Can Pharmacogenetic Variants in TPMT, MTHFR and SLCO1B1 Genes Be Used as Potential Markers of Outcome Prediction in Systemic Sclerosis Patients?
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics
2.2. Association of Genetic Variants with the Risk of Severe Disease Outcome
2.3. Polygenic Risk Score for Assessing Severe Disease Outcomes
3. Discussion
4. Materials and Methods
4.1. Subjects Demographics and Clinical Characteristics
4.2. Blood Sampling and DNA Extraction
4.3. Genotyping
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhao, M.; Wu, J.; Wu, H.; Sawalha, A.H.; Lu, Q. Clinical Treatment Options in Scleroderma: Recommendations and Comprehensive Review. Clin. Rev. Allerg. Immunol. 2022, 62, 273–291. [Google Scholar] [CrossRef]
- De Almeida Chaves, S.; Porel, T.; Mounié, M.; Alric, L.; Astudillo, L.; Huart, A.; Lairez, O.; Michaud, M.; Prévot, G.; Ribes, D.; et al. Sine Scleroderma, Limited Cutaneous, and Diffused Cutaneous Systemic Sclerosis Survival and Predictors of Mortality. Arthritis Res. Ther. 2021, 23, 295. [Google Scholar] [CrossRef]
- Zekovic, A.; Vreca, M.; Spasovski, V.; Andjelkovic, M.; Pavlovic, S.; Damjanov, N. Association between the -174 C/G Polymorphism in the Interleukin-6 (IL-6) Gene and Gastrointestinal Involvement in Patients with Systemic Sclerosis. Clin. Rheumatol. 2018, 37, 2447–2454. [Google Scholar] [CrossRef]
- Hughes, M.; Pauling, J.D.; Armstrong-James, L.; Denton, C.P.; Galdas, P.; Flurey, C. Gender-Related Differences in Systemic Sclerosis. Autoimmun. Rev. 2020, 19, 102494. [Google Scholar] [CrossRef]
- Freire, M.; Rivera, A.; Sopeña, B.; Vilella, C.T.; Castillo, A.G.-D.; Argüelles, D.C.; Rubio, J.L.C.; Rivas, M.R.; Martínez, L.T.; Parra, J.A.T.; et al. Clinical and Epidemiological Differences between Men and Women with Systemic Sclerosis: A Study in a Spanish Systemic Sclerosis Cohort and Literature Review. Clin. Exp. Rheumatol. 2017, 35, 89–97. [Google Scholar] [PubMed]
- Gourh, P.; Safran, S.A.; Alexander, T.; Boyden, S.E.; Morgan, N.D.; Shah, A.A.; Mayes, M.D.; Doumatey, A.; Bentley, A.R.; Shriner, D.; et al. HLA and Autoantibodies Define Scleroderma Subtypes and Risk in African and European Americans and Suggest a Role for Molecular Mimicry. Proc. Natl. Acad. Sci. USA 2020, 117, 552–562. [Google Scholar] [CrossRef]
- Barnes, J.; Mayes, M.D. Epidemiology of Systemic Sclerosis: Incidence, Prevalence, Survival, Risk Factors, Malignancy, and Environmental Triggers. Curr. Opin. Rheumatol. 2012, 24, 165–170. [Google Scholar] [CrossRef]
- Chung, M.P.; Dontsi, M.; Postlethwaite, D.; Kesh, S.; Simard, J.F.; Fiorentino, D.; Zaba, L.C.; Chung, L. Increased Mortality in Asians With Systemic Sclerosis in Northern California. ACR Open Rheumatol. 2020, 2, 197–206. [Google Scholar] [CrossRef]
- Pavlovic, S.; Kotur, N.; Stankovic, B.; Zukic, B.; Gasic, V.; Dokmanovic, L. Pharmacogenomic and Pharmacotranscriptomic Profiling of Childhood Acute Lymphoblastic Leukemia: Paving the Way to Personalized Treatment. Genes 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Kotur, N.; Lazic, J.; Ristivojevic, B.; Stankovic, B.; Gasic, V.; Dokmanovic, L.; Krstovski, N.; Milosevic, G.; Janic, D.; Zukic, B.; et al. Pharmacogenomic Markers of Methotrexate Response in the Consolidation Phase of Pediatric Acute Lymphoblastic Leukemia Treatment. Genes 2020, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, G.; Kotur, N.; Krstovski, N.; Lazic, J.; Zukic, B.; Stankovic, B.; Janic, D.; Katsila, T.; Patrinos, G.P.; Pavlovic, S.; et al. Variants in TPMT, ITPA, ABCC4 And ABCB1 Genes as Predictors of 6-Mercaptopurine Induced Toxicity in Children with Acute Lymphoblastic Leukemia. J. Med. Biochem. 2018, 37, 320–327. [Google Scholar] [CrossRef]
- Pavlovic, S.; Kotur, N.; Stankovic, B.; Gasic, V.; Lucafo, M.; Decorti, G.; Zukic, B. Clinical Application of Thiopurine Pharmacogenomics in Pediatrics. Curr. Drug Metab. 2020, 21, 53–62. [Google Scholar] [CrossRef]
- Adam de Beaumais, T.; Jacqz-Aigrain, E. Intracellular Disposition of Methotrexate in Acute Lymphoblastic Leukemia in Children. Curr. Drug Metab. 2012, 13, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Zaza, G.; Cheok, M.; Krynetskaia, N.; Thorn, C.; Stocco, G.; Hebert, J.M.; McLeod, H.; Weinshilboum, R.M.; Relling, M.V.; Evans, W.E.; et al. Thiopurine Pathway. Pharm. Genom. 2010, 20, 573–574. [Google Scholar] [CrossRef]
- Jančić, I.; Arsenović-Ranin, N.; Šefik-Bukilica, M.; Živojinović, S.; Damjanov, N.; Spasovski, V.; Srzentić, S.; Stanković, B.; Pavlović, S. -174G/C Interleukin-6 Gene Promoter Polymorphism Predicts Therapeutic Response to Etanercept in Rheumatoid Arthritis. Rheumatol. Int. 2013, 33, 1481–1486. [Google Scholar] [CrossRef]
- Jančić, I.; Šefik-Bukilica, M.; Živojinović, S.; Damjanov, N.; Spasovski, V.; Kotur, N.; Klaassen, K.; Pavlović, S.; Bufan, B.; Arsenović-Ranin, N. Influence of Promoter Polymorphisms Of The Tnf-α (-308g/A) And IL-6 (-174g/C) Genes On Therapeutic Response To Etanercept In Rheumatoid Arthritis. J. Med. Biochem. 2015, 34, 414–421. [Google Scholar] [CrossRef]
- Muller, I.B.; Hebing, R.F.; Jansen, G.; Nurmohamed, M.T.; Lems, W.F.; Peters, G.J.; de Jonge, R. Personalized Medicine in Rheumatoid Arthritis: Methotrexate Polyglutamylation Revisited. Expert Rev. Precis. Med. Drug Dev. 2018, 3, 331–334. [Google Scholar] [CrossRef]
- Jojic, N.; Urosevic, J.; Bojic, B.; Pavlovic, S. Determination of Thiopurine Methyltransferase Genotype in the Patients with Inflammatory Bowel Disease before and during Azathioprine Therapy. Arch. Gastroenterohepatol. 2003, 22, 5–9. [Google Scholar]
- van den Bosch, B.J.; Coenen, M.J. Pharmacogenetics of Inflammatory Bowel Disease. Pharmacogenomics 2021, 22, 55–66. [Google Scholar] [CrossRef]
- Denton, C.P.; Hughes, M.; Gak, N.; Vila, J.; Buch, M.H.; Chakravarty, K.; Fligelstone, K.; Gompels, L.L.; Griffiths, B.; Herrick, A.L.; et al. BSR and BHPR Guideline for the Treatment of Systemic Sclerosis. Rheumatology 2016, 55, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.L.; Dickson, A.L.; Chung, C.P. Precision Medicine for Rheumatologists: Lessons from the Pharmacogenomics of Azathioprine. Clin. Rheumatol. 2021, 40, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Lehr, H.A.; Wirtz, S.; Becker, C.; Atreya, R.; et al. CD28-Dependent Rac1 Activation Is the Molecular Target of Azathioprine in Primary Human CD4+ T Lymphocytes. J. Clin. Investig. 2003, 111, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M. Do Women Have More Adverse Drug Reactions? Am. J. Clin. Dermatol. 2001, 2, 349–351. [Google Scholar] [CrossRef]
- Campbell, J.M.; Bateman, E.; Stephenson, M.D.; Bowen, J.M.; Keefe, D.M.; Peters, M.D.J. Methotrexate-Induced Toxicity Pharmacogenetics: An Umbrella Review of Systematic Reviews and Meta-Analyses. Cancer Chemother. Pharmacol. 2016, 78, 27–39. [Google Scholar] [CrossRef]
- Radtke, S.; Zolk, O.; Renner, B.; Paulides, M.; Zimmermann, M.; Möricke, A.; Stanulla, M.; Schrappe, M.; Langer, T. Germline Genetic Variations in Methotrexate Candidate Genes Are Associated with Pharmacokinetics, Toxicity, and Outcome in Childhood Acute Lymphoblastic Leukemia. Blood 2013, 121, 5145–5153. [Google Scholar] [CrossRef]
- Shane, B.; Pangilinan, F.; Mills, J.L.; Fan, R.; Gong, T.; Cropp, C.D.; Kim, Y.; Ueland, P.M.; Bailey-Wilson, J.E.; Wilson, A.F.; et al. The 677C→T Variant of MTHFR Is the Major Genetic Modifier of Biomarkers of Folate Status in a Young, Healthy Irish Population. Am. J. Clin. Nutr. 2018, 108, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, W.; Pei, D.; Cheng, C.; Smith, C.; Landier, W.; Hageman, L.; Chen, Y.; Yang, J.; Crews, K.; et al. Genomewide Approach Validates Thiopurine Methyltransferase Activity Is a Monogenic Pharmacogenomic Trait. Clin. Pharmacol. Ther. 2017, 101, 373–381. [Google Scholar] [CrossRef]
- Treviño, L.R.; Shimasaki, N.; Yang, W.; Panetta, J.C.; Cheng, C.; Pei, D.; Chan, D.; Sparreboom, A.; Giacomini, K.M.; Pui, C.-H.; et al. Genetic Variation in an Organic Anion Transporter Polypeptide Associated with Methotrexate Pharmacokinetics and Clinical Effects. J. Clin. Oncol. 2009, 27, 5972–5978. [Google Scholar] [CrossRef] [PubMed]
- Tamm, R.; Mägi, R.; Tremmel, R.; Winter, S.; Mihailov, E.; Smid, A.; Möricke, A.; Klein, K.; Schrappe, M.; Stanulla, M.; et al. Polymorphic Variation in TPMT Is the Principal Determinant of TPMT Phenotype: A Meta-Analysis of Three Genome-Wide Association Studies. Clin. Pharmacol. Ther. 2017, 101, 684–695. [Google Scholar] [CrossRef]
- Łaczmańska, I.; Dębicka, I.; Gil, J.; Michałowska, D.; Pawlak, I.; Sąsiadek, M.M. Medycyna Personalizowana w Raku Płuca. Nowotw. J. Oncol. 2021, 71, 122–128. [Google Scholar] [CrossRef]
- Noor, N.M.; Verstockt, B.; Parkes, M.; Lee, J.C. Personalised Medicine in Crohn’s Disease. Lancet Gastroenterol. Hepatol. 2020, 5, 80–92. [Google Scholar] [CrossRef]
- Zhu, J.L.; Black, S.M.; Chen, H.W.; Jacobe, H.T. Emerging Treatments for Scleroderma/Systemic Sclerosis. Fac. Rev. 2021, 10, 43. [Google Scholar] [CrossRef]
- Di Battista, M.; Barsotti, S.; Orlandi, M.; Lepri, G.; Codullo, V.; Della Rossa, A.; Guiducci, S.; Del Galdo, F. One Year in Review 2021: Systemic Sclerosis. Clin. Exp. Rheumatol. 2021, 39, 3–12. [Google Scholar] [CrossRef]
- Dokmanovic, L.; Urosevic, J.; Janic, D.; Jovanovic, N.; Petrucev, B.; Tosic, N.; Pavlovic, S. Analysis of Thiopurine S-Methyltransferase Polymorphism in the Population of Serbia and Montenegro and Mercaptopurine Therapy Tolerance in Childhood Acute Lymphoblastic Leukemia. Ther. Drug Monit. 2006, 28, 800–806. [Google Scholar] [CrossRef]
- Park, R.; Nevskaya, T.; Baron, M.; Pope, J.E. Immunosuppression Use in Early Systemic Sclerosis May Be Increasing over Time. J. Scleroderma Relat. Disord. 2022, 7, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Vreca, M.; Andjelkovic, M.; Tosic, N.; Zekovic, A.; Damjanov, N.; Pavlovic, S.; Spasovski, V. Impact of Alterations in X-Linked IRAK1gene and MiR-146a on Susceptibility and Clinical Manifestations in Patients with Systemic Sclerosis. Immunol. Lett. 2018, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Sohn, I.; Kim, M.; Woo, H.I.; Lee, J.W.; Ma, Y.; Yi, E.S.; Koo, H.H.; Lee, S. Pathway Genes and Metabolites in Thiopurine Therapy in Korean Children with Acute Lymphoblastic Leukaemia. Br. J. Clin. Pharmacol. 2019, 85, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Zimdahl Kahlin, A.; Helander, S.; Wennerstrand, P.; Vikingsson, S.; Mårtensson, L.; Appell, M.L. Pharmacogenetic Studies of Thiopurine Methyltransferase Genotype-phenotype Concordance and Effect of Methotrexate on Thiopurine Metabolism. Basic Clin. Pharmacol. Toxicol. 2021, 128, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Taylor, Z.L.; Thompson, L.E.; Bear, H.; Mizuno, T.; Vinks, A.A.; Ramsey, L.B. Toward Pharmacogenetic SLCO1B1 -guided Dosing of Methotrexate in Arthritis Using a Murine Slco1b2 Knockout Model. Clin. Transl. Sci. 2021, 14, 2267–2277. [Google Scholar] [CrossRef]
- Wennerstrand, P.; Mårtensson, L.-G.; Söderhäll, S.; Zimdahl, A.; Appell, M.L. Methotrexate Binds to Recombinant Thiopurine S-Methyltransferase and Inhibits Enzyme Activity after High-Dose Infusions in Childhood Leukaemia. Eur. J. Clin. Pharmacol. 2013, 69, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Mizzi, C.; Dalabira, E.; Kumuthini, J.; Dzimiri, N.; Balogh, I.; Başak, N.; Böhm, R.; Borg, J.; Borgiani, P.; Bozina, N.; et al. A European Spectrum of Pharmacogenomic Biomarkers: Implications for Clinical Pharmacogenomics. PLoS ONE 2016, 11, e0162866. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Zhao, E.-Y.; Chen, C.-H.; Dong, L.-L. Relationship between MTHFR Gene Polymorphism and Susceptibility to Bronchial Asthma and Glucocorticoid Efficacy in Children. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-L.; Chen, G.-R.; Hsiao, P.-J.; Chiu, C.-C.; Tai, M.-C.; Kao, C.-C.; Tsai, D.-J.; Su, H.; Chen, Y.-H.; Chen, W.-T.; et al. Decisive Evidence Corroborates a Null Relationship between MTHFR C677T and Chronic Kidney Disease: A Case–Control Study and a Meta-Analysis. Medicine 2020, 99, e21045. [Google Scholar] [CrossRef]
- Karas-Kuzelicki, N.; Milek, M.; Mlinaric-Rascan, I. MTHFR and TYMS Genotypes Influence TPMT Activity and Its Differential Modulation in Males and Females. Clin. Biochem. 2010, 43, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tatebe, Y.; Kanamitsu, K.; Kanzaki, H.; Ishida, H.; Fujiwara, K.; Washio, K.; Kitamura, Y.; Sendo, T.; Shimada, A.; Tsukahara, H. Delayed Methotrexate Elimination after Administration of a Medium Dose of Methotrexate in a Patient with Genetic Variants Associated with Methotrexate Clearance. Acta Med. Okayama 2020, 74, 545–550. [Google Scholar]
- Ahern, T.P.; Damkier, P.; Feddersen, S.; Kjærsgaard, A.; Lash, T.L.; Hamilton-Dutoit, S.; Lythjohan, C.B.; Ejlertsen, B.; Christiansen, P.M.; Cronin-Fenton, D.P. Predictive Pharmacogenetic Biomarkers for Breast Cancer Recurrence Prevention by Simvastatin. Acta Oncol. 2020, 59, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Ragia, G.; Atzemian, N.; Maslarinou, A.; Manolopoulos, V.G. SLCO1B1 c.521T>C Gene Polymorphism Decreases Hypoglycemia Risk in Sulfonylurea-Treated Type 2 Diabetic Patients. Drug Metab. Pers. Ther. 2022, 37, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Smeijer, J.D.; Koomen, J.V.; Kohan, D.E.; McMurray, J.J.V.; Bakris, G.L.; Correa-Rotter, R.; Hou, F.; Kitzman, D.W.; Makino, H.; Mayer, G.; et al. Organic Anion Transporter Gene Variants Associated with Plasma Exposure and Long-Term Response to Atrasentan in Patients With Diabetic Kidney Disease. Clin. Pharmacol. Ther. 2022, 112, 1098–1107. [Google Scholar] [CrossRef]
- Turongkaravee, S.; Jittikoon, J.; Lukkunaprasit, T.; Sangroongruangsri, S.; Chaikledkaew, U.; Thakkinstian, A. A Systematic Review and Meta-Analysis of Genotype-Based and Individualized Data Analysis of SLCO1B1 Gene and Statin-Induced Myopathy. Pharm. J. 2021, 21, 296–307. [Google Scholar] [CrossRef]
- Merkel, M.; Schneider, C.; Greinert, R.; Zipprich, A.; Ripoll, C.; Lammert, F.; Reichert, M.C. Protective Effects of Statin Therapy in Cirrhosis Are Limited by a Common SLCO1B1 Transporter Variant. Hepatol. Commun. 2021, 5, 1755–1766. [Google Scholar] [CrossRef]
- Gibson, G. On the Utilization of Polygenic Risk Scores for Therapeutic Targeting. PLoS Genet. 2019, 15, e1008060. [Google Scholar] [CrossRef]
- Benton, M.L.; Abraham, A.; LaBella, A.L.; Abbot, P.; Rokas, A.; Capra, J.A. The Influence of Evolutionary History on Human Health and Disease. Nat. Rev. Genet. 2021, 22, 269–283. [Google Scholar] [CrossRef]
- Koido, M.; Kawakami, E.; Fukumura, J.; Noguchi, Y.; Ohori, M.; Nio, Y.; Nicoletti, P.; Aithal, G.P.; Daly, A.K.; Watkins, P.B.; et al. Polygenic Architecture Informs Potential Vulnerability to Drug-Induced Liver Injury. Nat. Med. 2020, 26, 1541–1548. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative: ACR/EULAR Classification Criteria for SSc. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Kiatchoosakun, S.; Wongvipaporn, C.; Nanagara, R.; Hoit, B.D. Right Ventricular Systolic Pressure Assessed by Echocardiography: A Predictive Factor of Mortality in Patients with Scleroderma. Clin. Cardiol. 2011, 34, 488–493. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mercurio, V.; Hsu, S.; Mayer, S.A.; Mathai, S.C.; Hummers, L.K.; Kass, D.A.; Hassoun, P.M.; Wigley, F.M.; Tedford, R.J.; et al. Assessment of Right Ventricular Reserve Utilizing Exercise Provocation in Systemic Sclerosis. Int. J. Cardiovasc. Imaging 2021, 37, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Oender, K.; Lanschuetzer, C.; Laimer, M.; Klausegger, A.; Paulweber, B.; Kofler, B.; Hintner, H.; Bauer, J. Introducing a Fast and Simple PCR-RFLP Analysis for the Detection of Mutant Thiopurine S-Methyltransferase Alleles TPMT*3A and TPMT*3C. J. Eur. Acad. Dermatol. Venerol. 2006, 20, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.M.; Lugemwa, P.R. Development of a Highly Accurate, Rapid PCR-RFLP Genotyping Assay for the Methylenetetrahydrofolate Reductase Gene. Genet. Test. 1999, 3, 287–289. [Google Scholar] [CrossRef] [PubMed]
| AZA | MTX | AZA + MTX | Other Medication | |
|---|---|---|---|---|
| No of patients (%) | 16 (15.7) | 43 (42.2) | 3 (2.9) | 40 (39.2) |
| Age, median [IQR] | 61 [58.75–63] | 57 [49.5–61.5] | 60 [51.5–62.5] | 60.5 [54.5–66] |
| No of women (%) | 14 (13.7) | 37 (36.6) | 3 (2.9) | 33 (32.2) |
| PF (%) | 6 (5.9) | 14 (13.7) | 0 | 19 (18.6) |
| Kidney insufficiency (%) | 4 (3.9) | 10 (9.8) | 0 | 14 (13.7) |
| RVSP > 35 mmHg (%) | 2 (1.9) | 8 (7.8) | 0 | 12 (11.8) |
| HUV (%) | 7 (6.9) | 12 (11.8) | 0 | 12 (11.8) |
| FVC/DLCO > 1.6 (%) | 0 | 4 (3.9) | 0 | 2 (1.9) |
| Genetic Variant (rs Number) | Disease Outcomes | Therapy | Dominant Genetic Model | p OR [CI 95%] | padj OR [CI 95%] |
|---|---|---|---|---|---|
| TPMT*3A (rs1800460 and rs1142345) | RVSP > 35 mmHg | MTX | GGR vs. GA + AA/ AAR vs. AG + AA | 0.11 2.09 [0.87–5.06] | 0.095 2.11 [0.897–4.984] |
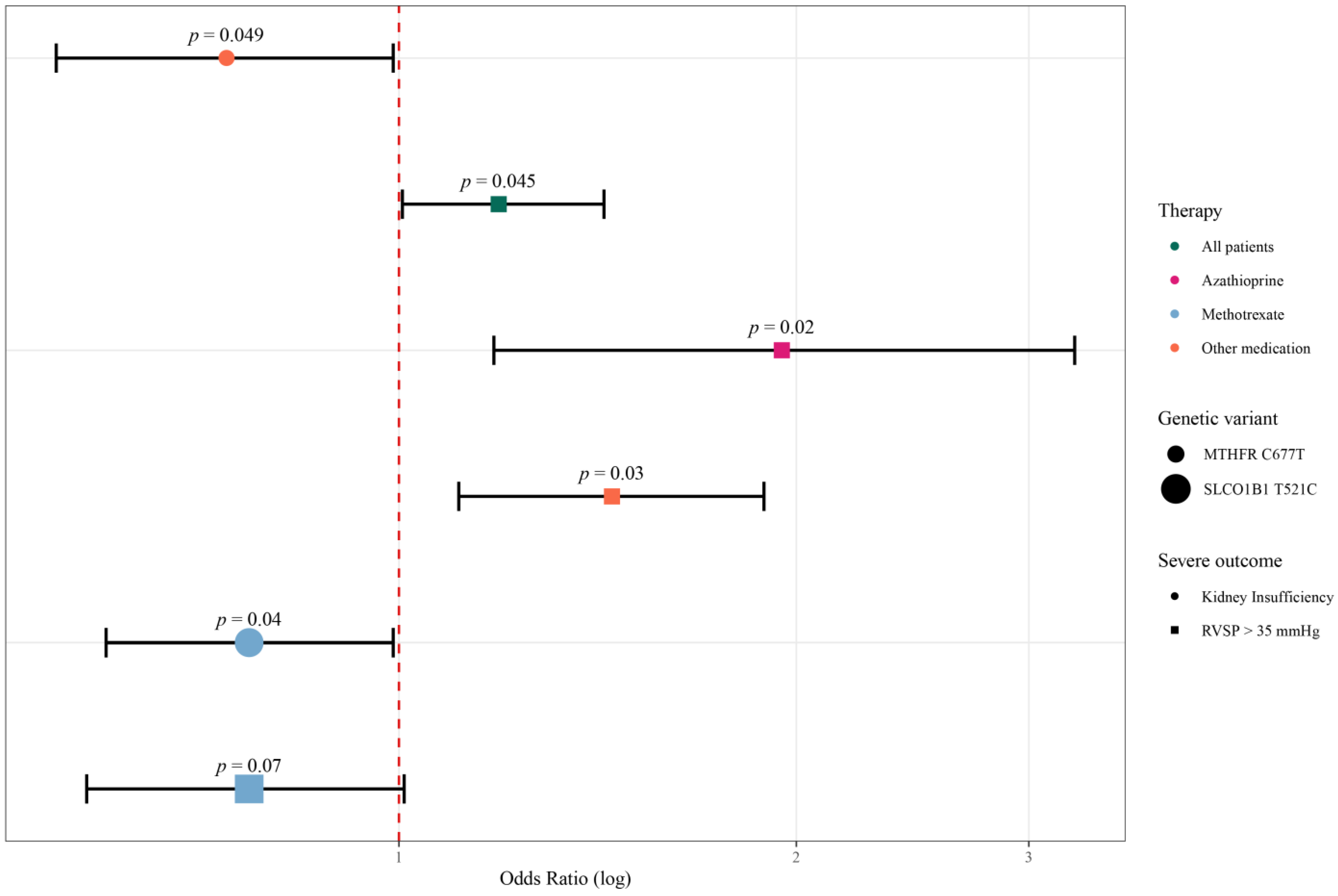
| MTHFR rs1801133 | Kidney insufficiency | Other | CCR vs. CT + TT | 0.12 0.79 [0.59–1.05] | 0.049 0.74 [0.55–0.989] |
| MTHFR rs1801133 | RVSP > 35 mmHg | All patients | CCR vs. CT + TT | 0.03 1.22 [1.02–1.45] | 0.045 1.19 [1.006–1.43] |
| MTHFR rs1801133 | RVSP > 35 mmHg | AZA | CCR vs. CT + TT | 0.04 1.69 [1.08–2.64] | 0.02 1.95 [1.18–3.25] |
| MTHFR rs1801133 | RVSP > 35 mmHg | Other | CCR vs. CT + TT | 0.009 1.45 [1.11–1.89] | 0.03 1.45 [1.11–1.89] |
| SLCO1B1 rs4149056 | PF | AZA | TTR vs. TC + CC | 0.04 0.64 [0.43–0.95] | 0.12 0.66 [0.41–1.08] |
| SLCO1B1 rs4149056 | Kidney insufficiency | MTX | TTR vs. TC + CC | 0.03 0.76 [0.59–0.96] | 0.04 0.77 [0.60–0.99] |
| SLCO1B1 rs4149056 | RVSP > 35 mmHg | MTX | TTR vs. TC + CC | 0.04 0.74 [0.56–0.97] | 0.07 0.77 [0.58–1.009] |
| SLCO1B1 rs4149056 | HUV | Other | TTR vs. TC + CC | 0.01 1.22 [1.05–1.41] | 0.197 1.11 [0.95–1.30] |
| Severe Outcome | p Values | |||
|---|---|---|---|---|
| All Patients | AZA | MTX | Other Medication | |
| PF | 0.4228 | 0.7585 | 0.6384 | 0.1409 |
| Kidney insufficiency | 0.1535 | 0.8091 | 0.3981 | 0.2699 |
| RVSP > 35 mmHg | 0.9052 | 0.5914 | 0.0838 | 0.1412 |
| HUV | 0.8329 | NaN | 0.5498 | 0.1144 |
| FVC/DLCO > 1.6 | 0.3346 | 0.2713 | 0.3472 | 0.757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelovac, M.; Kotur, N.; Ristivojevic, B.; Pavlovic, D.; Spasovski, V.; Damjanov, N.; Pavlovic, S.; Zukic, B. Can Pharmacogenetic Variants in TPMT, MTHFR and SLCO1B1 Genes Be Used as Potential Markers of Outcome Prediction in Systemic Sclerosis Patients? Int. J. Mol. Sci. 2023, 24, 8538. https://doi.org/10.3390/ijms24108538
Jelovac M, Kotur N, Ristivojevic B, Pavlovic D, Spasovski V, Damjanov N, Pavlovic S, Zukic B. Can Pharmacogenetic Variants in TPMT, MTHFR and SLCO1B1 Genes Be Used as Potential Markers of Outcome Prediction in Systemic Sclerosis Patients? International Journal of Molecular Sciences. 2023; 24(10):8538. https://doi.org/10.3390/ijms24108538
Chicago/Turabian StyleJelovac, Marina, Nikola Kotur, Bojan Ristivojevic, Djordje Pavlovic, Vesna Spasovski, Nemanja Damjanov, Sonja Pavlovic, and Branka Zukic. 2023. "Can Pharmacogenetic Variants in TPMT, MTHFR and SLCO1B1 Genes Be Used as Potential Markers of Outcome Prediction in Systemic Sclerosis Patients?" International Journal of Molecular Sciences 24, no. 10: 8538. https://doi.org/10.3390/ijms24108538
APA StyleJelovac, M., Kotur, N., Ristivojevic, B., Pavlovic, D., Spasovski, V., Damjanov, N., Pavlovic, S., & Zukic, B. (2023). Can Pharmacogenetic Variants in TPMT, MTHFR and SLCO1B1 Genes Be Used as Potential Markers of Outcome Prediction in Systemic Sclerosis Patients? International Journal of Molecular Sciences, 24(10), 8538. https://doi.org/10.3390/ijms24108538





