Adenosine Monophosphate-Activated Protein Kinase (AMPK) Phosphorylation Is Required for 20-Hydroxyecdysone Regulates Ecdysis in Apolygus lucorum
Abstract
1. Introduction
2. Results
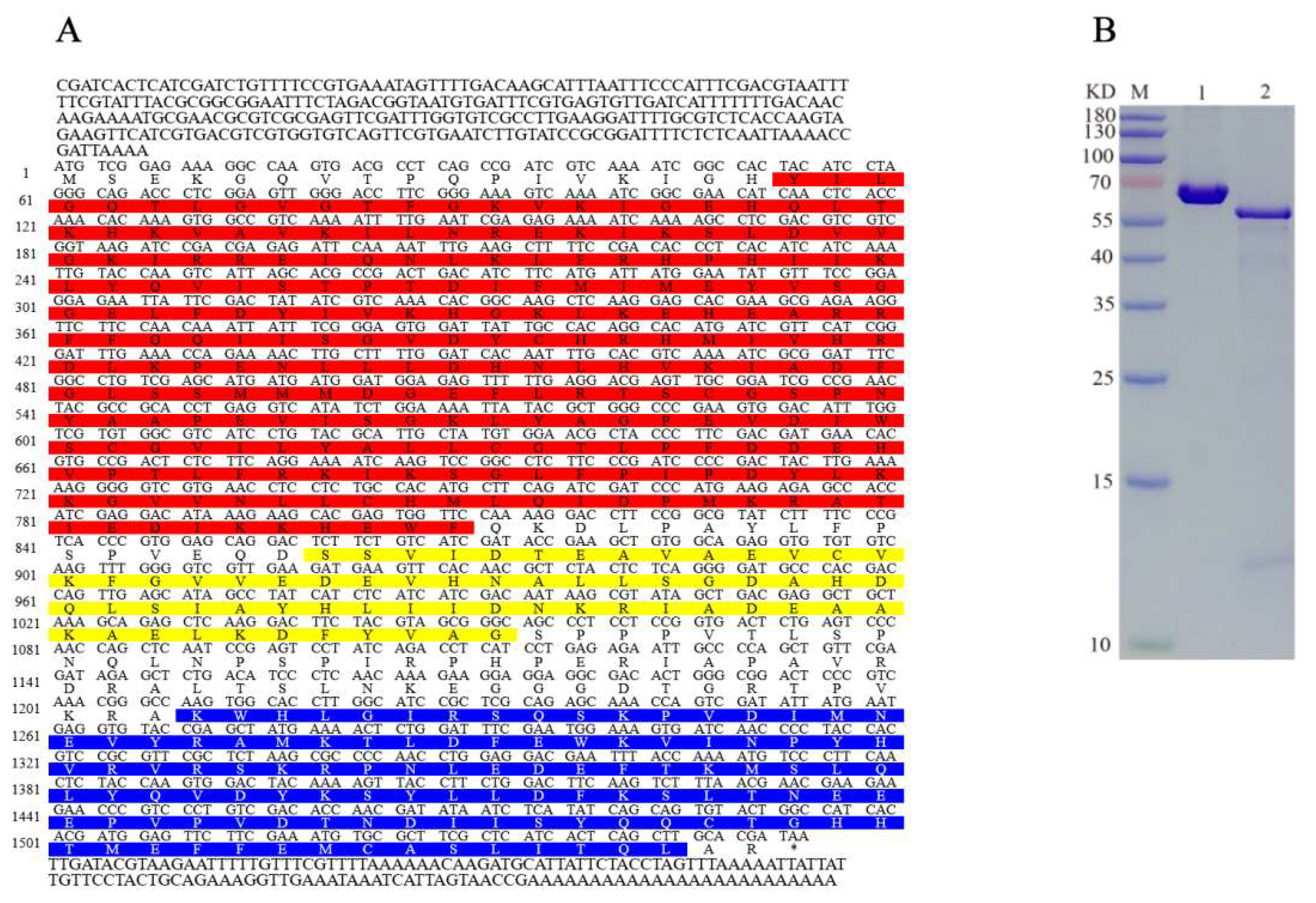
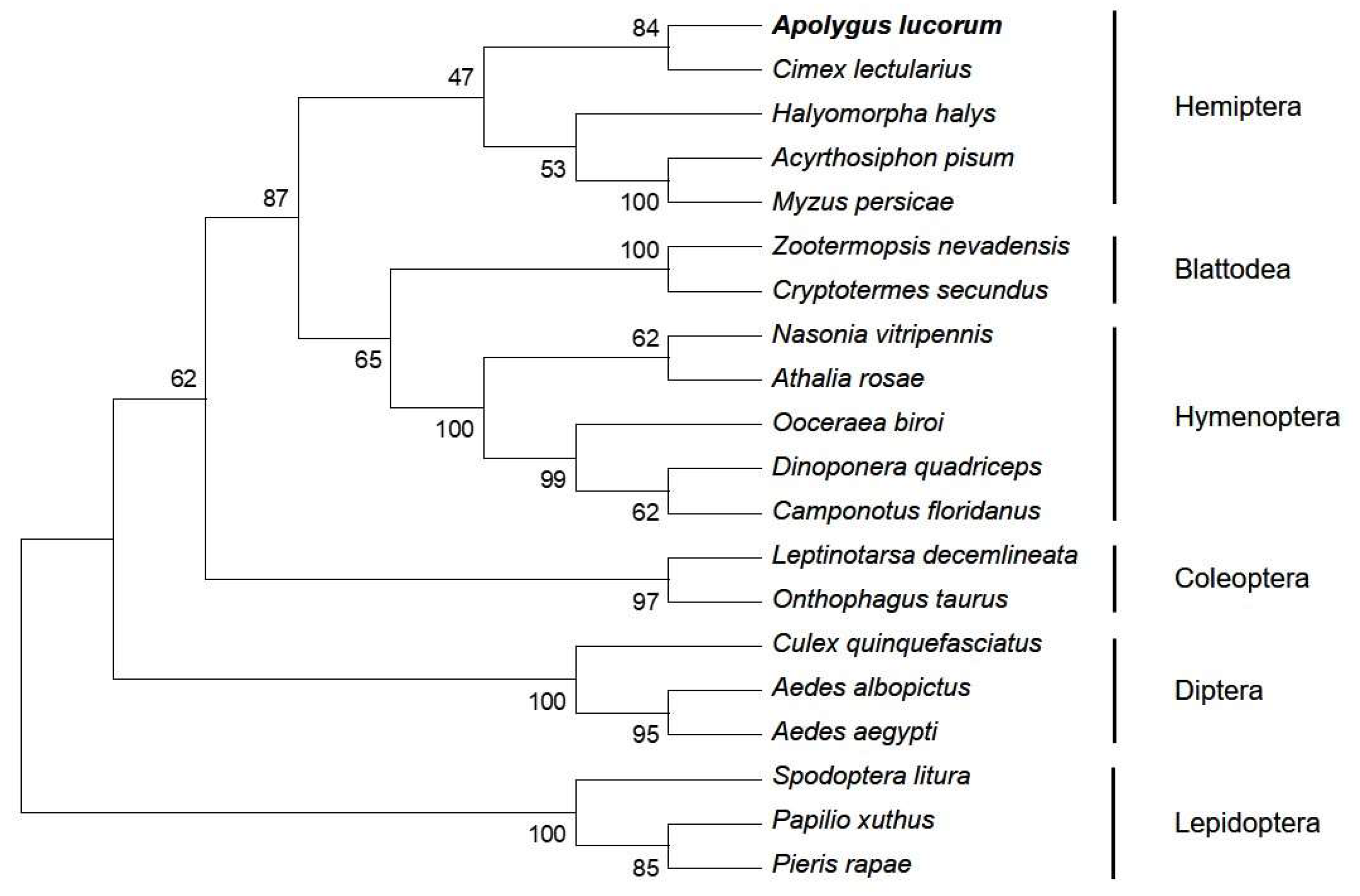
2.1. Characteristics of AlAMPK Cloned from A. lucorum
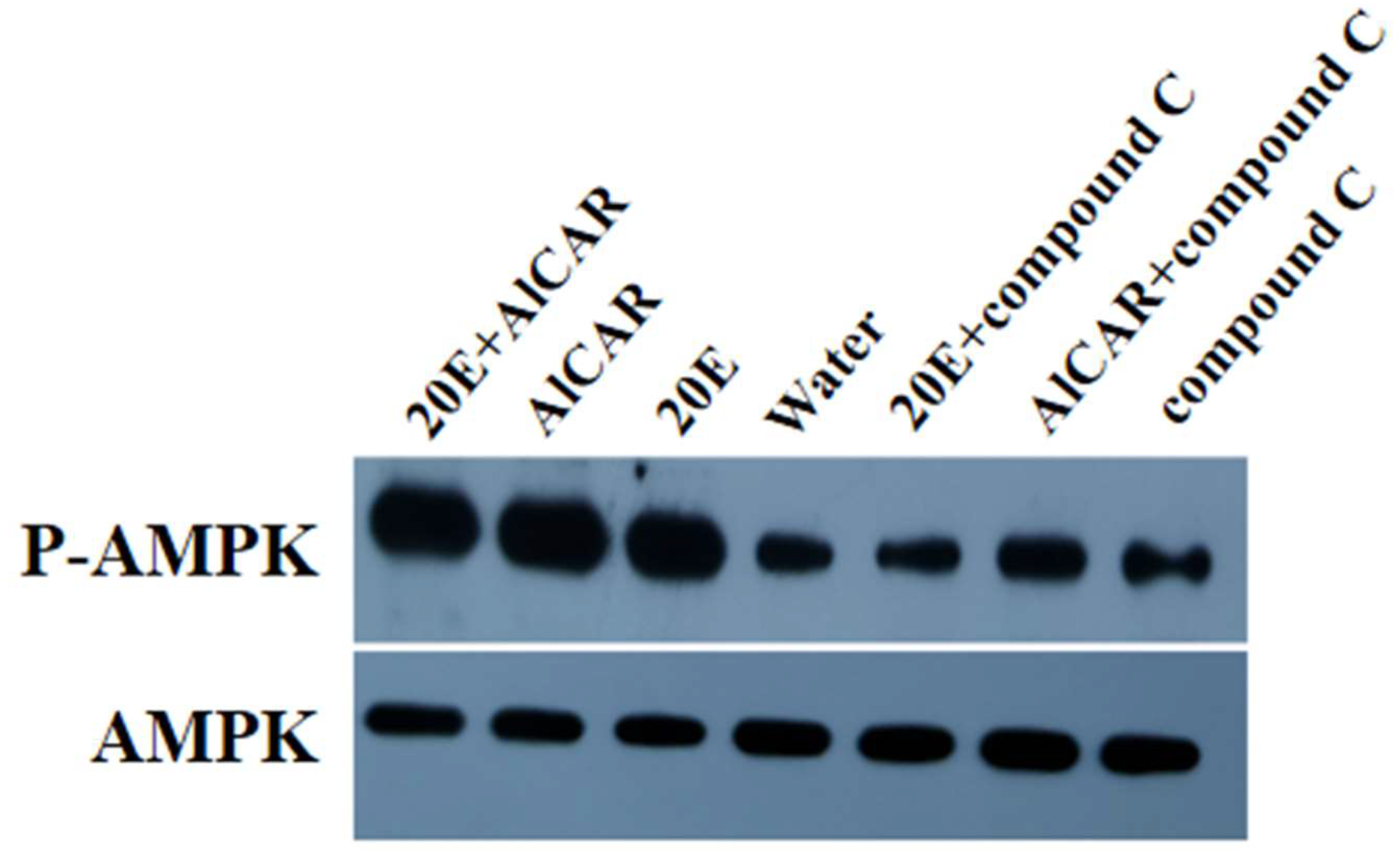
2.2. AlAMPK Was Phosphorylated by 20E
2.3. AlAMPK Expression Profile in the Ecdysis Stage
2.4. AlAMPK Expression and Phenotypes under Spraying and RNAi Experiments
2.5. The 20E-Induced Gene Expression under the Spray of Seven Different Compounds
2.6. The 20E-Induced Gene Expression under the AMPK RNAi
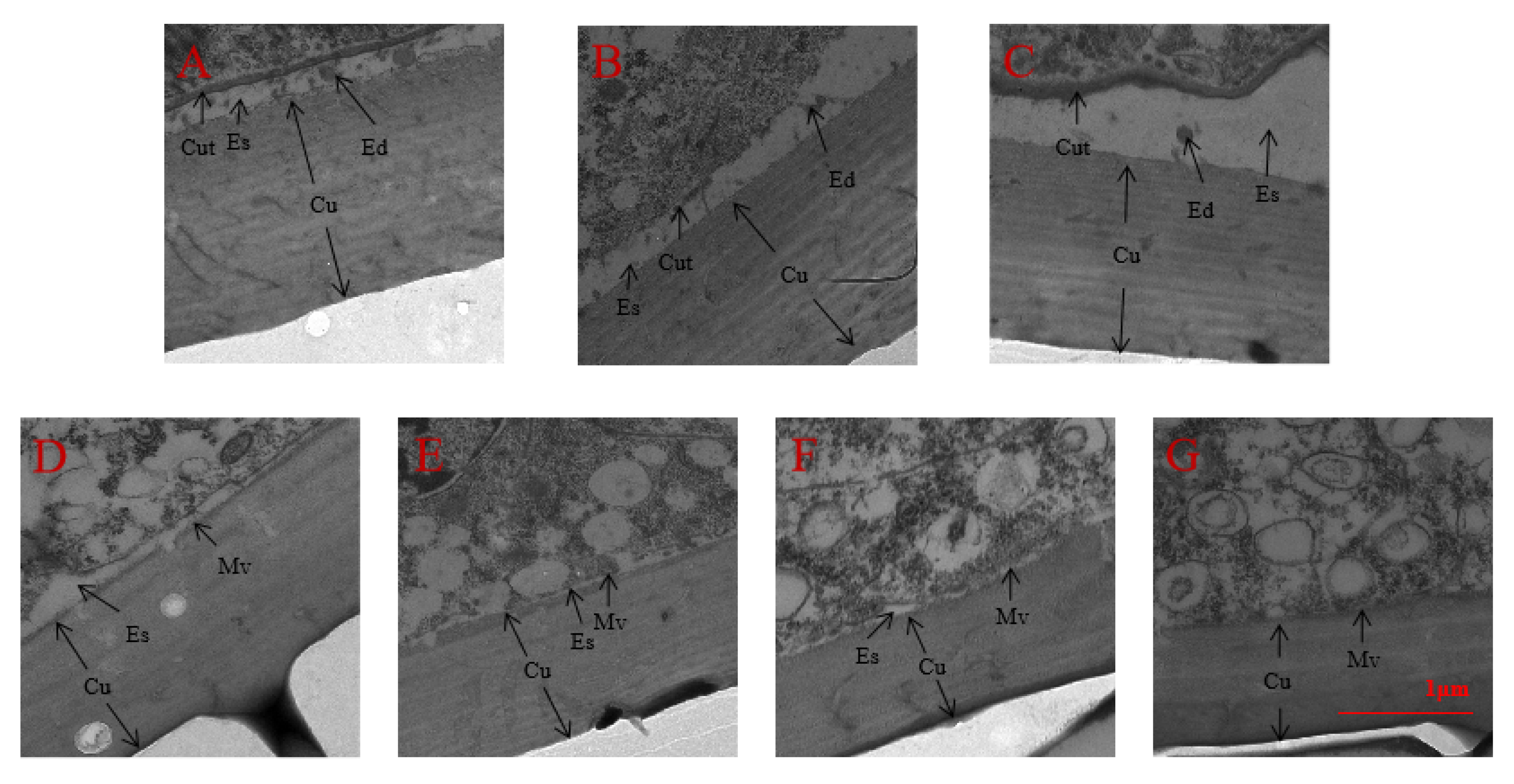
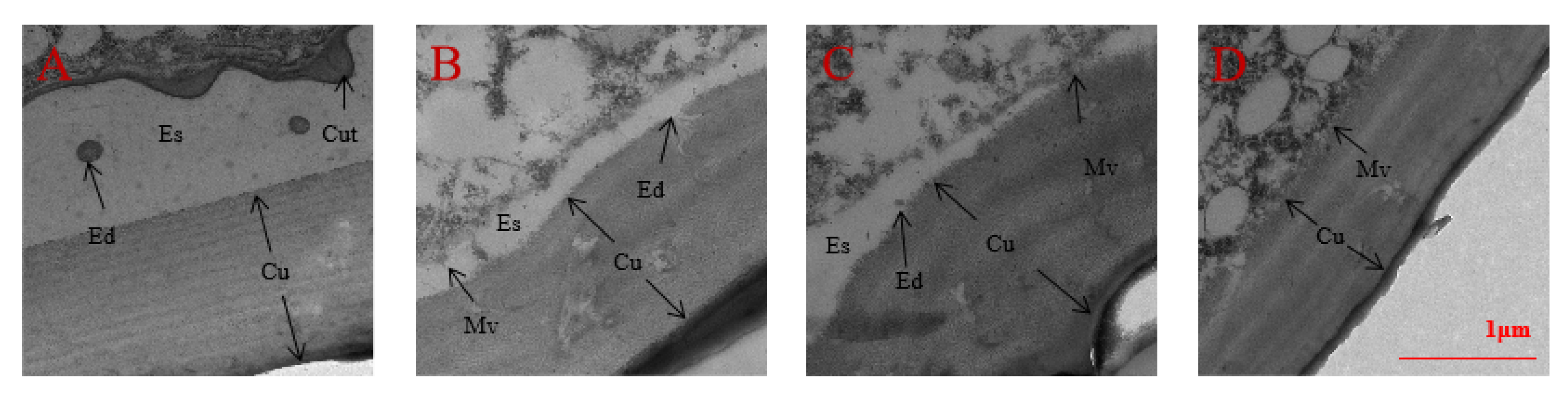
2.7. Observation of Epidermal Structure under Spray and RNAi by TEM
3. Discussion
3.1. AMPK Fine-Tune A. lucorum Physiology
3.2. 20E-Induced AMPK Phosphorylation Is Essential for Molting in A. lucorum
3.3. The Induction/Inhibition of AMPK Phosphorylation Sensitizes the Expression of 20E Downstream Genes
4. Materials and Methods
4.1. Experimental Insect Rearing
4.2. Cloning of AlAMPK Gene
4.3. In Vitro Translation
4.4. Reagents, Proteins, and Antibodies
4.5. Expression Profiles of AlAMPK mRNA Profile
4.6. Western Blot
4.7. dsRNA Synthesis and Application
4.8. Detection of the Effect of Spraying and RNAi
4.9. Transmission Electron Microscopy (TEM) after Spray and RNAi
4.10. Expression Profiles of Four 20E-Regulated Genes
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Liu, H.; Wang, H.; Huang, T.; Liu, B.; Yang, B.; Yin, L.; Li, B.; Zhang, Y.; Zhang, S.; et al. Apolygus lucorum genome provides insights into omnivorousness and mesophyll feeding. Mol. Ecol. Resourses 2021, 21, 287–300. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Jiang, Y.; Xia, B.; Li, P.; Feng, H.; Wyckhuys, K.A.; Guo, Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, W.; Feng, H.; Guo, Y. Seasonal abundance of the mirids, Lygus lucorum and Adelphocoris spp. (Hemiptera: Miridae) on Bt cotton in northern China. Crop Prot. 2002, 21, 997–1002. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, X.R.; Yang, T.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone promotes switching from autophagy to apoptosis by increasing intracellular calcium levels. Insect Biochem. Mol. Biol. 2016, 79, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Scieuzo, C.; Nardiello, M.; Salvia, R.; Pezzi, M.; Chicca, M.; Leis, M.; Bufo, S.A.; Vinson, S.B.; Rao, A.; Vogel, H.; et al. Ecdysteroidogenesis and development in Heliothis virescens (Lepidoptera: Noctuidae): Focus on PTTH-stimulated pathways. J. Insect Physiol. 2018, 107, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Kefi, M.; Balabanidou, V.; Douris, V.; Lycett, G.; Feyereisen, R.; Vontas, J. Two functionally distinct CYP4G genes of Anopheles gambiae contribute to cuticular hydrocarbon biosynthesis. Insect Biochem. Mol. Biol. 2019, 110, 52–59. [Google Scholar] [CrossRef]
- Moulos, P.; Samiotaki, M.; Panayotou, G.; Dedos, S.G. Combinatory annotation of cell membrane receptors and signalling pathways of Bombyx mori prothoracic glands. Sci. Data 2016, 3, 160073. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F. 20-hydroxyecdysone, what it can do. Science 1971, 174, 1041. [Google Scholar] [CrossRef]
- Horike, N.; Sonobe, H. Ecdysone 20-monooxygenase in eggs of the silkworm, Bombyx mori: Enzymatic properties and developmental changes. Arch. Insect Biochem. Physiol. 1999, 41, 9–17. [Google Scholar] [CrossRef]
- Li, K.; Tian, L.; Guo, Z.; Guo, S.; Zhang, J.; Gu, S.H.; Palli, S.R.; Cao, Y.; Li, S. 20-Hydroxyecdysone (20E) primary response gene E75 isoforms mediate steroidogenesis autoregulation and regulate developmental timing in bombyx. J. Biol. Chem. 2016, 291, 18163–18175. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Li, S. E93 predominantly transduces 20-hydroxyecdysone signaling to induce autophagy and caspase activity in Drosophila fat body. Insect Biochem. Mol. Biol. 2014, 45, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, F.; Guo, E.; Li, K.; Ma, L.; Tian, L.; Cao, Y.; Zhang, G.; Palli, S.R.; Li, S. 20-Hydroxyecdysone (20E) primary response gene E93 modulates 20E signaling to promote bombyx larval-pupal metamorphosis. J. Biol. Chem. 2015, 290, 27370–27383. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Guo, E.; Wang, S.; Liu, S.; Jiang, R.J.; Cao, Y.; Ling, E.; Li, S. Developmental regulation of glycolysis by 20-hydroxyecdysone and juvenile hormone in fat body tissues of the silkworm, Bombyx mori. J. Mol. Cell Biol. 2010, 2, 255–263. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dong, Y.; Luo, Y.; Jiang, S.; Meng, F.-L.; Tan, M.; Li, J.; Zang, Y. AMPK-mediated phosphorylation on 53BP1 promotes c-NHEJ. Cell Rep. 2021, 34, 108713. [Google Scholar] [CrossRef]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Lin, S.-C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Sonobe, H. Subchapter 98A-20-Hydroxyecdysone. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 560-e598A–562. [Google Scholar]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Sanz, P. AMP-activated protein kinase: Structure and regulation. Curr. Protein Pept. Sci. 2008, 9, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zou, H.; Li, X.; Qiu, Q.; Geng, N.; Zhang, B.; Yan, G.; Zhang, Z.; Zhang, S.; Yao, B.; et al. Metformin-induced AMPK activation suppresses larval growth and molting probably by disrupting 20E synthesis and glycometabolism in fall webworm, Hyphantria cunea Drury. Pestic. Biochem. Physiol. 2022, 183, 105083. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, X.; He, Y.; Shuai, J.; Chen, X.; Ling, E. Expression of antimicrobial peptide genes in Bombyx mori gut modulated by oral bacterial infection and development. Dev. Comp. Immunol. 2010, 34, 1191–1198. [Google Scholar] [CrossRef]
- Sinnett, S.E.; Brenman, J.E. The Role of AMPK in Drosophila melanogaster. Exp. Suppl. 2016, 107, 389–401. [Google Scholar] [CrossRef]
- Kim, M.; Shen, M.; Ngoy, S.; Karamanlidis, G.; Liao, R.; Tian, R. AMPK isoform expression in the normal and failing hearts. J. Mol. Cell. Cardiol. 2012, 52, 1066–1073. [Google Scholar] [CrossRef]
- Dominick, O.S.; Truman, J.W. The physiology of wandering behaviour in Manduca sexta. IV. Hormonal induction of wandering behaviour from the isolated nervous system. J. Exp. Biol. 1986, 121, 133–151. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Liu, H.; Wang, J.; Zhou, S.; Jiang, R.J.; Bendena, W.G.; Li, S. 20-hydroxyecdysone reduces insect food consumption resulting in fat body lipolysis during molting and pupation. J. Mol. Cell Biol. 2010, 2, 128–138. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Hou, Y.; Zhou, X.; Chen, Q.; Guo, C.; Xia, Q.; Zhang, Y.; Zhao, P. Integrative proteomics and metabolomics analysis of insect larva brain: Novel insights into the molecular mechanism of insect wandering behavior. J. Proteome Res. 2016, 15, 193–204. [Google Scholar] [CrossRef]
- Shang, L.; Chen, S.; Du, F.; Li, S.; Zhao, L.; Wang, X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc. Natl. Acad. Sci. USA 2011, 108, 4788–4793. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK—Sensing Energy while Talking to Other Signaling Pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef]
- Hindupur, S.K.; González, A.; Hall, M.N. The opposing actions of target of rapamycin and AMP-activated protein kinase in cell growth control. Cold Spring Harb. Perspect. Biol. 2015, 7, a019141. [Google Scholar] [CrossRef] [PubMed]
- Beckstead, R.B.; Lam, G.; Thummel, C.S. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 2005, 6, R99. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.S.; Colombani, J.; Léopold, P. Coordination of organ growth: Principles and outstanding questions from the world of insects. Trends Cell Biol. 2013, 23, 336–344. [Google Scholar] [CrossRef]
- Johnson, K.J.; Boekelheide, K. Dynamic testicular adhesion junctions are immunologically unique. I. Localization of p120 catenin in rat testis. Biol. Reprod. 2002, 66, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Laker, R.C.; Drake, J.C.; Wilson, R.J.; Lira, V.A.; Lewellen, B.M.; Ryall, K.A.; Fisher, C.C.; Zhang, M.; Saucerman, J.J.; Goodyear, L.J.; et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Long, S.; Liu, S.; Yuan, D.; Huang, D.; Xu, J.; Ma, Q.; Wang, G.; Wang, J.; Li, S.; et al. Atg1 phosphorylation is activated by AMPK and indispensable for autophagy induction in insects. Insect Biochem. Mol. Biol. 2023, 152, 103888. [Google Scholar] [CrossRef]
- Yuan, D.; Zhou, S.; Liu, S.; Li, K.; Zhao, H.; Long, S.; Liu, H.; Xie, Y.; Su, Y.; Yu, F.; et al. The AMPK-PP2A axis in insect fat body is activated by 20-hydroxyecdysone to antagonize insulin/IGF signaling and restrict growth rate. Proc. Natl. Acad. Sci. USA 2020, 117, 9292–9301. [Google Scholar] [CrossRef]
- Horike, N.; Sakoda, H.; Kushiyama, A.; Ono, H.; Fujishiro, M.; Kamata, H.; Nishiyama, K.; Uchijima, Y.; Kurihara, Y.; Kurihara, H.; et al. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J. Biol. Chem. 2008, 283, 33902–33910. [Google Scholar] [CrossRef]
- Chiba, Y.; Oshima, K.; Arai, H.; Ishii, M.; Igarashi, Y.J.J.o.B.C. Discovery and analysis of cofactor-dependent phosphoglycerate mutase homologs as novel phosphoserine phosphatases in Hydrogenobacter thermophilus. J. Biol. Chem. 2012, 287, 11934–11941. [Google Scholar] [CrossRef]
- Roy, A.; Shimizu, S.; Kiya, T.; Mita, K.; Iwami, M. Identification of 20-hydroxyecdysone-inducible genes from larval brain of the silkworm, Bombyx mori, and their expression analysis. Zool. Sci. 2012, 29, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Xiao, L.; Sun, Y.; Zhao, J.; Bai, L. Sublethal effects of the chitin synthesis inhibitor, hexaflumuron, in the cotton mirid bug, Apolygus lucorum (Meyer-Dür). Pestic. Biochem. Physiol. 2014, 111, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Xiao, L.; Sun, Y.; Zhao, J.; Bai, L.; Xiao, Y. Molecular characterization of soluble and membrane-bound trehalases in the cotton mirid bug, Apolygus lucorum. Arch. Insect Biochem. Physiol. 2014, 86, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Jiang, L.; Zheng, S.; Chen, Y.; Zhang, J.Y.; Stanley, D.; Miao, H.; Ge, L.Q. Silencing of a putative alanine aminotransferase (ALT) gene influences free amino acid composition in hemolymph and fecundity of the predatory bug, Cyrtorhinus lividipennis Reuter. Arch. Insect Biochem. Physiol. 2021, 108, e21836. [Google Scholar] [CrossRef]




| Experimental Treatment | 1st Nymph Instar | 2nd Nymph Instar | 3rd Nymph Instar | 4th Nymph Instar | 5th Nymph Instar | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dt (Days) | Molting (%) | Dt (Days) | Molting (%) | Dt (Days) | Molting (%) | Dt (Days) | Molting (%) | Dt (Days) | Molting (%) | ||
| Spraying | 20E + AlCAR | 2.66 ± 0.12 c | 98.5 | 2.04 ± 0.08 c | 97.4 | 2.09 ± 0.21 c | 95.2 | 2.22 ± 0.15 c | 87.8 | 2.53 ± 0.26 d | 84.8 |
| AlCAR | 2.71 ± 0.14 c | 99.2 | 2.11 ± 0.14 c | 96.8 | 2.14 ± 0.16 c | 95.8 | 2.19 ± 0.18 c | 89.1 | 2.48 ± 0.14 d | 86.2 | |
| 20E | 2.68 ± 0.08 c | 100 | 2.01 ± 0.12 c | 97.5 | 2.08 ± 0.15 c | 94.2 | 2.14 ± 0.29 c | 88.7 | 2.43 ± 0.16 d | 85.8 | |
| Water | 2.82 ± 0.13 b | 99.5 | 2.33 ± 0.16 b | 95.6 | 2.46 ± 0.14 b | 93.2 | 2.35 ± 0.24 b | 85.4 | 2.81 ± 0.23 c | 82.6 | |
| 20E + compound C | 2.99 ± 0.15 a | 85.6 | 2.76 ± 0.21 a | 68.5 | 2.98 ± 0.18 a | 55.6 | 3.07 ± 0.29 a | 52.2 | 3.92 ± 0.08 a | 42.8 | |
| AlCAR+ compound C | 2.96 ± 0.11 a | 83.2 | 2.81 ± 0.07 a | 73.2 | 3.01 ± 0.18 a | 50.8 | 2.92 ± 0.22 ab | 46.2 | 3.59 ± 0.32 b | 40.3 | |
| compound C | 3.07 ± 0.21 a | 73.6 | 2.88 ± 0.19 a | 62.8 | 3.42 ± 0.33 a | 51.2 | / | / | / | / | |
| Injection | 20E | - | - | 2.12 ± 0.11 a | 95.4 | 1.96 ± 0.15 a | 93.8 | 2.43 ± 0.27 a | 91.2 | 2.48 ± 0.26 a | 89.6 |
| Untreated | - | - | 2.35 ± 0.06 b | 96.2 | 2.15 ± 0.19 b | 94.2 | 2.47 ± 0.22 b | 92.4 | 2.68 ± 0.19 b | 88.4 | |
| dsGFP | - | - | 2.34 ± 0.08 b | 94.3 | 2.19 ± 0.07 b | 90.2 | 2.57 ± 0.19 b | 91.8 | 2.62 ± 0.18 b | 86.6 | |
| dsAMPK | - | - | 2.67 ± 0.28 c | 62.7 | 3.25 ± 0.22 c | 53.6 | / | / | / | / | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Xiao, L.; Zhao, J.; Zhang, J.; Ahmad, S.; Xu, D.; Xu, G.; Ge, L. Adenosine Monophosphate-Activated Protein Kinase (AMPK) Phosphorylation Is Required for 20-Hydroxyecdysone Regulates Ecdysis in Apolygus lucorum. Int. J. Mol. Sci. 2023, 24, 8587. https://doi.org/10.3390/ijms24108587
Tan Y, Xiao L, Zhao J, Zhang J, Ahmad S, Xu D, Xu G, Ge L. Adenosine Monophosphate-Activated Protein Kinase (AMPK) Phosphorylation Is Required for 20-Hydroxyecdysone Regulates Ecdysis in Apolygus lucorum. International Journal of Molecular Sciences. 2023; 24(10):8587. https://doi.org/10.3390/ijms24108587
Chicago/Turabian StyleTan, Yongan, Liubin Xiao, Jing Zhao, Jieyu Zhang, Sheraz Ahmad, Dejin Xu, Guangchun Xu, and Linquan Ge. 2023. "Adenosine Monophosphate-Activated Protein Kinase (AMPK) Phosphorylation Is Required for 20-Hydroxyecdysone Regulates Ecdysis in Apolygus lucorum" International Journal of Molecular Sciences 24, no. 10: 8587. https://doi.org/10.3390/ijms24108587
APA StyleTan, Y., Xiao, L., Zhao, J., Zhang, J., Ahmad, S., Xu, D., Xu, G., & Ge, L. (2023). Adenosine Monophosphate-Activated Protein Kinase (AMPK) Phosphorylation Is Required for 20-Hydroxyecdysone Regulates Ecdysis in Apolygus lucorum. International Journal of Molecular Sciences, 24(10), 8587. https://doi.org/10.3390/ijms24108587







