 2023, 24, 8594. https://doi.org/10.3390/ijms24108594
Markina E, Andreeva E, Buravkova L. Stromal Lineage Precursors from Rodent Femur and Tibia Bone Marrows after Hindlimb Unloading: Functional Ex Vivo Analysis. International Journal of Molecular Sciences. 2023; 24(10):8594. https://doi.org/10.3390/ijms24108594
Chicago/Turabian StyleMarkina, Elena, Elena Andreeva, and Ludmila Buravkova. 2023. "Stromal Lineage Precursors from Rodent Femur and Tibia Bone Marrows after Hindlimb Unloading: Functional Ex Vivo Analysis" International Journal of Molecular Sciences 24, no. 10: 8594. https://doi.org/10.3390/ijms24108594
APA StyleMarkina, E., Andreeva, E., & Buravkova, L. (2023). Stromal Lineage Precursors from Rodent Femur and Tibia Bone Marrows after Hindlimb Unloading: Functional Ex Vivo Analysis. International Journal of Molecular Sciences, 24(10), 8594. https://doi.org/10.3390/ijms24108594





