Cold-Adapted Proteases: An Efficient and Energy-Saving Biocatalyst
Abstract
1. Introduction
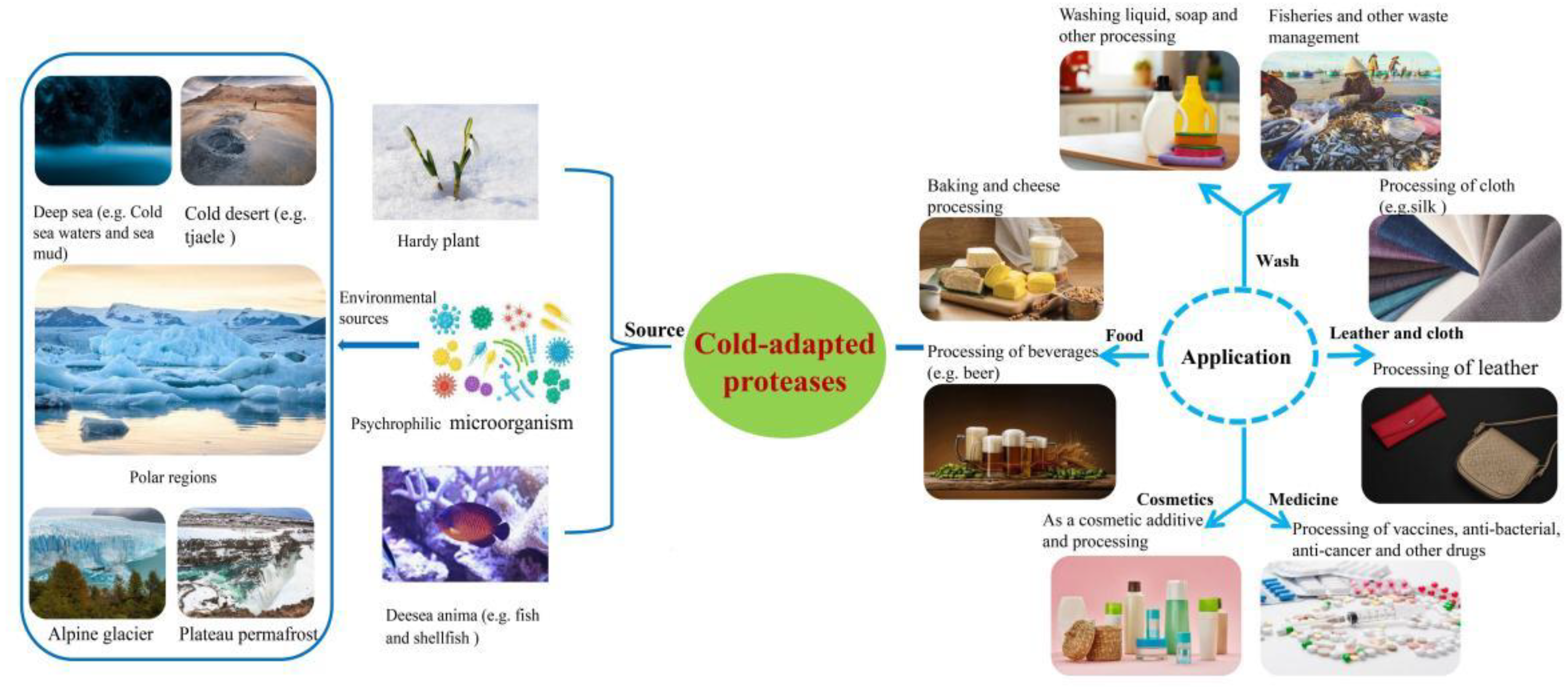
2. Sources and Mining of Cold-Adapted Protease
3. Classification of Cold-Adapted Proteases
4. Enzymatic Characteristics of Cold-Adapted Proteases
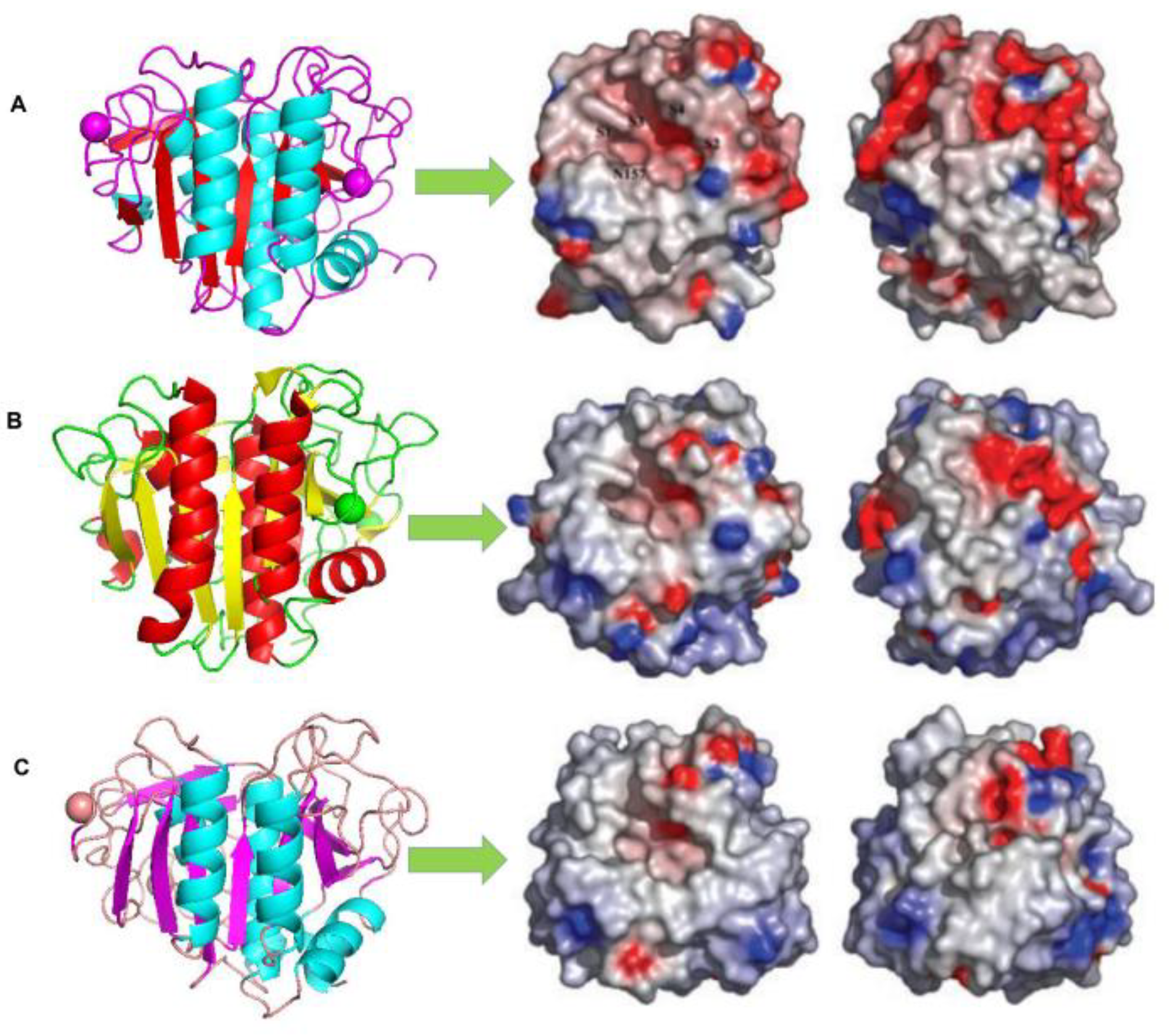
5. Structural Adaptations of Cold-Adapted Protease
6. Thermal Stability Modification of Cold-Adapted Proteases
7. Production of Cold-Adapted Proteases
8. Medical Applications of Cold-Active Protease
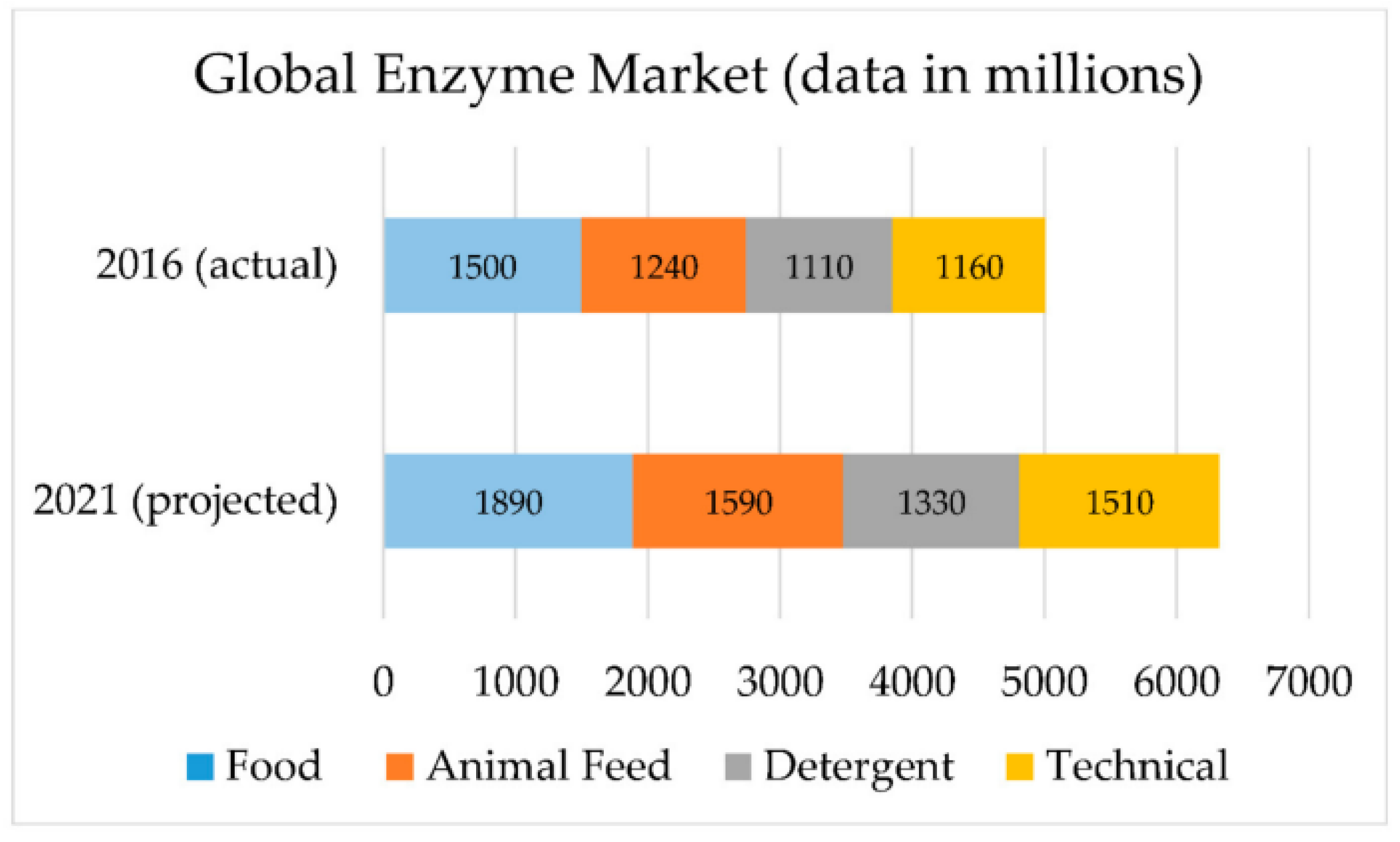
9. Other Industrial Applications of Cold-Adapted Proteases
10. Cold-Adapted Proteases and Detergents
11. Cold-Adapted Proteases and the Food Industry
12. Other Applications
13. Expectations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furhan, J. Adaptation, production, and biotechnological potential of cold-adapted proteases from psychrophiles and psychrotrophs: Recent overview. J. Genet. Eng. Biotechnol. 2020, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mukhia, S.; Kumar, R. Industrial applications of cold-adapted enzymes: Challenges, innovations and future perspective. 3 Biotech 2021, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Margesin, R. Psychrophilic lifestyles: Mechanisms of adaptation and biotechnological tools. Appl. Microbiol. Biotechnol. 2019, 103, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Shen, l.-J.; Zhang, S.-T.; Chen, G. Regulated strategies of cold-adapted microorganisms in response to cold: A review. Environ. Sci. Pollut. Res. 2021, 28, 68006–68024. [Google Scholar] [CrossRef] [PubMed]
- Gounot, A.M. Bacterial life at low temperature: Physiological aspects and biotechnological implications. J. Appl. Bacteriol. 1991, 71, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Khiari, Z. Sustainable Upcycling of Fisheries and Aquaculture Wastes Using Fish-Derived Cold-Adapted Proteases. Front. Nutr. 2022, 9, 875697. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.H.; Jepsen, S.J.; Outtrup, H. Enzymes for low temperature washing. J. Am. Oil Chem. Soc. 1981, 58, 644–649. [Google Scholar] [CrossRef]
- Craik, C.S.; Page, M.J.; Madison, E.L. Proteases as therapeutics. Biochem. J. 2011, 435, 1–16. [Google Scholar] [CrossRef]
- Morani, A.D. Trypsin therapy in the management of chronic surface ulcers. Plast. Reconstr. Surg. 1953, 11, 372–379. [Google Scholar] [CrossRef]
- Rapoport, C. The use of trypsin in the therapy of tuberculous lymphadenitis and tuberculous fistulae. Dis. Chest. 1958, 34, 154–161. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.; Palsdottir, H.M. Atlantic cod trypsins: From basic research to practical applications. Mar. Biotechnol. 2005, 7, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and Hot Extremozymes: Industrial Relevance and Current Trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Fornbacke, M.; Clarsund, M. Cold-adapted proteases as an emerging class of therapeutics. Infect. Dis. Ther. 2013, 2, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Perfumo, A.; Banat, I.M.; Marchant, R. Going Green and Cold: Biosurfactants from Low-Temperature Environments to Biotechnology Applications. Trends Biotechnol. 2018, 36, 277–289. [Google Scholar] [CrossRef]
- Morita, R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [CrossRef]
- Kralova, S. Role of fatty acids in cold adaptation of Antarctic psychrophilic Flavobacterium spp. Syst. Appl. Microbiol. 2017, 40, 329–333. [Google Scholar] [CrossRef]
- Dhakar, K.; Pandey, A. Microbial Ecology from the Himalayan Cryosphere Perspective. Microorganisms 2020, 8, 257. [Google Scholar] [CrossRef]
- Rafiq, M.; Nadeem, S.; Hassan, N.; Hayat, M.; Sajjad, W.; Zada, S.; Sajjad, W.; Hasan, F. Fungal recovery and characterization from Hindu Kush mountain range, Tirich Mir glacier, and their potential for biotechnological applications. J. Basic. Microbiol. 2020, 60, 444–457. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, Y.M.; Kim, S.; Wi, A.R.; Han, S.J.; Kim, H.W.; Kim, I.C.; Yim, J.H.; Kim, D. Identification of proteolytic bacteria from the Arctic Chukchi Sea expedition cruise and characterization of cold-active proteases. J. Microbiol. 2014, 52, 825–833. [Google Scholar] [CrossRef]
- Kim, E.H.; Cho, K.H.; Lee, Y.M.; Yim, J.H.; Lee, H.K.; Cho, J.C.; Hong, S.G. Diversity of cold-active protease-producing bacteria from arctic terrestrial and marine environments revealed by enrichment culture. J. Microbiol. 2010, 48, 426–432. [Google Scholar] [CrossRef]
- Bhat, A.; Riyaz-Ul-Hassan, S.; Ahmad, N.; Srivastava, N.; Johri, S. Isolation of cold-active, acidic endocellulase from Ladakh soil by functional metagenomics. Extremophiles 2013, 17, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.N.; Park, D.; Seong, H.J.; Kim, D.; Sul, W.J. Antarctic tundra soil metagenome as useful natural resources of cold-active lignocelluolytic enzymes. J. Microbiol. 2019, 57, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Paixao, D.; Tomazetto, G.; Sodre, V.R.; Goncalves, T.A.; Uchima, C.A.; Buchli, F.; Alvarez, T.M.; Persinoti, G.F.; Da, S.M.; Bragatto, J.; et al. Microbial enrichment and meta-omics analysis identify Cazymes from mangrove sediments with unique properties. Enzyme Microb. Technol. 2021, 148, 109820. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Kim, J.H.; Lee, Y.M.; Lee, J.S.; Shin, D.H.; Ku, B.H.; Son, K.H.; Park, H.Y. Identification and Characterization of a Novel, Cold-Adapted D-Xylobiose- and D-Xylose-Releasing Endo-β-1,4-Xylanase from an Antarctic Soil Bacterium, Duganella sp. PAMC 27433. Biomolecules 2021, 11, 680. [Google Scholar] [CrossRef]
- Kumar, R.; Acharya, V.; Mukhia, S.; Singh, D.; Kumar, S. Complete genome sequence of Pseudomonas frederiksbergensis ERDD5:01 revealed genetic bases for survivability at high altitude ecosystem and bioprospection potential. Genomics 2019, 111, 492–499. [Google Scholar] [CrossRef]
- Liu, K.; Ding, H.-T.; Yu, Y.; Chen, B. A Cold-Adapted Chitinase-Producing Bacterium from Antarctica and Its Potential in Biocontrol of Plant Pathogenic Fungi. Mar. Drugs. 2019, 17, 695. [Google Scholar] [CrossRef]
- Nakagawa, T.; Nagaoka, T.; Taniguchi, S.; Miyaji, T.; Tomizuka, N. Isolation and characterization of psychrophilic yeasts producing cold-adapted pectinolytic enzymes. Lett. Appl. Microbiol. 2004, 38, 383–387. [Google Scholar] [CrossRef]
- See-Too, W.S.; Convey, P.; Pearce, D.A.; Chan, K.G. Characterization of a novel N-acylhomoserine lactonase, AidP, from Antarctic Planococcus sp. Microb. Cell Fact. 2018, 17, 179. [Google Scholar] [CrossRef]
- Kryukova, M.V.; Petrovskaya, L.E.; Kryukova, E.A.; Lomakina, G.Y.; Yakimov, S.A.; Maksimov, E.G.; Boyko, K.M.; Popov, V.O.; Dolgikh, D.A.; Kirpichnikov, M.P. Thermal Inactivation of a Cold-Active Esterase PMGL3 Isolated from the Permafrost Metagenomic Library. Biomolecules 2019, 9, 880. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, M.; Zhang, M.; Wang, C.; Liu, Y.; Fan, X.; Li, H. Characterization of a Novel, Cold-Adapted, and Thermostable Laccase-Like Enzyme with High Tolerance for Organic Solvents and Salt and Potent Dye Decolorization Ability, Derived from a Marine Metagenomic Library. Front. Microbiol. 2018, 9, 2998. [Google Scholar] [CrossRef]
- Berlemont, R.; Pipers, D.; Delsaute, M.; Angiono, F.; Feller, G.; Galleni, M.; Power, P. Exploring the Antarctic soil metagenome as a source of novel cold-adapted enzymes and genetic mobile elements. Rev. Argent. Microbiol. 2011, 43, 94–103. [Google Scholar] [PubMed]
- Fan, X.J.; Liang, M.J.; Wang, L.; Chen, R.; He, L.; Liu, X.L. Aii810, a Novel Cold-Adapted N-Acylhomoserine Lactonase Discovered in a Metagenome, Can Strongly Attenuate Pseudomonas aeruginosa Virulence Factors and Biofilm Formation. Front. Microbiol. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Tchigvintsev, A.; Tran, H.; Popovic, A.; Kovacic, F.; Brown, G.; Flick, R.; Hajighasemi, M.; Egorova, O.; Somody, J.C.; Tchigvintsev, D.; et al. The environment shapes microbial enzymes: Five cold-active and salt-resistant carboxylesterases from marine metagenomes. Appl. Microbiol. Biol. 2015, 99, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Arnórsdottir, J.; Smáradóttir, R.B.; Magnússon, O.T.; Thorbjarnardóttir, S.H.; Eggertsson, G.; Kristjánsson, M.M. Characterization of a cloned subtilisin-like serine proteinase from a psychrotrophic Vibrio species. Eur. J. Biochem. 2002, 269, 5536–5546. [Google Scholar] [CrossRef]
- Oskarsson, K.R.; Kristjansson, M.M. Improved expression, purification and characterization of VPR, a cold active subtilisin-like serine proteinase and the effects of calcium on expression and stability. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 152–162. [Google Scholar] [CrossRef]
- Lario, L.D.; Pillaca-Pullo, O.S.; Duraes, S.L.; Converti, A.; Casati, P.; Spampinato, C.; Pessoa, A. Optimization of protease production and sequence analysis of the purified enzyme from the cold adapted yeast Rhodotorula mucilaginosa CBMAI 1528. Biotechnol. Rep. 2020, 28, e546. [Google Scholar] [CrossRef]
- Farooq, S.; Nazir, R.; Ganai, S.A.; Ganai, B.A. Isolation and characterization of a new cold-active protease from psychrotrophic bacteria of Western Himalayan glacial soil. Sci. Rep. 2021, 11, 12768. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Karbancioglu-Guler, F.; Ozcelik, B. Cold active pectinase, amylase and protease production by yeast isolates obtained from environmental samples. Extremophiles 2018, 22, 599–606. [Google Scholar] [CrossRef]
- Perfumo, A.; Freiherr, V.S.G.; Nordmann, E.L.; Budisa, N.; Wagner, D. Discovery and Characterization of a New Cold-Active Protease From an Extremophilic Bacterium via Comparative Genome Analysis and in vitro Expression. Front. Microbiol. 2020, 11, 881. [Google Scholar] [CrossRef]
- Mageswari, A.; Subramanian, P.; Chandrasekaran, S.; Karthikeyan, S.; Gothandam, K.M. Systematic functional analysis and application of a cold-active serine protease from a novel Chryseobacterium sp. Food Biochem. 2017, 217, 18–27. [Google Scholar] [CrossRef]
- Singh, D.; Thakur, S.; Thayil, S.M.; Kesavan, A.K. Characterization of a cold-active, detergent-stable metallopeptidase purified from Bacillus sp. S1DI 10 using Response Surface Methodology. PLoS ONE. 2019, 14, e216990. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Tian, Y.; Hou, Y.H.; Wang, T.H. Purification and characterization of the cold-active alkaline protease from marine cold-adaptive Penicillium chrysogenum FS010. Mol. Biol. Rep. 2009, 36, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.F.; Miao, J.L.; Hou, Y.H.; Ding, Y.; Wang, G.D.; Li, G.Y. Purification and characterization of an extracellular cold-active serine protease from the psychrophilic bacterium Colwellia sp. NJ341. Biotechnol. Lett. 2005, 27, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Mo, Q.; Liu, H.; Yuan, F.; Chai, H.; Lu, F.; Zhang, H. Identification and characterization of a novel cold-tolerant extracellular protease from Planococcus sp. CGMCC 8088. Extrenophiles 2018, 22, 473–484. [Google Scholar] [CrossRef]
- Damare, C.; Raghukuma, C.; Muraleedharan, U.D.; Raghukumar, S. Deep-sea fungi as a source of alkaline and cold-tolerant proteases. Enzyme Microb. Technol. 2006, 39, 172–181. [Google Scholar] [CrossRef]
- Joshi, G.K.; Kumar, S.; Sharma, V. Production of moderately halotolerant, SDS stable alkaline protease from Bacillus cereus MTCC 6840 isolated from lake Nainital, Uttaranchal state, India. Braz. J. Microbiol. 2007, 38, 773–779. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, R.; Zhao, J.; Lin, N. Cold-active serine alkaline protease from the psychrophilic bacterium Pseudomonas strain DY-A: Enzyme purification and characterization. Extremophiles 2003, 7, 335–337. [Google Scholar] [CrossRef]
- Morita, Y.; Kondoh, K.; Hasan, Q.; Sakaguchi, T.; Murakami, Y.; Yokoyama, K.; Tamiya, E. Purification and characterization of a cold-active protease from psychrotrophic Serratia marcescens AP3801. J. Am. Oil Chem. Soc. 1997, 74, 1377–1383. [Google Scholar] [CrossRef]
- Raymond-Bouchard, I.; Goordial, J.; Zolotarov, Y.; Ronholm, J.; Stromvik, M.; Bakermans, C.; Whyte, L.G. Conserved genomic and amino acid traits of cold adaptation in subzero-growing Arctic permafrost bacteria. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, S.M.; Choi, J.I. Purification, Characterization, and Cloning of a Cold-Adapted Protease from Antarctic Janthinobacterium lividum. J. Microbiol. Biotechn. 2018, 28, 448–453. [Google Scholar] [CrossRef]
- Kuddus, M.; Ramteke, P.W. A cold-active extracellular metalloprotease from Curtobacterium luteum (MTCC 7529): Enzyme production and characterization. J. Gen. Appl. Microbiol. 2008, 54, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.Q.; Ambrosini, A.; Passaglia, L.M.P.; Brandelli, A. A new coldadapted serine peptidase from Antarctic Lysobacter sp. A03: Insights about enzyme activity at low temperatures. Int. J. Biol. Macromol. 2017, 103, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Secades, P.; Alvarez, B.; Guijarro, J.A. Purification and properties of a new psychrophilic metalloprotease (Fpp2) in the fish pathogen Flavobacterium psychrophilum. FEMS Microbiol. Lett. 2003, 226, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, M.; Pazgier, M.; Kalinowska, H.; Bielecki, S. A cold-adapted extracellular serine proteinase of the yeast Leucosporidium antarcticum. Extremophiles 2003, 7, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, S.M.; Dhakephalkar, P. Diversity, cold active enzymes and adaptation strategies of bacteria inhabiting glacier cryoconite holes of High Arctic. Extremophiles 2014, 18, 229–242. [Google Scholar] [CrossRef]
- Yang, C.; Wang, F.; Hao, J.; Zhang, K.; Yuan, N.; Sun, M. Identification of a proteolytic bacterium, HW08, and characterization of its extracellular coldactive alkaline metalloprotease Ps5. Biosci. Biotechnol. Biochem. 2010, 74, 1220–1225. [Google Scholar] [CrossRef]
- Suzuki, S.; Odagami, T. Low-temperature-active thiol protease from marine bacterium Alteromonas haloplanktis. J. Biotechnol. 1997, 5, 230–233. [Google Scholar]
- Shi, J.S.; Wu, Q.F.; Xu, Z.H.; Tao, W.Y. Identification of psychrotrophs SYP-A2-3 producing cold-adapted protease from the No. 1 Glacier of China and study on its fermentation conditions. Wei Sheng Wu Xue Bao 2005, 45, 258–263. [Google Scholar]
- Park, H.J.; Lee, C.W.; Kim, D.; Do, H.; Han, S.J.; Kim, J.E.; Koo, B.H.; Lee, J.H.; Yim, J.H. Crystal structure of a cold-active protease (Pro21717) from the psychrophilic bacterium, Pseudoalteromonas arctica PAMC 21717, at 1.4 A resolution: Structural adaptations to cold and functional analysis of a laundry detergent enzyme. PLoS ONE 2018, 13, e191740. [Google Scholar] [CrossRef]
- Ray, M.K.; Devi, K.U.; Kumar, G.S.; Shivaji, S. Extracellular protease from the antarctic yeast Candida humicola. Appl. Environ. Microbiol. 1992, 58, 1918–1923. [Google Scholar] [CrossRef]
- Nakajima, M.; Mizusawa, K.; Yoshida, F. Purification and properties of an extracellular proteinase of psychrophilic Escherichia freundii. Eur. J. Biochem. 1974, 44, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Dieplinger, H.; Hofmann, J.; Sarg, B.; Lindner, H. A cold-active extracellular metalloprotease from Pedobacter cryoconitis-production and properties. Res. Microbiol. 2005, 156, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Honig, B.; Nicholls, A. Classical electrostatics in biology and chemistry. Science 1995, 268, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Saba, I.; Qazi, P.H.; Rather, S.A.; Dar, R.A.; Qadri, Q.A.; Ahmad, N.; Johri, S.; Taneja, S.C.; Shawl, S. Purification and characterization of a cold active alkaline protease from Stenotrophomonas sp. isolated from Kashmir, India. World J. Microb. Biot. 2012, 28, 1071–1079. [Google Scholar] [CrossRef]
- Turkiewicz, M.; Gromek, E.; Kalinowska, H.; Zielinska, M. Biosynthesis and properties of an extracellular metalloprotease from the Antarctic marine bacterium Sphingomonas paucimobilis. J. Biotechnol. 1999, 70, 53–60. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Mai, Z.; Tian, X.; Zhang, S. Purification, characterization, and gene cloning of a cold-adapted thermolysin-like protease from Halobacillus sp. SCSIO 20089. J. Biosci. Bioeng. 2013, 115, 628–632. [Google Scholar] [CrossRef]
- Szilagyi, A.; Zavodszky, P. Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: Results of a comprehensive survey. Structure 2000, 8, 493–504. [Google Scholar] [CrossRef]
- Haney, P.J.; Badger, J.H.; Buldak, G.L.; Reich, C.I.; Woese, C.R.; Olsen, G.J. Thermal adaptation analyzed by comparison of protein sequences from mesophilic and extremely thermophilic Methanococcus species. Proc. Natl. Acad. Sci. USA 1999, 96, 3578–3583. [Google Scholar] [CrossRef]
- Bialkowska, A.M.; Morawski, K.; Florczak, T. Extremophilic proteases as novel and efficient tools in short peptide synthesis. J. Ind. Microbiol. Biol. 2017, 44, 1325–1342. [Google Scholar] [CrossRef]
- Aghajari, N.; Feller, G.; Gerday, C.; Haser, R. Structures of the psychrophilic Alteromonas haloplanctis alpha-amylase give insights into cold adaptation at a molecular level. Structure 1998, 6, 1503–1516. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hwang, K.Y.; Kim, S.H.; Sung, H.C.; Han, Y.S.; Cho, Y. Structural basis for cold adaptation. Sequence, biochemical properties, and crystal structure of malate dehydrogenase from a psychrophile Aquaspirillium arcticum. J. Biol. Chem. 1999, 274, 11761–11767. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.J.; Gerike, U.; Danson, M.J.; Hough, D.W.; Taylor, G.L. Structural adaptations of the cold-active citrate synthase from an Antarctic bacterium. Structure 1998, 6, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Arnorsdottir, J.; Kristjansson, M.M.; Ficner, R. Crystal structure of a subtilisin-like serine proteinase from a psychrotrophic Vibrio species reveals structural aspects of cold adaptation. FEBS J. 2005, 272, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Wintrode, P.L.; Grayling, R.A.; Rubingh, D.N.; Arnold, F.H. Directed evolution study of temperature adaptation in a psychrophilic enzyme. J. Mol. Biol. 2000, 297, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Isaksen, G.V.; Aqvist, J.; Brandsdal, B.O. Protein surface softness is the origin of enzyme cold-adaptation of trypsin. PLoS Comput. Biol. 2014, 10, e1003813. [Google Scholar] [CrossRef]
- Isaksen, G.V.; Aqvist, J.; Brandsdal, B.O. Enzyme surface rigidity tunes the temperature dependence of catalytic rates. Proc. Natl. Acad. Sci. USA 2016, 113, 7822–7827. [Google Scholar] [CrossRef]
- Matthews, B.W. Solvent content of protein crystals. J. Mol. Biol. 1968, 33, 491–497. [Google Scholar] [CrossRef]
- Yu, H.-R.; Yan, Y.-H.; Zhang, C.; Dalby, P.A. Two strategies to engineer flexible loops for improved enzyme thermostability. Sci. Rep. 2017, 7, 41212. [Google Scholar] [CrossRef]
- Pantoliano, M.W.; Whitlow, M.; Wood, J.F.; Dodd, S.W.; Hardman, K.D.; Rollence, M.L.; Bryan, P.N. Large increases in general stability for subtilisin BPN’ through incremental changes in the free energy of unfolding. Biochemistry 1989, 28, 7205–7213. [Google Scholar] [CrossRef]
- Strausberg, S.L.; Alexander, P.A.; Gallagher, D.T.; Gilliland, G.L.; Barnett, B.L.; Bryan, P.N. Directed evolution of a subtilisin with calcium-independent stability. Biotechnology 1995, 13, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Narinx, E.; Baise, E.; Gerday, C. Subtilisin from psychrophilic antarctic bacteria: Characterization and site-directed mutagenesis of residues possibly involved in the adaptation to cold. Protein Eng. 1997, 10, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Sigurdardottir, A.G.; Arnorsdottir, J.; Thorbjarnardottir, S.H.; Eggertsson, G.; Suhre, K.; Kristjansson, M.M. Characteristics of mutants designed to incorporate a new ion pair into the structure of a cold adapted subtilisin-like serine proteinase. Biochim. Biophys. Acta 2009, 1794, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, X.L.; Shun, C.Y.; He, H.L.; Zhang, Y.Z. Stabilization of cold-adapted protease MCP-01 promoted by trehalose: Prevention of the autolysis. Protein Pept. Lett. 2005, 12, 375–378. [Google Scholar] [CrossRef] [PubMed]
- He, H.L.; Chen, X.L.; Zhang, X.Y.; Sun, C.Y.; Zou, B.C.; Zhang, Y.Z. Novel use for the osmolyte trimethylamine N-oxide: Retaining the psychrophilic characters of cold-adapted protease deseasin MCP-01 and simultaneously improving its thermostability. Mar. Biotechnol. 2009, 11, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Tu, T.; Zheng, J.; Bai, Y.-G.; Huang, H.-Q.; Su, X.-Y.; Wang, Y.; Wang, Y.-R.; Yao, B.; Luo, H.-Y. Improvement of BsAPA aspartic protease thermostability via autocatalysis-resistant mutation. J. Agric. Food Chem. 2019, 67, 10505–10512. [Google Scholar] [CrossRef]
- Dube, S.; Singh, L.; Alam, S.I. Proteolytic anaerobic bacteria from lake sediments of Antarctica. Enzyme Microb. Technol. 2001, 28, 114–121. [Google Scholar] [CrossRef]
- Vazquez, S.C.; Mac, C.W.; Rios, M.L.; Fraile, E.R. Factors influencing protease production by two Antarctic strains of Stenotrophomonas maltophilia. Rev. Argent. Microbiol. 2000, 32, 53–62. [Google Scholar]
- Han, S.J.; Park, H.; Kim, S.; Kim, D.; Park, H.J.; Yim, J.H. Enhanced production of protease by Pseudoalteromonas arctica PAMC 21717 via statistical optimization of mineral components and fed-batch fermentation. Prep. Biochem. Biotechnol. 2016, 46, 328–335. [Google Scholar]
- Bialkowska, A.M.; Krysiak, J.; Florczak, T.; Szulczewska, K.M.; Wanarska, M.; Turkiewicz, M. The psychrotrophic yeast Sporobolomyces roseus LOCK 1119 as a source of a highly active aspartic protease for the in vitro production of antioxidant peptides. Biotechnol. Appl. Biochem. 2018, 65, 726–738. [Google Scholar] [CrossRef]
- Macouzet, M.; Simpson, B.K.; Lee, B.H. Expression of a cold-adapted fish trypsin in Pichia pastoris. FEMS Yeast Res. 2005, 5, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, G.; Bjarnason, J.B.; Gudmundsdottir, A. Recombinant cold-adapted trypsin I from Atlantic cod-expression, purification, and identification. Protein Expres. Purif. 2004, 33, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Palsdottir, H.M.; Gudmundsdóttir, A. Expression and purification of a cold-adapted group III trypsin in Escherichia coli. Protein Expres. Purif. 2007, 51, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kurata, A.; Uchimura, K.; Shimamura, S.; Kobayashi, T.; Horikoshi, K. Nucleotide and deduced amino acid sequences of a subtilisin-like serine protease from a deep-sea bacterium, Alkalimonas collagenimarina AC40(T). Appl. MicrobiolI. Biol. 2007, 77, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, L.; Roda, S.; Gonzalez-Alfonso, J.L.; Plou, F.J.; Guallar, V.; Ferrer, M. Design and Characterization of In-One Protease-Esterase PluriZyme. Int. J. Mol. Sci. 2022, 23, 13337. [Google Scholar] [CrossRef]
- Berg, C.H.; Kalfas, S.; Malmsten, M.; Arnebrant, T. Proteolytic degradation of oral biofilms in vitro and in vivo: Potential of proteases originating from Euphausia superba for plaque control. Eur. J. Oral. Sci. 2001, 109, 316–324. [Google Scholar] [CrossRef]
- Potter, A.S.; Steven, P.S. Dissociation of Tissues for Single-Cell Analysis. Methods Mol. Biol. 2019, 1926, 55–62. [Google Scholar]
- Lee, S.G.; Koh, H.Y.; Lee, H.K.; Yim, J.H. Possible roles of Antarctic krill proteases for skin regeneration. Ocean Polar Res. 2008, 30, 467–472. [Google Scholar] [CrossRef]
- Mekkes, J.R.; Le Poole, I.C.; Das, P.K.; Bos, J.D.; Westerhof, W. Efficient debridement of necrotic wounds using proteolytic enzymes derived from Antarctic krill: A double-blind, placebo- controlled study in a standardized animal wound model. Wound Repair. Regen. 1998, 6, 50–57. [Google Scholar] [CrossRef]
- Hellgren, K. Krill enzymes (Krillase) an important factor to improve oral hygiene. In Oral Health Care-Pediatric, Research, Epidemiology and Clinical Practice; Virdi, M.S., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- ColdZyme [Product Information]; Enzymatica AB: Lund, Sweden, 2011.
- O’Flanagan, C.H.; Campbell, K.R.; Zhang, A.W.; Kabeer, F.; Lim, J.; Biele, J.; Eirew, P.; Lai, D.; McPherson, A.; Kong, E.; et al. Dissociation of solid tumor tissues with cold active protease for single-cell RNA-seq minimizes conserved collagenase-associated stress responses. Genome Biol. 2019, 20, 210. [Google Scholar] [CrossRef]
- Kasana, R.C. Proteases from psychrotrophs: An overview. Crit. Rev. Microbiol. 2010, 36, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.S.; Soria, M.; Rojas, A.M.; Fissore, E.N.; Gerschenson, L.N. Protease and Hemicellulase Assisted Extraction of Dietary Fiber from Wastes of Cynara cardunculus. Int. J. Mol. Sci. 2015, 16, 6057–6075. [Google Scholar] [CrossRef] [PubMed]
- Karamac, M.; Kosinska-Cagnazzo, A.; Kulczyk, A. Use of Different Proteases to Obtain Flaxseed Protein Hydrolysates with Antioxidant Activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef] [PubMed]
- Dvoryakova, E.A.; Klimova, M.A.; Simonyan, T.R.; Dombrovsky, I.A.; Serebryakova, M.V.; Tereshchenkova, V.F.; Dunaevsky, Y.E.; Belozersky, M.A.; Filippova, I.Y.; Elpidina, E.N. Recombinant Cathepsin L of Tribolium castaneum and Its Potential in the Hydrolysis of Immunogenic Gliadin Peptides. Int. J. Mol. Sci. 2022, 23, 7001. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts. 2018, 8, 238. [Google Scholar] [CrossRef]
- Baweja, M.; Nain, L.; Kawarabayasi, Y.; Shukla, P. Current Technological Improvements in Enzymes toward Their Biotechnological Applications. Front. Microbiol. 2016, 7, 965. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Joseph, B. Current prospective in using cold-active enzymes as eco-friendly detergent additive. Appl. Microbiol. Biol. 2020, 104, 2871–2882. [Google Scholar] [CrossRef]
- Joshi, S.; Satyanarayana, T. Biotechnology of cold-active proteases. Biology 2013, 2, 755–783. [Google Scholar] [CrossRef]
- Baghel, V.S.; Tripathi, R.D.; Ramteke, R.W.; Gopal, K.; Dwivedi, S.; Jain, R.K.; Rai, U.N.; Singh, S.N. Psychrotrophic proteolytic bacteria from cold environments of Gangotri glacier, Westren Himalaya India. Enzyme Microb. Technol. 2005, 36, 654–659. [Google Scholar] [CrossRef]
- De Gobba, C.; Tompa, G.; Otte, J. Bioactive peptides from caseins released by cold active proteolytic enzymes from Arsukibacterium ikkense. Food Chem. 2014, 165, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Zambare, V.; Nilegaonkar, S.; Kanekar, P. A novel extracellular protease from Pseudomonas aeruginosa MCMB-327: Enzyme production and its partial characterization. New Biotechnol. 2011, 28, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Cho, J. Production of an extracellular protease by an Antarctic bacterial isolate (Bacillus sp. JSP1) as a potential feed additive. Rev. Colom Cienc. Pecua. 2011, 24, 11–18. [Google Scholar]
| Source/Protease | Temp Optima (°C) | pH Optima | Molecular Weight (kDa) | Inhibitors | Activators | Refs. |
|---|---|---|---|---|---|---|
| Alteromonas haloplanktis | 20 | 8.0–9.0 | 74–76 | Leupeptin, PCMB | - | [57] |
| Aspergillus ustus | 45 | 9.0 | 32 | PMSF, DFP, Cu2+ | - | [45] |
| Bacillus subtilis PAMC 26541 | 40 | 7.0–7.5 | 107 | PMSF | K+, Na+ | [50] |
| Bacillus sp. S1DI 10 | 10 | 8 | 40 | EDTA, PMSF, Leucopeptin | Fe2+, Mn2+, Co2+, Twain 80 | [41] |
| Bacillus subtilis WLCP1 | 15 | 10 | 38 | PMSF | Ca2+, Cu2+ | [49] |
| Bacillus cereus | 20 | 9.0 | - | EDTA, PMSF, Ca2+, Cu2+, K+ | Co2+, Fe2+ | [46] |
| Bacillus cereus | 42 | 7.0–8.5 | 34.2 | Mg2+, Mn2+ | - | [58] |
| Curtobacterium luteum | 20 | 7 | 115 | EDTA, EGTA | Zn2+, Cr2+ | [51] |
| Colwellia sp. NJ341 | 35 | 8.0–9.0 | 60 | PMSF, Fe2+ | - | [43] |
| Colwellia psychrerythraea | 19 | 6–8.5 | 71 | EDTA, Zn2+, Mn2+ | Na+, Mg2+ | [55] |
| Candida humicola | 37 | 1–1.2 | 36 | - | - | [60] |
| Chryseobacterium sp. IMDY | 10 | 7.0–8.0 | 27 | PMSF, Hg2+, Zn2+ | Na+, Ca2+ | [40] |
| Escherichia freundii | 25 | 10.0 | 55 | Iodoacetamide, SDS | - | [61] |
| Flavobacterium psychrophilum | 24 | 6.0–7.0 | 62 | Benzalkonium chloride, Na+, Phenanthroline, | - | [53] |
| Pseudomonas lundensis | 40 | 10.4 | 46 | EDTA, PMSF Cu2+, Zn2+, Hg2+, EDTA, SDS | Mg2+, Ca2+ | [56] |
| Halobillus sp. SCSIO 20089 | 30 | 8 | 35 | EDTA | Ca2+, Mg2+, Mn2+ | [55] |
| Leucosporidium antarcticum | 25 | 6.7–7.1 | 34.4 | - | - | [54] |
| Lysobacter sp. | 40 | 9.0 | 35 | PMSF, EDTA, Zn2+ | Ca2+, Mg2+, Ba2+, Na+, NH4+, isopropyl alcohol | [52] |
| Penicillium chrysogenum FS010 | 35 | 9 | 41 | PMSF, DFP, Cu2+, Co2+ | Mg2+, Ca2+ | [42] |
| Psychrobacter sp. 94–6 PB | 30 | 9 | 80 | - | - | [39] |
| Planococcus sp. M7 | 35 | 10 | 43 | PMSF, TNBS, EDAC, EDTA, Cu2+, Ni2+ | Fe3+, Ca2+ | [44] |
| Pseudomonas DY | 40 | 10 | 25 | PMSF, DFP, AEBSF | Ca2+, Mg2+ | [43] |
| Pseudoalteromonas arctica PAMC 21717 | 30 | 9 | 37 | LAS, SDS | Ca2+ | [59] |
| Pedobacter cryoconitis | 40 | 8.0 | 27 | - | - | [62] |
| Pseudoaltermonas. sp. | 30 | 8.0 | 47 |
PMSF, Chymostatin, Trypsin | - | [63] |
| Pseudomonas strain DY-A | 40 | 10.0 | - | EDTA, EGTA, SDS | - | [47] |
| Stenotrophomonas IIIM-ST045 | 15 | 10 | 55 | Zn2+, Cu2+, Co2+ | Mg2+, Mn2+, Ca2+ | [64] |
| Serratia marcescens | 40 | 6.5–8.0 | 58 | Phenanthroline, PSFM, EGTA, DTT | - | [48] |
| Sphingomonas paucimobilis | 20–30 | 6.5–7.0 | - | DFP, PMSF, AEBSF | - | [65] |
| Trichoderma Atroviride | 25 | 6.2 | 24 | SDS, Urea | - | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Huang, Z.; Wu, Q.; Tang, X.; Huang, Z. Cold-Adapted Proteases: An Efficient and Energy-Saving Biocatalyst. Int. J. Mol. Sci. 2023, 24, 8532. https://doi.org/10.3390/ijms24108532
Yang Z, Huang Z, Wu Q, Tang X, Huang Z. Cold-Adapted Proteases: An Efficient and Energy-Saving Biocatalyst. International Journal of Molecular Sciences. 2023; 24(10):8532. https://doi.org/10.3390/ijms24108532
Chicago/Turabian StyleYang, Zhengfeng, Zhendi Huang, Qian Wu, Xianghua Tang, and Zunxi Huang. 2023. "Cold-Adapted Proteases: An Efficient and Energy-Saving Biocatalyst" International Journal of Molecular Sciences 24, no. 10: 8532. https://doi.org/10.3390/ijms24108532
APA StyleYang, Z., Huang, Z., Wu, Q., Tang, X., & Huang, Z. (2023). Cold-Adapted Proteases: An Efficient and Energy-Saving Biocatalyst. International Journal of Molecular Sciences, 24(10), 8532. https://doi.org/10.3390/ijms24108532




